Synergy of the Bacteriocin AS-48 and Antibiotics against Uropathogenic Enterococci
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterization of Uropathogenic Enterococcal Strains
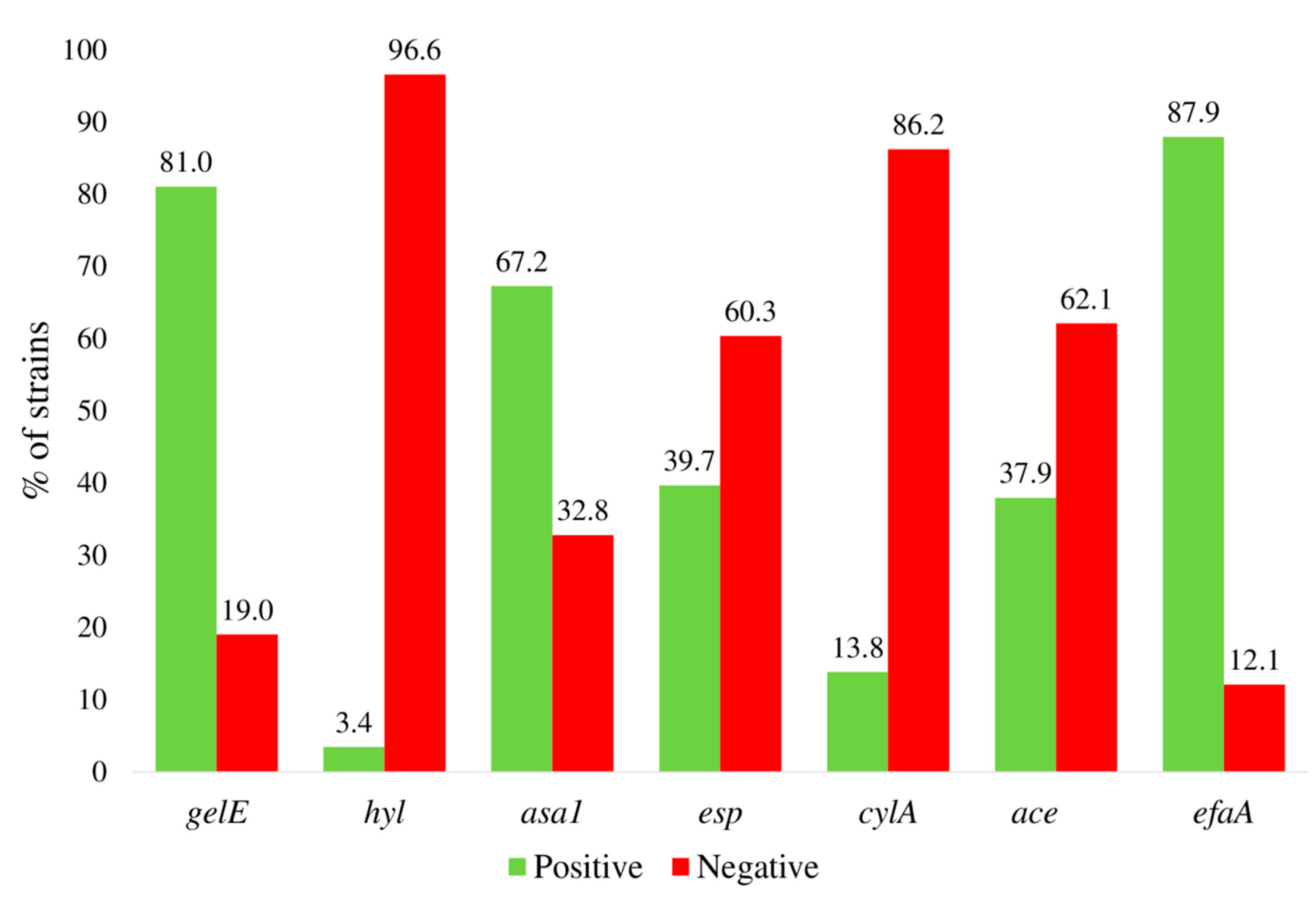
2.2. Determination of Virulence Factors in the Clinical Isolates
2.3. Antibiotic Sensitivity of the Clinical Isolates
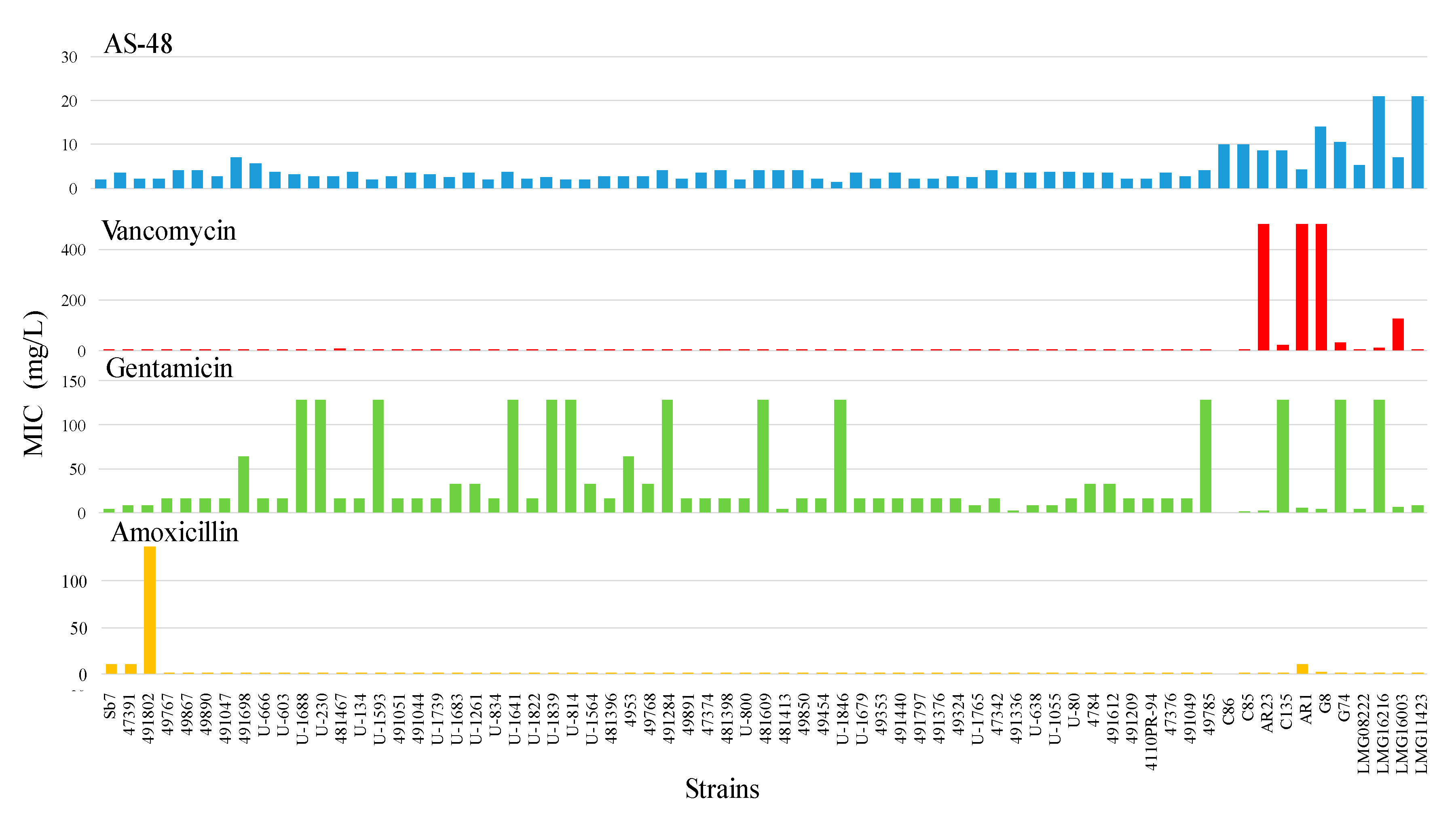
2.4. Broth Microdilution MIC Determination
2.5. Synergy between AS-48 and Antibiotics of Clinical Use
2.5.1. Combination of AS-48 and Vancomycin
2.5.2. Combination of AS-48 and Gentamicin
2.5.3. Combination of AS-48 and Amoxicillin/Clavulanate
3. Discussion
4. Materials and Methods
4.1. Isolation of Clinical Bacterial Strains and Growth Conditions
4.2. Genotyping and Molecular Identification
4.2.1. RAPD
4.2.2. Species Identification
4.3. Purification of the Bacteriocin AS-48
4.4. Determination of the Minimal Inhibitory Concentration (MIC)
4.5. Determination of the Synergy between AS-48 and Antibiotics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Brien, V.P.; Hannan, T.J.; Nielsen, H.V.; Hultgren, S.J. Drug and vaccine development for the treatment and prevention of urinary tract infections. Microbiol. Spectr. 2016, 4. UTI-0013-2012. [Google Scholar] [CrossRef]
- Abat, C.; Huart, M.; Garcia, V.; Dubourg, G.; Raoult, D. Enterococcus faecalis urinary-tract infections: Do they have a zoonotic origin? J. Infect. 2016, 73, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Potretzke, A.; Schreiber, H.L.; Pinkner, J.S.; Bauman, T.M.; Park, A.M.; Desai, A.; Hultgren, S.J.; Caparon, M.G. Antibody-based therapy for enterococcal catheter-associated urinary tract infections. mBio 2016, 7, e01653-16. [Google Scholar] [CrossRef] [PubMed]
- Souhail, B.; Le Maréchal, M.; Manuello, R.; Chrétien, R.; Charlot, P.; Déroudilhes, G.; Della Guardia, M.; Blanc, V.; Fribourg, A.; Degand, N.; et al. Antibiotic therapy for Enterococcus bacteraemia: Warning for the antimicrobial stewardship team. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2087–2095. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef]
- Abbas, M.; Paul, M.; Huttner, A. New and improved? A review of novel antibiotics for Gram-positive bacteria. Clin. Microbiol. Infect. 2017, 23, 697–703. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting pathogenic bacteria on two fronts: Phages and antibiotics as combined strategy. Front. Cell Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: Resistance is futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef]
