Mycogenic Metal Nanoparticles for the Treatment of Mycobacterioses
Abstract
:1. Introduction
1.1. Mycobacterioses
1.2. Tuberculosis
1.2.1. TB and Co-Infections
1.2.2. TB and COVID-19
1.3. Current Solutions
2. Methods
3. Results
3.1. Silver Nanoparticles (AgNPs)
- AgNPs against mycobacteria
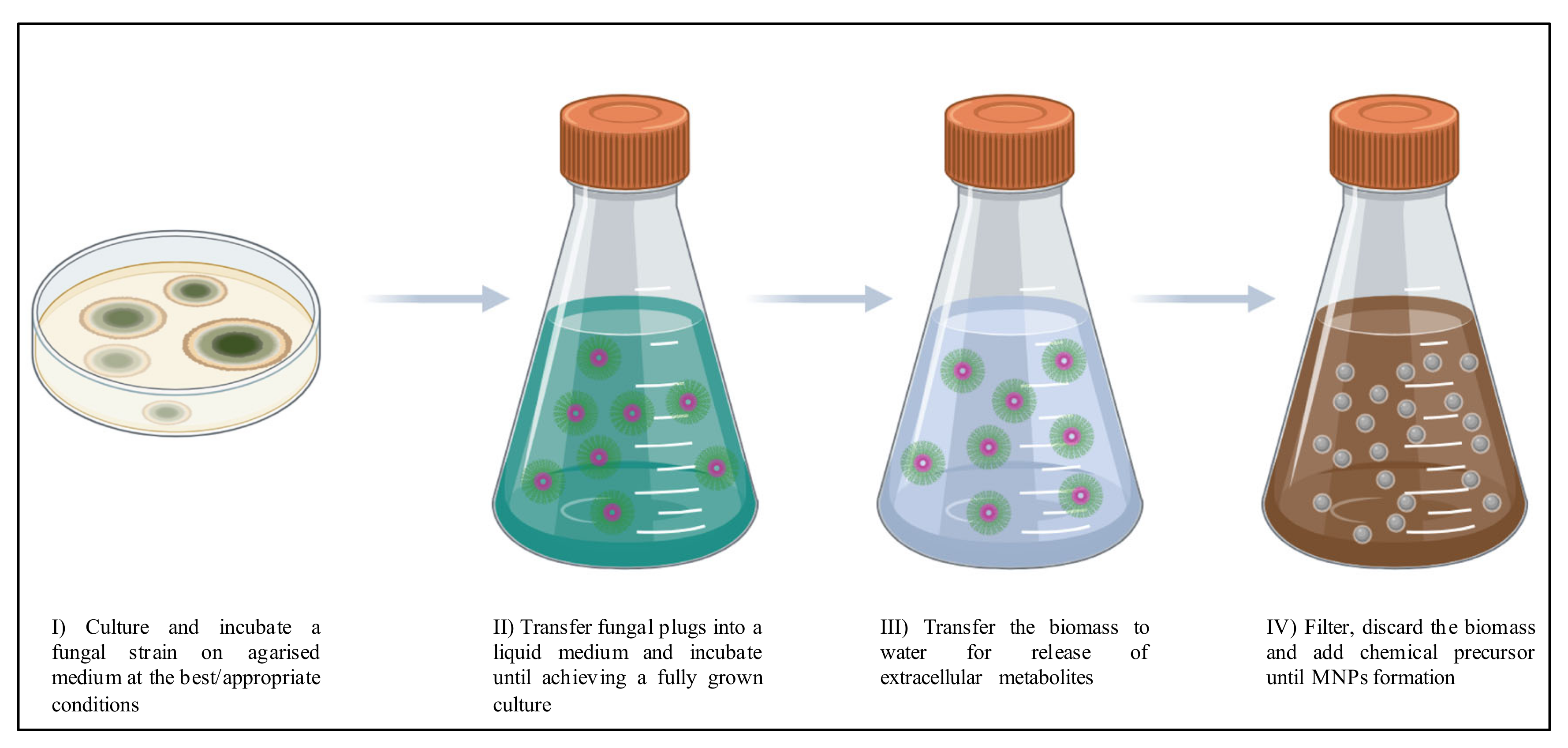
3.2. Mycogenic MNPs
- Mycogenic AgNPs against mycobacteria
3.3. MNPs (Other Than AgNPs) against Mycobacteria
3.4. Synergistic and Complementing Effects of MNPs against Mycobacteria
4. Discussion
4.1. Advantages of Using Mycogenic MNPs
4.2. Future Focus of Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Clarke, T.; Brinkac, L.; Manoranjan, J.; García-Basteiro, A.; Grewal, H.; Kiyimba, A.; Lopez, E.; Macaden, R.; Respeito, d.; Ssengooba, W.; et al. Typing and classification of non-tuberculous mycobacteria isolates. F1000Research 2020, 9, 249. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. On behalf of the ATS Mycobacterial Diseases Subcommittee. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Lezcano, O.M.; González-Cortés, C.; Mirsaeidi, M. The unexplained increase of nontuberculous mycobacteriosis. Int. J. Mycobacteriol. 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Azadi, D.; Motallebirad, T.; Ghaffari, K.; Shojaei, H. Mycobacteriosis and tuberculosis: Laboratory diagnosis. Open Microbiol. J. 2018, 12, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, P.J. Patogénesis de la tuberculosis y otras micobacteriosis. Enferm. Infecc. Microbiol. Clin. 2018, 36, 38–46. [Google Scholar] [CrossRef]
- Davey, P.; Wilcox, M.H.; Irving, W.; Thwaites, G. Chapter 30, tuberculosis and other mycobacterial diseases. In Antimicrobial Chemotherapy, 7th ed.; OUP Oxford: Oxford, UK, 2015; pp. 326–337. [Google Scholar]
- Tăbăran, A.F.; Matea, C.T.; Mocan, T.; Tăbăran, A.; Mihaiu, M.; Iancu, C.; Mocan, L. Silver nanoparticles for the therapy of tuberculosis. Int. J. Nanomed. 2020, 15, 2231–2258. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.M.; Samir, T.M.; Azzazy, H.M. Unmodified gold nanoparticles for direct and rapid detection of Mycobacterium tuberculosis complex. Clin. Biochem. 2013, 46, 633–637. [Google Scholar] [CrossRef]
- Kaneko, T.; Cooper, C.; Mdluli, K. Challenges and opportunities in developing novel drugs for TB. Future Med. Chem. 2011, 3, 1373–1400. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Tuberculosis Report 2019. 17 October 2019. Available online: www.who.int/tb/publications/global_report/en/ (accessed on 18 June 2020).
