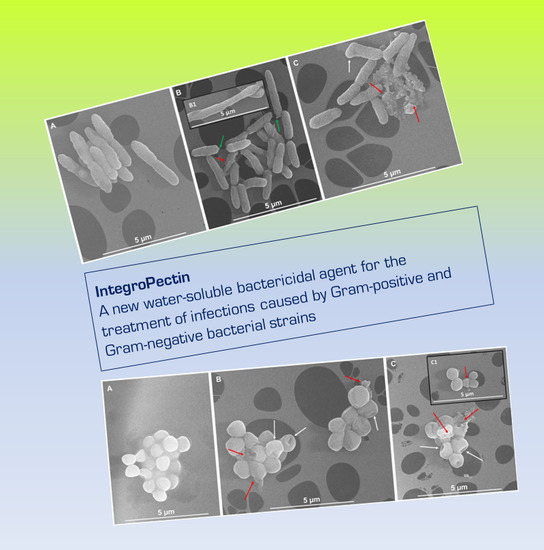
A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains
Abstract
1. Introduction
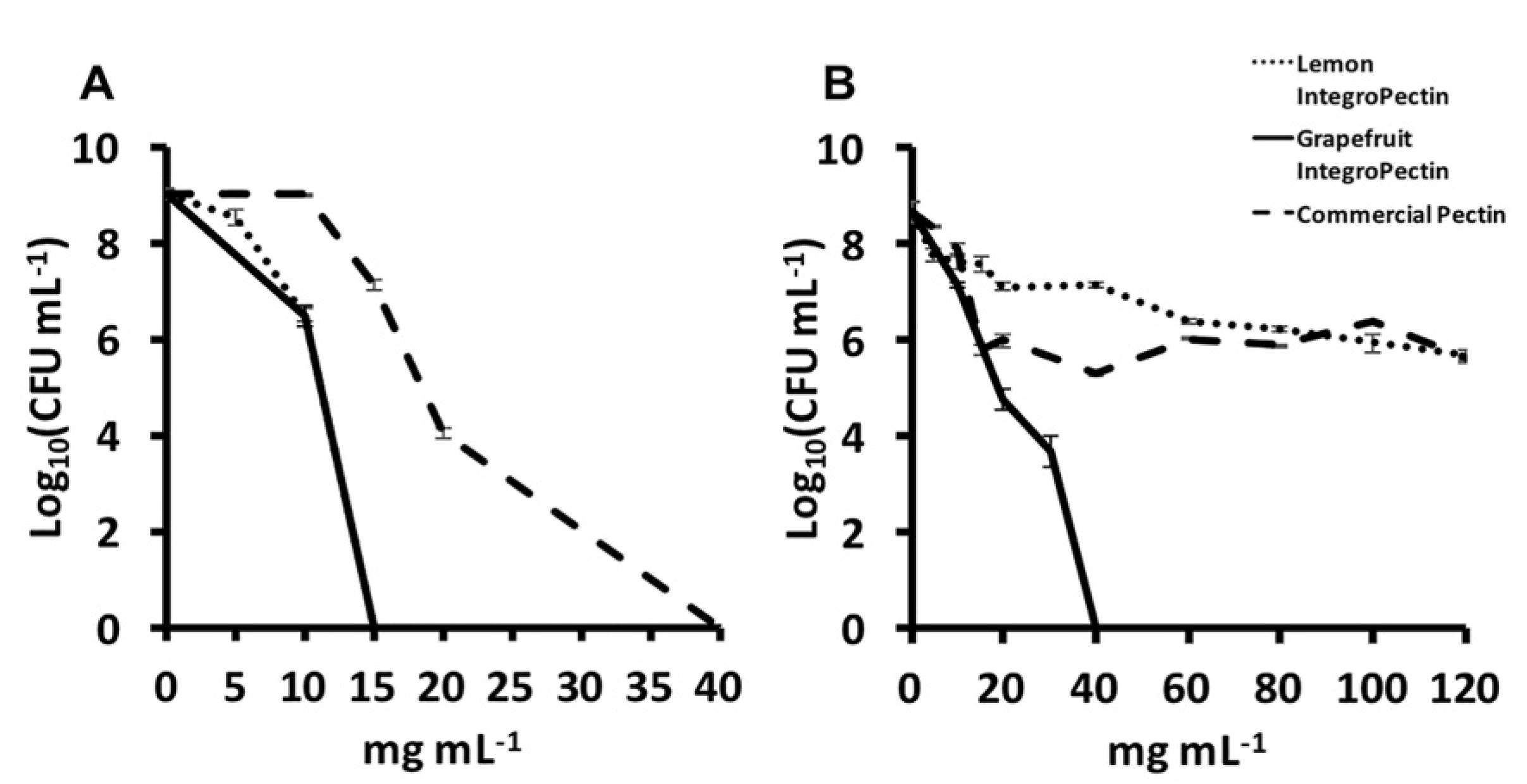
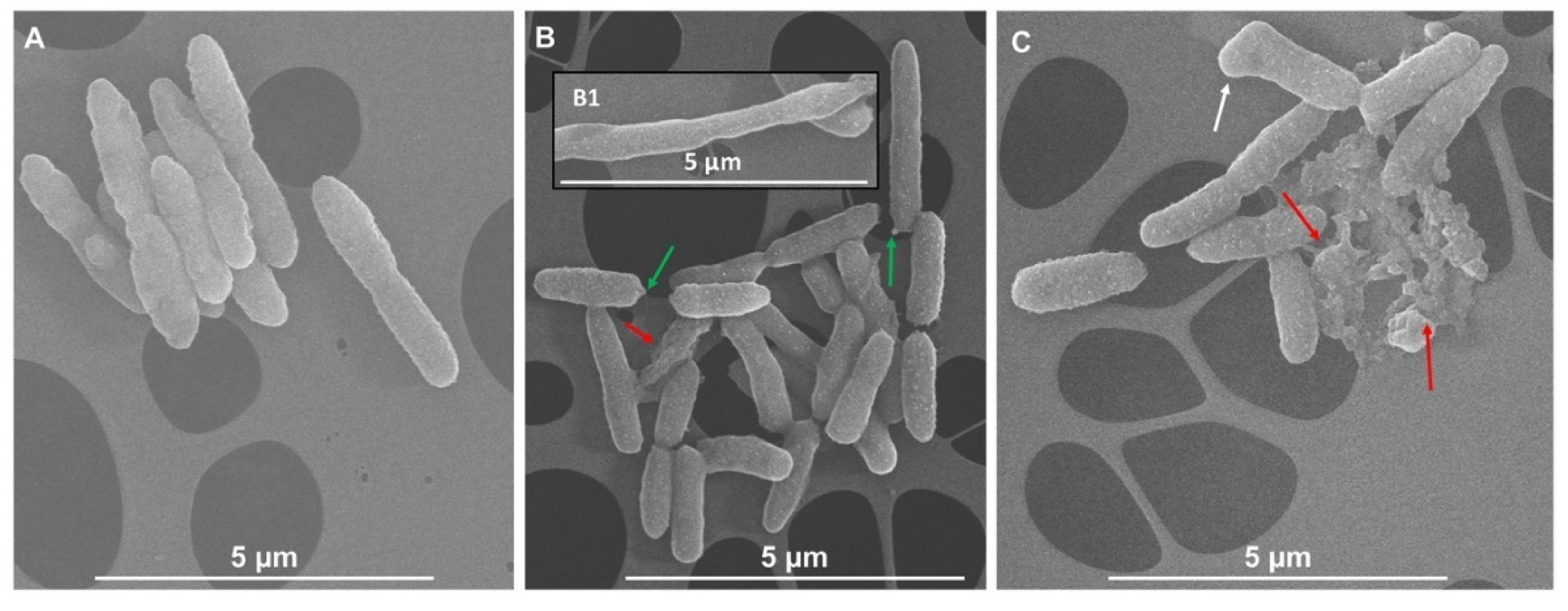
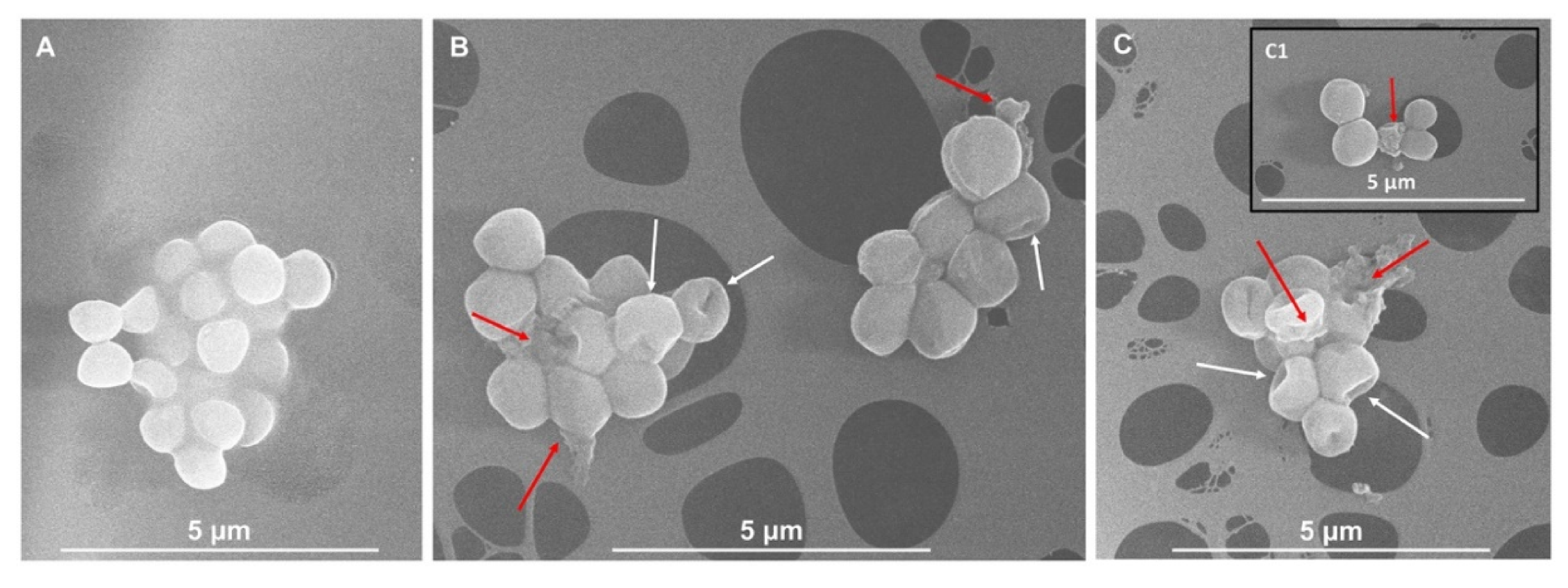
2. Results and Discussion
3. Materials and Methods
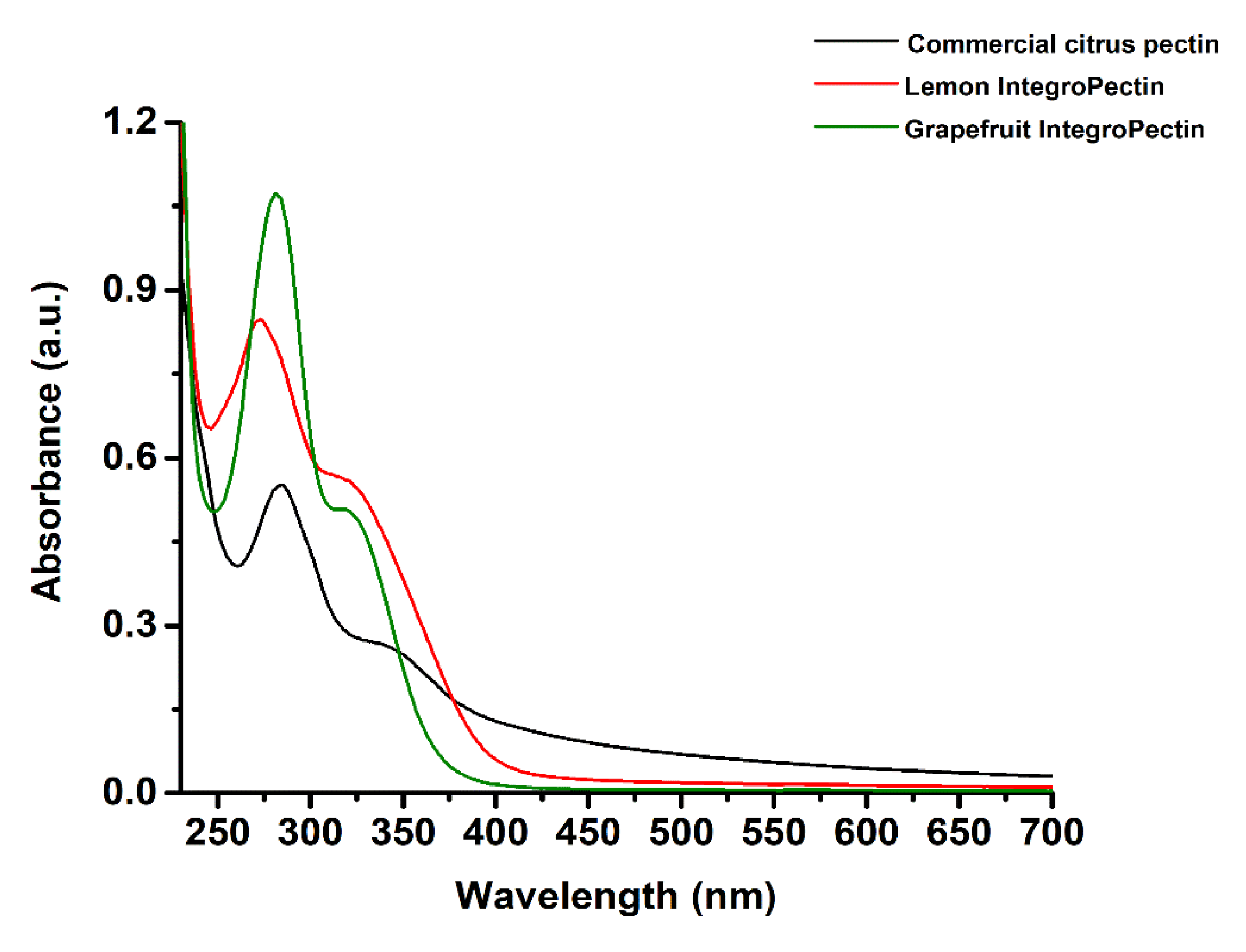
