A Trypsin Inhibitor from Moringa oleifera Flowers Modulates the Immune Response In Vitro of Trypanosoma cruzi-Infected Human Cells
Abstract
1. Introduction
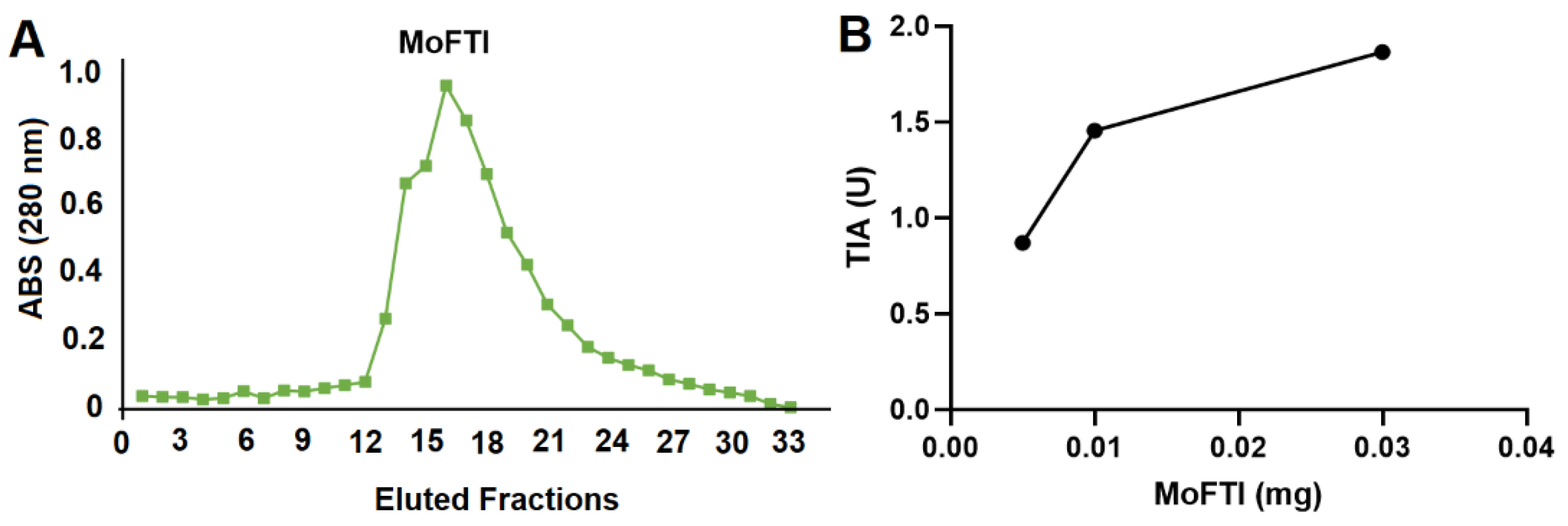
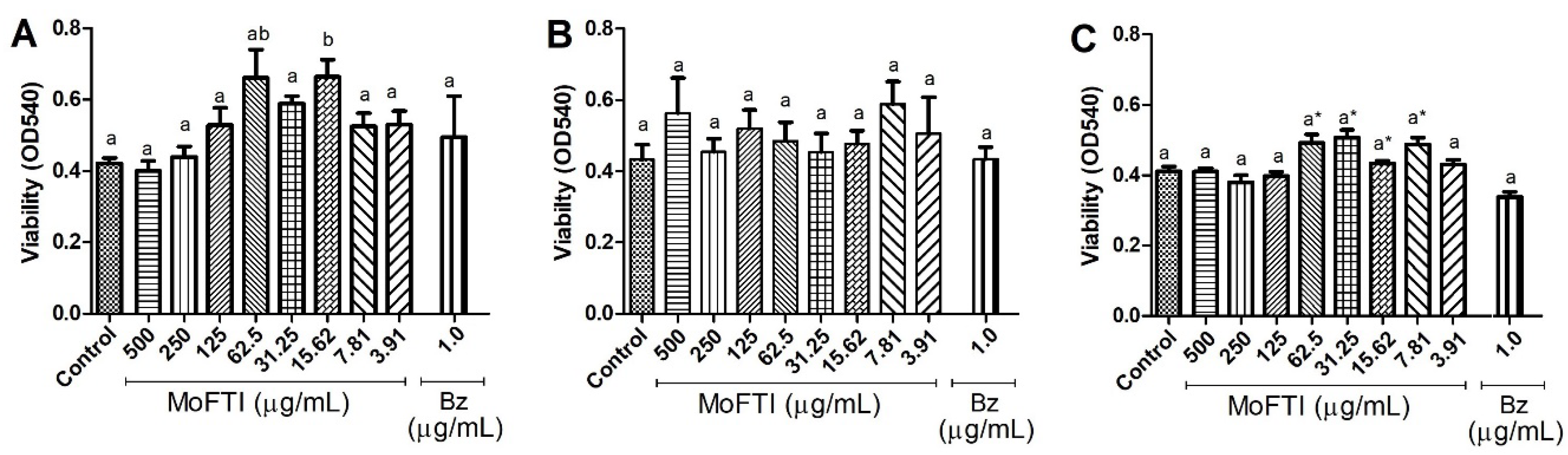
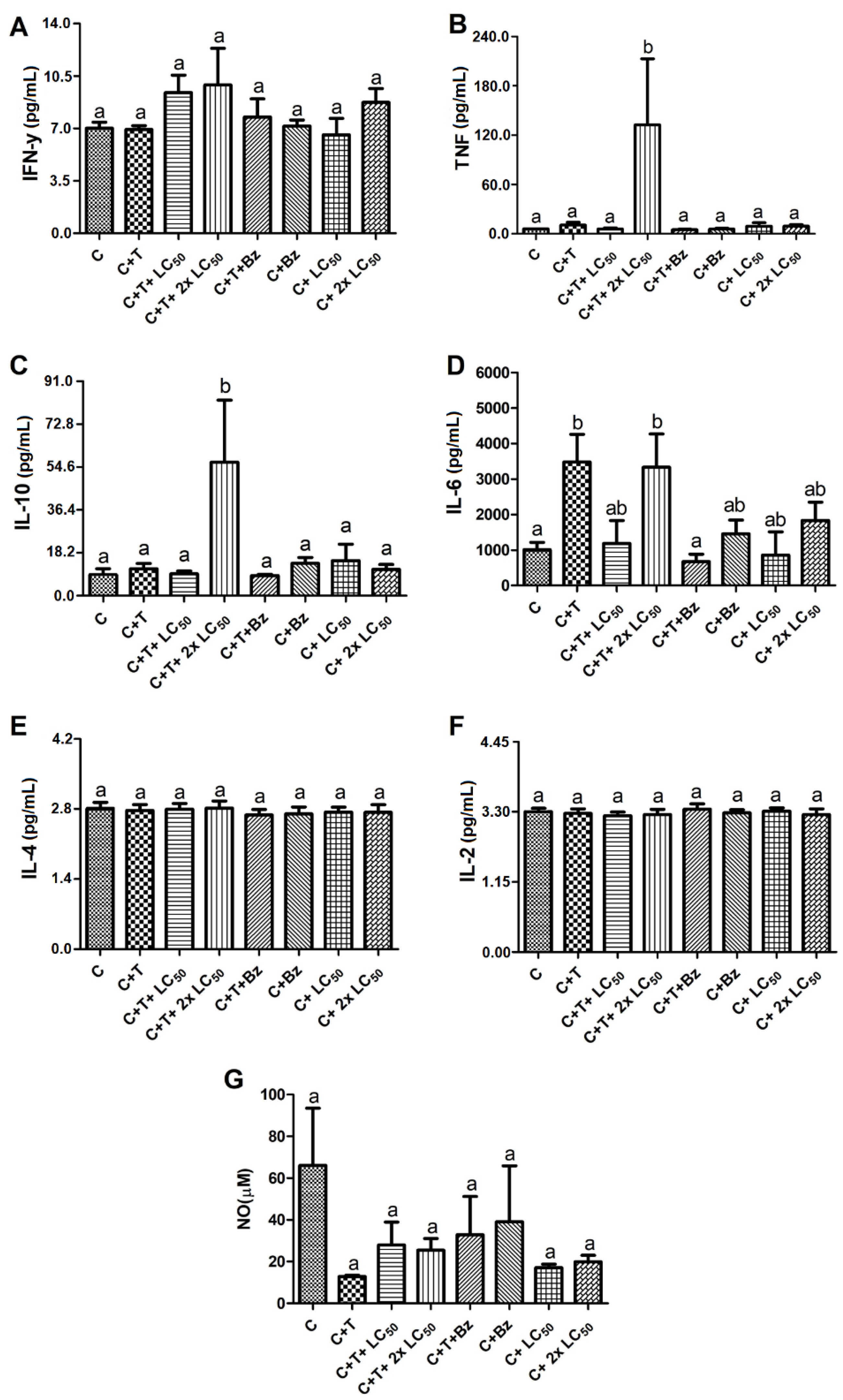
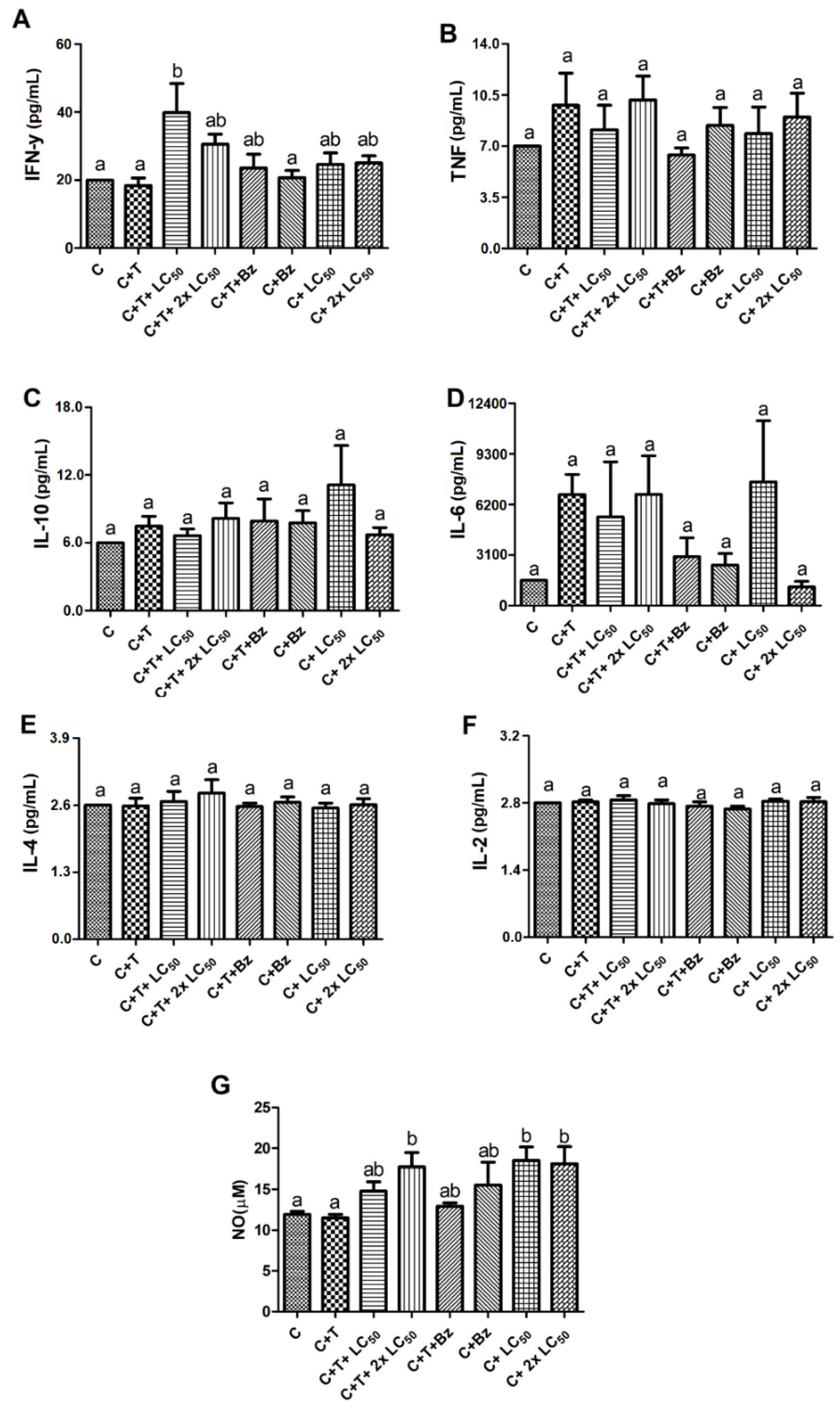
2. Results and Discussion
3. Materials and Methods
3.1. Isolation of MoFTI
3.2. Trypsin Inhibitory Activity
3.3. Obtaining of T. cruzi Trypomastigotes
3.4. Trypanocidal Activity of MoFTI
3.5. Isolation of Human PBMCs
3.6. Effect of MoFTI on PBMCs Viability
3.7. Treatment of T. cruzi-Infected PBMCs with MoFTI
3.8. Effect of MoFTI on Cytokine Release by T. cruzi-Infected PBMCs
3.9. Effect of MoFTI on NO Production by T. cruzi-Infected PBMCs
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Chagas Disease. Available online: http://www.who.int/en/news-room/factsheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 20 June 2020).