- Gordon, S.V.; Parish, T. Microbe Profile: Mycobacterium tuberculosis: Humanity’s deadly microbial foe. Microbiology 2018, 164, 437–439. [Google Scholar] [CrossRef]
- Machado, D.; Girardini, M.; Viveiros, M.; Pieroni, M. Challenging the drug-likeness dogma for new drug discovery in tuberculosis. Front. Microbiol. 2018, 9, 1367. [Google Scholar] [CrossRef] [Green Version]
- Sterling, T.R.; Njie, G.; Zenner, D.; Cohn, D.L.; Reves, R.; Ahmed, A.; Menzies, D.; Horsburgh, C.R., Jr.; Crane, C.M.; Burgos, M.; et al. Guidelines for the treatment of latent tuberculosis infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. Am. J. Transplant. 2020, 20, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Nasiruddin, M.; Neyaz, M.; Das, S. Nanotechnology-based approach in tuberculosis treatment. Tuberc. Res. Treat. 2017, 2017, 4920209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Health Organization (WHO) Information Note. Tuberculosis and COVID-19. 12 May 2020. Available online: www.who.int/docs/default-source/documents/tuberculosis/infonote-tb-covid-19.pdf (accessed on 18 June 2020).
- WHO. World Tuberculosis Day 2020. 24 March 2020. Available online: www.who.int/news-room/campaigns/world-tb-day/world-tb-day-2020 (accessed on 8 June 2020).
- Singh, A.; Gupta, A.K.; Singh, S. Molecular mechanisms of drug resistance in Mycobacterium tuberculosis: Role of nanoparticles against multi-drug-resistant tuberculosis (MDR-TB). In NanoBioMedicine; Springer: Singapore, 2020; pp. 285–314. [Google Scholar]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Bostanabad, S.Z.; Amini, S.M.; Jafari, A.; Nobar, M.G.; Ghodousi, A.; Kamalzadeh, M.; Darban-Sarokhalil, D. The anti-mycobacterial activity of Ag, ZnO, and Ag-ZnO nanoparticles against MDR- and XDR-Mycobacterium tuberculosis. Infect. Drug Resist. 2019, 12, 3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, P.M.; Furrer, H. Think tuberculosis—but is thinking enough? Lancet Infect. Dis. 2020, 20, 639–640. [Google Scholar] [CrossRef] [Green Version]
- Amala, S.E.; Hanson, A.; Wokem, G.N. Candida co-infection with Mycobacterium tuberculosis in tuberculosis patients and antifungal susceptibility of the isolates. J. Tuberc. Res. 2020, 8, 53–65. [Google Scholar] [CrossRef]
- Hosseini, M.; Shakerimoghaddam, A.; Ghazalibina, M.; Khaledi, A. Aspergillus coinfection among patients with pulmonary tuberculosis in Asia and Africa countries; a systematic review and meta-analysis of cross-sectional studies. Microb. Pathog. 2020, 141, 104018. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Bhagat, R.; Panchal, N.; Shah, A. Allergic bronchopulmonary aspergillosis with aspergilloma mimicking fibrocavitary pulmonary tuberculosis. Asian Pac. J. Allergy Immunol. 1996, 14, 5–8. [Google Scholar]
- Al-Moudi, O.S. Allergic bronchopulmonary aspergillosis mimicking pulmonary Tuberculosis. Saudi Med. J. 2001, 22, 708–713. [Google Scholar]
- Ashraf, M.; Zaidi, A.; Alam, M.F. Allergic bronchopulmonary aspergillosis (ABPA) commonly misdiagnosed as pulmonary tuberculosis. Pak. J. Chest Med. 2007, 13, 3–8. [Google Scholar]
- Selim, A.; Elhaig, M.M.; Taha, S.A.; Nasr, E.A. Antibacterial activity of silver nanoparticles against field and reference strains of Mycobacterium tuberculosis, Mycobacterium bovis and multiple-drug resistant tuberculosis strains. Rev. Off. Int. Epizoot 2018, 37, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Aziz, M.M.; Elella, M.H.A.; Mohamed, R.R. Green synthesis of quaternized chitosan/silver nanocomposites for targeting Mycobacterium tuberculosis and lung carcinoma cells (A-549). Int. J. Biol. Macromol. 2020, 142, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, L.; Fu, H.; Vesga, J.F.; Dowdy, D.; Pretorius, C.; Ahmedov, S.; Nair, S.A.; Mosneaga, A.; Masini, E.; Sahu, S.; et al. The potential impact of the COVID-19 pandemic on tuberculosis: A modelling analysis. MedRxiv 2020. [Google Scholar] [CrossRef]
- Saunders, M.J.; Evans, C.A. COVID-19, tuberculosis, and poverty: Preventing a perfect storm. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Amimo, F.; Lambert, B.; Magit, A. What does the COVID-19 pandemic mean for HIV, tuberculosis, and malaria control? Trop. Med. Health 2020, 48, 1–4. [Google Scholar] [CrossRef]
- Togun, T.; Kampmann, B.; Stoker, N.G.; Lipman, M. Anticipating the impact of the COVID-19 pandemic on TB patients and TB control programmes. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–6. [Google Scholar] [CrossRef]
- Kumar, R.; Bhattacharya, B.; Meena, V.; Soneja, M.; Wig, N. COVID-19 and TB co-infection-’Finishing touch’’ in perfect recipe to ‘severity’ or ‘death’. J. Infect. 2020, 81, e39–e40. [Google Scholar] [CrossRef]
- Visca, D.; Tiberi, S.; Pontali, E.; Spanevello, A.; Migliori, G.B. Tuberculosis in the time of COVID-19: Quality of life and digital innovation. Eur. Respir. J. 2020, 56, 2001998. [Google Scholar] [CrossRef]
- Manyazewal, T.; Woldeamanuel, Y.; Blumberg, H.M.; Fekadu, A.; Marconi, V.C. The fight to end tuberculosis must not be forgotten in the COVID-19 outbreak. Nat. Med. 2020, 26, 811–812. [Google Scholar] [CrossRef]
- Maciel, E.L.N.; Gonçalves Júnior, E.; Dalcolmo, M.M.P. Tuberculosis and coronavirus: What do we know? Epidemiol. Serviços Saúde 2020, 29, e2020128. [Google Scholar]
- Stop TB Partnership; Imperial College; Avenir Health; Johns Hopkins University; USAID. The Potential Impact of the COVID-19 Response on Tuberculosis in High-Burden Countries: A Modelling Analysis. 2020. Available online: www.stoptb.org/assets/documents/news/Modeling%20Report_1%20May%202020_FINAL.pdf (accessed on 2 August 2020).