3.1. UV-visible and Fourier Transform Infrared (FTIR) Spectroscopies of Commercial Citrus Pectin, Lemon, and Grapefruit IntegroPectins
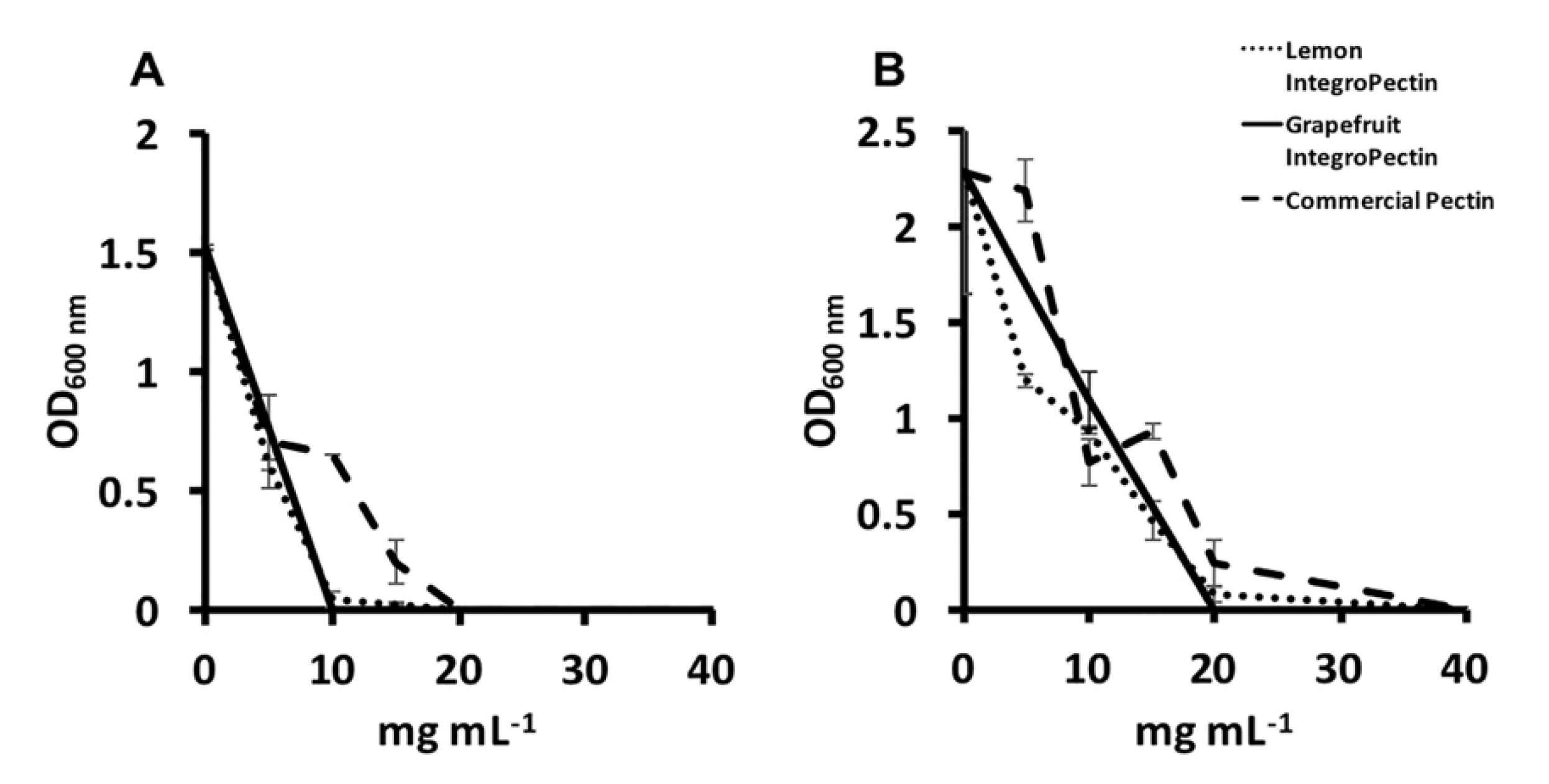
3.2. MBC and MIC Evaluation of Lemon and Grapefruit IntegroPectin
3.3. Scanning Electron Microscopy (SEM) Imaging of Bacterial Cells Exposed to IntegroPectins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AMR Industry Alliance. 2020 Progress Report. January 2020. Available online: https://www.amrindustryalliance.org/wp-content/uploads/2020/01/AMR-2020-Progress-Report.pdf (accessed on 27 August 2020).
- Dadgostra, P. Antimicrobial resistance: Implication and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Wellcome Trust. Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 27 August 2020).
- The World Bank. By 2050, Drug-Resistant Infections Could Cause Global Economic Damage on Par with 2008 Financial Crisis. September 2016. Available online: https://www.worldbank.org/en/news/press-release/2016/09/18/by-2050-drug-resistant-infections-could-cause-global-economic-damage-on-par-with-2008-financial-crisis (accessed on 27 August 2020).