- Souza, W. Basic cell biology of Trypanosoma cruzi. Curr. Pharm. Des. 2002, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, L.E.; Morillo, C.A. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. North Am. 2019, 33, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Damasceno, R.F.; Monteiro-Junior, R.S.; Oliveira, I.A.C.D.; Prates, T.E.C.; Nunes, M.C.P.; Haikal, D.S.A. Reações adversas ao benzonidazol no tratamento da Doença de Chagas: Revisão sistemática de ensaios clínicos randomizados e controlados. Cad. Saúde Colet. 2019, 27, 354–362. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Olmo, F.; Lewis, M.D.; Wilkinson, S.R.; Taylor, M.C.; Kelly, J.M. Challenges in Chagas Disease drug development. Molecules 2020, 25, 2799. [Google Scholar] [CrossRef]
- Molina-Morant, D.; Fernández, M.L.; Bosch-Nicolau, P.; Sulleiro, E.; Bangher, M.; Salvador, F.; Sanchez-Montalva, A.; Ribeiro, A.L.P.; de Paula, A.M.B.; Eloi, S.; et al. Efficacy and safety assessment of different dosage of benznidazol for the treatment of Chagas disease in chronic phase in adults (MULTIBENZ study): Study protocol for a multicenter randomized Phase II non-inferiority clinical trial. Trials 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Juárez-Saldivar, A.; Schroeder, M.; Salentin, S.; Haupt, V.J.; Saavedra, E.; Vázquez, C.; Reyes-Espinosa, F.; Herrera-Mayorga, V.; Villalobos-Rocha, J.C.; García-Pérez, C.A.; et al. Computational drug repositioning for Chagas Disease using protein-ligand interaction profiling. Int. J. Mol. Sci. 2020, 21, 4270. [Google Scholar] [CrossRef]
- Cazzulo, J.J.; Couso, R.; Raimondi, A.; Wernstedt, C.; Hellman, U. Further characterization and partial amino acid sequence of a cysteine proteinase from Trypanosoma cruzi. Mol. Biochem. Parasitol. 1989, 33, 33–42. [Google Scholar] [CrossRef]
- Cazzulo, J. Proteinases of Trypanosoma cruzi: Potential targets for the chemotherapy of Chagas disease. Curr. Top. Med. Chem. 2002, 2, 1261–1271. [Google Scholar] [CrossRef]
- Ferreira, R.A.A.; Pauli, I.; Sampaio, T.S.; Souza, M.L.; Ferreira, L.L.G.; Magalhães, L.G.; Rezende, C.O., Jr.; Ferreira, R.S.; Krogh, R.; Dias, L.C.; et al. Structure-based and molecular modeling studies for the discovery of cyclic imides as reversible cruzain inhibitors with potent anti-Trypanosoma cruzi activity. Front. Chem. 2019, 7, 798. [Google Scholar] [CrossRef]
- Pontual, E.V.; Pires-Neto, D.F.; Fraige, K.; Higino, T.M.M.; Carvalho, B.E.A.; Alves, N.M.P.; Lima, T.A.; Zingali, R.B.; Coelho, L.C.B.B.; Bolzani, V.S.; et al. A trypsin inhibitor from Moringa oleifera flower extract is cytotoxic to Trypanosoma cruzi with high selectivity over mammalian cells. Nat. Prod. Res. 2018, 32, 2940–2944. [Google Scholar] [CrossRef]
- Marathe, K.R.; Patil, R.H.; Vishwakarma, K.S.; Chaudhari, A.B.; Maheshwari, V.L. Protease inhibitors and their applications: An overview. In Studies Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62, pp. 211–242. [Google Scholar]
- Agbowuro, A.A.; Huston, W.M.; Gamble, A.B.; Tyndall, J.D. Proteases and protease inhibitors in infectious diseases. Med. Res. Rev. 2018, 38, 1295–1331. [Google Scholar] [CrossRef] [PubMed]