- Nahid, P.; Dorman, S.E.; Alipanah, N.; Barry, P.M.; Brozek, J.L.; Cattamanchi, A.; Chaisson, L.H.; Chaisson, R.E.; Daley, C.L.; Grzemska, M.; et al. Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: Treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 2016, 63, e147–e195. [Google Scholar] [CrossRef] [PubMed]
- Somoskovi, A.; Salfinger, M. Mycobacteria: Tuberculosis 64. In Antimicrobial Drug Resistance: Clinical and Epidemiological Aspects; Mayers, D.L., Sobel, J.D., Ouellette, M., Kaye, K.S., Marchaim, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1041–1059. [Google Scholar]
- Hakkimane, S.S.; Shenoy, V.P.; Gaonkar, S.L.; Bairy, I.; Guru, B.R. Antimycobacterial susceptibility evaluation of rifampicin and isoniazid benz-hydrazone in biodegradable polymeric nanoparticles against Mycobacterium tuberculosis H37Rv strain. Int. J. Nanomed. 2018, 13, 4303. [Google Scholar] [CrossRef] [Green Version]
- Costa-Gouveia, J.; Ainsa, J.A.; Brodin, P.; Lucia, A. How can nanoparticles contribute to antituberculosis therapy? Drug Discov. Today 2017, 22, 600–607. [Google Scholar] [CrossRef]
- Praba, V.L.; Kathirvel, M.; Vallayyachari, K.; Surendar, K.; Muthuraj, M.; Jesuraj, P.J.; Govindarajan, S.; Raman, K.V. Bactericidal effect of silver nanoparticles against Mycobacterium tuberculosis. J. Bionanosci. 2013, 7, 282–287. [Google Scholar] [CrossRef]
- Vale, N.; Correia, A.; Silva, S.; Figueiredo, P.; Mäkilä, E.; Salonen, J.; Hirvonen, J.; Pedrosa, J.; Santos, H.A.; Fraga, A. Preparation and biological evaluation of ethionamide-mesoporous silicon nanoparticles against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2017, 27, 403–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, M.F.; Valente, E.; Gómez, M.J.R.; Anes, E.; Constantino, L. Lipophilic pyrazinoic acid amide and ester prodrugs: Stability, activation and activity against Mycobacterium tuberculosis. Eur. J. Pharm. Sci. 2009, 37, 257–263. [Google Scholar] [CrossRef]
- Pardhi, D.M.; Karaman, D.Ş.; Timonen, J.; Wu, W.; Zhang, Q.; Satija, S.; Mehta, M.; Charbe, N.; McCarron, P.; Tambuwala, M.; et al. Anti-bacterial activity of inorganic nanomaterials and their antimicrobial peptide conjugates against resistant and non-resistant pathogens. Int. J. Pharm. 2020, 586, 119531. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.M.; Jo, E.K. Antimicrobial peptides in innate immunity against mycobacteria. Immune Netw. 2011, 11, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Raghav, R.; Priyanka, K.; Rishi, P.; Sharma, S.; Srivastava, S.; Verma, I. Exploiting chitosan and gold nanoparticles for antimycobacterial activity of in silico identified antimicrobial motif of human neutrophil peptide-1. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Simões, M.F.; Fernandes, S.; dos Santos, J.G.; da Silva, E.S.; de Souza, R.F.B.; Maiorano, A.E. Screening of filamentous fungi for antimicrobial silver nanoparticles synthesis. AMB Express 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 2017, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.F.; Ottoni, C.A.; Antunes, A. Biogenic metal nanoparticles: A new approach to detect life on Mars? Life 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Larimer, C.; Ojha, A.; Nettleship, I. Antimycobacterial efficacy of silver nanoparticles as deposited on porous membrane filters. Mater. Sci. Eng. C 2013, 33, 4575–4581. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Rajoka, M.S.R.; Yan, L.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H.; et al. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2018, 38, 817–835. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.; Nada, A.A.; O’Donovan, A.; Thakur, V.K.; Elkelish, A. Mycogenic silver nanoparticles from endophytic Trichoderma atroviride with antimicrobial activity. J. Renew. Mater. 2020, 8, 171–185. [Google Scholar] [CrossRef]
- Singh, R.; Nawale, L.U.; Arkile, M.; Shedbalkar, U.U.; Wadhwani, S.A.; Sarkar, D.; Chopade, B.A. Chemical and biological metal nanoparticles as antimycobacterial agents: A comparative study. Int. J. Antimicrob. Agents 2015, 46, 183–188. [Google Scholar] [CrossRef]
- Kote, J.R.; Mulani, R.M.; Kadam, A.S.; Solankar, B.M. Anti-mycobacterial activity of nanoparticles from Psidium guajava L. J. Microbiol. Biotechnol. Res. 2014, 4, 14–17. [Google Scholar]
- Gumel, A.M.; Surayya, M.M.; Yaro, M.N.; Waziri, I.Z.; Amina, A.A. Biogenic synthesis of silver nanoparticles and its synergistic antimicrobial potency: An overview. J. Appl. Biotechnol. Bioeng. 2019, 6, 22–28. [Google Scholar]
- Sweet, M.J.; Chessher, A.; Singleton, I. Metal-based nanoparticles; size, function, and areas for advancement in applied microbiology. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2012; Volume 80, pp. 113–142. [Google Scholar]
- Singh, R.; Nawale, L.; Arkile, M.; Wadhwani, S.; Shedbalkar, U.; Chopade, S.; Sarkar, D.; Chopade, B.A. Phytogenic silver, gold, and bimetallic nanoparticles as novel antitubercular agents. Int. J. Nanomed. 2016, 11, 1889–1897. [Google Scholar]
- Ranjitha, J.; Rajan, A.; Shankar, V. Features of the biochemistry of Mycobacterium smegmatis, as a possible model for Mycobacterium tuberculosis. J. Infect. Public Health 2020, 1393. [Google Scholar] [CrossRef]
- Shiloh, M.U.; Champion, P.A.D. To catch a killer. What can mycobacterial models teach us about Mycobacterium tuberculosis pathogenesis? Curr. Opin. Microbiol. 2010, 13, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Nino, N.; Sanchez, E.M.; Ruiz, f.; Bach, H.; Av-Gay, Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 681–688. [Google Scholar] [CrossRef]