- Haynes, E.; Tompkins, C.A.; Washburn, G.; Winters, M. Bactericidal action of pectin. Exp. Boil. Med. 1937, 36, 839–840. [Google Scholar] [CrossRef]
- Men’Shikov, D.D.; Lazareva, E.B.; Popova, T.S.; Shramko, L.U.; Tokaev, I.S.; Zalogueva, G.V.; Gaponova, I.N. Antimicrobial properties of pectins and their effects on antibiotics. Antibiot. Khimioterapiia Antibiot. Chemoterapy 1997, 42, 10–15. [Google Scholar]
- Noreen, A.; Nazli, Z.-I.-H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int. J. Boil. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Krayushkina, A.V.; Mironova, L.; Zakirova, Y.M.; Miluykov, V.A. Biological activity and pharmacological application of pectic polysaccharides: A review. Carbohydr. Polym. 2018, 10, 1407. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Carnaroglio, D.; Tamburino, A.; Cravotto, G.; Grillo, G.; Ilharco, L.M.; Pagliaro, M. Eco-friendly extraction of pectin and essential oils from orange and lemon peels. ACS Sustain. Chem. Eng. 2016, 4, 2243–2251. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; DeLisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Nascimento, L.B.D.S.; De Carlo, A.; et al. Real-scale integral valorization of waste orange peel via hydrodynamic cavitation. Process 2019, 7, 581. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior antibacterial activity of integral lemon pectin extracted via hydrodynamic cavitation. ChemistryOpen 2020, 9, 628–630. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Jr, V.G.F. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Vitale, M.; Gaglio, S.; Galluzzo, P.; Cascone, G.; Piraino, C.; Presti, V.D.M.L.; Alduina, R. Antibiotic resistance profiling, analysis of virulence aspects and molecular genotyping of Staphylococcus aureus isolated in Sicily, Italy. Foodborne Pathog. Dis. 2017, 15, 177–185. [Google Scholar] [CrossRef]
- Vitale, M.; Galluzzo, P.; Buffa, P.G.; Carlino, E.; Spezia, O.; Alduina, R. Comparison of antibiotic resistance profile and biofilm production of Staphylococcus aureus isolates derived from human specimens and animal-derived samples. Antibiotics 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the ability of Perfluorohexane Sulfonate (PFHxS) Bioaccumulation by two pseudomonas sp. strains isolated from PFAS-contaminated environmental matrices. Microorganism 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, H.; McMullan, R.; Filloux, A. The pseudomonas aeruginosa reference strain PA14 displays increased virulence due to a mutation in ladS. PLoS ONE 2011, 6, e29113. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Hayes, D.; Wozniak, D.J. Cystic fibrosis and Pseudomonas aeruginosa: The host-microbe interface. Clin. Microbiol. Rev. 2019, 32, e00138-18. [Google Scholar] [CrossRef]
- Ferreiro, J.L.L.; Otero, J.Á.; González, L.G.; Lamazares, L.N.; Blanco, A.A.; Sanjurjo, J.R.B.; Conde, I.R.; Soneira, M.F.; Aguado, J.D.L.F. Pseudomonas aeruginosa urinary tract infections in hospitalized patients: Mortality and prognostic factors. PLoS ONE 2017, 12, e0178178. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guéry, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 1–18. [Google Scholar] [CrossRef]
- Bassetti, M.; Carnelutti, A.; Castaldo, N.; Peghin, M. Important new therapies for methicillin-resistant Staphylococcus aureus. Expert Opin. Pharmacother. 2019, 20, 2317–2334. [Google Scholar] [CrossRef]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is Caretta Caretta a carrier of antibiotic resistance in the Mediterranean sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef]
