Efficacy and Mechanisms of Flavonoids against the Emerging Opportunistic Nontuberculous Mycobacteria
Abstract
1. Introduction
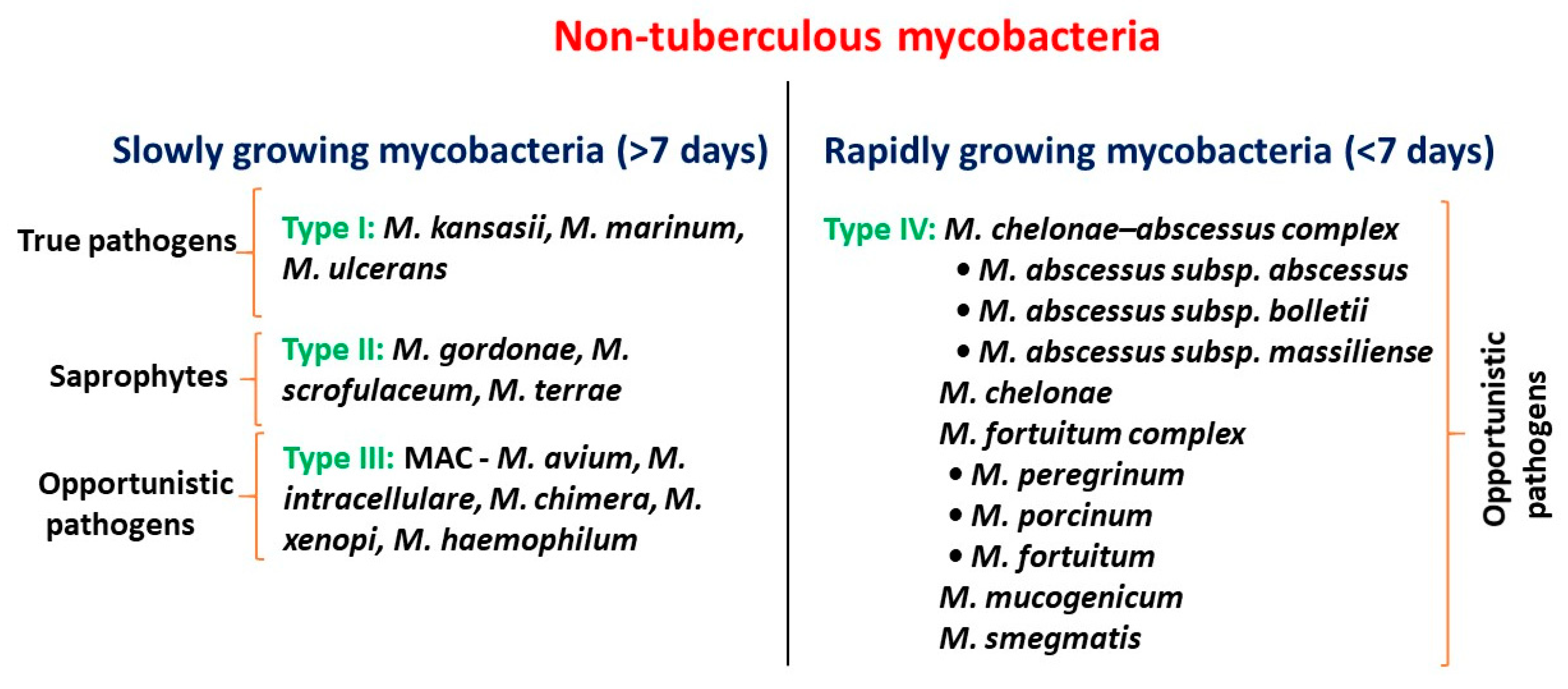
2. Clinical Epidemiology of NTM
3. Challenges in Diagnosing and Treatment of NTM Diseases
4. Flavonoids
5. Anti-Nontuberculous Mycobacterial Efficacy and Mechanisms
5.1. Inhibition of Cell Wall Formation
5.2. Inhibition of Biofilm Formation
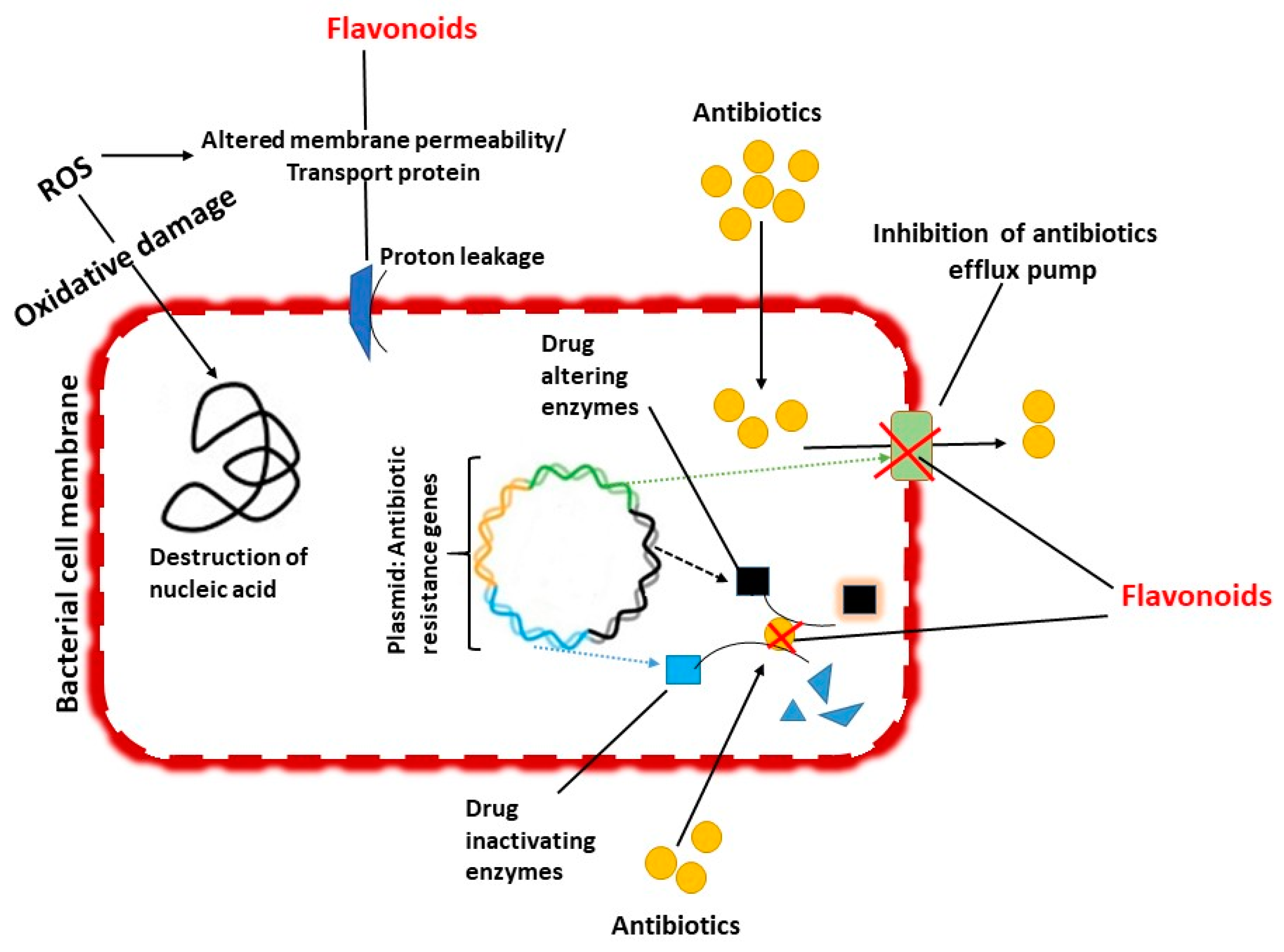
5.3. Inhibition of Efflux Mediated Pumping System
5.4. Inhibition of Bacterial DNA Synthesis
5.5. Synergistic Action of Flavonoids with Antimycobacterial Agents
6. Future Directions and Remarks
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peyrani, P.; Ramirez, J.A. Nontuberculous mycobacterial pulmonary infections. In Pulmonary Complications of HIV; European Respiratory Society: Sheffield, UK, 2014; pp. 128–137. [Google Scholar] [CrossRef]
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Anti-mycobacterial effect of leaf extract of Centella asiatica. Res. J. Pharm. Technol. 2010, 3, 872–876. [Google Scholar]
- Lim, S.S.; Selvaraj, A.; Ng, Z.Y.; Palanisamy, M.; Mickmaray, S.; Cheong, P.C.H.; Lim, R.L.H. Isolation of actinomycetes with antibacterial activity against multi-drug resistant bacteria. Malays. J. Microbiol. 2018, 14, 293–305. [Google Scholar] [CrossRef]
- Devi, C.A.; Dhanasekaran, D.; Suresh, M.; Thajuddin, N. Diagnostic value of real time PCR and associated bacterial and fungal infections in female genital tuberculosis. Biomed. Pharm. J. 2015, 3, 73–79. [Google Scholar]
- Tortoli, E.; Fedrizzi, T.; Meehan, C.J.; Trovato, A.; Grottola, A.; Giacobazzi, E.; Serpini, G.F.; Tagliazucchi, S.; Fabio, A.; Bettua, C.; et al. The new phylogeny of the genus mycobacterium: The old and the news. Infect. Genet. Evol. 2017, 56, 19–25. [Google Scholar] [CrossRef]
- Catherinot, E.; Roux, A.-L.; Vibet, M.-A.; Bellis, G.; Ravilly, S.; Lemonnier, L.; Le Roux, E.; Bernède-Bauduin, C.; Le Bourgeois, M.; Herrmann, J.-L.; et al. Mycobacterium avium and mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J. Cyst. Fibros. 2013, 12, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.L.; Larsen, S.E.; Ordway, D.; Cassell, G.; Coler, R.N. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Negl. Trop. Dis. 2019, 13, e0007083. [Google Scholar] [CrossRef]
- Johansen, M.D.; Herrmann, J.-L.; Kremer, L. Non-tuberculous mycobacteria and the rise of mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef]
- Turenne, C.Y. Nontuberculous mycobacteria: Insights on taxonomy and evolution. Infect. Genet. Evol. 2019, 72, 159–168. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Marcos, L.A.; Henao-Martínez, A.F.; Rodríguez-Morales, A.J.; Villamil-Gómez, W.E.; Gotuzzo, E.; Bonifaz, A. Cutaneous mycobacterial infections. Clin. Microbiol. Rev. 2018, 32, e00069-18. [Google Scholar] [CrossRef]
- Kim, B.-J.; Kim, B.-R.; Jeong, J.; Lim, J.-H.; Park, S.H.; Lee, S.-H.; Kim, C.K.; Kook, Y.-H.; Kim, B.-J. A description of mycobacterium chelonae subsp. gwanakae subsp. nov., a rapidly growing mycobacterium with a smooth colony phenotype due to glycopeptidolipids. Int. J. Syst. Evol. Microbiol. 2018, 68, 3772–3780. [Google Scholar] [CrossRef]
- Jankovic, M.; Sabol, I.; Zmak, L.; Jankovic, V.K.; Jakopovic, M.; Obrovac, M.; Ticac, B.; Bulat, L.K.; Grle, S.P.; Marekovic, I.; et al. Microbiological criteria in non-tuberculous mycobacteria pulmonary disease: A tool for diagnosis and epidemiology. Int. J. Tuberc. Lung Dis. 2016, 20, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Olivier, K.N.; Weber, D.J.; Wallace, R.J.; Faiz, A.R.; Lee, J.-H.; Zhang, Y.; Brown-Elliot, B.A.; Handler, A.; Wilson, R.W.; Schechter, M.S.; et al. Nontuberculous Mycobacteria. Am. J. Respir. Crit. Care Med. 2003, 167, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.L.; Catherinot, E.; Ripoll, F.; Soismier, N.; Macheras, E.; Ravilly, S.; Bellis, G.; Vibet, M.A.; Le Roux, E.; Lemonnier, L.; et al. Multicenter study of prevalence of nontuberculous Mycobacteria in patients with cystic fibrosis in France. J. Clin. Microbiol. 2009, 47, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.; Perry, A.; Appleby, M.R.; Lee, D.; Davison, J.; Johnston, A.; Jones, A.L.; Nelson, A.; Bourke, S.J.; Thomas, M.F.; et al. An evaluation of methods for the isolation of nontuberculous mycobacteria from patients with cystic fibrosis, bronchiectasis and patients assessed for lung transplantation. BMC Pulm. Med. 2019, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.W.; Wee, W.Y.; Ngeow, Y.F.; Mitchell, W.; Tan, J.L.; Wong, G.J.; Zhao, Y.; Xiao, J. Genomic reconnaissance of clinical isolates of emerging human pathogen mycobacterium abscessus reveals high evolutionary potential. Sci. Rep. 2014, 4, 4061. [Google Scholar] [CrossRef] [PubMed]
- Sapriel, G.; Konjek, J.; Orgeur, M.; Bouri, L.; Frézal, L.; Roux, A.-L.; Dumas, E.; Brosch, R.; Bouchier, C.; Brisse, S.; et al. Genome-wide mosaicism within mycobacterium abscessus: Evolutionary and epidemiological implications. BMC Genom. 2016, 17, 118. [Google Scholar] [CrossRef]
- Viljoen, A.; Gutiérrez, A.V.; Dupont, C.; Ghigo, E.; Kremer, L. A simple and rapid gene disruption strategy in mycobacterium abscessus: On the design and application of glycopeptidolipid mutants. Front. Cell Infect. Microbiol. 2018, 8, 69. [Google Scholar] [CrossRef]
- Gutiérrez, A.V.; Viljoen, A.; Ghigo, E.; Herrmann, J.-L.; Kremer, L. Glycopeptidolipids, a double-edged sword of the mycobacterium abscessus complex. Front. Microbiol. 2018, 9, 1145. [Google Scholar] [CrossRef]
- Wallace, J.R.; Mangas, K.M.; Porter, J.L.; Marcsisin, R.; Pidot, S.J.; Howden, B.; Omansen, T.F.; Zeng, W.; Axford, J.K.; Johnson, P.D.R.; et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl. Trop. Dis. 2017, 11, e0005553. [Google Scholar] [CrossRef]
- Wu, U.-I.; Holland, S.M. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect. Dis. 2015, 15, 968–980. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, M.; He, J.-Q. Disseminated mycobacterium kansasii infection with cutaneous lesions in an immunocompetent patient. Int. J. Infect. Dis. 2017, 62, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.L.; Limaye, A.; Pottinger, P.; Whimbey, E.; Goss, C.H.; Tonelli, M.R.; Cangelosi, G.A.; Dirac, M.A.; Olivier, K.N.; Brown-Elliott, B.A.; et al. Respiratory outbreak of mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am. J. Respir. Crit. Care Med. 2012, 185, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.M.; Grogono, D.M.; Greaves, D.; Foweraker, J.; Roddick, I.; Inns, T.; Reacher, M.; Haworth, C.S.; Curran, M.D.; Harris, S.R.; et al. Whole-genome sequencing to identify transmission of mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet 2013, 381, 1551–1560. [Google Scholar] [CrossRef]