- Seth, D.; Choudhury, S.R.; Pradhan, S.; Gupta, S.; Palit, D.; Das, S.; Debnat, N.; Goswami, A. Nature-inspired novel drug design paradigm using nanosilver: Efficacy on multi-drug-resistant clinical isolates of tuberculosis. Curr. Microbiol. 2011, 62, 715–726. [Google Scholar] [CrossRef]
- Jena, P.; Mohanty, S.; Mallick, R.; Jacob, B.; Sonawane, A. Toxicity and antibacterial assessment of chitosan coated silver nanoparticles on human pathogens and macrophage cells. Int. J. Nanomed. 2012, 7, 1805–1818. [Google Scholar]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnol. 2012, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Mishra, S.; Jena, P.; Jacob, B.; Sarkar, B.; Sonawane, A. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 916–924. [Google Scholar] [CrossRef]
- Daniel, S.K.; Banu, B.N.; Harshiny, M.; Nehru, K.; Ganesh, P.S.; Kumaran, S.; Sivakumar, M. Ipomea carnea-based silver nanoparticle synthesis for antibacterial activity against selected human pathogens. J. Exp. Nanosci. 2014, 9, 197–209. [Google Scholar] [CrossRef]
- Donnellan, S.; Tran, L.; Johnston, H.; McLuckie, J.; Stevenson, K.; Stone, V. A rapid screening assay for identifying mycobacteria targeted nanoparticle antibiotics. Nanotoxicology 2016, 10, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Salique, S.M.; Gajalakshmi, P.; James, A. Antibacterial and hemolytic activity of green silver nanoparticles from Catharanthus roseus. Int. J. Pharm. Sci. Nanotechnol. 2016, 9, 7. [Google Scholar] [CrossRef]
- Agarwal, P.; Mehta, A.; Kachhwaha, S.; Kothari, S.L. Green synthesis of silver nanoparticles and their activity against Mycobacterium tuberculosis. Adv. Sci. Eng. Med. 2013, 5, 709–714. [Google Scholar] [CrossRef]
- Bello, A.J.; Adams, L.A.; Onyepeju, N.N.; Igbinehi, J.I.; Igbari, O.F.; Okpuzor, J. Nanosilver biosynthesis by Moringa oleifera and Allium cepa and antimycobacterial study. In Proceedings of the Poster presented in 10th UNILAG Annual Research Conference and Fair, Akoka, Lagos, 24–26 November 2015. [Google Scholar]
- Sarkar, S.; Leo, B.F.; Carranza, C.; Chen, S.; Rivas-Santiago, C.; Porter, A.E.; Ryan, M.P.; Gow, A.; Chung, K.F.; Tetley, T.D.; et al. Modulation of human macrophage responses to Mycobacterium tuberculosis by silver nanoparticles of different size and surface modification. PLoS ONE 2015, 10, e0143077. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, P.M. Anti-tubercular activity of silver nanoparticle synthesized from the fruits of Coriandrum sativum Linn. World J. Pharm. Pharm. Sci. 2017, 6, 1720–1727. [Google Scholar] [CrossRef] [Green Version]
- Jaryal, N.; Kaur, H. Plumbago auriculata leaf extract-mediated AgNPs and its activities as antioxidant, anti-TB and dye degrading agents. J. Biomater. Sci. Polym. Ed. 2017, 28, 1847–1858. [Google Scholar] [CrossRef]
- Patel, S.I.; Gohil, T.G. Biogenic silver nanoparticles as potential agent against Mycobacterium tuberculosis. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 505–511. [Google Scholar] [CrossRef]
- Ghotekar, S.; Savale, A.; Pansambal, S. Phytofabrication of fluorescent silver nanoparticles from Leucaena leucocephala L. leaves and their biological activities. J. Water Environ. Nanotechnol. 2018, 3, 95–105. [Google Scholar]
- Punjabi, K.; Mehta, S.; Chavan, R.; Chitalia, V.; Deogharkar, D.; Deshpande, S. Efficiency of biosynthesized silver and zinc nanoparticles against multi-drug resistant pathogens. Front. Microbiol. 2018, 9, 2207. [Google Scholar] [CrossRef] [Green Version]
- Donnellan, S.; Giardiello, M. Nanomedicines towards targeting intracellular Mtb for the treatment of tuberculosis. J. Interdiscip. Nanomed. 2019, 4, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, K.S.; Husen, A. Fabrication of metal nanoparticles from fungi and metal salts: Scope and application. Nanoscale Res. Lett. 2016, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Devi Rajeswari, V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef] [PubMed]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostruct. Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Saravanakumar, K.; Jin, T.; Wang, M.H. Mycosynthesis, characterization, anticancer and antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces purpureogenus. Int. J. Nanomed. 2019, 14, 3427–3438. [Google Scholar] [CrossRef] [Green Version]
- Jaidev, L.R.; Narasimha, G. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. Coll. Surf. B Biointerfaces 2010, 81, 430–433. [Google Scholar] [CrossRef]
- Das, S.K.; Khan, M.M.; Guha, A.K.; Das, A.R.; Mandal, A.B. Silver-nano biohybride material: Synthesis, characterization and application in water purification. Bioresour. Technol. 2012, 124, 495–499. [Google Scholar] [CrossRef]
- Devi, T.P.; Kulanthaivel, S.; Kamil, D.; Borah, J.L.; Prabhakaran, N.; Srinivasa, N. Biosynthesis of silver nanoparticles from Trichoderma species. Indian J. Exp. Biol. 2013, 51, 543–547. [Google Scholar]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galsiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar]
- Singh, D.; Rathod, V.; Ninganagouda, S.; Hiremath, J.; Singh, A.K.; Mathew, J. Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg. Chem. Appl. 2014, 2014, 408021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.R. Antibacterial activity of silver nanoparticles synthesized from Fusarium semitectum and green extracts. Int. J. Sci. Eng. Res. 2014, 2, 140–145. [Google Scholar]
- Rahimi, G.; Alizadeh, F.; Khodavandi, A. Mycosynthesis of silver nanoparticles from Candida albicans and its antibacterial activity against Escherichia coli and Staphylococcus aureus. Trop. J. Pharm. Res. 2016, 15, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Nano-silver biosynthesis using culture supernatant of Penicillium politans NRC510: Optimization, characterization and its antimicrobial activity. Int. J. Chem. Tech. Res. 2016, 9, 433–444. [Google Scholar]
