The Anti-Microbial Peptide (Lin-SB056-1)2-K Reduces Pro-Inflammatory Cytokine Release through Interaction with Pseudomonas aeruginosa Lipopolysaccharide
Abstract
:1. Introduction
2. Results
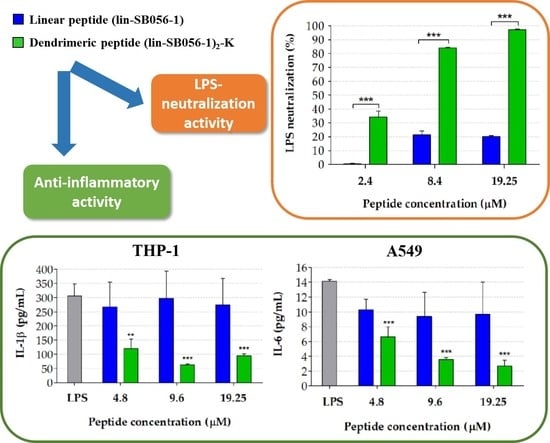
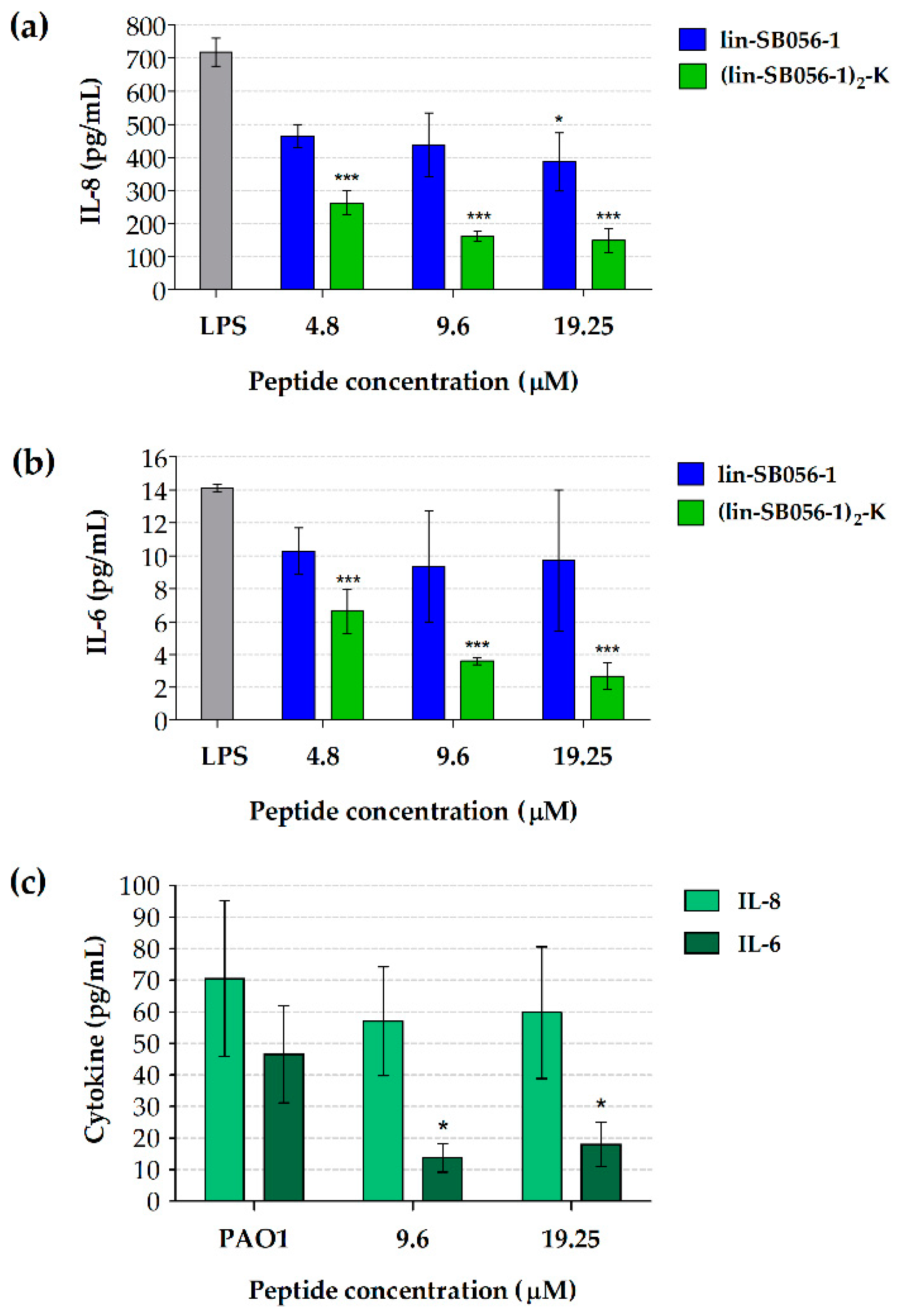
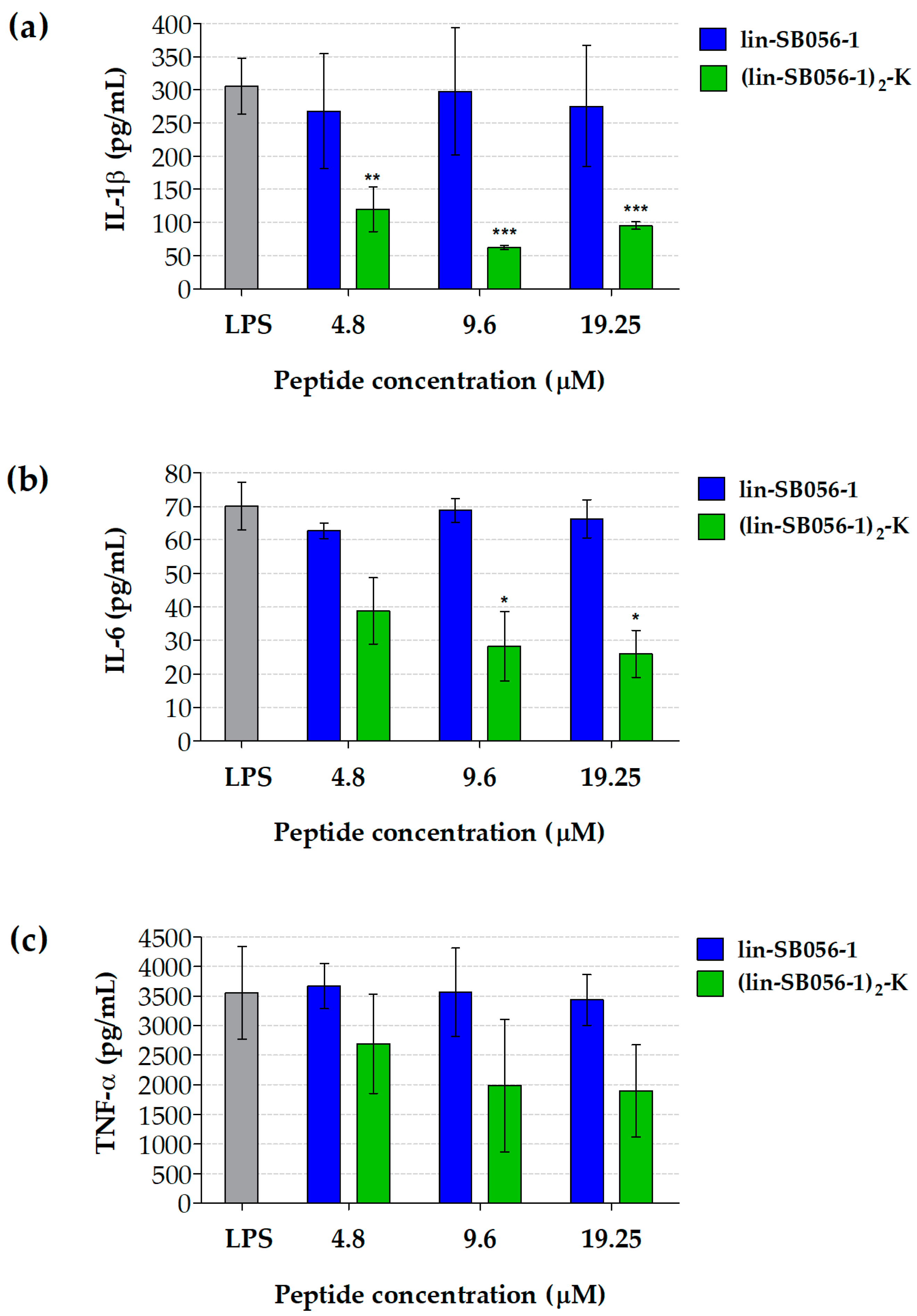
2.1. The Dendrimeric Peptide (Lin-SB056-1)2-K Significantly Reduces Cytokine Production by Lung Epithelial Cells and Macrophages Stimulated with LPS and/or P. aeruginosa
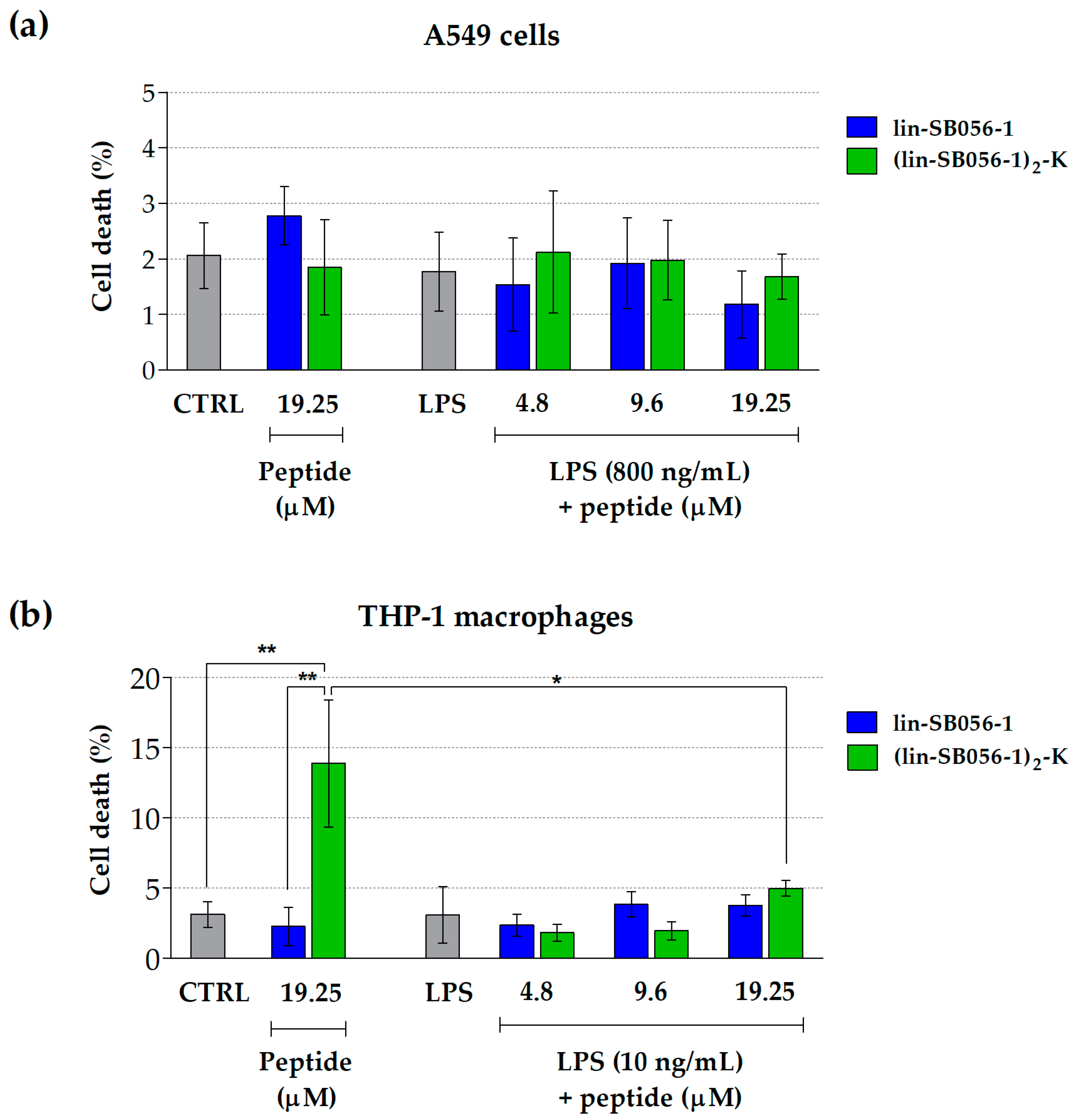
2.2. Lin-SB056-1 and Its Dendrimeric Derivative Do Not Affect Cell Viability
2.3. The Dimeric Derivative (Lin-SB056-1)2-K Exhibits Higher LPS-Binding Affinity than Its Monomeric Counterpart
2.4. The Dimeric Derivative (Lin-SB056-1)2-K Exerts a Stronger LPS-Neutralizing Activity than Its Monomeric Counterpart
3. Discussion
4. Materials and Methods
4.1. Peptides
4.2. Bacterial Strains and Culture Conditions
4.3. Cell Lines
4.4. Cell Stimulation and Peptide Treatment
4.5. Cytokine Assay
4.6. Cytotoxicity Assay
4.7. Fluorescent Displacement Assay
4.8. LAL Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hancock, R.E.W.; Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–8861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Greber, K.E.; Dawgul, M. Antimicrobial peptides under clinical trials. Curr. Top. Med. Chem. 2017, 17, 620–628. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Gellatly, S.L.; Hancock, R.E. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Bezzerri, V.; Avitabile, C.; Dechecchi, M.C.; Lampronti, I.; Borgatti, M.; Montagner, G.; Cabrini, G.; Gambari, R.; Romanelli, A. Antibacterial and anti-inflammatory activity of a Temporin B peptide analogue on an in vitro model of cystic fibrosis. J. Pept. Sci. 2014, 20, 822–830. [Google Scholar] [CrossRef]
- Brunetti, J.; Roscia, G.; Lampronti, I.; Gambari, R.; Quercini, L.; Falciani, C.; Bracci, L.; Pini, A. Immunomodulatory and anti-inflammatory activity in vitro and in vivo of a novel antimicrobial candidate. J. Biol. Chem. 2016, 291, 25742–25748. [Google Scholar] [CrossRef] [Green Version]