- Pedrero, S.; Tabernero, E.; Arana-Arri, E.; Urra, E.; Larrea, M.; Zalacain, R. Changing epidemiology of nontuberculous mycobacterial lung disease over the last two decades in a region of the Basque country. ERJ Open Res. 2019, 5, 00110–02018. [Google Scholar] [CrossRef] [PubMed]
- Ringshausen, F.C.; Wagner, D.; de Roux, A.; Diel, R.; Hohmann, D.; Hickstein, L.; Welte, T.; Rademacher, J. Prevalence of nontuberculous Mycobacterial pulmonary disease, Germany, 2009–2014. Emerg. Infect. Dis. 2016, 70, 1102. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of Nontuberculous Mycobacterial lung disease in U.S. medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar] [CrossRef]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Falkinham, J.O.; Holland, S.M.; Prevots, D.R. Spatial clusters of Nontuberculous Mycobacterial lung disease in the United States. Am. J. Respir. Crit. Care Med. 2012, 186, 553–558. [Google Scholar] [CrossRef]
- Henkle, E.; Hedberg, K.; Schafer, S.; Novosad, S.; Winthrop, K.L. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann. Am. Thorac. Soc. 2015, 12, 642–647. [Google Scholar] [CrossRef]
- Morimoto, K.; Iwai, K.; Uchimura, K.; Okumura, M.; Yoshiyama, T.; Yoshimori, K.; Ogata, H.; Kurashima, A.; Gemma, A.; Kudoh, S. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann. Am. Thorac. Soc. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Cassidy, P.M.; Hedberg, K.; Saulson, A.; McNelly, E.; Winthrop Kevin, L. Nontuberculous mycobacterial disease prevalence and risk factors: A changing epidemiology. Clin. Infect. Dis. 2009, 49, e124–e129. [Google Scholar] [CrossRef]
- Larsson, L.-O.; Polverino, E.; Hoefsloot, W.; Codecasa, L.R.; Diel, R.; Jenkins, S.G.; Loebinger, M.R. Pulmonary disease by non-tuberculous mycobacteria—Clinical management, unmet needs and future perspectives. Expert Rev. Respir. Med. 2017, 11, 977–989. [Google Scholar] [CrossRef]
- Wang, S.-H.; Pancholi, P. Mycobacterial skin and soft tissue infection. Curr. Infect. Dis. Rep. 2014, 16, 438. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, E.P.; Leung, J.M.; Fowler, C.J.; Haney, C.; Hsu, A.P.; Chen, F.; Duggal, P.; Oler, A.J.; McCormack, R.; Podack, E.; et al. Pulmonary nontuberculous mycobacterial infection. A multisystem, multigenic disease. Am. J. Respir. Crit. Care Med. 2015, 192, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.J.; Koh, W.-J.; Daley, C.L. Diagnosis and treatment of nontuberculous mycobacterial lung disease: Clinicians’ perspectives. Tuberc. Respir. Dis. 2016, 79, 74. [Google Scholar] [CrossRef] [PubMed]
- Henkle, E.; Winthrop, K.L. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin. Chest Med. 2015, 36, 91–99. [Google Scholar] [CrossRef]
- Mokaddas, E.; Ahmad, S. Species spectrum of nontuberculous mycobacteria isolated from clinical specimens in Kuwait. Curr. Microbiol. 2008, 56, 413–417. [Google Scholar] [CrossRef]
- Al-Mahruqi, S.H.; van Ingen, J.; Al-Busaidy, S.; Boeree, M.J.; Al-Zadjali, S.; Patel, A.; Dekhuijzen, P.N.R.; van Soolingen, D. Clinical relevance of nontuberculous mycobacteria, Oman. Emerg. Infect. Dis. 2009, 15, 292–294. [Google Scholar] [CrossRef]
- Varghese, B.; Memish, Z.; Abuljadayel, N.; Al-Hakeem, R.; Alrabiah, F.; Al-Hajoj, S.A. Emergence of clinically relevant non-tuberculous mycobacterial infections in Saudi Arabia. PLoS Negl. Trop. Dis. 2013, 7, e2234. [Google Scholar] [CrossRef]
- Al-Harbi, A.; Al-Jahdali, H.; Al-Johani, S.; Baharoon, S.; Bin Salih, S.; Khan, M. Frequency and clinical significance of respiratory isolates of non-tuberculous mycobacteria in Riyadh, Saudi Arabia. Clin. Respir. J. 2014, 10, 198–203. [Google Scholar] [CrossRef]
- Russell, C.D.; Claxton, P.; Doig, C.; Seagar, A.L.; Rayner, A.; Laurenson, I.F. Non-tuberculous mycobacteria: A retrospective review of Scottish isolates from 2000 to 2010. Thorax 2014, 69, 593–595. [Google Scholar] [CrossRef][Green Version]
- Jankovic, M.; Samarzija, M.; Sabol, I.; Jakopovic, M.; Katalinic Jankovic, V.; Zmak, L.; Ticac, B.; Marusic, A.; Obrovac, M.; van Ingen, J. Geographical distribution and clinical relevance of non-tuberculous mycobacteria in Croatia. Int. J. Tuberc. Lung Dis. 2013, 17, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, N.; Simşek, H.; Sezen, F.; Arslantürk, A.; Tarhan, G.; Ceyhan, I. Evaluation of the distribution of non-tuberculous mycobacteria strains isolated in National Tuberculosis Reference Laboratory in 2009–2010, Turkey. Mikrobiyol. Bul. 2012, 46, 560–567. [Google Scholar] [PubMed]
- Simons, S.; van Ingen, J.; Hsueh, P.R.; Van Hung, N.; Dekhuijzen, P.N.; Boeree, M.J.; van Soolingen, D. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg. Infect. Dis. 2011, 17, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Hsueh, P.R. Diseases caused by nontuberculous mycobacteria in Asia. Future Microbiol. 2014, 9, 93–106. [Google Scholar] [CrossRef]
- Baron, E.J. Clinical Microbiology in Underresourced Settings. Clin. Lab. Med. 2019, 39, 359–369. [Google Scholar] [CrossRef]
- Wu, M.-L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519. [Google Scholar] [CrossRef]
- Fleshner, M.; Olivier, K.N.; Shaw, P.A.; Adjemian, J.; Strollo, S.; Claypool, R.J.; Folio, L.; Zelazny, A.; Holland, S.M.; Prevots, D.R. Mortality among patients with pulmonary non-tuberculous mycobacteria disease. Int. J. Tuberc. Lung Dis. 2016, 20, 582–587. [Google Scholar] [CrossRef]
- Winthrop, K.L.; McNelley, E.; Kendall, B.; Marshall-Olson, A.; Morris, C.; Cassidy, M.; Saulson, A.; Hedberg, K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features. Am. J. Respir. Crit. Care Med. 2010, 182, 977–982. [Google Scholar] [CrossRef]
- Martínez González, S.; Cano Cortés, A.; Sota Yoldi, L.A.; García García, J.M.; Alba Álvarez, L.M.; Palacios Gutiérrez, J.J. Non-tuberculous mycobacteria. An emerging threat? Arch. Bronconeumol. 2017, 53, 554–560. [Google Scholar] [CrossRef]
- Novosad, S.A.; Beekmann, S.E.; Polgreen, P.M.; Mackey, K.; Winthrop, K.L. Treatment of Mycobacterium abscessus Infection. Emerg. Infect. Dis. 2016, 22, 511–514. [Google Scholar] [CrossRef]
- Wolinsky, E. Mycobacterial Diseases Other Than Tuberculosis. Clin. Infect. Dis. 1992, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Redelman-Sidi, G.; Sepkowitz, K.A. Rapidly growing mycobacteria infection in patients with cancer. Clin. Infect. Dis. 2010, 51, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; García-Coca, M. Mycobacterium biofilms. Front. Microbiol. 2017, 8, 2651. [Google Scholar] [CrossRef]
