Clinical Experience with High-Dose Polymyxin B against Carbapenem-Resistant Gram-Negative Bacterial Infections—A Cohort Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Setting and Population
2.2. Ethics
2.3. Data Collection
2.4. Study Outcomes
2.5. Microbiological Methods
2.6. Statistical Analysis
3. Results
3.1. Baseline and Infection Characteristics
3.2. Characteristics of Polymyxin B Use
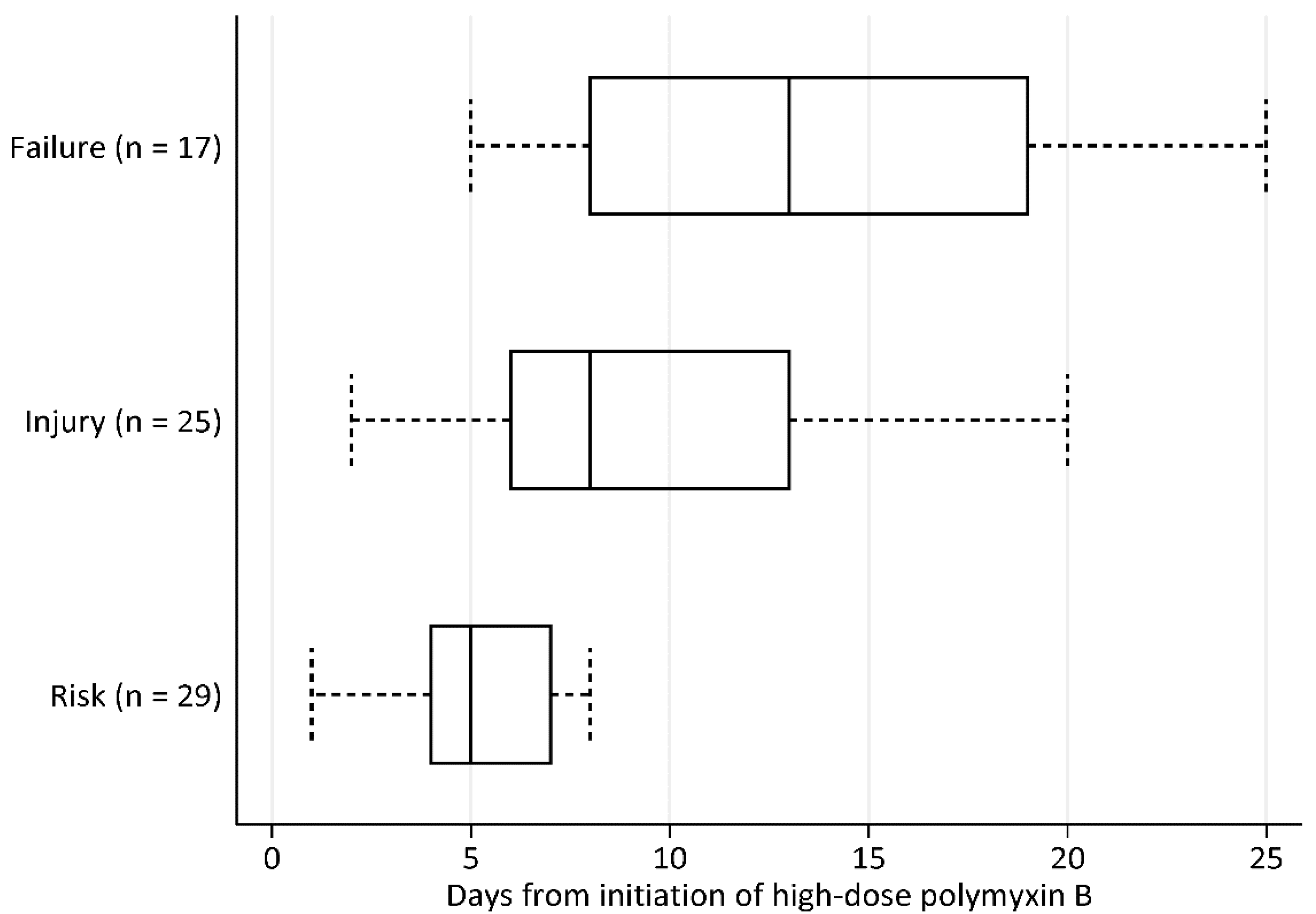
3.3. Efficacy Outcomes
3.4. Nephrotoxicity Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kassamali, Z.; Rotschafer, J.C.; Jones, R.N.; Prince, R.A.; Danziger, L.H. Polymyxins: Wisdom does not always come with age. Clin. Infect. Dis. 2013, 57, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs. no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lee, W.; Kwa, A.L. Polymyxin B versus colistin: An update. Expert Rev. Anti-Infect. Ther. 2015, 13, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
- Onufrak, N.J.; Rao, G.G.; Forrest, A.; Pogue, J.M.; Scheetz, M.H.; Nation, R.L.; Li, J.; Kaye, K.S. Critical Need for Clarity in Polymyxin B Dosing. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Kwa, A.L.; Tam, V.H.; Falagas, M.E. Polymyxins: A review of the current status including recent developments. Ann. Acad. Med. Singap. 2008, 37, 870–883. [Google Scholar]
- Sandri, A.M.; Landersdorfer, C.B.; Jacob, J.; Boniatti, M.M.; Dalarosa, M.G.; Falci, D.R.; Behle, T.F.; Bordinhao, R.C.; Wang, J.; Forrest, A.; et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: Implications for selection of dosage regimens. Clin. Infect. Dis. 2013, 57, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Thamlikitkul, V.; Dubrovskaya, Y.; Manchandani, P.; Ngamprasertchai, T.; Boonyasiri, A.; Babic, J.T.; Tam, V.H. Dosing and Pharmacokinetics of Polymyxin B in Patients with Renal Insufficiency. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Chambers, H.F.; Eliopoulos, G.M.; Gilbert, D.N.; Pavia, A.; Saag, M.S. (Eds.) Sanford Guide to Antimicrobial Therapy 2018, 48th ed.; Antimicrobial Therapy: Sperryville, VA, USA, 2018. [Google Scholar]
- Wynn, F.L. Drug Information Handbook, 27th ed.; Wolters Kluwer Clinical Drug Information Inc.: Hudson, OH, USA, 2013; pp. 1143–1147. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals; ECDC: Stockholm, Sweden, 2013.
- Nelson, B.C.; Eiras, D.P.; Gomez-Simmonds, A.; Loo, A.S.; Satlin, M.J.; Jenkins, S.G.; Whittier, S.; Calfee, D.P.; Furuya, E.Y.; Kubin, C.J. Clinical outcomes associated with polymyxin B dose in patients with bloodstream infections due to carbapenem-resistant Gram-negative rods. Antimicrob. Agents Chemother. 2015, 59, 7000–7006. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Acute Dialysis Quality Initiative Workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2014; Volume M100-S24E. [Google Scholar]