- Blasi, M.; Migliore, L.; Mattei, D.; Rotini, A.; Thaller, M.; Alduina, R. Antibiotic resistance of gram-negative bacteria from wild captured loggerhead sea turtles. Antibiotics 2020, 9, 162. [Google Scholar] [CrossRef]
- Nisly, S.A.; McClain, D.L.; Fillius, A.G.; Davis, K. Oral antibiotics for the treatment of Gram-negative bloodstream infections: A retrospective comparison of three antibiotic classes. J. Glob. Antimicrob. Resist. 2020, 20, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovic, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Petrus, D.R.; Dougherty, M.H. Spectrophotometric analyses of orange juices and corresponding orange pulp washes. J. Food Sci. 1973, 38, 913–914. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Du Toit, W. The role of UV-visible spectroscopy for phenolic compounds quantification in winemaking. In Frontiers and New Trends in the Size of Fermented Food and Beverages; Solis-Oviedo, R.L., De La Cruz Pech-Canul, A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Cho, C. Concentration and purification of soluble pectin from mandarin peels using crossflow microfiltration system. Carbohydr. Polym. 2003, 54, 21–26. [Google Scholar] [CrossRef]
- Murkovic, M. Phenolic compounds. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L., Finglas, P.M., Eds.; Academic Press: London, UK, 2003; pp. 4507–4514. [Google Scholar]
- Heredia-Guerrero, J.A.; Benitez, J.J.; Dominguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A.; Benitez, J.J.; Domínguez, E. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Boil. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analyses of the degree of methyl esterification of pectin in different fruit and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- La Cava, E.L.; Gerbino, E.; Sgroppo, S.C.; Gomez-Zavaglia, A. Characterization of pectins extracted from different varieties of pink/red and white grapefruits [Citrus Paradisi (Macf.)] by thermal treatment and thermosonication. J. Food Sci. 2018, 83, 1613–1621. [Google Scholar] [CrossRef]
- Lóránd, T.; Deli, J.; Molnár, P.; Toth, G. FT-IR study of some carotenoids. Helvetica Chim. Acta 2002, 85, 1691–1697. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fuorier transform infrared (FT-IR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Aburto, J.; Moran, M.; Galano, A.; Torres-García, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Bichara, L.C.; Alvarez, P.E.; Bimbi, M.V.F.; Vaca, H.; Gervasi, C.; Brandán, S.A. Structural and spectroscopic study of a pectin isolated from citrus peel by using FTIR and FT-Raman spectra and DFT calculations. Infrared Phys. Technol. 2016, 76, 315–327. [Google Scholar] [CrossRef]
- Zeier, J.; Schreiber, L. Fourier transform infrared-spectroscopic characterization of isolated endodermal cell walls from plant roots: Chemical nature in relation to anatomical development. Planta 1999, 209, 537–542. [Google Scholar] [CrossRef]
- Rashid, T.; Kait, C.F.; Murugesan, T. A fourier transformed infrared compound study of lignin recovered from a formic acid process. Procedia Eng. 2016, 148, 1312–1319. [Google Scholar] [CrossRef]
- Espana, L.; Heredia-Guerrero, J.A.; Segado, P.; Benitez, J.J.; Heredia, A.; Dominguez, E. Biochemical properties of the tomato (Solanum lycopersicum) fruit cuticle during development are modulated by changes in the relative amounts of its components. New Phytol. 2014, 202, 790–802. [Google Scholar] [CrossRef]
- Morris, E.; Gidley, M.; Murray, E.; Powell, D.; Rees, D. Characterization of pectin gelation under conditions of low water activity, by circular dichroism, competitive inhibition and mechanical properties. Int. J. Boil. Macromol. 1980, 2, 327–330. [Google Scholar] [CrossRef]