- Forbes, B.A.; Hall, G.S.; Miller, M.B.; Novak, S.M.; Rowlinson, M.-C.; Salfinger, M.; Somoskövi, A.; Warshauer, D.M.; Wilson, M.L. Practice guidelines for clinical microbiology laboratories: Mycobacteria. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Mougari, F.; Guglielmetti, L.; Raskine, L.; Sermet-Gaudelus, I.; Veziris, N.; Cambau, E. Infections caused by mycobacterium abscessus: Epidemiology, diagnostic tools and treatment. Expert Rev. Anti-Infect. Ther. 2016, 14, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Marion, E.; Song, O.-R.; Christophe, T.; Babonneau, J.; Fenistein, D.; Eyer, J.; Letournel, F.; Henrion, D.; Clere, N.; Paille, V.; et al. Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. Cell 2014, 157, 1565–1576. [Google Scholar] [CrossRef]
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Antifungal activity of selected Indian medicinal plant salts. J. Glob. Pharm. Technol. 2010, 2, 71–74. [Google Scholar]
- Prabakar, K.; Sivalingam, P.; Mohamed Rabeek, S.I.; Muthuselvam, M.; Devarajan, N.; Arjunan, A.; Karthick, R.; Suresh, M.M.; Wembonyama, J.P. Evaluation of antibacterial efficacy of phyto fabricated silver nanoparticles using Mukia scabrella (Musumusukkai) against drug resistance nosocomial gram negative bacterial pathogens. Colloids Surf. B Biointerfaces 2013, 104, 282–288. [Google Scholar] [CrossRef]
- Mickymaray, S.; Al Aboody, M.S.; Rath, P.K.; Annamalai, P.; Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 185–191. [Google Scholar] [CrossRef]
- Moorthy, K.; Punitha, T.; Vinodhini, R.; Mickymaray, S.; Shonga, A.; Tomass, Z.; Thajuddin, N. Efficacy of different solvent extracts of Aristolochia krisagathra and Thottea ponmudiana for potential antimicrobial activity. J. Pharm. Res. 2015, 9, 35–40. [Google Scholar]
- Mickymaray, S.; Alturaiki, W. Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases. Molecules 2018, 23, 3032. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, M.; Manuel, V.N.; Raja, V.; Thambidurai, P.; Mickymaray, S.; Nooruddin, T. Antimicrobial activity of the ethanolic and aqueous extracts of Salacia chinensis Linn. against human pathogens. Asian Pac. J. Trop. Dis. 2012, 2, S416–S420. [Google Scholar] [CrossRef]
- Mickymaray, S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S.; Al Aboody, M.S. In vitro antioxidant and bactericidal efficacy of 15 common spices: Novel therapeutics for urinary tract infections? Medicina 2019, 55, 289. [Google Scholar] [CrossRef]
- Suresh, M.; Alfonisan, M.; Alturaiki, W.; Aboody, M.S.A.; Alfaiz, F.A.; Premanathan, M.; Vijayakumar, R.; Umamagheswari, K.; Ghamdi, S.A.; Alsagaby, S.A. Investigations of bioactivity of Acalypha indica (L.), Centella asiatica (L.) and croton bonplandianus (Baill) against multidrug resistant bacteria and cancer cells. J. Herb. Med. 2020, 100359. [Google Scholar] [CrossRef]
- Kumar, G.; Murugesan, A.G.; Rajasekara Pandian, M. Effect of Helicteres isora bark extract on blood glucose and hepatic enzymes in experimental diabetes. Pharmazie 2006, 61, 353–355. [Google Scholar]
- Ganesan, K.; Xu, B. Ethnobotanical studies on folkloric medicinal plants in Nainamalai, Namakkal District, Tamil Nadu, India. Trends Phytochem. Res. 2017, 1, 153–168. [Google Scholar]
- Kumar, G.; Banu, G.S.; Murugesan, A.G.; Pandian, M.R. Hypoglycaemic effect of Helicteres isora bark extract in rats. J. Ethnopharmacol. 2006, 107, 304–307. [Google Scholar] [CrossRef]
- Sinaga, M.; Ganesan, K.; Kumar Nair, S.K.P.; Gani, S.B. Preliminary phytochemical analysis and in vitro antibacterial activity of bark and seeds of Ethiopian neem (Azadirachta indica A. Juss). World J Pharm. Pharma. Sci. 2016, 5, 1714–1723. [Google Scholar] [CrossRef]
- Zhang, T.; Jayachandran, M.; Ganesan, K.; Xu, B. Black truffle aqueous extract attenuates oxidative stress and inflammation in STZ-induced hyperglycemic rats via Nrf2 and NF-κB pathways. Front. Pharmacol. 2018, 9, 1257. [Google Scholar] [CrossRef]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): Phytochemistry and pharmacological properties. Phytochem. Rev. 2017, 16, 461–478. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Sandle, T.; Al-Aboody, M.S.; AlFonaisan, M.K.; Alturaiki, W.; Mickymaray, S.; Premanathan, M.; Alsagaby, S.A. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii—A first report from the Kingdom of Saudi Arabia. J. Infect. Public Health 2018, 11, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, R.; Moorthy, K.; Suresh, M. Incidence and virulence traits of Candida dubliniensis isolated from clinically suspected patients. Asian J. Pharm. Clin. Res. 2016, 9, 77. [Google Scholar] [CrossRef]
- Chandran, R.P.; Kumar, S.N.; Manju, S.; Kader, S.A.; Dileep Kumar, B.S. In vitro α-glucosidase inhibition, antioxidant, anticancer, and antimycobacterial properties of ethyl acetate extract of Aegle tamilnadensis Abdul Kader (Rutaceae) leaf. Appl. Biochem. Biotechnol. 2015, 175, 1247–1261. [Google Scholar] [CrossRef]
- Vianna, J.S.; Machado, D.; Ramis, I.B.; Silva, F.P.; Bierhals, D.V.; Abril, M.A.; von Groll, A.; Ramos, D.F.; Lourenço, M.C.S.; Viveiros, M.; et al. The Contribution of Efflux Pumps in Mycobacterium abscessus Complex Resistance to Clarithromycin. Antibiotics 2019, 8, 153. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front. Pharmacol. 2015, 6, 205. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-diabetic effects and mechanisms of dietary polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich lentils and their health promoting effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Taylor, P.W.; Nagaoka, Y.; Uesato, S.; Hara, Y.; Lamb, A.J. Investigation of the antibacterial activity of 3-O-octanoyl-(–)-epicatechin. J. Appl. Microbiol. 2008, 105, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Aboody, M.S.A.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Ganesan, K.; Jayachandran, M.; Xu, B. Diet-derived phytochemicals targeting colon cancer stem cells and microbiota in colorectal cancer. Int. J. Mol. Sci. 2020, 21, 3976. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. New insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Ganesan Murugesan, A. Effect of Helicteres isora bark extracts on heart antioxidant status and lipid peroxidation in streptozotocin diabetic rats. J. Appl. Biomed. 2008, 6, 89–95. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Sharmila Banu, G.; Ganesan Murugesan, A.; Pandian, M.R. Antihyperglycaemic and antiperoxidative effect of Helicteres isora L. bark extracts in streptozotocin-induced diabetic rats. J. Appl. Biomed. 2007, 5, 97–104. [Google Scholar] [CrossRef]
- Kumar, G.; Murugesan, A.G. Hypolipidaemic activity of Helicteres isora L. bark extracts in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2008, 116, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ganesan, K.; Mickymaray, S.; Alfaiz, F.A.; Thatchinamoorthi, R.; Aboody, M.S.A. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. Bioact. Compd. Health Dis. 2020, 3, 15. [Google Scholar] [CrossRef]