- WHO|Critically Important Antimicrobials for Human Medicine, 5th Revision. Available online: http://www.who.int/foodsafety/publications/antimicrobials-fifth/en/ (accessed on 6 July 2020).
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://apo.org.au/node/63983 (accessed on 19 May 2016).
- Loose, M.; Link, I.; Naber, K.G.; Wagenlehner, F.M.E. Carbapenem-containing combination antibiotic therapy against carbapenem-resistant uropathogenic Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 64, e01839-19. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Maqueda, M.; Gálvez, A.; Bueno, M.M.; Sanchez-Barrena, M.J.; González, C.; Albert, A.; Rico, M.; Valdivia, E. Peptide AS-48: Prototype of a new class of cyclic bacteriocins. Curr. Protein Pept. Sci. 2004, 5, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hidalgo, M.; Montalbán-López, M.; Cebrián, R.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. AS-48 bacteriocin: Close to perfection. Cell. Mol. Life Sci. 2011, 68, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barrena, M.J.; Martınez-Ripoll, M.; Gálvez, A.; Valdivia, E.; Maqueda, M.; Cruz, V.; Albert, A. Structure of bacteriocin AS-48: From soluble state to membrane bound state. J. Mol. Biol. 2003, 334, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Abengózar, M.Á.; Cebrián, R.; Saugar, J.M.; Gárate, T.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M.; Rivas, L. Enterocin AS-48 as evidence for the use of bacteriocins as new leishmanicidal agents. Antimicrob. Agents Chemother. 2017, 61, e02288. [Google Scholar] [CrossRef]
- Cebrián, R.; Arévalo, S.; Rubiño, S.; Arias-Santiago, S.; Rojo, M.D.; Montalbán-López, M.; Martínez-Bueno, M.; Valdivia, E.; Maqueda, M. Control of Propionibacterium acnes by natural antimicrobial substances: Role of the bacteriocin AS-48 and lysozyme. Sci. Rep. 2018, 8, 11766. [Google Scholar] [CrossRef]
- Martínez-García, M.; Bart, J.-M.; Campos-Salinas, J.; Valdivia, E.; Martínez-Bueno, M.; González-Rey, E.; Navarro, M.; Maqueda, M.; Cebrián, R.; Pérez-Victoria, J.M. Autophagic-related cell death of Trypanosoma brucei induced by bacteriocin AS-48. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 203–212. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Cebrián, R.; Martín-Escolano, J.; Rosales, M.J.; Maqueda, M.; Sánchez-Moreno, M.; Marín, C. Insights into Chagas treatment based on the potential of bacteriocin AS-48. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Cebrián, R.; Maqueda, M.; Romero, D.; Rosales, M.J.; Sánchez-Moreno, M.; Marín, C. Assessing the effectiveness of AS-48 in experimental mice models of Chagas’ disease. J. Antimicrob. Chemother. 2020, 75, 1537–1545. [Google Scholar] [CrossRef]