- Govindappa, M.; Farheen, H.; Chandrappa, C.P.; Rai, R.V.; Raghavendra, V.B. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035014. [Google Scholar] [CrossRef]
- Shende, S.; Gade, A.; Rai, M. Large-scale synthesis and antibacterial activity of fungal-derived silver nanoparticles. Environ. Chem. Lett. 2016, 15, 427–434. [Google Scholar] [CrossRef]
- Borthakur, M.; Gogoi, J.; Joshi, S.R. Macro and Micro-fungi mediated synthesis of silver. Adbu J. Eng. Technol. 2017, 6, A5. [Google Scholar]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Application of response surface methodology to optimize the extracellular fungal mediated nanosilver green synthesis. J. Genet. Eng. Biotechnol. 2017, 15, 497–504. [Google Scholar] [CrossRef]
- Singh, D.K.; Kumar, J.; Sharma, V.K.; Verma, S.K.; Singh, A.; Kumari, P.; Kharwar, R.N. Mycosynthesis of bactericidal silver and polymorphic gold nanoparticles–physicochemical variation effects and mechanism. Nanomedicine 2018, 13, 191–207. [Google Scholar] [CrossRef]
- Bhangale, H.; Patil, D. Mycosynthesis of silver nanoparticles, their characterization and antimicrobial activity. IOSR J. Appl. Chem. 2018, 11, 49–52. [Google Scholar]
- Elsayed, M.A.; Othman, A.M.; Hassan, M.M.; Elshafei, A.M. Optimization of silver nanoparticles biosynthesis mediated by Aspergillus niger NRC1731 through application of statistical methods: Enhancement and characterization. 3 Biotech 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.M.; Elsayed, M.A.; Al-Balakocy, N.G.; Hassan, M.M.; Elshafei, A.M. Biosynthesis and characterization of silver nanoparticles induced by fungal proteins and its application in different biological activities. J. Genet. Eng. Biotechnol. 2019, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Qaralleh, H.; Khleifat, K.M.; Al-Limoun, M.O.; Alzedaneen, F.Y.; Al-Tawarah, N. Antibacterial and synergistic effect of biosynthesized silver nanoparticles using the fungi Tritirachium oryzae W5H with essential oil of Centaurea damascena to enhance conventional antibiotics activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025016. [Google Scholar] [CrossRef]
- Hassan, S.A.; Hanif, E.; Asif, E.; Anis, H.; Hussain, H.M. Mycobiosynthesis and characterization of silver nanoparticles and its antimicrobial activity. Int. J. Biol. Biotechnol. 2019, 16, 255–259. [Google Scholar]
- Mohamed, N.H.; Ismail, M.A.; Abdel-Mageed, W.M.; Shoreit, A.A.M. Antimicrobial activity of green silver nanoparticles from endophytic fungi isolated from Calotropis procera (Ait) latex. Microbiology 2019, 165, 967–975. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Germano-Costa, T.; Pasquoto-Stigliani, T.; Fraceto, L.F.; de Lima, R. Biosynthesis of silver nanoparticles employing Trichoderma harzianum with enzymatic stimulation for the control of Sclerotinia sclerotiorum. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Banu, A.; Rathod, V. Biosynthesis of monodispersed silver nanoparticles and their activity against Mycobacterium tuberculosis. J. Nanomed. Biother. Discov. 2013, 3, 2. [Google Scholar] [CrossRef]
- Mohanty, S.; Jena, P.; Mehta, R.; Pati, R.; Banerjee, B.; Patil, S.; Sonawane, A. Cationic antimicrobial peptides and biogenic silver nanoparticles kill mycobacteria without eliciting DNA damage and cytotoxicity in mouse macrophages. Antimicrob. Agents Chemother. 2013, 57, 3688–3698. [Google Scholar] [CrossRef] [Green Version]
- Sivaraj, A.; Kumar, V.; Sunder, R.; Parthasarathy, K.; Kasivelu, G. Commercial yeast extracts mediated green synthesis of silver chloride nanoparticles and their anti-mycobacterial activity. J. Clust. Sci. 2020, 31, 287–291. [Google Scholar] [CrossRef]
- De Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef]
- Ramachandran, R.; Krishnaraj, C.; Kumar, V.A.; Harper, S.L.; Kalaichelvan, T.P.; Yun, S.I. In vivo toxicity evaluation of biologically synthesized silver nanoparticles and gold nanoparticles on adult zebrafish: A comparative study. 3 Biotech 2018, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; MubarakAli, D.; Velusamy, P.; Vadivelu, J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2017, 10, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Narayanasamy, P.; Switzer, B.L.; Britigan, B.E. Prolonged-acting, multi-targeting gallium nanoparticles potently inhibit growth of both HIV and mycobacteria in co-infected human macrophages. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, B.N.; Taranath, T.C. Limonia acidissima L. leaf mediated synthesis of zinc oxide nanoparticles: A potent tool against Mycobacterium tuberculosis. Int. J. Mycobacteriol. 2016, 5, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.R.; Britigan, B.E.; Narayanasamy, P. Ga (III) nanoparticles inhibit growth of both Mycobacterium tuberculosis and HIV and release of interleukin-6 (IL-6) and IL-8 in coinfected macrophages. Antimicrob. Agents Chemother. 2017, 61, e02505–e02516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingam, V.; Sundaramahalingam, S.; Rajaram, R. Size-dependent antimycobacterial activity of titanium oxide nanoparticles against Mycobacterium tuberculosis. J. Mater. Chem. B 2019, 7, 4338–4346. [Google Scholar] [CrossRef]
- Estevez, H.; Palacios, A.; Gil, D.; Anguita, J.; Vallet-Regi, M.; González, B.; Prados-Rosales, R.; Luque-Garcia, J.L. Antimycobacterial effect of selenium nanoparticles on Mycobacterium tuberculosis. Front. Microbiol. 2020, 11, A800. [Google Scholar] [CrossRef]
- Kreytsberg, G.N.; Gracheva, I.E.; Kibrik, B.S.; Golikov, I.V. Antituberculous effect of silver nanoparticles. J. Phys. Conf. Ser. 2011, 291, 012030. [Google Scholar] [CrossRef]