- Pandian, M.R.; Banu, G.S.; Kumar, G.; Smila, K.H. Screening of antibacterial activity of fruit extract of citrus medica against bacteria involved in typhoid fever. Nat. J. Life Sci. 2006, 3, 289–292. [Google Scholar]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. CellsNanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Pandian, M.R.; Banu, G.S.; Kumar, G. A study of the antimicrobial activity of Alangium salviifolium. Indian J. Pharmacol. 2006, 38, 203. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase inhibitors from natural products and their anticancer potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef]
- Ganesan, K.; Guo, S.; Fayyaz, S.; Zhang, G.; Xu, B. Targeting Programmed Fusobacterium nucleatum Fap2 for Colorectal Cancer Therapy. Cancers 2019, 11, 1592. [Google Scholar] [CrossRef]
- Chedraui, P.; Pérez-López, F.R. Nutrition and health during mid-life: Searching for solutions and meeting challenges for the aging population. Climacteric 2013, 16, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Bojić, M.; Maleš, Ž.; Antolić, A.; Babić, I.; Tomičić, M. Antithrombotic activity of flavonoids and polyphenols rich plant species. Acta Pharm. 2019, 69, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2017, 58, 1165–1229. [Google Scholar] [CrossRef] [PubMed]
- Sukalingam, K.; Ganesan, K.; Xu, B. Protective effect of aqueous extract from the leaves of justicia tranquebariesis against thioacetamide-induced oxidative stress and hepatic fibrosis in rats. Antioxidants 2018, 7, 78. [Google Scholar] [CrossRef]
- Ganesan, K.; Sukalingam, K.; Xu, B. Solanum trilobatum L. Ameliorate thioacetamide-induced oxidative stress and hepatic damage in albino rats. Antioxidants 2017, 6, 68. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Pappa, P.V.; Sundararajan, M.; Pandian, M.R. Hepatoprotective activity of Trianthema portulacastrum L. against paracetamol and thioacetamide intoxication in albino rats. J. Ethnopharmacol. 2004, 92, 37–40. [Google Scholar] [CrossRef]
- Gabrielová, E.; Bartošíková, L.; Nečas, J.; Modrianský, M. Cardioprotective effect of 2,3-dehydrosilybin preconditioning in isolated rat heart. Fitoterapia 2019, 132, 12–21. [Google Scholar] [CrossRef]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E.; Nabavi, S.F. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in alzheimer’s and parkinson’s disease. CNS Neurol. Disord. Drug Targets 2017, 16. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Murugesan, A.G.A. Influence of Helicteres isora L. bark extracts on glycemic control and renoprotective activity in streptozotocin-induced diabetic rats. Int. J. Pharma Sci. Nanotechnol. 2008, 1, 275–280. [Google Scholar]
- Ye, J.; Guan, M.; Lu, Y.; Zhang, D.; Li, C.; Li, Y.; Zhou, C. Protective effects of hesperetin on lipopolysaccharide-induced acute lung injury by targeting MD2. Eur. J. Pharmacol. 2019, 852, 151–158. [Google Scholar] [CrossRef]
- Murphy, K.J.; Walker, K.M.; Dyer, K.A.; Bryan, J. Estimation of daily intake of flavonoids and major food sources in middle-aged Australian men and women. Nutr. Res. 2019, 61, 64–81. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and inflammation in cognitive ageing and alzheimer’s disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, L.; Fornasaro, S.; Čvorović, J.; Tramer, F.; Passamonti, S. Bioavailability of flavonoids. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2014; pp. 489–511. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Murugesan, A.G. Attenuation of Helicteres isora L. bark extracts on streptozotocin-induced alterations in glycogen and carbohydrate metabolism in albino rats. Hum. Exp. Toxicol. 2009, 28, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Banu, G.S.; Pandian, M.R. Biochemical activity of selenium and glutathione on country made liquor (CML) induced hepatic damage in rats. Indian J. Clin. Biochem. 2007, 22, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Banu, G.S.; Kannan, V.; Pandian, M.R. Antihepatotoxic effect of beta-carotene on paracetamol induced hepatic damage in rats. Indian J. Exp. Biol. 2005, 43, 351–355. [Google Scholar] [PubMed]
- Yadav, S.S.; Singh, M.K.; Singh, P.K.; Kumar, V. Traditional knowledge to clinical trials: A review on therapeutic actions of Emblica officinalis. Biomed. Pharm. 2017, 93, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, C.; Lu, W.; Wei, L. Phytochemistry, pharmacology, and clinical use of Panax notoginseng flowers buds. Phytother. Res. 2018, 32, 2155–2163. [Google Scholar] [CrossRef]
- Chan, E.W.; Lye, P.Y.; Wong, S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [CrossRef]
- Yusook, K.; Weeranantanapan, O.; Hua, Y.; Kumkrai, P.; Chudapongse, N. Lupinifolin from Derris reticulata possesses bactericidal activity on Staphylococcus aureus by disrupting bacterial cell membrane. J. Nat. Med. 2017, 71, 357–366. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.; Pandian, M. Evaluation of the antioxidant activity of Trianthema portulacastrum L. Indian J. Pharmacol. 2005, 37, 331–333. [Google Scholar] [CrossRef]
- Marini, E.; Di Giulio, M.; Magi, G.; Di Lodovico, S.; Cimarelli, M.E.; Brenciani, A.; Nostro, A.; Cellini, L.; Facinelli, B. Curcumin, an antibiotic resistance breaker against a multiresistant clinical isolate of mycobacterium abscessus. Phytother. Res. 2017, 32, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.E.; Griffiths, W.J.; Taylor, P.W. Components derived fromPelargoniumstimulate macrophage killing of mycobacterium species. J. Appl. Microbiol. 2009, 106, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Di Giulio, M.; Ginestra, G.; Magi, G.; Di Lodovico, S.; Marino, A.; Facinelli, B.; Cellini, L.; Nostro, A. Efficacy of carvacrol against resistant rapidly growing mycobacteria in the planktonic and biofilm growth mode. PLoS ONE 2019, 14, e0219038. [Google Scholar] [CrossRef] [PubMed]
- Sharmila Banu, G.; Kumar, G.; Murugesan, A.G. Effect of ethanolic leaf extract of Trianthema portulacastrum L. on aflatoxin induced hepatic damage in rats. Indian J. Clin. Biochem. 2009, 24, 414–418. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Selective toxicity of Catechin-a natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Friedman, M.; Sum, A.K. Molecular binding of catechins to biomembranes: Relationship to biological activity. J. Agric. Food. Chem. 2009, 57, 6720–6728. [Google Scholar] [CrossRef]
- Stepanović, S.; Antić, N.; Dakić, I.; Svabić-Vlahović, M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharm. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Ollila, F.; Halling, K.; Vuorela, P.; Vuorela, H.; Slotte, J.P. Characterization of flavonoid-biomembrane interactions. Arch. Biochem. Biophys. 2002, 399, 103–108. [Google Scholar] [CrossRef]