- Heinbockel, L.; Weindl, G.; Martinez-de-Tejada, G.; Correa, W.; Sanchez-Gomez, S.; Bárcena-Varela, S.; Goldmann, T.; Garidel, P.; Gutsmann, T.; Brandenburg, K. Inhibition of lipopolysaccharide- and lipoprotein-induced inflammation by antitoxin peptide Pep19-2.5. Front. Immunol. 2018, 9, 1704. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, G.; Kim, E.Y.; Shin, S.Y. LL-37-derived membrane-active FK-13 analogs possessing cell selectivity, anti-biofilm activity and synergy with chloramphenicol and anti-inflammatory activity. Biochim. Biophys. Acta 2017, 1859, 722–733. [Google Scholar] [CrossRef]
- Wuerth, K.C.; Falsafi, R.; Hancock, R.E.W. Synthetic host defense peptide IDR-1002 reduces inflammation in Pseudomonas aeruginosa lung infection. PLoS ONE 2017, 12, e0187565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzo, G.; Scorciapino, M.A.; Wadhwani, P.; Bürck, J.; Montaldo, N.P.; Pintus, M.; Sanna, R.; Casu, M.; Giuliani, A.; Pirri, G.; et al. Enhanced amphiphilic profile of a short β-stranded peptide improves its antimicrobial activity. PLoS ONE 2015, 10, e0116379. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, A.; Liu, Y.; Matthijs, N.; Rigole, P.; De La Fuente-Nùñez, C.; Davis, R.; Ledesma, M.A.; Sarker, S.; Van Houdt, R.; Hancock, R.E.W.; et al. Antimicrobial efficacy against Pseudomonas aeruginosa biofilm formation in a three-dimensional lung epithelial model and the influence of fetal bovine serum. Sci. Rep. 2017, 7, 43321. [Google Scholar] [CrossRef] [Green Version]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorschner, R.A.; Lopez-Garcia, B.; Peschel, A.; Kraus, D.; Morikawa, K.; Nizet, V.; Gallo, R.L. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 2006, 20, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Maisetta, G.; Di Luca, M.; Esin, S.; Florio, W.; Brancatisano, F.L.; Bottai, D.; Campa, M.; Batoni, G. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta-defensin 3. Peptides 2008, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Grassi, L.; Esin, S.; Serra, I.; Scorciapino, M.A.; Rinaldi, A.C.; Batoni, G. The semi-synthetic peptide lin-SB056-1 in combination with EDTA exerts strong antimicrobial and antibiofilm activity against Pseudomonas aeruginosa in conditions mimicking cystic fibrosis sputum. Int. J. Mol. Sci. 2017, 18, 1994. [Google Scholar] [CrossRef]