- El Zowalaty, M.E.; Al Ali, S.H.H.; Husseiny, M.I.; Geilich, B.M.; Webster, T.J.; Hussein, M.Z. The ability of streptomycin-loaded chitosan-coated magnetic nanocomposites to possess antimicrobial and antituberculosis activities. Int. J. Nanomed. 2015, 10, 3269–3274. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Sil, A.K. Synthesis of chiral gold nanoparticle by direct reduction with L and D-serine and enhanced anti-mycobacterial activity by D-serine protected gold nanoparticle. Mod. Chem. Appl. 2015, 3, 4. [Google Scholar] [CrossRef]
- Jafari, A.R.; Mosavi, T.; Mosavari, N.; Majid, A.; Movahedzade, F.; Tebyaniyan, M.; Kamalzadeh, M.; Dehgan, M.; Jafari, S.; Arastoo, S. Mixed metal oxide nanoparticles inhibit growth of Mycobacterium tuberculosis into THP-1 cells. Int. J. Mycobacteriol. 2016, 5, S181–S183. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Oh, S.; Kim, J.; Kato, T.; Kim, H.J.; Lee, J.; Park, E.Y. Enhanced internalization of macromolecular drugs into Mycobacterium smegmatis with the assistance of silver nanoparticles. J. Microbiol. Biotechnol. 2017, 27, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; Chiappi, M.; García-Trenco, A.; Al-Ejji, M.; Sarkar, S.; Georgiou, T.K.; Shaffer, M.S.; Tetley, T.D.; Schwander, S.; Ryan, M.P.; et al. Multimetallic microparticles increase the potency of rifampicin against intracellular Mycobacterium tuberculosis. ACS Nano 2018, 12, 5228–5240. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, P.; Ghosh, M.; Brar, B.; Kumar, R.; Lambe, U.P.; Ranjan, K.; Manoj, J.; Prasad, G. Nano-antimicrobials: A new paradigm for combating mycobacterial resistance. Curr. Pharm. Des. 2019, 25, 1554–1579. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Verma, A.; Yadav, K. Fungal nanoparticles: An emerging tool in medical biology. In Advance Appliance through Fungal Nanobiotechnol; Springer: Cham, Switzerland, 2016; pp. 213–240. [Google Scholar]
- Simões, M.F.; Pereira, L.; Santos, C.; Lima, N. Polyphasic identification and preservation of fungal diversity: Concepts and applications. In Management of Microbial Resources in the Environment; Springer: Dordrecht, The Netherlands, 2013; pp. 91–117. [Google Scholar]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. In Fungal Kingd; Willey: Hoboken, NJ, USA, 2017; pp. 79–95. [Google Scholar]
- Larimer, C.; Islam, M.S.; Ojha, A.; Nettleship, I. Mutation of environmental mycobacteria to resist silver nanoparticles also confers resistance to a common antibiotic. BioMetals 2014, 27, 695–702. [Google Scholar] [CrossRef]
- Mourato, A.; Gadanho, M.; Lino, A.R.; Tenreiro, R. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg. Chem. Appl. 2011, 2011, 546074. [Google Scholar] [CrossRef]
- Jelenko, C. Silver nitrate resistant E. coli: Report of case. Ann. Surg. 1969, 170, 296. [Google Scholar] [CrossRef]
- Glaziou, P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. MedRxiv 2020. [Google Scholar] [CrossRef]
| AgNPs Size | AgNPs Shape | Synthesis Process | Highlights | Ref. |
|---|---|---|---|---|
| 20–25 nm | Spherical | Chemically synthesized | Activity against M. smegmatis (strain mc2155, MIC = 0.46 μg/mL) and M. bovis (strain BCG, MIC = 1.1 μg/mL) | [63] |
| 5–45 nm | Spherical | Capped with bovine serum albumin (BSA), and poly-n-vinyl-pyrrolidone (PVP) | Activity of BSA-AgNPs and PVP-AgNPs against M. xenopi | [64] |
| 55 and 278 nm | Spherical | Stabilized with chitosan | In vitro activity against M. smegmatis mc2155 and antibiofilm activity, ex vivo (raw 264.7 macrophages) antimycobacterial activity, noncytotoxic to macrophages, disruption of mycobacteria cell wall | [65] |
| 25–45 nm | Spherical and monodispersed | Chemically synthesized with photo-irradiation | Activity against M. bovis (strain BCG) | [66] |
| ≈20 nm | Spherical and monodispersed | Synthesized with 1% starch | In vitro antimycobacterial activity against M. smegmatis mc2155, ex vivo (raw 264.7 macrophages) antimycobacterial activity, noncytotoxic to macrophages, inhibition of biofilm formation | [67] |
| ≈70 nm | Spherical with agglomeration | Chemically synthesized | Bactericidal effect against M. smegmatis when the AgNPs were complemented with 2% chloroform | [41] |
| 6.9–18.3 nm | Spherical | Commercially acquired | Bactericidal effect when applied as coatings on polycarbonate membranes, against biofilms of M. smegmatis, M. avium, and M. marinum | [53] |
| 30–130 nm | Spherical-oval | Biologically synthesized from leafs of Ipomea carnea | Activity against M. smegmatis | [68] |
| NR | NR | Biologically synthesized from leaves of Psidium guajava L. | Activity against M. smegmatis and M. pheli | [57] |
| 1–5 nm | Spherical-oval | Chemically synthesized | Activity against M. bovis (strain BCG) | [56] |
| 8–12 nm | Spherical | Biologically synthesized from Acinetobacter sp. | Activity against M. bovis (strain BCG) | [56] |
| <50 nm | Spherical | Chemically synthesized | Activity against M. avium subsp. paratuberculosis | [69] |
| 38–52 nm | Spherical | Biologically synthesized from flowers of Catharanthus roseus L (apocyanaceae) | Activity against M. smegmatis, with 71% of growth inhibition | [70] |
| ≈50 nm | Tetrahedral | Chemically synthesized | Activity against M. bovis (strain BCG, MIC = 4 µg/mL) and clinical isolates of M. bovis (MIC = 4–32 µg/mL) | [26] |
| AgNPs Size | AgNPs Shape | Synthesis Process | Highlights | Ref. |
|---|---|---|---|---|
| 5–9 nm | Spherical | Conjugated with bovine serum albumin (BSA) | Activity against several drug-sensitive MTb (strain H37Rv) and clinical isolates of MTb | [64] |
| 6–45 nm | Spherical | Capped with poly-n-vinyl-pyrrolidone (PVP) | Activity against several drug-sensitive MTb (strain H37Rv) and clinical isolates of MTb | [64] |