- Masoko, P.; Masiphephethu, M.V. Phytochemical investigation, antioxidant and antimycobacterial activities of schkuhria pinnata (Lam) thell extracts against mycobacterium smegmatis. J. Evid. Based Integr. Med. 2019, 24, 2515690x19866104. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, H.; Yuan, M.; Zhou, J.; Tu, Q.; Liu, J.-J.; Wang, J. Synthesis and biological evaluation of apigenin derivatives as antibacterial and antiproliferative agents. Molecules 2013, 18, 11496–11511. [Google Scholar] [CrossRef] [PubMed]
- Boonsai, P.; Phuwapraisirisan, P.; Chanchao, C. Antibacterial activity of a cardanol from Thai Apis mellifera propolis. Int. J. Med. Sci. 2014, 11, 327–336. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Luo, X.; Yang, Z. Advances in Biosynthesis, Pharmacology, and Pharmacokinetics of Pinocembrin, a Promising Natural Small-Molecule Drug. Molecules 2019, 24, 2323. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Bae, E.A.; Han, M.J. Anti-Helicobacter pylori activity of the metabolites of poncirin from Poncirus trifoliata by human intestinal bacteria. Biol. Pharm. Bull. 1999, 22, 422–424. [Google Scholar] [CrossRef]
- Lucarini, R.; Tozatti, M.G.; Silva, M.L.A.; Gimenez, V.M.M.; Pauletti, P.M.; Groppo, M.; Turatti, I.C.C.; Cunha, W.R.; Martins, C.H.G. Antibacterial and anti-inflammatory activities of an extract, fractions, and compounds isolated from Gochnatia pulchra aerial parts. Braz. J. Med. Biol. Res. 2015, 48, 822–830. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, antifungal and cytotoxic activities of two flavonoids from retama raetam flowers. Molecules 2012, 17, 7284–7293. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial activity of polyphenols: Structure-activity relationship and influence of hyperglycemic condition. Molecules 2017, 22, 1913. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Coronado-Aceves, E.W.; Sánchez-Escalante, J.J.; López-Cervantes, J.; Robles-Zepeda, R.E.; Velázquez, C.; Sánchez-Machado, D.I.; Garibay-Escobar, A. Antimycobacterial activity of medicinal plants used by the Mayo people of Sonora, Mexico. J. Ethnopharmacol. 2016, 190, 106–115. [Google Scholar] [CrossRef]
- Yeung, M.-F.; Lau, C.B.S.; Chan, R.C.Y.; Zong, Y.; Che, C.-T. Search for antimycobacterial constituents from a Tibetan medicinal plant, Gentianopsis paludosa. Phytother. Res. 2009, 23, 123–125. [Google Scholar] [CrossRef]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant phenolic compounds as ethidium bromide efflux inhibitors in mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef]
- Kuete, V.; Nono, E.C.N.; Mkounga, P.; Marat, K.; Hultin, P.G.; Nkengfack, A.E. Antimicrobial activities of the CH2Cl2–CH3OH (1:1) extracts and compounds from the roots and fruits ofPycnanthus angolensis(Myristicaceae). Nat. Prod. Res. 2011, 25, 432–443. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Hariri, B.M.; McMahon, D.B.; Chen, B.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Lee, R.J. Plant flavones enhance antimicrobial activity of respiratory epithelial cell secretions against Pseudomonas aeruginosa. PLoS ONE 2017, 12, e0185203. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complementary Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Bisignano, C.; Filocamo, A.; La Camera, E.; Zummo, S.; Fera, M.T.; Mandalari, G. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013, 13, 103. [Google Scholar] [CrossRef]
- Eerdunbayaer; Orabi, M.A.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of two new flavonoids and effects of licorice phenolics on vancomycin-resistant Enterococcus species. Molecules 2014, 19, 3883–3897. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and antibacterial properties of anthocyanins and flavonols from fruits of black and non-black mulberries. Molecules 2017, 23, 4. [Google Scholar] [CrossRef]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; de Koning, C.B. A new cinnamoylglycoflavonoid, antimycobacterial and antioxidant constituents from Heritiera littoralis leaf extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef]
- Reshma, M.V.; Jacob, J.; Syamnath, V.L.; Habeeba, V.P.; Dileep Kumar, B.S.; Lankalapalli, R.S. First report on isolation of 2,3,4-trihydroxy-5-methylacetophenone from palmyra palm (Borassus flabellifer Linn.) syrup, its antioxidant and antimicrobial properties. Food Chem. 2017, 228, 491–496. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Kuete, V.; McGaw, L.J.; Eloff, J.N. The 15-lipoxygenase inhibitory, antioxidant, antimycobacterial activity and cytotoxicity of fourteen ethnomedicinally used African spices and culinary herbs. J. Ethnopharmacol. 2014, 156, 1–8. [Google Scholar] [CrossRef]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The antibacterial activity of coriolus versicolor methanol extract and its effect on ultrastructural changes of staphylococcus aureus and salmonella enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, A.; Kehri, H.K.; Sharma, B.; Pandey, A.K. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 9. [Google Scholar] [CrossRef]
- Shah, S.; Stapleton, P.D.; Taylor, P.W. The polyphenol (-)-epicatechin gallate disrupts the secretion of virulence-related proteins by Staphylococcus aureus. Lett. Appl. Microbiol. 2008, 46, 181–185. [Google Scholar] [CrossRef]
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, M.A.; Cho, H.J.; Oh, S.K.; Lee, I.K.; Kim, U.K.; Lee, K.Y. Galangin prevents aminoglycoside-induced ototoxicity by decreasing mitochondrial production of reactive oxygen species in mouse cochlear cultures. Toxicol. Lett. 2016, 245, 78–85. [Google Scholar] [CrossRef]
- Safwat, N.A.; Kashef, M.T.; Aziz, R.K.; Amer, K.F.; Ramadan, M.A. Quercetin 3-O-glucoside recovered from the wild Egyptian Sahara plant, Euphorbia paralias L., inhibits glutamine synthetase and has antimycobacterial activity. Tuberculosis 2018, 108, 106–113. [Google Scholar] [CrossRef]
- Mowbray, S.L.; Kathiravan, M.K.; Pandey, A.A.; Odell, L.R. Inhibition of glutamine synthetase: A potential drug target in mycobacterium tuberculosis. Molecules 2014, 19, 13161–13176. [Google Scholar] [CrossRef]
- Harth, G.; Clemens, D.L.; Horwitz, M.A. Glutamine synthetase of mycobacterium tuberculosis: Extracellular release and characterization of its enzymatic activity. Proc. Natl. Acad. Sci. USA 1994, 91, 9342–9346. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; Papaemmanouil, A.; Bhowruth, V.; Bhatt, A.; Dover, L.G.; Besra, G.S. Flavonoid inhibitors as novel antimycobacterial agents targeting Rv0636, a putative dehydratase enzyme involved in mycobacterium tuberculosis fatty acid synthase II. Microbiology 2007, 153, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xiang, X.; Xie, J. Crucial components of mycobacterium type II fatty acid biosynthesis (Fas-II) and their inhibitors. Fems. Microbiol. Lett. 2014, 360, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Korabliovienė, J.; Mauricas, M.; Ambrozevičienė, Č.; Valius, M.; Kaupinis, A.; Čaplinskas, S.; Korabliov, P. Mycobacteria produce proteins involved in biofilm formation and growth-affecting processes. Acta Microbiol. Immunol. Hung. 2018, 65, 405–418. [Google Scholar] [CrossRef]