- Perales-Adán, J.; Rubiño, S.; Martínez-Bueno, M.; Valdivia, E.; Montalbán-López, M.; Cebrián, R.; Maqueda, M. LAB bacteriocins controlling the food isolated (drug-resistant) Staphylococci. Front. Microbiol. 2018, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, R.; Rodríguez-Cabezas, M.E.; Martín-Escolano, R.; Rubiño, S.; Garrido-Barros, M.; Montalbán-López, M.; Rosales, M.J.; Sánchez-Moreno, M.; Valdivia, E.; Martínez-Bueno, M.; et al. Preclinical studies of toxicity and safety of the AS-48 bacteriocin. J. Adv. Res. 2019, 20, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Hasanpour, S.; Ebrahim-Saraie, H.S.; Motamedifar, M. High incidence of virulence factors among clinical Enterococcus faecalis isolates in southwestern Iran. Infect. Chemother. 2017, 49, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Kwok, L.-Y.; Hou, Q.; Sun, Y.; Li, W.; Zhang, H.; Sun, Z. Comparative genomic analysis revealed great plasticity and environmental adaptation of the genomes of Enterococcus faecium. BMC Genom. 2019, 20, 602. [Google Scholar] [CrossRef]
- Kajihara, T.; Nakamura, S.; Iwanaga, N.; Oshima, K.; Takazono, T.; Miyazaki, T.; Izumikawa, K.; Yanagihara, K.; Kohno, N.; Kohno, S. Clinical characteristics and risk factors of enterococcal infections in Nagasaki, Japan: A retrospective study. BMC Infect. Dis. 2015, 15, 426. [Google Scholar] [CrossRef]
- Kline, K.A.; Lewis, A.L. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol. Spectr. 2016, 4, UTI-0012-2012. [Google Scholar] [CrossRef]
- Kathirvel, S.; Mani, M.; Gopala Krishnan, G.K.; Sethumadhavan, A.; Vijayalakshmi, T.; Ponnan, S.M.; Hanna, L.E.; Mathaiyan, M. Molecular characterization of Enterococcus faecalis isolates from urinary tract infection and interaction between Enterococcus faecalis encountered Dendritic and Natural Killer cells. Microb. Pathog. 2020, 140, 103944. [Google Scholar] [CrossRef]
- Bittencourt De Marques, E.B.; Suzart, S. Occurrence of virulence-associated genes in clinical Enterococcus faecalis strains isolated in Londrina, Brazil. J. Med. Microbiol. 2004, 53, 1069–1073. [Google Scholar] [CrossRef]
- Zalipour, M.; Esfahani, B.N.; Halaji, M.; Azimian, A.; Havaei, S.A. Molecular characterization of vancomycin-resistant Enterococcus faecalis among inpatients at Iranian University Hospitals: Clonal dissemination of ST6 And ST422. Infect. Drug Resist. 2019, 12, 3039–3047. [Google Scholar] [CrossRef]