- Venzon, S.S.; Canteri, M.H.G.; Granato, D.; Junior, B.D.; Maciel, G.M.; Stafussa, A.P.; Haminiuk, C.W.I. Physicochemical properties of modified citrus pectins extracted from orange pomace. J. Food Sci. Technol. 2014, 52, 4102–4112. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; DeLisi, R.; Tamburino, A.; Carnaroglio, D.; Cravotto, G.; Ilharco, L.M.; Pagliaro, M. Controlling the degree of esterification of citrus pectin for demanding applications by selection of the source. ACS Omega 2017, 2, 7991–7995. [Google Scholar] [CrossRef]
- Wang, W.; Wu, X.; Chantapakul, T.; Wang, D.; Zhang, S.; Ma, X.; Ding, T.; Ye, X.; Liu, D. Acoustic cavitation assisted extraction of pectin from waste grapefruit peels: A green two-stage approach and its general mechanism. Food Res. Int. 2017, 102, 101–110. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.I.; Araujo-Andrade, C.; Tymczyszyn, E.; Gomez-Zavaglia, A. Determination of amorphous/rubbery states in freeze-dried prebiotic sugars using a combined approach of near-infrared spectroscopy and multivariate analysis. Food Res. Int. 2014, 64, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Sene, C.; McCann, M.C.; Wilson, R.H.; Grinter, R. Fourier- transform Raman and fourier-transform infrared spectroscopy: An investigation of five higher plant cell walls and their components. Plant Physiol. 1994, 106, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Balakumar, R.; Krishnakumar, N.; Saleem, H.; Subashchandrabose, S. Structural geometry, vibrational and electronic spectra investigation on narginin molecules using experimental and density functional theory calculation. Int. J. Mol. Theor. Phys. 2018, 2, 1–22. [Google Scholar]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Consuelo Díaz-Maroto, M.; Jordán, J.; Galindo, M.F.; Arroyo-Jiménez, M.d.M. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (Citrus paradisi Macf.). Oxid. Med. Cell. Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef]
- Mitzner, B.M.; Theimer, E.T. β-Terpinene and β-Pellandrene from the pyrolysis of Sabinene. J. Org. Chem. 1962, 27, 3359. [Google Scholar] [CrossRef]
- Tracanna, M.I.; Romano, E.; Raschi, A.B.; Fortuna, A.M.; Brandan, S.A. Structural and FT-IR studies of phenolic 2,8-dihydroxy-7H-furo [2,3-f] chromen-7-one derivative isolated from Tibouchina paratropica. J. Mater. Environ. Sci. 2019, 10, 818–831. [Google Scholar]
- Editorial Secretary (for the Judicial Commission of the International Committee on Nomenclature of Bacteria), Opinion 36. Designation of strain ATCC 10145 as the neotype strain of Pseudomonas aeruginosa (Schroeter) Migula. Int. J. Syst. Bacteriol. 1970, 20, 15. [Google Scholar] [CrossRef]
- Treangen, T.J.; Maybank, R.A.; Enke, S.; Friss, M.B.; Diviak, L.F.; Karaolis, D.K.R.; Koren, S.; Ondov, B.; Phillippy, A.M.; Bergman, N.H.; et al. Complete genome sequence of the quality control strain Staphylococcus aureus subsp. aureus ATCC 25923. Genome Announc. 2014, 2, e01110-14. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Yao, X.; Zhu, X.; Pan, S.; Fang, Y.; Jiang, F.; Phillips, G.O.; Xu, X. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem. 2012, 132, 1883–1890. [Google Scholar] [CrossRef]
- Negy, P.; Jayaprakasha, G.K. Antibacterial activity of grapefruit (Citrus paradisi) peel extracts. Eur. Food Res. Technol. 2001, 213, 484–487. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- El–Nakeeb, M.A.; Yousef, R.T. Study of antimicrobial action of pectin. Planta Med. 1970, 18, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.u.; Wang, P.; Wang, N.; Chen, Y. A key bacterial cytoskeletal cell division protein FtsZ as a novel therapeutic antibacterial drug target. Bosn. J. Basic Med. Sci. 2020, 20, 310–318. [Google Scholar]