- Munayco, C.V.; Grijalva, C.G.; Culqui, D.R.; Bolarte, J.L.; Suárez-Ognio, L.A.; Quispe, N.; Calderon, R.; Ascencios, L.; Del Solar, M.; Salomón, M.; et al. Outbreak of persistent cutaneous abscesses due to mycobacterium chelonae after mesotherapy sessions, Lima, Peru. Rev. Saude Publica 2008, 42, 146–149. [Google Scholar] [CrossRef][Green Version]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Falkinham, J.O., 3rd. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg. Infect. Dis. 2011, 17, 419–424. [Google Scholar] [CrossRef]
- Williams, M.M.; Yakrus, M.A.; Arduino, M.J.; Cooksey, R.C.; Crane, C.B.; Banerjee, S.N.; Hilborn, E.D.; Donlan, R.M. Structural analysis of biofilm formation by rapidly and slowly growing nontuberculous mycobacteria. Appl. Env. Microbiol. 2009, 75, 2091–2098. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Zhou, Y.; Flavin, M.T.; Zhou, L.-M.; Nie, W.; Chen, F.-C. Chalcones and flavonoids as anti-Tuberculosis agents. Bioorganic Med. Chem. 2002, 10, 2795–2802. [Google Scholar] [CrossRef]
- Bhunu, B.; Mautsa, R.; Mukanganyama, S. Inhibition of biofilm formation in mycobacterium smegmatis by Parinari curatellifolia leaf extracts. BMC Complementary Altern. Med. 2017, 17, 285. [Google Scholar] [CrossRef]
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G. Current perspectives in drug discovery against tuberculosis from natural products. Int. J. Mycobacteriol. 2015, 4, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, B.; Wang, D.E.; Yao, T.; Pang, L.; Tu, Q.; Ahmed, S.M.; Liu, J.J.; Wang, J. Nitrogen-containing apigenin analogs: Preparation and biological activity. Molecules 2012, 17, 14748–14764. [Google Scholar] [CrossRef] [PubMed]
- Prawat, U.; Chairerk, O.; Phupornprasert, U.; Salae, A.W.; Tuntiwachwuttikul, P. Two new C-benzylated dihydrochalcone derivatives from the leaves of Melodorum siamensis. Planta Med. 2013, 79, 83–86. [Google Scholar] [CrossRef]
- Gumula, I.; Heydenreich, M.; Derese, S.; Ndiege, I.O.; Yenesew, A. Four isoflavanones from the stem bark of Platycelphium voënse. Phytochem. Lett. 2012, 5, 150–154. [Google Scholar] [CrossRef]
- Moreira, R.R.D.; Martins, G.Z.; Pietro, R.C.L.R.; Sato, D.N.; Pavan, F.R.; Leite, S.R.A.; Vilegas, W.; Leite, C.Q.F. Paepalanthus spp.: Antimycobacterial activity of extracts, methoxylated flavonoids and naphthopyranone fractions. Rev. Bras. Farmacogn. 2013, 23, 268–272. [Google Scholar] [CrossRef]
- Alcalde-Rico, M.; Hernando-Amado, S.; Blanco, P.; Martínez, J.L. Multidrug efflux pumps at the crossroad between antibiotic resistance and bacterial virulence. Front. Microbiol. 2016, 7, 1483. [Google Scholar] [CrossRef]
- Kumar, S.; Varela, M.F. Biochemistry of bacterial multidrug efflux pumps. Int. J. Mol. Sci. 2012, 13, 4484–4495. [Google Scholar] [CrossRef]
- Gröblacher, B.; Kunert, O.; Bucar, F. Compounds of Alpinia katsumadai as potential efflux inhibitors in mycobacterium smegmatis. Bioorg. Med. Chem. 2012, 20, 2701–2706. [Google Scholar] [CrossRef]
- Solnier, J.; Martin, L.; Bhakta, S.; Bucar, F. Flavonoids as novel efflux pump inhibitors and antimicrobials against both environmental and pathogenic intracellular mycobacterial species. Molecules 2020, 25, 734. [Google Scholar] [CrossRef]
- Gröblacher, B.; Maier, V.; Kunert, O.; Bucar, F. Putative mycobacterial efflux inhibitors from the seeds of Aframomum melegueta. J. Nat. Prod. 2012, 75, 1393–1399. [Google Scholar] [CrossRef]
- Roy, S.K.; Pahwa, S.; Nandanwar, H.; Jachak, S.M. Phenylpropanoids of Alpinia galanga as efflux pump inhibitors in mycobacterium smegmatis mc2 155. Fitoterapia 2012, 83, 1248–1255. [Google Scholar] [CrossRef]
- Suriyanarayanan, B.; Sarojini Santhosh, R. Docking analysis insights quercetin can be a non-antibiotic adjuvant by inhibiting Mmr drug efflux pump in mycobacterium sp. and its homologue EmrE in Escherichia coli. J. Biomol. Struct. Dyn. 2015, 33, 1819–1834. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, Y.; Zang, X.; Wu, T.; Qi, X.; Pan, S.; Xu, X. 3D-QSAR and docking studies of flavonoids as potent Escherichia coli inhibitors. Sci. Rep. 2016, 6, 23634. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Sahar Traoré, M.; Aliou Baldé, M.; Camara, A.; Saïdou Baldé, E.; Diané, S.; Telly Diallo, M.S.; Keita, A.; Cos, P.; Maes, L.; Pieters, L.; et al. The malaria co-infection challenge: An investigation into the antimicrobial activity of selected Guinean medicinal plants. J. Ethnopharmacol. 2015, 174, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Phytochemical and antimicrobial investigations of stilbenoids and flavonoids isolated from three species of Combretaceae. Fitoterapia 2012, 83, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. Fems. Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Van Acker, H.; Coenye, T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017, 25, 456–466. [Google Scholar] [CrossRef]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharm. 2018, 829, 112–120. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Murugesan, A.G.; Rajasekara Pandian, M. Effect of Helicteres isora. Bark Extracts on Brain Antioxidant Status and Lipid Peroxidation in Streptozotocin Diabetic Rats. Pharm. Biol. 2007, 45, 753–759. [Google Scholar] [CrossRef]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Ezraty, B.; Vergnes, A.; Banzhaf, M.; Duverger, Y.; Huguenot, A.; Brochado, A.R.; Su, S.Y.; Espinosa, L.; Loiseau, L.; Py, B.; et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 2013, 340, 1583–1587. [Google Scholar] [CrossRef]
- Farha, M.A.; Brown, E.D. Discovery of antibiotic adjuvants. Nat. Biotechnol. 2013, 31, 120–122. [Google Scholar] [CrossRef]
- Nøhr-Meldgaard, K.; Ovsepian, A.; Ingmer, H.; Vestergaard, M. Resveratrol enhances the efficacy of aminoglycosides against Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 390–396. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Shakya, T.; Stogios, P.J.; Waglechner, N.; Evdokimova, E.; Ejim, L.; Blanchard, J.E.; McArthur, A.G.; Savchenko, A.; Wright, G.D. A small molecule discrimination map of the antibiotic resistance kinome. Chem. Biol. 2011, 18, 1591–1601. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Hertel, W.; Peschel, G.; Ozegowski, J.-H.; Müller, P.-J. Inhibitory effects of triterpenes and flavonoids on the enzymatic activity of hyaluronic acid-splitting enzymes. Arch. Pharm. Int. J. Pharm. Med. Chem. 2006, 339, 313–318. [Google Scholar] [CrossRef]