- Rostkowska, O.M.; Kuthan, R.; Burban, A.; Salińska, J.; Ciebiera, M.; Młynarczyk, G.; Durlik, M. Analysis of susceptibility to selected antibiotics in Klebsiella pneumoniae, Escherichia coli, Enterococcus faecalis and Enterococcus faecium causing urinary tract infections in kidney transplant tecipients over 8 Years: Single-center study. Antibiotics 2020, 9, 284. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hamasuna, R.; Uehara, S.; Yasuda, M.; Yamamoto, S.; Hayami, H.; Takahashi, S.; Matsumoto, T.; Minamitani, S.; Kadota, J.; et al. Japanese nationwide surveillance in 2011 of antibacterial susceptibility patterns of clinical isolates from complicated urinary tract infection cases. J. Infect. Chemother. 2015, 21, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.B.; Baral, R.; Poudel, P.; Khanal, B. Clinical, etiological and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatr. 2019, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Maqueda, M.; Sánchez-Hidalgo, M.; Fernández, M.; Montalbán-López, M.; Valdivia, E.; Martínez-Bueno, M. Genetic features of circular bacteriocins produced by Gram-positive bacteria. FEMS Microbiol. Rev. 2008, 32, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Pérez, C.; Gracia, B.; Rodrigues, L.; Vitoria, A.; Cebrián, R.; Deboosère, N.; Song, O.-R.; Brodin, P.; Maqueda, M.; Aínsa, J.A. Synergy between circular bacteriocin AS-48 and ethambutol against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e00359-18. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Baah, J.; Hober, D.; Jouy, E.; Rubrecht, C.; Sané, F.; Drider, D. Synergistic effect between colistin and bacteriocins in controlling Gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob. Agents Chemother. 2013, 57, 2719–2725. [Google Scholar] [CrossRef]
- Jorge, P.; Pérez-Pérez, M.; Pérez Rodríguez, G.; Pereira, M.O.; Lourenço, A. A network perspective on antimicrobial peptide combination therapies: The potential of colistin, polymyxin B and nisin. Int. J. Antimicrob. Agents 2017, 49, 668–676. [Google Scholar] [CrossRef]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-antimicrobial synergy: A medical and food perspective. Front. Microbiol. 2017, 8, 1205. [Google Scholar] [CrossRef]
- Mühlberg, E.; Umstätter, F.; Kleist, C.; Domhan, C.; Mier, W.; Uhl, P. Renaissance of vancomycin: Approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can. J. Microbiol. 2019, 66, 11–16. [Google Scholar] [CrossRef]