- Grassi, L.; Batoni, G.; Ostyn, L.; Rigole, P.; Van den Bossche, S.; Rinaldi, A.C.; Maisetta, G.; Esin, S.; Coenye, T.; Crabbé, A. The antimicrobial peptide lin-SB056-1 and its dendrimeric derivative prevent Pseudomonas aeruginosa biofilm formation in physiologically relevant models of chronic infections. Front. Microbiol. 2019, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- Maisetta, G.; Grassi, L.; Esin, S.; Kaya, E.; Morelli, A.; Puppi, D.; Piras, M.; Chiellini, F.; Pifferi, M.; Batoni, G. Targeting Pseudomonas aeruginosa in the sputum of primary ciliary dyskinesia patients with a combinatorial strategy having antibacterial and anti-virulence potential. Int. J. Mol. Sci. 2020, 21, 69. [Google Scholar] [CrossRef] [Green Version]
- Pier, G.B. Pseudomonas aeruginosa lipopolysaccharide: A major virulence factor, initiator of inflammation and target for effective immunity. Int. J. Med. Microbiol. 2007, 297, 277–295. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.; Zhang, T. Optimization of fixation methods for observation of bacterial cell morphology and surface ultrastructures by atomic force microscopy. Appl. Microbiol. Biotechnol. 2011, 92, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, S.J.; Miller, K.A.; David, S.A. Anti-endotoxin agents. 1. Development of a fluorescent probe Dis. placement method optimized for the rapid identification of lipopolysaccharide-binding agents. Combin. Chem. High Throughput Screen 2004, 7, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Ried, C.; Wahl, C.; Miethke, T.; Wellnhofer, G.; Landgraf, C.; Schneider-Mergener, J.; Hoess, A. High affinity endotoxin-binding and neutralizing peptides based on the crystal structure of recombinant Limulus anti-lipopolysaccharide factor. J. Biol. Chem. 1996, 271, 28120–28127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afacan, N.J.; Yeung, A.T.; Pena, O.M.; Hancock, R.E. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Velden, W.J.; van Iersel, T.M.; Blijlevens, N.M.; Donnelly, J.P. Safety and tolerability of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11). BMC Med. 2009, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Dong, F.; Shi, C.; Liu, S.; Sun, J.; Chen, J.; Li, H.; Xu, H.; Lao, X.; Zheng, H. DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci. Data 2019, 6, 148. [Google Scholar] [CrossRef] [Green Version]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of persister cells of Pseudomonas aeruginosa and Staphylococcus aureus by Chem. ical treatment and evaluation of their susceptibility to membrane-targeting agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Lavoie, E.G.; Wangdi, T.; Kazmierczak, B.I. Innate immune responses to Pseudomonas aeruginosa infection. Microbes. Infect. 2011, 13, 1133–1145. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.P.; Chmiel, J.F. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 2015, 40, S39–S56. [Google Scholar] [CrossRef]
- Courtney, J.M.; Ennis, M.; Elborn, J.S. Cytokines and inflammatory mediators in cystic fibrosis. J. Cyst. Fibros 2004, 3, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Moskowitz, S.M.; Ernst, R.K. The role of Pseudomonas lipopolysaccharide in cystic fibrosis airway infection. Subcell Bio. Chem. 2010, 53, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Kirikae, T.; Kirikae, F.; Saito, S.; Tominaga, K.; Tamura, H.; Uemura, Y.; Yokochi, T.; Nakano, M. Biological characterization of endotoxins released from antibiotic-treated Pseudomonas aeruginosa and Escherichia coli. Antimicrob. Agents Chemother. 1998, 42, 1015–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.S.; Lee, J.; Jang, S.C.; Kim, S.R.; Jang, M.H.; Lötvall, J.; Kim, Y.K.; Gho, Y.S. Pulmonary inflammation induced by bacteria-free outer membrane vesicles from Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 2013, 49, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Leeson, M.C.; Morrison, D.C. Induction of proinflammatory responses in human monocytes by particulate and soluble forms of lipopolysaccharide. Shock 1994, 2, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Datta, A.; Schmidtchen, A.; Bhunia, A.; Malmsten, M. Tryptophan end-tagging for promoted lipopolysaccharide interactions and anti-inflammatory effects. Sci. Rep. 2017, 7, 212. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Rinaldi, A.C. Antimicrobial peptides: The LPS connection. Methods Mol. Biol. 2010, 618, 137–154. [Google Scholar] [CrossRef]
- Pulido, D.; Nogués, M.V.; Boix, E.; Torrent, M. Lipopolysaccharide neutralization by antimicrobial peptides: A gambit in the innate host defense strategy. J. Innate. Immun. 2012, 4, 327–336. [Google Scholar] [CrossRef]
- Bhattacharjya, S. NMR structures and interactions of antimicrobial peptides with lipopolysaccharide: Connecting structures to functions. Curr. Top. Med. Chem. 2016, 16, 4–15. [Google Scholar] [CrossRef]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Rev. Cell Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Brandenburg, K. Current understanding of polymyxin B applications in bacteraemia/sepsis therapy prevention: Clinical, pharmaceutical, structural and mechanistic aspects. Anti-Inf. Agents Med. Chem. 2009, 8, 367–385. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Shai, Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta 2009, 1788, 1610–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Serrano Luna, J.; Campos-Rodríguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Rev. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bahl, N.; Du, R.; Winarsih, I.; Ho, B.; Tucker-Kellogg, L.; Tidor, B.; Ding, J.L. Delineation of lipopolysaccharide (LPS)-binding sites on hemoglobin: From in silico predictions to biophysical characterization. J. Biol. Chem. 2011, 286, 37793–37803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Mao, X.; Guan, Y.; Kang, Y.; Shang, D. Antimicrobial and anti-inflammatory activities of three chensinin-1 peptides containing mutation of glycine and histidine residues. Sci. Rep. 2017, 7, 40228. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.P.; Lu, Y.A.; Yang, J.L. Antimicrobial dendrimeric peptides. Eur. J. Biol. Chem. 2002, 269, 923–932. [Google Scholar] [CrossRef]