- Cotabarren, J.; Lufrano, D.; Parisi, M.G.; Obregón, W.D. Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Trends Plant. Sci. 2020, 262, 110398. [Google Scholar] [CrossRef]
- Shamsi, T.N.; Parveen, R.; Afreen, S.; Azam, M.; Sen, P.; Sharma, Y.; Haque, Q.M.R.; Fatma, T.; Manzoor, N.; Fatima, S. Trypsin inhibitors from Cajanus cajan and Phaseolus limensis possess antioxidant, anti-inflammatory, and antibacterial activity. J. Diet. Suppl. 2018, 15, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Blisnick, A.; Šimo, L.; Grillon, C.; Fasani, F.; Brûlé, S.; Le Bonniec, B.; Prina, E.; Marsot, M.; Relmy, A.; Blaise-Boisseau, S.; et al. The immunomodulatory effect of IrSPI, a tick salivary gland serine protease inhibitor involved in Ixodes ricinus tick feeding. Vaccines 2019, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.N.; Reddy, M.V.; Prasad, D.T. Plant serine protease inhibitor (SPI): A potent player with bactericidal, fungicidal, nematicidal and antiviral properties. Int. J. Cardiovasc. Sci. 2020, 8, 2985–2993. [Google Scholar] [CrossRef][Green Version]
- Pramanik, A.; Paik, D.; Pramanik, P.K.; Chakraborti, T. Serine protease inhibitors rich Coccinia grandis (L.) Voigt leaf extract induces protective immune responses in murine visceral leishmaniasis. Biomed. Pharmacother. 2019, 111, 224–235. [Google Scholar] [CrossRef]
- Sangenito, L.S.; Menna-Barreto, R.F.; Oliveira, A.C.; d’Avila-Levy, C.M.; Branquinha, M.H.; Santos, A.L. Primary evidence of the mechanisms of action of HIV aspartyl peptidase inhibitors on Trypanosoma cruzi trypomastigote forms. Int. J. Antimicrob. Agents. 2018, 52, 185–194. [Google Scholar] [CrossRef]
- Makkar, H.A.; Becker, K. Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera. Anim. Feed Sci. Technol. 1996, 63, 211–228. [Google Scholar] [CrossRef]
- Karadi, R.V.; Gadge, N.B.; Alagawadi, K.R.; Savadi, R.V. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J. Ethnopharmacol. 2006, 105, 306–311. [Google Scholar] [CrossRef]
- Pontual, E.V.; Santos, N.D.L.; Moura, M.C.; Coelho, L.C.B.B.; Navarro, D.M.A.F.; Napoleão, T.H.; Paiva, P.M.G. Trypsin inhibitor from Moringa oleifera flowers interferes with survival and development of Aedes aegypti larvae and kills bacteria inhabitant of larvae midgut. Parasitol. Res. 2014, 113, 727–733. [Google Scholar] [CrossRef]
- Bermudez, J.; Davies, C.; Simonazzi, A.; Real, J.P.; Palma, S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016, 156, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Patriota, L.L.S.; Procópio, T.F.; Souza, M.F.D.; Oliveira, A.P.S.; Carvalho, L.V.N.; Pitta, M.G.R.; Rego, M.J.B.M.; Paiva, P.M.G.; Pontual, E.V.; Napoleão, T.H. A trypsin inhibitor from Tecoma stans leaves inhibits growth and promotes ATP depletion and lipid peroxidation in Candida albicans and Candida krusei. Front. Microbiol. 2016, 7, 611. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant. Sci. 2015, 6, 655. [Google Scholar] [CrossRef] [PubMed]