- Vrieze, S.I. Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol. Methods 2012, 17, 228–243. [Google Scholar] [CrossRef]
- Phe, K.; Lee, Y.; McDaneld, P.M.; Prasad, N.; Yin, T.; Figueroa, D.A.; Musick, W.L.; Cottreau, J.M.; Hu, M.; Tam, V.H. In Vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob. Agents Chemother. 2014, 58, 2740–2746. [Google Scholar] [CrossRef]
- John, J.F.; Falci, D.R.; Rigatto, M.H.; Oliveira, R.D.; Kremer, T.G.; Zavascki, A.P. Severe Infusion-Related Adverse Events and Renal Failure in Patients Receiving High-Dose Intravenous Polymyxin B. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Crusio, R.; Rao, S.; Changawala, N.; Paul, V.; Tiu, C.; van Ginkel, J.; Chapnick, E.; Kupfer, Y. Epidemiology and outcome of infections with carbapenem-resistant Gram-negative bacteria treated with polymyxin B-based combination therapy. Scand. J. Infect. Dis. 2014, 46, 1–8. [Google Scholar] [CrossRef]
- Kassamali, Z.; Danziger, L. To B or not to B, that is the question: Is it time to replace colistin with polymyxin B? Pharmacotherapy 2015, 35, 17–21. [Google Scholar] [CrossRef]
- Nation, R.L.; Velkov, T.; Li, J. Colistin and polymyxin B: Peas in a pod, or chalk and cheese? Clin. Infect. Dis. 2014, 59, 88–94. [Google Scholar] [CrossRef]
- Azad, M.A.; Akter, J.; Rogers, K.L.; Nation, R.L.; Velkov, T.; Li, J. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob. Agents Chemother. 2015, 59, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Ortwine, J.K.; Kaye, K.S. Clinical considerations for optimal use of the polymyxins: A focus on agent selection and dosing. Clin. Microbiol. Infect. 2017, 23, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Ortwine, J.K.; Kaye, K.S. Are there any ways around the exposure-limiting nephrotoxicity of the polymyxins? Int. J. Antimicrob. Agents 2016, 48, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Rigatto, M.H.; Oliveira, M.S.; Perdigao-Neto, L.V.; Levin, A.S.; Carrilho, C.M.; Tanita, M.T.; Tuon, F.F.; Cardoso, D.E.; Lopes, N.T.; Falci, D.R.; et al. Multicenter Prospective Cohort Study of Renal Failure in Patients Treated with Colistin versus Polymyxin B. Antimicrob. Agents Chemother. 2016, 60, 2443–2449. [Google Scholar] [CrossRef]
- Tuon, F.F.; Rigatto, M.H.; Lopes, C.K.; Kamei, L.K.; Rocha, J.L.; Zavascki, A.P. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int. J. Antimicrob. Agents 2014, 43, 349–352. [Google Scholar] [CrossRef]
- Dubrovskaya, Y.; Prasad, N.; Lee, Y.; Esaian, D.; Figueroa, D.A.; Tam, V.H. Risk factors for nephrotoxicity onset associated with polymyxin B therapy. J. Antimicrob. Chemother. 2015, 70, 1903–1907. [Google Scholar] [CrossRef]