- Du, T.A. Bacterial physiology: FtsZ and FtsA find the right place. Nat. Rev. Microbiol. 2014, 13, 67. [Google Scholar]
- Somerville, G.A.; Saïd-Salim, B.; Wickman, J.M.; Raffel, S.J.; Kreiswirth, B.N.; Musser, J.M. Correlation of acetate catabolism and growth yield in Staphylococcus aureus: Implications for host-pathogen interactions. Infect. Immun. 2003, 71, 4724–4732. [Google Scholar] [CrossRef]
- Patton, T.G.; Rice, K.C.; Foster, M.K.; Bayles, K.W. The Staphylococcus aureus cidCgene encodes a pyruvate oxidase that affects acetate metabolism and cell death in stationary phase. Mol. Microbiol. 2005, 56, 1664–1674. [Google Scholar] [CrossRef]
- Rice, K.C.; Bayles, K.W. Molecular control of bacterial death and lysis. Microbiol. Mol. Boil. Rev. 2008, 72, 85–109. [Google Scholar] [CrossRef]
- Rice, K.C.; Nelson, J.B.; Patton, T.G.; Yang, S.-J.; Bayles, K.W. Acetic acid induces expression of the Staphylococcus aureus cidABC and lrgAB murein hydrolase regulator operons. J. Bacteriol. 2005, 187, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2006, 63, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Cappelletti, M.; Sansone, A.; Ferreri, C.; Piacenza, E.; Demeter, M.A.; Crognale, S.; Petruccioli, M.; Milazzo, G.; Fedi, S.; et al. Aerobic growth of Rhodococcus aetherivorans BCP1 using selected naphthenic acids as the sole carbon and energy sources. Front. Microbiol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Murínová, S.; Dercová, K. Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane. Int. J. Microbiol. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Sikkema, J.; De Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Ambrosi, E.; Speghini, A.; Turner, R.J.; Vallini, G.; Lampis, S. Physical–chemical properties of biogenic selenium nanostructures produced by Stenotrophomonas maltophilia SeITE02 and Ochrobactrum sp. MPV1. Front. Microbiol. 2018, 9, 3178. [Google Scholar] [CrossRef]
- Ciabocco, M.; Cancemi, P.; Saladino, M.L.; Caponetti, E.; Alduina, R.; Berrettoni, M. Synthesis and antibacterial activity of iron-hexacyanocobaltate nanoparticles. JBIC J. Boil. Inorg. Chem. 2018, 23, 385–398. [Google Scholar] [CrossRef]
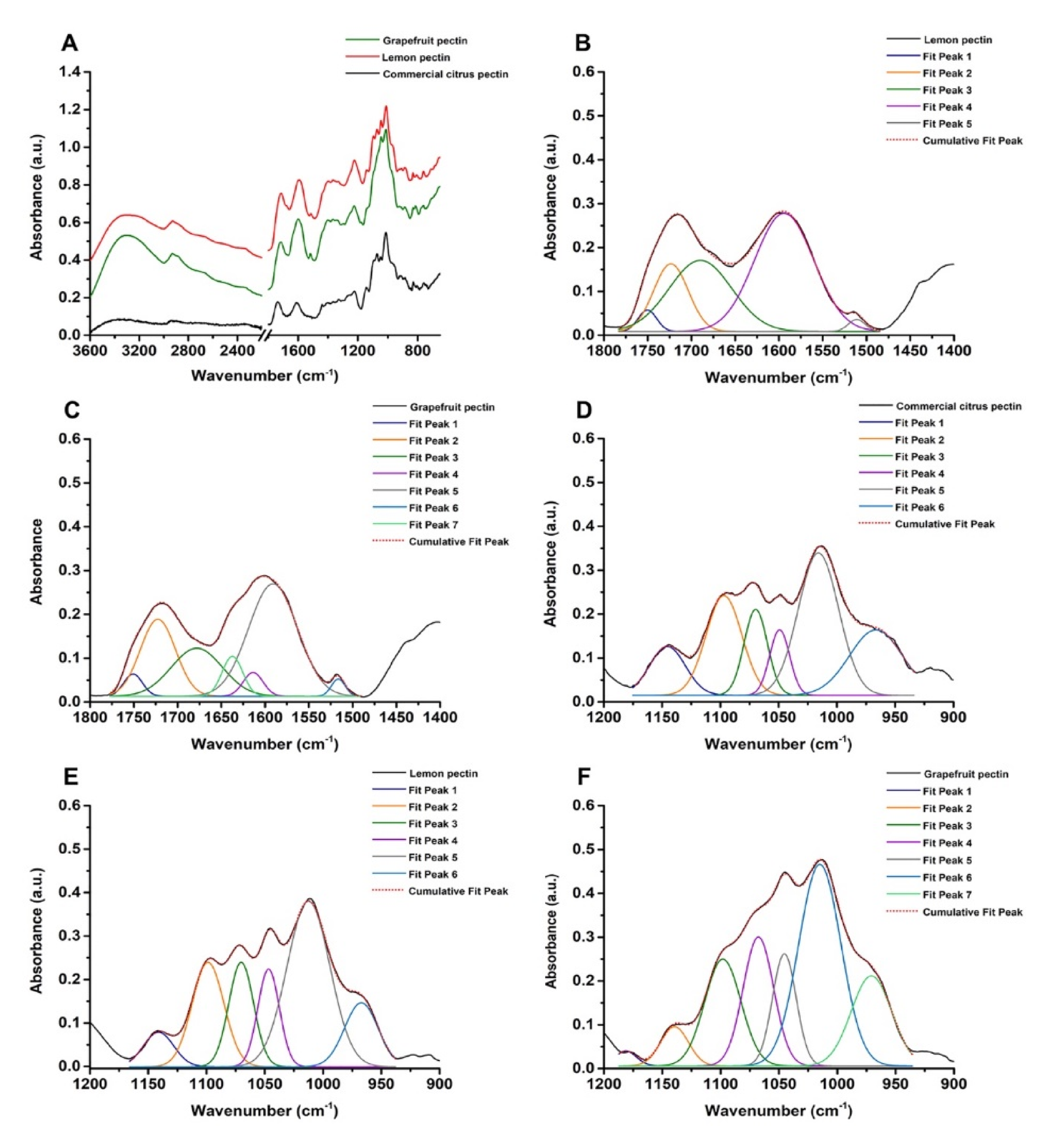
| Equation Member | Commercial Citrus Pectin | Lemon IntegroPectin | Grapefruit IntegroPectin |
|---|---|---|---|
| ∑Aν(C = O)ester | 7.80 | 1.29 | 1.35 |
| Aν(C = O)acid + Aνas(COO-) | 0 + 8.38 | 14.53 + 0 | 8.01 + 1.55 |
| DE (%) | 48 | 8 | 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presentato, A.; Piacenza, E.; Scurria, A.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Martino, D.C.; Alduina, R.; et al. A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains. Antibiotics 2020, 9, 586. https://doi.org/10.3390/antibiotics9090586
Presentato A, Piacenza E, Scurria A, Albanese L, Zabini F, Meneguzzo F, Nuzzo D, Pagliaro M, Martino DC, Alduina R, et al. A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains. Antibiotics. 2020; 9(9):586. https://doi.org/10.3390/antibiotics9090586
Chicago/Turabian StylePresentato, Alessandro, Elena Piacenza, Antonino Scurria, Lorenzo Albanese, Federica Zabini, Francesco Meneguzzo, Domenico Nuzzo, Mario Pagliaro, Delia Chillura Martino, Rosa Alduina, and et al. 2020. "A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains" Antibiotics 9, no. 9: 586. https://doi.org/10.3390/antibiotics9090586
APA StylePresentato, A., Piacenza, E., Scurria, A., Albanese, L., Zabini, F., Meneguzzo, F., Nuzzo, D., Pagliaro, M., Martino, D. C., Alduina, R., & Ciriminna, R. (2020). A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains. Antibiotics, 9(9), 586. https://doi.org/10.3390/antibiotics9090586