| 10–20 nm | Spherical | Biological synthesis from extracts of cucumber (Cucumis sativus). | Activity against several drug-sensitive MTb (MIC = 7.8–12.5 µg/mL), MDR strains (MIC = 12.5 µg/mL), a XDR strain (MIC = 15.6 µg/mL), and NTM strains (MIC = 25 µg/mL) | [71] |
| NR | NR | Biologically synthesized from leaves of Psidium guajava L. | Activity against an avirulent MTb | [57] |
| ≈70 nm | Spherical with agglomeration | Chemically synthesized | Bactericidal effect against MTb, only observed when complemented with 2% chloroform | [41] |
| >200 nm | Spherical and cubic | Biological synthesis from aqueous extracts of fresh Moringa oleifera leaves and Allium cepa bulbs | Activity against MTb strains (H37Ra, a wild type drug-sensitive, and a MDR strain) | [72] |
| 1–5 nm | Spherical-oval | Chemically synthesized | In vitro activity against active MTb (strain H37Ra, MIC = 1.31 µg/mL) and dormant (MIC = 2.04 µg/mL), as well as ex vivo (in THP-1 cells) active MTb (MIC = 1.97 µg/mL) and dormant MTb (MIC = 2.18 µg/mL) | [56] |
| 8–12 nm | Spherical | Biologically synthesized from Acinetobacter sp. | In vitro activity against active and dormant MTb (strain H37Ra), as well as ex vivo (in THP-1 cells) MTb (MIC > 2.56 µg/mL for all conditions tested) | [56] |
| 20–110 nm | Spherical | Commercial AgNPs with surface modifications (citrate or poly-n-vinyl-pyrrolidone (PVP) coated) | Ex vivo (in human monocyte-derived macrophages) activity against MTb (strain H37Ra) Suppression of MTb-induced expression of IL-1β, IL-10, and TNF-α mRNA | [73] |
| 38–52 nm | Spherical | Biologically synthesized from flowers of Catharanthus roseus L (apocyanaceae) | Activity against MTb, with 57% of growth inhibition | [70] |
| 50–200 nm | Spherical and polygonal | Biologically synthesized from fruits of Coriandrum sativum | Activity against MTb (strain H37Rv, MIC = 1.56 μg/mL) | [74] |
| 15–45 nm | Spherical and with face centerd cubic geometry | Biologically synthesized from leaf extract of Plumbago auriculata | In vitro activity against MTb (MIC = 1.6 μg/mL) | [75] |
| 20–56 nm | Spherical | Biologically synthesized from flower extracts of Sesbania grandiflora | In vitro activity against MTb (strain H37Rv, MIC = 12 µg/mL) | [76] |
| 32–50 nm | Quasi-spherical | Biologically synthesized from leaves of Leucaena leucocephala L. | Monodispersed with activity against obtained for MTb (strain H37Rv, MIC = 125 μg/mL). | [77] |
| 10–70 nm | Spherical and polyhedral | Biologically synthesized from Pseudomonas hibiscicola | Polydispersed, with activity against MTb (strain H37Rv) and a clinical MDR MTb strain (MIC = 1.25 mg/mL for both strains) | [78] |
| ≈50 nm | Tetrahedral | Chemically synthesized | Activity against MTb (MIC = 1 µg/mL), clinical isolates of MTb (MIC = 1–16 µg/mL), and a MDR MTb strain (MIC = 16 µg/mL) | [26] |
| 2.8–8 nm | Spherical | Chemically synthesized | Activity against XDR MTb and MTb (strain H37Rv) (MIC = 1 μg/mL for both strains), and against a MDR-MTb strain (MIC = 4 μg/mL) | [19] |
| 11–17.5 nm | Spherical | Chemically synthesized as nanocomposites of chitosan | Activity against MTb (strain H37Ra, MIC = 1.95 µg/mL) | [27] |
| Parameter | Effects | Consequences |
|---|---|---|
| pH | Formation of nucleation centers, time of reaction and morphology (size). |
|
| Temperature | Resultant morphology (size and shape), synthesis rate, and formation of nucleation centers. |
|
| Time of reaction | Resultant morphology (size). |
|
| Concentration of chemical precursors | Resultant morphology (size). |
|
| Culture media | Quantity of MNPs. |
|
| Quantity of fungal biomass | Quantity of MNPs. |
|
| Agitation | Resultant morphology (size), quantity of MNPs, and synthesis rate. |
|
| Light intensity | Quantity of MNPs and synthesis rate. |
|
| AgNPs Size | AgNPs Shape | Fungal Species Involved in the Mycogenic Synthesis | Highlights | Ref. |
|---|---|---|---|---|
| 3–20 nm | Spherical | Rhizopus stolonifer (filamentous fungus; family Mucoraceae) | Activity against clinical isolates of MDR MTb (MIC = 6.25–12.5 µg/mL) | [107] |
| 22–50 nm | Spherical with agglomeration | Trichoderma sp. (filamentous fungus; family Hypocreaceae) | Activity at all concentrations tested (0.1, 0.5 and 1 ppm) against M. smegmatis (strain mc2155), and M. marinum, being higher for M. smegmatis. Reduced the survival of intracellular (RAW264.7 macrophages) M. smegmatis (in 35%), and M. marinum (in 5%) | [108] |
| ≈17 nm sized silver chloride (AgCl) NPs | Spherical | Commercial yeast extract | AgClNPs (37 μg/mL), with activity against M. smegmatis (strain mc2155) and MTb (strain H37Rv) | [109] |
| MNP Type | MNP Morphology | Synthesis Process | Highlights | Ref. |
|---|---|---|---|---|
| Gold (AuNPs) | 15–30 nm sized, spherical and monodispersed | Chemically synthesized, stabilized with citrate, and Poly-allylamine hydrochloride (PAH) | Both citrate-AuNPs and PAH-AuNPs have activity against M. bovis (strain BCG), lower than tested AgNPs | [66] |
| Copper (CuNPs) | NR | Biologically synthesized from leaves of Psidium guajava L. | Activity against MTb, M. smegmatis, and M. pheli, but lower than other MNPs | [57] |
| Gallium (GaNPs) | 305 nm sized and cylindrical | Chemically synthesized by double emulsification and sonication | Polydispersed, with prolonged activity against intracellular M. smegmatis | [113] |
| Copper oxide and zinc oxide (Cu(II)ONPs and ZnONPs) | Spherical | Chemically synthesized | Activity against M. avium subsp. paratuberculosis | [69] |