- Ochensberger, S.; Alperth, F.; Mitić, B.; Kunert, O.; Mayer, S.; Mourão, M.F.; Turek, I.; Luca, S.V.; Skalicka-Woźniak, K.; Maleš, Ž.; et al. Phenolic compounds of Iris adriatica and their antimycobacterial effects. Acta Pharm. 2019, 69, 673–681. [Google Scholar] [CrossRef]
- Anakok, O.F.; Ndi, C.P.; Barton, M.D.; Griesser, H.J.; Semple, S.J. Antibacterial spectrum and cytotoxic activities of serrulatane compounds from the Australian medicinal plant Eremophila neglecta. J. Appl. Microbiol. 2011, 112, 197–204. [Google Scholar] [CrossRef]
- Zgoda-Pols, J.R.; Freyer, A.J.; Killmer, L.B.; Porter, J.R. Antimicrobial Resveratrol Tetramers from the Stem Bark ofVaticaoblongifoliassp.oblongifolia. J. Nat. Prod. 2002, 65, 1554–1559. [Google Scholar] [CrossRef]
- Roy, S.K.; Kumari, N.; Gupta, S.; Pahwa, S.; Nandanwar, H.; Jachak, S.M. 7-Hydroxy-(E)-3-phenylmethylene-chroman-4-one analogues as efflux pump inhibitors against mycobacterium smegmatis mc2 155. Eur. J. Med. Chem. 2013, 66, 499–507. [Google Scholar] [CrossRef]
- Okemo, P.; Kirimuhuzya, C.; Otieno, J.; Magadula, J.; Mariita, R.; Orodho, J. Methanolic extracts of Aloe secundiflora Engl. inhibits in vitro growth of tuberculosis and diarrhea-causing bacteria. Pharmacogn. Res. 2011, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Okemo, P.; Mbugua, P.; Mariita, R.; Orodho, J. Antifungal, antibacterial and antimycobacterial activity of Entada abysinnica Steudel ex A. Rich (Fabaceae) methanol extract. Pharmacogn. Res. 2010, 2, 163. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.G.; Pendland, S.L.; Prause, J.L.; Danzinger, L.H.; Schunke Vigo, J.; Cabieses, F.; Farnsworth, N.R. Antimycobacterial evaluation of Peruvian plants. Phytomedicine 2003, 10, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Dutta, P.; Sharma, M.; Rajput, N.K.; Dodiya, B.; Georrge, J.J.; Kholia, T.; Bhardwaj, A. BioPhytMol: A drug discovery community resource on anti-mycobacterial phytomolecules and plant extracts. J. Cheminf. 2014, 6, 1–10. [Google Scholar] [CrossRef]
- Cantrell, C.; Franzblau, S.; Fischer, N. Antimycobacterial plant terpenoids. Antimycobact. Planta Med. 2001, 67, 685–694. [Google Scholar] [CrossRef]
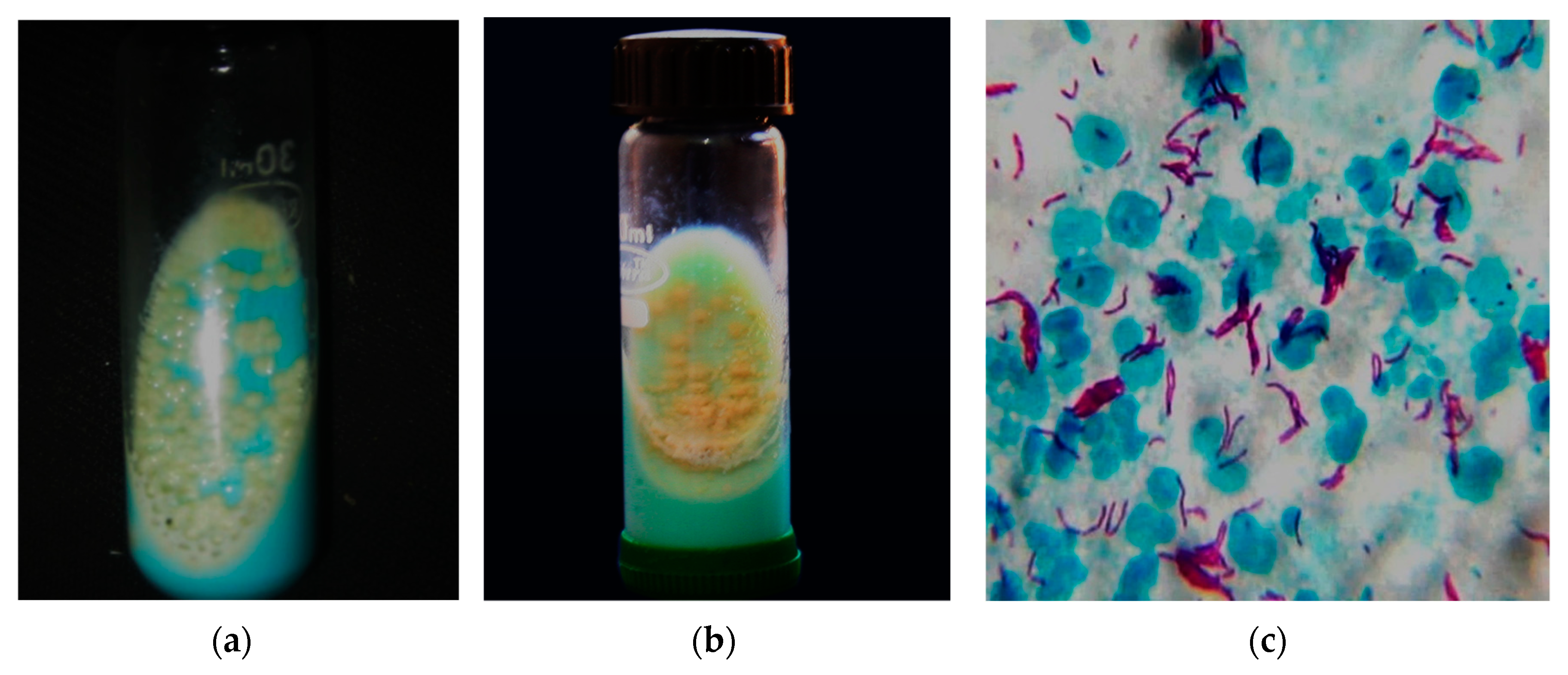
| List of NTM Species | Clinical Relevance and Possible Site of Infection | Reference |
|---|---|---|
| M. abscessus | Peripheral blood, peritoneal biopsy, pulmonary and permanent catheter tip. | [2,3,37,38,39,40,41,42,43,44,45] |
| M. asiaticum | Pulmonary | |
| M. avium | Pulmonary | |
| M. celatum | Pulmonary | |
| M. chelonae | Breast abscesses, blood and peritoneal fluid, pleural fluid | |
| M. flavescens | Pulmonary | |
| M. fortuitum | Ascetic fluid, peritoneal dialysis fluid, pulmonary, lipoid pneumonia, mediastinal infection, a myocardial and abdominal abscess. | |
| M. gastri | Pulmonary | |
| M. gordonae | Urinary tract and rarely liver biopsies | |
| M. intracellulare | Pulmonary and extrapulmonary | |
| M. kansasii | Appendiceal abscess | |
| M. lentiflavum | Extrapulmonary | |
| M. marinum | Wound-elbow and nasal cavity | |
| M. riyadhense | Pulmonary infection, sclerotic lesions, maxillary sinus, dural lesion | |
| M. scrofulaceum | Extrapulmonary | |
| M. simiae | Pulmonary | |
| M. smegmatis | Pulmonary | |
| M. szulgai | Joints/synovial aspiration | |
| M. terrae | Pulmonary | |
| M. xenopi | Pulmonary |
| Mycobacterium Species | Established Regimens | Additional or Suggested Agents |
|---|---|---|
| M. avium complex | rifampin, ethambutol, isoniazid, streptomycin or amikacin | clarithromycin (azithromycin), ciprofloxacin, clofazimine |
| M. scrofulaceum | - | clarithromycin (azithromycin), ciprofloxacin, clofazimine |
| M. kansasii | rifampin, ethambutol, isoniazid | streptomycin, ciprofloxacin, clarithromycin |
| M. marinum | rifampin, ethambutol, doxycycline or trimethoprim-sulfamethoxazole | streptomycin, ciprofloxacin |
| M. xenopi | rifampin, ethambutol, isoniazid | streptomycin |
| M. malmoense | - | clarithromycin (azithromycin), ciprofloxacin, clofazimine |
| M. simiae | - | clarithromycin (azithromycin), ciprofloxacin, clofazimine |
| M. szulgai | - | streptomycin, ciprofloxacin, clarithromycin |
| M. hemophilum | - | rifampin, cefoxitin, doxycycline, trimethoprim-sulfamethoxazole |
| M. fortuitum | amikacin, ciprofloxacin, sulfonamides | clofazimine, cefoxitin, imipenem, a cocktail of azithromycin or clarithromycin, doxycycline, fluoroquinolones, trimethoprim-sulfamethoxazole |
| M. abscessus | amikacin, streptomycin, cefoxitin | clofazimine, clarithromycin, a cocktail of azithromycin, imipenem, clarithromycin, |
| M. chelonae | tobramycin, amikacin | clofazimine, clarithromycin, doxycycline, a cocktail of azithromycin, imipenem, cefoxitin, clarithromycin, fluoroquinolones |
| Class of Flavonoids | Plant Source (Family) | Compounds | Chemical Structure | NTM | MIC (mg/L) | References |
|---|---|---|---|---|---|---|
| Flavonoid | Euphorbia paralias L (Euphorbiaceaea) | quercetin-3-O-β-d-glucoside | 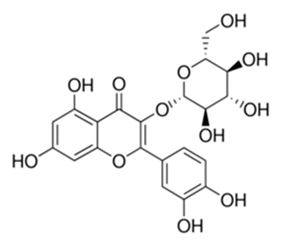 | M. fortuitum and M. chelonae | 3.13 | [161] |
| Flavonoid | Adonis dentate (Delile) (Ranunculaceae) | quercetin-3-O-β-d-glucoside | 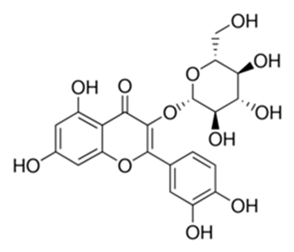 | M. abscessus | 5 | [161] |
| Flavonoid | Jasoniac andicans (Delile) Botsch (Asteraceae) | quercetin-3-O-β-d-glucoside | 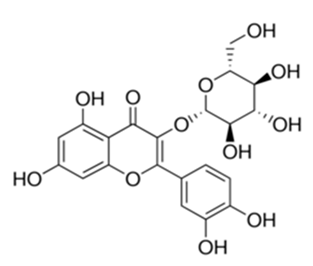 | M. fortuitum and M. chelonae | 6.25 | [161] |
| Flavone | Galenia africana (Aizoaceae) | 5,7,2′-trihydroxyflavone | 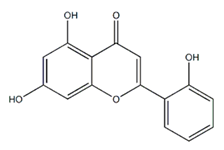 | M. abscessus | 10 | [209] |
| Flavonoid | Moltkiopsis ciliate (Forssk.) I.M (Boraginaceae) | quercetin-3-O-β-d-glucoside | 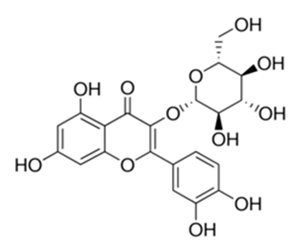 | M. fortuitum and M. chelonae | 10 | [161] |
| Flavonoid | Terminalia albida (Combretaceae) | gallic acid, flavogallonic acid isomer i, gallagic acid | 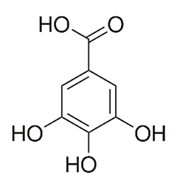 | M. chelonae | 11.81 | [193] |
| Flavonoids | Pelargonium reniforme (Geraniaceae) | myricetin and quercitin-3-O-β-d-glucoside | 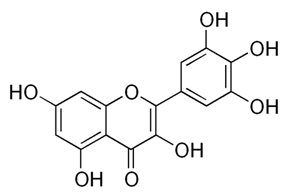 | M. fortuitum | 12.5 | [124] |
| Flavonoid | Eremophila sturtii (Myoporaceae) | 8,19-dihydroxyserrulat-14-ene and 8-hydroxyserrulat-14-en-19-oic acid | 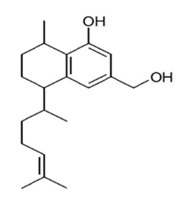 | M. fortuitum and M. chelonae | 12.5 | [210] |
| Flavonoid | Isatis microcarpa J. Gay ex Boiss. (Brassicaceae) | quercetin-3-O-β-d-glucoside | 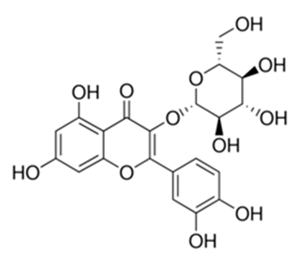 | M. fortuitum and M. chelonae | 12.5 | [161] |
| Flavonoid | Piper nigrum L (Piperaceae) | quercetin-3-O-β-d-glucoside | 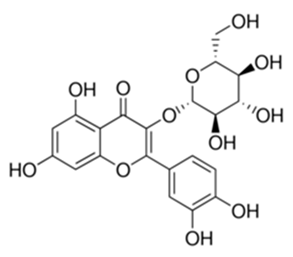 | M. smegmatis | 12.5 | [161] |
| Eugenol | Alpinia galanga (Zingiberaceae) | 1′-s-1′-acetoxychavicol acetate, trans-p-coumaryl diacetate and 1′-s-1′-acetoxyeugenol acetate | 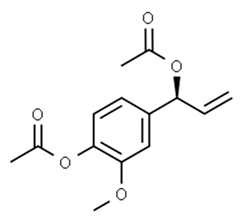 | M. smegmatis | 2.5, 6.25 and 5.0 | [187] |