- Dutra, W.O.; Menezes, C.A.; Magalhães, L.M.; Gollob, K.J. Immunoregulatory networks in human Chagas disease. Parasite Immunol. 2014, 36, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, A.; Monge-Maillo, B.; Gutiérrez, C.; Norman, F.F.; López-Vélez, R.; Pérez-Molina, J.A. Changes in the immune response after treatment with benznidazole versus no treatment in patients with chronic indeterminate Chagas disease. Acta Trop. 2016, 164, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, J.M.; Fonseca, R.; Silva, H.B.; Marinho, C.R.; Bortoluci, K.R.; Sardinha, L.R.; D’império-Lima, M.R. Chagas disease: Still many unsolved issues. Mediators Inflamm. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Interleukin-6: Discovery of a pleiotropic cytokine. Arthritis Res. Ther. 2006, 8, S2. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Roffê, E.; Rothfuchs, A.G.; Santiago, H.C.; Marino, A.P.M.P.; Ribeiro-Gomes, F.L.; Eckhaus, M.; Antonelli, L.R.V.; Murphy, P.M. IL-10 limits parasite burden and protects against fatal myocarditis in a mouse model of Trypanosoma cruzi infection. J. Immunol. 2012, 188, 649–660. [Google Scholar] [CrossRef]
- Cardoso, M.S.; Reis-Cunha, J.L.; Bartholomeu, D.C. Evasion of the immune response by Trypanosoma cruzi during acute infection. Front. Immunol. 2016, 6, 659. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Resende, L.A.; Roatt, B.M.; Aguiar-Soares, R.D.O.; Viana, K.F.; Mendonca, L.Z.; Lanna, M.F.; Silveira-Lemos, D.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Fujiwara, R.T.; et al. Cytokine and nitric oxide patterns in dogs immunized with LBSap vaccine, before and after experimental challenge with Leishmania chagasi plus saliva of Lutzomyia longipalpis. Vet. Parasitol. 2013, 198, 371–381. [Google Scholar] [CrossRef] [PubMed][Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nova, I.C.V.; Moreira, L.R.; Torres, D.J.L.; Oliveira, K.K.d.S.; Patriota, L.L.d.S.; Coelho, L.C.B.B.; Paiva, P.M.G.; Napoleão, T.H.; Lorena, V.M.B.d.; Pontual, E.V. A Trypsin Inhibitor from Moringa oleifera Flowers Modulates the Immune Response In Vitro of Trypanosoma cruzi-Infected Human Cells. Antibiotics 2020, 9, 515. https://doi.org/10.3390/antibiotics9080515
Nova ICV, Moreira LR, Torres DJL, Oliveira KKdS, Patriota LLdS, Coelho LCBB, Paiva PMG, Napoleão TH, Lorena VMBd, Pontual EV. A Trypsin Inhibitor from Moringa oleifera Flowers Modulates the Immune Response In Vitro of Trypanosoma cruzi-Infected Human Cells. Antibiotics. 2020; 9(8):515. https://doi.org/10.3390/antibiotics9080515
Chicago/Turabian StyleNova, Isabella Coimbra Vila, Leyllane Rafael Moreira, Diego José Lira Torres, Kamila Kássia dos Santos Oliveira, Leydianne Leite de Siqueira Patriota, Luana Cassandra Breitenbach Barroso Coelho, Patrícia Maria Guedes Paiva, Thiago Henrique Napoleão, Virgínia Maria Barros de Lorena, and Emmanuel Viana Pontual. 2020. "A Trypsin Inhibitor from Moringa oleifera Flowers Modulates the Immune Response In Vitro of Trypanosoma cruzi-Infected Human Cells" Antibiotics 9, no. 8: 515. https://doi.org/10.3390/antibiotics9080515
APA StyleNova, I. C. V., Moreira, L. R., Torres, D. J. L., Oliveira, K. K. d. S., Patriota, L. L. d. S., Coelho, L. C. B. B., Paiva, P. M. G., Napoleão, T. H., Lorena, V. M. B. d., & Pontual, E. V. (2020). A Trypsin Inhibitor from Moringa oleifera Flowers Modulates the Immune Response In Vitro of Trypanosoma cruzi-Infected Human Cells. Antibiotics, 9(8), 515. https://doi.org/10.3390/antibiotics9080515