- Jaimee, G.; Halami, P.M. Emerging resistance to aminoglycosides in lactic acid bacteria of food origin-an impending menace. Appl. Microbiol. Biotechnol. 2016, 100, 1137–1151. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Ben Omar, N.; Castro, A.; Lucas, R.; Abriouel, H.; Yousif, N.M.K.; Franz, C.M.A.P.; Holzapfel, W.H.; Pérez-Pulido, R.; Martínez-Cañamero, M.; Gálvez, A. Functional and safety aspects of Enterococci isolated from different Spanish foods. Syst. Appl. Microbiol. 2004, 27, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Ruiz-Larrea, F.; Zarazaga, M.; Alonso, A.; Martinez, J.L.; Torres, C. Macrolide resistance genes in Enterococcus spp. Antimicrob. Agents Chemother. 2000, 44, 967–971. [Google Scholar] [CrossRef]
- Eaton, T.J.; Gasson, M.J. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 2001, 67, 1628–1635. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Martín-Platero, A.M.; Valdivia, E.; Maqueda, M.; Martínez-Bueno, M. Fast, convenient, and economical method for isolating genomic DNA from lactic acid bacteria using a modification of the protein “salting-out” procedure. Anal. Biochem. 2007, 366, 102–104. [Google Scholar] [CrossRef]
- Martín-Platero, A.M.; Maqueda, M.; Valdivia, E.; Purswani, J.; Martínez-Bueno, M. Polyphasic study of microbial communities of two Spanish farmhouse goats’ milk cheeses from Sierra de Aracena. Food Microbiol. 2009, 26, 294–304. [Google Scholar] [CrossRef]
- Michener, C.D.; Sokal, R.R. A quantitative approach to a problem in classification. Evolution 1957, 11, 130–162. [Google Scholar] [CrossRef]
- Deasy, B.M.; Rea, M.C.; Fitzgerald, G.F.; Cogan, T.M.; Beresford, T.P. A rapid PCR based method to distinguish between Lactococcus and Enterococcus. Syst. Appl. Microbiol. 2000, 23, 510–522. [Google Scholar] [CrossRef]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Fernández-Cuenca, F.; Martínez-Martínez, L.; Pascual, A.; De Cueto, M.; Gutiérrez, O.; Nieto, J.; Perea, E.J. Evaluation of the WIDER I system for antimicrobial susceptibility testing of clinical isolates of Haemophilus influenzae and Streptococcus pneumoniae. Clin. Microbiol. Infect. 2003, 9, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, M.-C.; Park, S.-J.; Kim, H.S.; Sung, H.; Kim, M.-N.; Kim, S.-H.; Lee, S.-O.; Choi, S.-H.; Woo, J.H.; et al. In vitro synergistic activity of antimicrobial agents in combination against clinical isolates of colistin-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 6774–6779. [Google Scholar] [CrossRef]
- Eckert, R. Road to clinical efficacy: Challenges and novel strategies for antimicrobial peptide development. Future Microbiol. 2011, 6, 635–651. [Google Scholar] [CrossRef] [PubMed]