| Flavonoid | Rhynchosia precatoria (Willd.) DC. (Fabaceae) | β-sitosterol, daucosterol, tricin, gallic acid, daidzein, 5,7,3′-trihydroxy-4′-methoxyisoflavone, epicatechin, stigmast-5-ene-3β,7α-diol, quercetin, apigenin-7-O-β-d-glucoside, luteolin-7-O-β-d-glucoside, and calycosin | 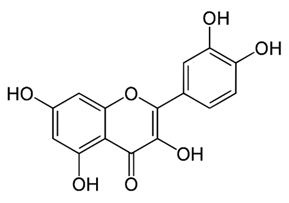 | M. fortuitum and M. chelonae | 15.6 | [142,143,144,145] |
| Flavonoid | Lawsonia inermis (Lythraceae) | lawsonicin | 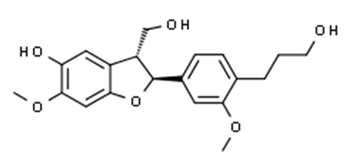 | M. chelonae | 16 | [193] |
| Flavonoid | Zingiber officinale Rosc. (Zingiberaceae) and Curcuma longa L. (Zingiberaceae) | flavonoid | 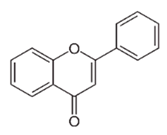 | M. abscessus | 25 | [181] |
| Flavonoid | Combretumhereroense,C.apiculatum and C. collinum (Combretaceae) | pinocembrin | 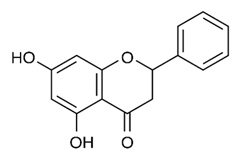 | M. fortuitum | 25 | [194] |
| Flavonoid | Cistanche tubulosa (Schrenk) Hoof.f (Orobanchaceae) | quercetin-3-O-β-d-glucoside | 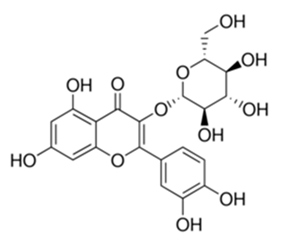 | M. fortuitum and M. chelonae | 25 | [161] |
| Flavonoid | Morcandias nites (Viv) E.A. Durand & Barratte (Brassicaceae) | quercetin-3-O-β-d-glucoside | 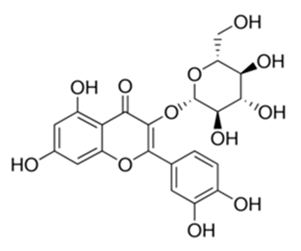 | M. fortuitum and M. chelonae | 25 | [161] |
| Flavonoid | Onopordum acanthium L (Asteraceae) | quercetin-3-O-β-d-glucoside | 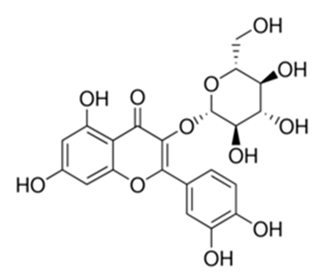 | M. smegmatis | 25 | [161] |
| Flavonoid | Phlomis fraticosa L (Lamiaceae) | quercetin-3-O-β-d-glucoside | 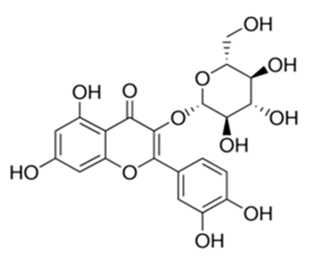 | M. smegmatis | 25 | [161] |
| O-Methylated isoflavone | Trifolium pretense (Fabaceae) | biochanin A | 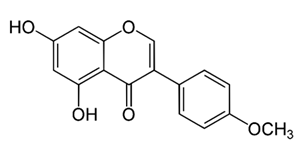 | M. smegmatis | 32 | [144] |
| Stilbene | Vatica oblongifolia ssp. Oblongifolia (Dipterocarpaceae) | resveratrol hopeaphenol A, isohopeaphenol A, vaticaphenol A | 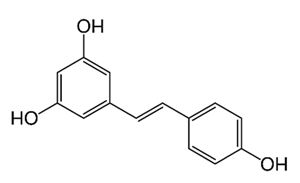 | M. abscessus | 32 | [211] |
| Flavone | – | luteolin | 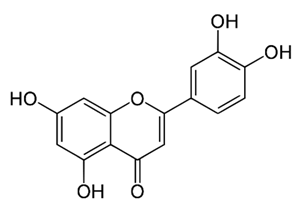 | M. smegmatis | 32 | [144] |
| Flavonoid | – | myricetin | 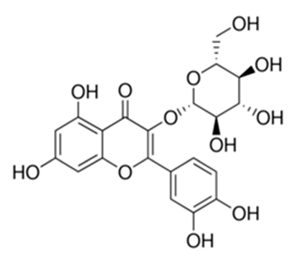 | M. smegmatis | 32 | [144] |
| Flavonoid | Thymelea hirsute L (Thymelaeaceae) | quercetin-3-O-β-d-glucoside | 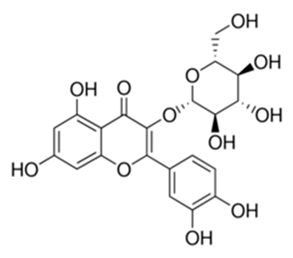 | M. smegmatis | 40 | [161] |
| Methoxylated Flavonoid | Paepalanthus Latipes (Eriocaulaceae) | 7-methyl quercetagetin-4′-O-β-d-glucopyranoside, 7-methylquercetagetin | 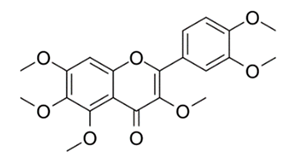 | M. abscessus | 50 | [181] |
| Flavonoid | Nasturtium africanum (Braun-Blanq) (Brassicaceae) | quercetin-3-O-β-d-glucoside | 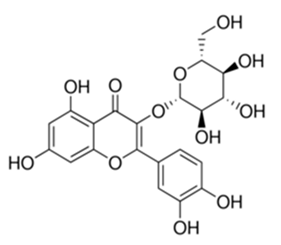 | M. smegmatis | 50 | [161] |
| Flavonoid | Cesalpinia digyna (Fabaceae) | Bonducellin | 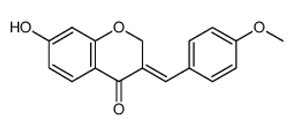 | M. abscessus | 62.5 | [212] |
| Flavonoid | - | carvacrol | 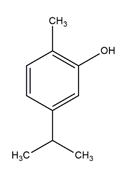 | M. abscessus,M. chelonae, M. fortuitum, M. mucogenicum, M. smegmatis | 64 | [125] |
| Isoflavones | Iris adriatica (Iridaceae) | Irigenin, irilone, methoxylated benzophenone |  | M. abscessus | 64 | [209] |
| Flavone | - | baicalein |  | M. abscessus | 64 | [144] |
| Stilbenoid | - | resveratrol | 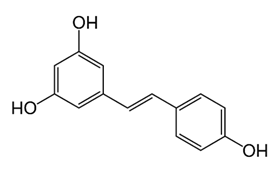 | M. smegmatis | 64 | [144] |
| Flavonoid | Alpinia katsumadai (Zingiberaceae) | pinocembrin | 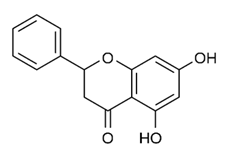 | M. abscessus | ≥ 64 | [184] |
| Flavonoid | Curcuma longa L. (Zingiberaceae) | curcumin | 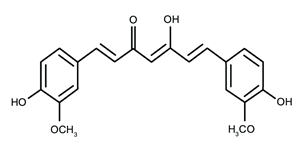 | M. abscessus, | 128 | [123] |
| Flavonoid | Aloe secundiflora Engl. (Asphodelaceae) | Flavonoids | 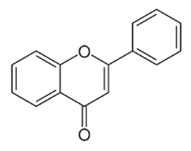 | M. fortuitum and M. smegmatis | 150 | [213] |
| Flavonoid | Colletotrichum tofieldiae and Magnaporthe grisea | 2,4-diacetyl phloroglucinol, phloretin | 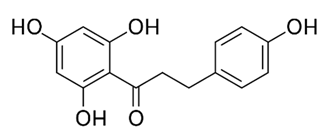 | M. abscessus | 100, 150 | [193] |
| Flavonoid | Entada abysinnica steudel ex. A. Rich (Fabaceae) | Flavonoids | 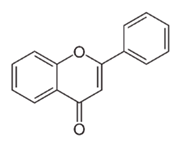 | M. fortuitum and M. smegmatis | 250 | [214] |
| Flavonoid | Euphorbia albomarginata Torr. (Euphorbiaceae) | Gallic acid methylester, 7-O-galloylcatechin, 1,6-di-O-galloylglucose, 1-O-galloylglucose, trigalloylgallic acid and gallic acid | 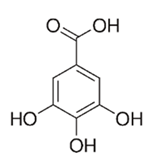 | M. fortuitum and M. chelonae | 250 | [142,215,216] |
| Flavonoid | Helianthus annuus L. (Asteraceae) | Gallic acid, daidzein and calycosin |  | M. fortuitum and M. chelonae | 250 | [142,217] |
| Cinnamolyglico flavonoids | Heritiera littoralis (Sterculiaceae) | 3-cinnamoyl tribuloside | 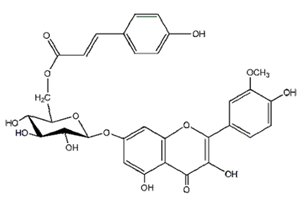 | M. fortuitum | 256 | [189] |
| Flavonoid | Dorstenia barteri (Moraceae) | Isobavachalcone, kanzanol C, 4-hydroxylonchocarpin, stipulin, amentoflavone |  | M. smegmatis | 256 | [214] |
| Flavone glycoside | - | Baicalin | 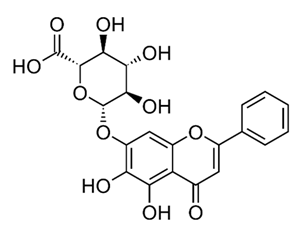 | M. abscessus | 256 | [144] |
| O-methylated isoflavone | - | biochanin A | 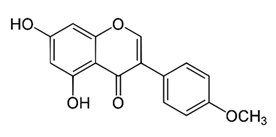 | M. abscessus, | 256 | [144] |
| Isoflavone | - | Daidzein | 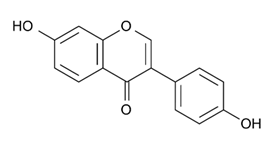 | M. smegmatis | >256 | [144] |
| O-methylated isoflavone | - | Formononetin |  | M. smegmatis | 256 | [144] |
| Isoflavone | - | Genistein |  | M. smegmatis | 256 | [144] |
| Flavonoid | Pelargonium reniforme and Pelargonium sidoides (Geraniaceae) | Gallic acid, methyl gallate, myricetin and quercitin-3-O-beta-d-glucoside, 1-O-(2-(4-methoxyphenyl)ethyl-6-O-galloyl-glucopyranoside | 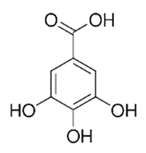 | M. fortuitum | 250, 150 | [115] |
| Polymethoxy flavones | - | Skullcapflavone II and nobiletin, tangeretin, baicalein and wogonin. | 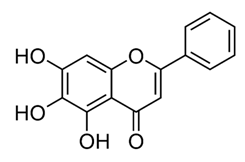 | M. fortuitum and M. chelonae | 128, 128, 128, 32, 128 | [177] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickymaray, S.; Alfaiz, F.A.; Paramasivam, A. Efficacy and Mechanisms of Flavonoids against the Emerging Opportunistic Nontuberculous Mycobacteria. Antibiotics 2020, 9, 450. https://doi.org/10.3390/antibiotics9080450
Mickymaray S, Alfaiz FA, Paramasivam A. Efficacy and Mechanisms of Flavonoids against the Emerging Opportunistic Nontuberculous Mycobacteria. Antibiotics. 2020; 9(8):450. https://doi.org/10.3390/antibiotics9080450
Chicago/Turabian StyleMickymaray, Suresh, Faiz Abdulaziz Alfaiz, and Anand Paramasivam. 2020. "Efficacy and Mechanisms of Flavonoids against the Emerging Opportunistic Nontuberculous Mycobacteria" Antibiotics 9, no. 8: 450. https://doi.org/10.3390/antibiotics9080450
APA StyleMickymaray, S., Alfaiz, F. A., & Paramasivam, A. (2020). Efficacy and Mechanisms of Flavonoids against the Emerging Opportunistic Nontuberculous Mycobacteria. Antibiotics, 9(8), 450. https://doi.org/10.3390/antibiotics9080450