| Bimetallic Silver-Gold (Au-AgNPs) | 10–70 nm sized and polydispersed | Phyto-synthesized from Barleria prionitis | In vitro activity against MTb (strain H37Ra, active–MIC = 0.06–0.12 μg/mL, and dormant–MIC = 1.05–2.53 μg/mL) and M. bovis (strain BCG, active–MIC = 0.32–0.42 μg/mL, and dormant–MIC = 0.32–0.64 μg/mL), as well as ex vivo in THP-1 cells infected with MTb (active–MIC = 0.63–1.46 μg/mL, and dormant–MIC = 0.56–2.16 μg/mL) Higher activity than AuNPs or AgNPs (MIC ≈ 2.5 μg/mL), and more specific for mycobacteria with a higher selectivity index In addition, the smaller MNPs (from S. cumini) are more effective | [60] |
| 90 nm sized and hexagonal | Phyto-synthesized from Plumbago zeylanica | |||
| 10–20 nm sized and spherical | Phyto-synthesized from Syzygium cumini | |||
| Zinc oxide (ZnONPs) | 12–53 nm sized and spherical | Biologically synthesized from leaves of Limonia acidissima Linn. also known as Feronia elephantum Correa or wood apple | Activity against MTb (strain H37Rv) | [114] |
| GaNPs | ≈300 nm sized and cylindrical | Chemically synthesized by double emulsification and sonication | Activity against intracellular MTb (strain H37Ra) in monocyte-derived macrophage (MDMs) and THP-1 macrophages (up to 70% MTb growth inhibition) | [115] |
| Zinc (ZnNPs) | ≈60 nm sized and variable shapes, mostly spherical | Biologically synthesized from Pseudomonas hibiscicola | Activity against MTb (strain H37Rv) and a clinical MDR MTb strain, (MIC = 1.25 mg/mL for both strains) | [78] |
| Titanium dioxide (TiO2NPs) | 16 nm sized and spherical | Chemically synthesized | Inhibited the growth of a clinical isolate of MTb (61%), and a clinical isolate of M. bovis (74%), at a concentration of 100 μg/mL. Effective surface coaters on inhibiting mycobacterial biofilm formation | [116] |
| Zinc oxide (ZnONPs) | 5.4–13.2 nm sized and spherical | Chemically synthesized | Activity against MTb (strain H37Rv) and XDR MTb strains (MIC = 1 μg/mL for both), and MDR MTb (MIC = 4 μg/mL) | [19] |
| Selenium (SeNPs) | Spherical | Chemically synthesized | Activity against M. smegmatis (MIC = 0.4 μg/mL), and MTb (MIC = 0.195 μg/mL). Low toxicity (compared to other MNPs) and involvement in reduction of the integrity of the mycobacterial cell envelope. Colloidally stable | [117] |
| MNP Type | MNP Morphology | Combination | Highlights | Ref. |
|---|---|---|---|---|
| AgNPs | 250–300 nm sized and spherical | With commercial Titanium dioxide (TiO2) NPs | Activity against M. smegmatis mc2155 (MIC > 100 μg/mL for 10:1 ratio, and MIC = 5 ± 2.4μg/mL for 50:1 ratio), and M. bovis (strain BCG, MIC = 11 μg/mL for 50:1 ratio) | [63] |
| AgNPs | 5–50 nm sized | 25 mg/Kg of AgNPs with 50 mg/Kg isoniazid | In vivo activity, in mice infected with a MDR MTb strain, led to a higher survival rate of 95% | [118] |
| AgNPs | 22–50 nm sized, spherical with agglomeration | With cationic antimicrobial peptides, NK-2 (7 μg/mL) and LLKK-18 (1 μg/mL) at sub-lethal doses | Activity against M. smegmatis mc2155 and M. marinum increased after conjugation | [108] |
| With rifampicin (RIF; 0.7 μg/mL) | Activity against M. smegmatis mc2155 and M. marinum increased after conjugation | |||
| AgNPs | ≈70 nm sized | With chloroform | Increased antimycobacterial activity when compared to AgNPs without chloroform | [41] |
| FeNPs nanocomposites | Magnetic | With chitosan and loaded with streptomycin | Successfully used against MTb and other microorganisms, showing higher activity than FeNPs and even FeNPs nanocomposites with chitosan | [119] |
| AuNPs | 52.8 ± 5.33 nm sized and hexagonal | With both D- and L-enantiomeric forms of the amino acid serine | Active against M. smegmatis (strain mc2155) showing higher activity than D-serine alone, which is also known to be active against other mycobacterial species | [120] |
| AgNPs | 30–80 nm sized | With ZnONPs | Increased MIC, when compared with the individual NPs, against MTb (strain H37Rv) both in vitro and ex vivo assays using THP-1 cells | [121] |
| AgNPs | 17 ± 3 nm sized and spherical | Conjugated with vancomycin (VAN) (increased size of 30 ± 3 nm) | Activity against M. smegmatis with improved cell internalization by the conjugate in comparison with AgNPs and VAN on their own | [122] |
| AgNPs | 20 nm sized and spherical | With ZnONPs | Ex vivo (in THP-1 macrophages) activity against MTb | [123] |
| With ZnNPs and RIF | Increased (76% more than RIF on its own) ex vivo (in THP-1 macrophages) activity against MTb | |||
| AuNPs | 15 ±2 nm sized and spherical | With partial peptide tagged on the surface | Increased activity when compared to the peptide or the AuNPs on their own The attachment of the peptide increased the size of the NPs (to 20 ± 4 nm) and the inhibition of intracellular MTb (strain H37Rv) growth, from 45% for the peptide, to 59% for the AuNPs, to 91% for the peptide-AuNPs (all at 1 μg/mL) | [46] |
| AgNPs | 2.8–8 nm sized and spherical | With ZnONPs (ratios 5:5, 2:8, 8:2, 3:7 and 7:3) | Activity against XDR MTb (MIC = 1 μg/mL for all ratios tested), MTb (strain H37Rv, MIC = 1–32 μg/mL), and against a MDR MTb strain (MIC = 4–64 μg/mL) | [19] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões, M.F.; Ottoni, C.A.; Antunes, A. Mycogenic Metal Nanoparticles for the Treatment of Mycobacterioses. Antibiotics 2020, 9, 569. https://doi.org/10.3390/antibiotics9090569
Simões MF, Ottoni CA, Antunes A. Mycogenic Metal Nanoparticles for the Treatment of Mycobacterioses. Antibiotics. 2020; 9(9):569. https://doi.org/10.3390/antibiotics9090569
Chicago/Turabian StyleSimões, Marta Filipa, Cristiane Angélica Ottoni, and André Antunes. 2020. "Mycogenic Metal Nanoparticles for the Treatment of Mycobacterioses" Antibiotics 9, no. 9: 569. https://doi.org/10.3390/antibiotics9090569
APA StyleSimões, M. F., Ottoni, C. A., & Antunes, A. (2020). Mycogenic Metal Nanoparticles for the Treatment of Mycobacterioses. Antibiotics, 9(9), 569. https://doi.org/10.3390/antibiotics9090569