| Genotype | No. Strains | % of Strains | Total % of Strains |
|---|---|---|---|
| gelE | 1 | 1.72 | 6.89 (1) a |
| hyl | 1 | 1.72 | |
| asa1 | 1 | 1.72 | |
| efaA | 1 | 1.72 | |
| gelE + ace | 1 | 1.72 | 19.96 (2) a |
| gelE + efaA | 6 | 10.34 | |
| asa1 + esp | 3 | 5.17 | |
| esp + efaA | 1 | 1.72 | |
| gelE + hyl + efaA | 1 | 1.72 | 27.58 (3) a |
| gelE + asa1 + efaA | 9 | 15.52 | |
| gelE + esp + efaA | 2 | 3.45 | |
| gelE + ace + efaA | 2 | 3.45 | |
| asa1 + esp + efaA | 1 | 1.72 | |
| asa1 + ace + efaA | 1 | 1.72 | |
| gelE + asa1 + esp + efaA | 6 | 10.34 | 27.58 (4) a |
| gelE + asa1 + cylA + efaA | 2 | 3.45 | |
| gelE + asa1 + ace + efA | 5 | 8.62 | |
| gelE + esp + ace + efaA | 2 | 3.45 | |
| asa1 + cylA + ace + efaA | 1 | 1.72 | |
| asa1 + esp + cy l+ ace + efaA | 1 | 1.72 | 13.79 (5) a |
| gelE + asa1 + esp + ace + efaA | 5 | 8.62 | |
| gelE + asa1 + cylA + ace + efaA | 2 | 3.45 | |
| gelE + asa1 + esp + cylA + ace + efaA | 3 | 5.17 | 5.17 (6) a |
| MIC (mg/L) | ||||
|---|---|---|---|---|
| AS-48 | Van | Gent | Amo/Cla | |
| E. gallinarum C86 | 10 | 7.8 | 1.5 | 2.7 |
| E. casseliflavus C85 | 10 | 1.9 | 0.5 | 0.3 |
| E. durans AR23 | 8.7 | >500 | 2 | 0.7 |
| E. faecium C135 | 8.7 | 20.8 | >128 | 0.3 |
| E. faecium AR1 | 4.4 | >500 | 5 | 10.9 |
| E. faecium G8 | 14 | >500 | 4 | 2.7 |
| E. faecium LMG11423 | 21 | 1.6 | 8 | 1.4 |
| E. faecalis LMG08222 | 5.2 | 3.9 | 4 | 1.4 |
| E. faecalis LMG16216 | 21 | 12 | >128 | 0.7 |
| E. faecalis LMG16003 | 7 | 125 | 6 | 1.4 |
| E. faecalis G74 | 10.5 | 31.3 | >128 | 0.7 |
| Strain | VanR Gen | Van (1) | AS-48 (1) | Van (2) | AS-48 (2) | FICI |
|---|---|---|---|---|---|---|
| U-666 | - | 3.91 | 5.67 | 4.7 | 0.02 | 1.21 |
| U-1055 | - | 3.91 | 3.78 | 3.9 | 4.25 | 2.12 |
| U-1641 | - | 3.91 | 3.78 | 3.9 | 4.25 | 2.12 |
| U-230 | - | 3.91 | 2.83 | 1.95 | 4.25 | 2 |
| 481467 | - | 7.81 | 2.83 | 3.9 | 0.27 | 0.59 |
| 47374 | - | 4.56 | 3.54 | 0.03 | 1.05 | 0.3 |
| 481413 | - | 2.6 | 4.25 | 0.03 | 2.1 | 0.51 |
| 49324 | - | 3.91 | 2.83 | 0.03 | 2.1 | 0.75 |
| 49768 | - | 3.91 | 2.83 | 3.9 | 1.45 | 1.51 |
| 49785 | - | 2.6 | 4.25 | 0.03 | 0.5 | 0.13 |
| 49890 | - | 2.6 | 4.25 | 0.03 | 0.5 | 0.13 |
| 491698 | - | 3.26 | 7.08 | 0.03 | 1 | 0.15 |
| E. gallinarum C86 | vanC1 | 7.8 | 10 | 0.97 | 1.3 | 0.25 |
| E. casseliflavus C85 | vanC2 | 1.9 | 10 | 0.48 | 0.16 | 0.27 |
| E. durans AR23 | vanA | >500 | 8.7 | 62.5 | 1.3 | <0.27 * |
| E. faecium C135 | vanB | 20.8 | 8.7 | 1.9 | 0.02 | 0.09 |
| E. faecium AR1 | vanA | >500 | 4.4 | 250 | 0.65 | <0.65 * |