| Variable Median (IQR) or No. (%) | All Patients (n = 43) |
|---|---|
| Demographics and Admission Characteristics | |
| Age (years) | 54 (33–66) |
| Male gender | 30 (69.8) |
| Length of hospital stay | 55 (39–96) |
| Admission to ICU | 12 (27.9) |
| Total body weight (kg) | 60 (50–71) |
| Comorbidities | |
| Congestive heart failure | 4 (9.3) |
| Chronic kidney disease | 4 (9.3) |
| Diabetes mellitus | 8 (18.6) |
| Solid and haematological malignancy | 14 (32.6) |
| Age-adjusted Charlson comorbidity index | 3 (0–4) |
| Infection Characteristics and Clinical Presentation | |
| Time to infection onset (days) | 10 (4–28) |
| APACHE II score at infection onset | 17 (11–22) |
| Presence of sepsis | 43 (100.0) |
| Septic shock | 38 (88.4) |
| Types of Infection | |
| Bloodstream infections | 24 (55.8) |
| Complicated intra-abdominal infections | 1 (2.3) |
| Complicated skin and soft tissue infections | 8 (18.6) |
| Complicated urinary tract infections | 3 (7.0) |
| Pneumonia | 2 (4.7) |
| Multiple infection types | 5 (11.3) |
| Types of CR-GNB (Total No. of CR-GNB = 58) a | |
| Acinetobacter spp. | 31 (53.5) |
| Enterobacteriaceae | 14 (24.1) |
| P. aeruginosa | 13 (22.4) |
| Patients with concurrent non-CR-GNB infections | 29 (67.4) |
| Characteristics of Treatment | |
| APACHE II score at time of high-dose PMB initiation | 21 (12–24) |
| Use of PMB loading dose (>25,000 IU/kg) | 6 (14.0) |
| Daily high-dose PMB dose (IU/kg) | 32 051 (29,340–34,884) |
| Duration of high-dose PMB (days) | 14 (7–28) |
| Cumulative high-dose PMB (MIU) | 24.5 (13.8–52.8) |
| Dose reduction or use of standard-dose PMB (≤25,000 IU/kg/day) prior to high-dose PMB | 22 (51.6) |
| Overall average daily PMB dose (IU/kg) b | 29 412 (28,070–33,751) |
| Overall cumulative PMB (MIU) b | 27.0 (18.4–67.5) |
| Use of combination therapy | 40 (93.0) |
| Presence of source control | 25 (58.1) |
| Organisms | Acinetobacter spp. (n = 31) | P. aeruginosa (n = 13) | Enterobacteriaceae (n = 14) |
|---|---|---|---|
| Susceptibility Profiles a | |||
| Antimicrobial Agents | No. of non-susceptible isolates (%) | No. of non-susceptible isolates (%) | No. of non-susceptible isolates (%) |
| Ampicillin/sulbactam | 31 (100) | ND | ND |
| Piperacillin/tazobactam | 31 (100) | 12 (92.3) | 14 (100) |
| Cefepime | 31 (100) | 11 (84.6) | 13 (92.9) |
| Meropenem | 31 (100) | 13 (100) | 14 (100) |
| Aztreonam | ND | 12 (92.3) | 14 (100) |
| Ciprofloxacin | 31 (100) | 11 (84.6) | 14 (100) |
| Tigecycline | 14 (45.2) | ND | 1 (7.1) |
| Gentamicin | 29 (93.5) | 12 (92.3) | 11 (78.6) |
| Polymyxin B | 0 (0) | 0 (0) | 1 (7.1) |
| Minimum Inhibitory Concentrations | |||
| MIC range (mg/l) | MIC range (mg/l) | MIC range (mg/l) | |