- Scorciapino, M.A.; Serra, I.; Manzo, G.; Rinaldi, A.C. Antimicrobial dendrimeric peptides: Structure, activity and new therapeutic applications. Int. J. Mol. Sci. 2017, 18, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, T.K.; Polcyn, P.; Zielinska, P.; Cárdenas, M.; Urbanczyk-Lipkowska, Z. On the antimicrobial activity of various peptide-based dendrimers of similar architecture. Molecules 2015, 20, 738–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batoni, G.; Casu, M.; Giuliani, A.; Luca, V.; Maisetta, G.; Mangoni, M.L.; Manzo, G.; Pintus, M.; Pirri, G.; Rinaldi, A.C.; et al. Rational modification of a dendrimeric peptide with antimicrobial activity: Consequences on membrane-binding and biological properties. Amino Acids 2016, 48, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shang, D. Inhibitory effects of antimicrobial peptides on lipopolysaccharide-induced inflammation. Mediat. Inflamm. 2015, 2015, 167572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaconis, Y.; Kowalski, I.; Howe, J.; Brauser, A.; Richter, W.; Razquin-Olazaran, I.; Iñigo-Pestaña, M.; Garidel, P.; Rössle, M.; Martinez de Tejada, G.; et al. Biophysical mechanisms of endotoxin neutralization by cationic amphiphilic peptides. Biophys. J. 2011, 100, 2652–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, K.; Howe, J.; Sanchez-Gomez, S.; Garidel, P.; Roessle, M.; Andra, J.; Jerala, R.; Zweytick, D.; Lohner, K.; Rappolt, M.; et al. Effective antimicrobial and anti-endotoxin activity of cationic peptides based on lactoferricin: A biophysical and microbiological study. Antiinfect. Agents Med. Chem. 2010, 9, 9. [Google Scholar] [CrossRef]
- Lee, J.K.; Seo, C.H.; Luchian, T.; Park, Y. Antimicrobial peptide CMA3 derived from the CA-MA hybrid peptide: Antibacterial and anti-inflammatory activities with low cytotoxicity and mechanism of action in Escherichia coli. Antimicrob. Agents Chemother. 2015, 60, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Gan, B.H.; Siriwardena, T.N.; Javor, S.; Darbre, T.; Reymond, J.L. Fluorescence imaging of bacterial killing by antimicrobial peptide dendrimer G3KL. ACS Infect. Dis. 2019, 5, 2164–2173. [Google Scholar] [CrossRef]