| E. faecium G8 | vanA | >500 | 14 | 31 | 0.32 | <0.08 * |
| E. faecium LMG11423 | ND | 1.6 | 21 | 0.12 | 0.32 | 0.09 |
| E. faecalis G74 | vanB | 31.3 | 10.5 | 15.1 | 0.32 | 0.51 |
| E. faecalis LMG08222 | ND | 3.9 | 5.2 | 0.24 | 0.16 | 0.09 |
| E. faecalis LMG16216 | vanB | 12 | 21 | 0.97 | 0.65 | 0.11 |
| E. faecalis LMG16003 | ND | 125 | 7 | 7.8 | 0.04 | 0.07 |
| Strains | Gent (1) | AS-48 (1) | Gent (2) | AS-48 (2) | FICI |
|---|---|---|---|---|---|
| U-1688 | 128 | 3.15 | 0.25 | 3.15 | 1 |
| U-1593 | 128 | 1.9 | 0.25 | 1.9 | 1 |
| U-1641 | 128 | 3.8 | 0.25 | 3.8 | 1 |
| U-230 | 128 | 2.83 | 0.5 | 2.83 | 1 |
| U-1839 | 128 | 4.25 | 0.25 | 4.25 | 1 |
| U-814 | 128 | 1.89 | 128 | 1.89 | 2 |
| U-1846 | 128 | 1.89 | 64 | 1.89 | 1.51 |
| 481609 | 128 | 4.25 | 32 | 1.06 | 0.5 |
| 491284 | 128 | 2.5 | 0.25 | 2.5 | 1 |
| 49785 | 128 | 4.25 | 16 | 1.15 | 0.4 |
| E. faecium C135 | 128 | 20.8 | 8 | 2.6 | 0.19 |
| E. faecalis G74 | 128 | 31.3 | 0.25 | 3.9 | 0.13 |
| E. faecalis LMG16216 | 128 | 21 | 2 | 10.5 | 0.52 |
| Strain | Amo/Cla (1) | AS-48 (1) | Amo/Cla (2) | AS-48 (2) | FICI |
|---|---|---|---|---|---|
| Sb-7 | 10.9 | 1.9 | 0.01 | 1.9 | 1 |
| 47391 | 10.93 | 3.54 | 5.47 | 3.5 | 1.49 |
| 491802 | 136.71 | 2.12 | 17 | 0.25 | 0.24 |
| E. faecium AR1 | 10.9 | 4.4 | 0.09 | 0.34 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalbán-López, M.; Cebrián, R.; Galera, R.; Mingorance, L.; Martín-Platero, A.M.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. Synergy of the Bacteriocin AS-48 and Antibiotics against Uropathogenic Enterococci. Antibiotics 2020, 9, 567. https://doi.org/10.3390/antibiotics9090567
Montalbán-López M, Cebrián R, Galera R, Mingorance L, Martín-Platero AM, Valdivia E, Martínez-Bueno M, Maqueda M. Synergy of the Bacteriocin AS-48 and Antibiotics against Uropathogenic Enterococci. Antibiotics. 2020; 9(9):567. https://doi.org/10.3390/antibiotics9090567
Chicago/Turabian StyleMontalbán-López, Manuel, Rubén Cebrián, Rosa Galera, Lidia Mingorance, Antonio M. Martín-Platero, Eva Valdivia, Manuel Martínez-Bueno, and Mercedes Maqueda. 2020. "Synergy of the Bacteriocin AS-48 and Antibiotics against Uropathogenic Enterococci" Antibiotics 9, no. 9: 567. https://doi.org/10.3390/antibiotics9090567
APA StyleMontalbán-López, M., Cebrián, R., Galera, R., Mingorance, L., Martín-Platero, A. M., Valdivia, E., Martínez-Bueno, M., & Maqueda, M. (2020). Synergy of the Bacteriocin AS-48 and Antibiotics against Uropathogenic Enterococci. Antibiotics, 9(9), 567. https://doi.org/10.3390/antibiotics9090567