| Meropenem | 4—≥32 | 4—≥32 | 2—≥32 |
| Polymyxin B | 0.25—1 | 0.5—2 | 0.25—16 |
| Variable Median (IQR) or No. (%) | Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| No AKI (n = 18) | AKI (n = 25) | p Value | Adjusted OR (95% CI) | p Value | |
| Demographics and Infection Characteristics | |||||
| Age (years) | 55 (27–66) | 54 (36–66) | 0.89 | ||
| Male gender | 12 (66.7) | 18 (72.0) | 0.71 | ||
| Age-adjusted Charlson comorbidity index | 3 (1–6) | 3 (0–4) | 0.21 | ||
| APACHE II at infection onset | 16 (12–22) | 19 (11–21) | 0.74 | ||
| APACHE II at high-dose PMB initiation | 17 (12–22) | 22 (15–24) | 0.22 | ||
| Renal insufficiency at high-dose PMB initiation | 5 (27.8) | 3 (12.0) | 0.20 | ||
| In-hospital 30-day all-cause mortality | 3 (16.7) | 5 (20.0) | 0.78 | ||
| Details of PMB dosing prior to time at risk a | |||||
| Duration of PMB (days) | 12 (7–20) | 12 (8–14) | 0.68 | ||
| Overall average daily PMB dose (IU/kg) | 30 273 (29 126–33 333) | 33 708 (30 000–37 037) | 0.04 | 1.01 (1.00–1.02) | 0.04 |
| Overall cumulative PMB (MIU) | 20 (12–30) | 23 (16–27) | 0.95 | ||
| Use of combination therapy | 17 (94.4) | 23 (92.0) | 0.76 | ||
| Use of concomitant nephrotoxins | |||||
| Diuretics | 5 (27.8) | 12 (48.0) | 0.19 | ||
| Vancomycin | 16 (88.9) | 24 (96.0) | 0.39 | ||
| Aminoglycosides | 7 (38.9) | 14 (56.0) | 0.27 | ||
| Intravenous contrast | 4 (22.2) | 11 (44.0) | 0.15 | ||
| Total number of nephrotoxins | 2 (1–2) | 3 (2–3) | 0.04 | 2.14 (1.03–4.45) | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Leck, H.; Tan, R.W.; Teo, J.Q.; Lim, T.-P.; Lee, W.; Chlebicki, M.P.; Kwa, A.L. Clinical Experience with High-Dose Polymyxin B against Carbapenem-Resistant Gram-Negative Bacterial Infections—A Cohort Study. Antibiotics 2020, 9, 451. https://doi.org/10.3390/antibiotics9080451
Cai Y, Leck H, Tan RW, Teo JQ, Lim T-P, Lee W, Chlebicki MP, Kwa AL. Clinical Experience with High-Dose Polymyxin B against Carbapenem-Resistant Gram-Negative Bacterial Infections—A Cohort Study. Antibiotics. 2020; 9(8):451. https://doi.org/10.3390/antibiotics9080451
Chicago/Turabian StyleCai, Yiying, Hui Leck, Ray W. Tan, Jocelyn Q. Teo, Tze-Peng Lim, Winnie Lee, Maciej Piotr Chlebicki, and Andrea L. Kwa. 2020. "Clinical Experience with High-Dose Polymyxin B against Carbapenem-Resistant Gram-Negative Bacterial Infections—A Cohort Study" Antibiotics 9, no. 8: 451. https://doi.org/10.3390/antibiotics9080451
APA StyleCai, Y., Leck, H., Tan, R. W., Teo, J. Q., Lim, T.-P., Lee, W., Chlebicki, M. P., & Kwa, A. L. (2020). Clinical Experience with High-Dose Polymyxin B against Carbapenem-Resistant Gram-Negative Bacterial Infections—A Cohort Study. Antibiotics, 9(8), 451. https://doi.org/10.3390/antibiotics9080451