- Bruschi, M.; Pirri, G.; Giuliani, A.; Nicoletto, S.F.; Basterb, I.; Scorciapino, M.A.; Casu, M.; Rinaldi, A.C. Synthesis, characterization, antimicrobial activity and LPS-interaction properties of SB041, a novel dendrimeric peptide with antimicrobial properties. Peptides 2010, 31, 1459–1467. [Google Scholar] [CrossRef]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef]
- Darveau, R.P.; Hancock, R.E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 1983, 155, 831–838. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, L.; Pompilio, A.; Kaya, E.; Rinaldi, A.C.; Sanjust, E.; Maisetta, G.; Crabbé, A.; Di Bonaventura, G.; Batoni, G.; Esin, S. The Anti-Microbial Peptide (Lin-SB056-1)2-K Reduces Pro-Inflammatory Cytokine Release through Interaction with Pseudomonas aeruginosa Lipopolysaccharide. Antibiotics 2020, 9, 585. https://doi.org/10.3390/antibiotics9090585
Grassi L, Pompilio A, Kaya E, Rinaldi AC, Sanjust E, Maisetta G, Crabbé A, Di Bonaventura G, Batoni G, Esin S. The Anti-Microbial Peptide (Lin-SB056-1)2-K Reduces Pro-Inflammatory Cytokine Release through Interaction with Pseudomonas aeruginosa Lipopolysaccharide. Antibiotics. 2020; 9(9):585. https://doi.org/10.3390/antibiotics9090585
Chicago/Turabian StyleGrassi, Lucia, Arianna Pompilio, Esingül Kaya, Andrea C. Rinaldi, Enrico Sanjust, Giuseppantonio Maisetta, Aurélie Crabbé, Giovanni Di Bonaventura, Giovanna Batoni, and Semih Esin. 2020. "The Anti-Microbial Peptide (Lin-SB056-1)2-K Reduces Pro-Inflammatory Cytokine Release through Interaction with Pseudomonas aeruginosa Lipopolysaccharide" Antibiotics 9, no. 9: 585. https://doi.org/10.3390/antibiotics9090585
APA StyleGrassi, L., Pompilio, A., Kaya, E., Rinaldi, A. C., Sanjust, E., Maisetta, G., Crabbé, A., Di Bonaventura, G., Batoni, G., & Esin, S. (2020). The Anti-Microbial Peptide (Lin-SB056-1)2-K Reduces Pro-Inflammatory Cytokine Release through Interaction with Pseudomonas aeruginosa Lipopolysaccharide. Antibiotics, 9(9), 585. https://doi.org/10.3390/antibiotics9090585