Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits?
Abstract
1. Introduction
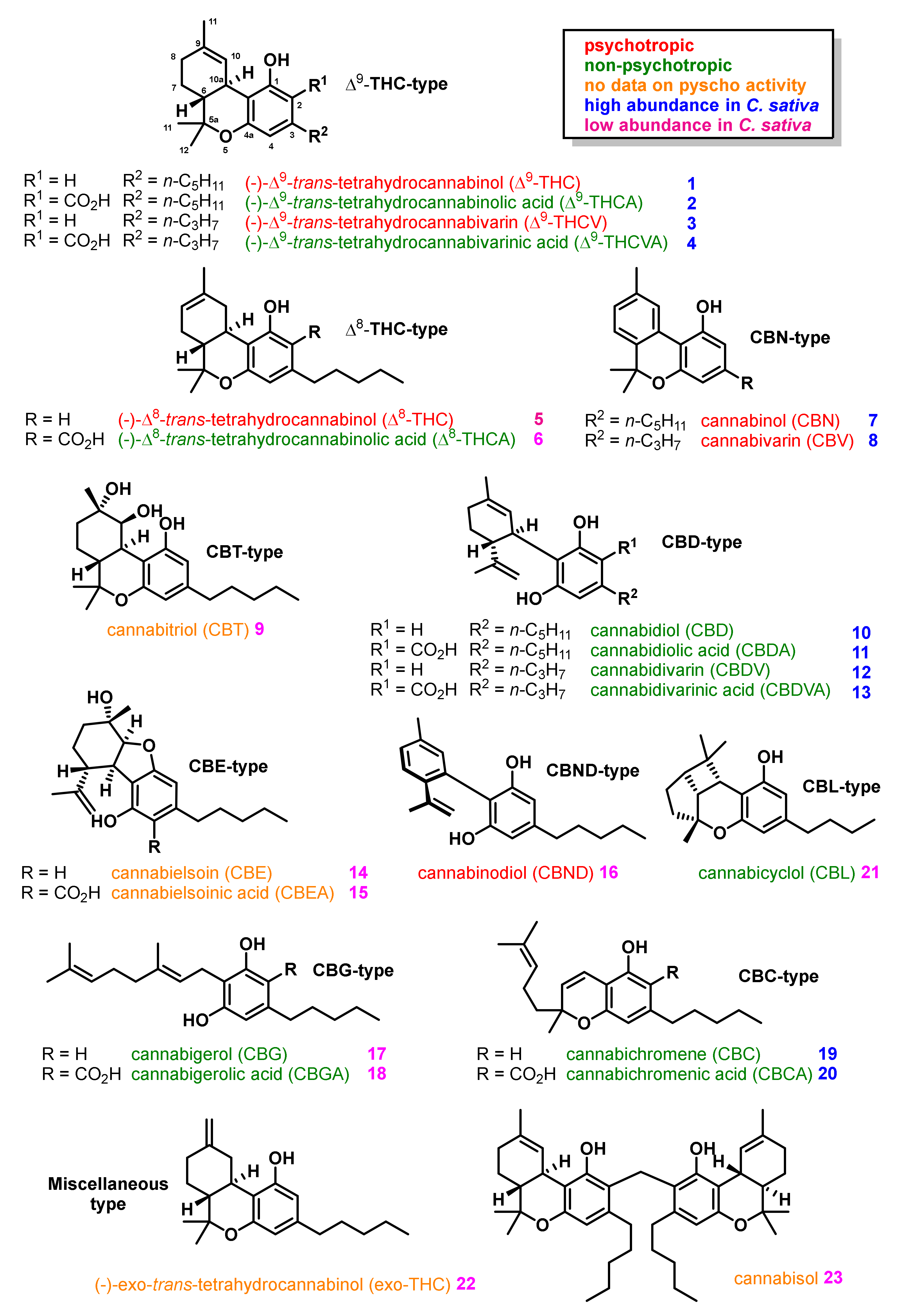
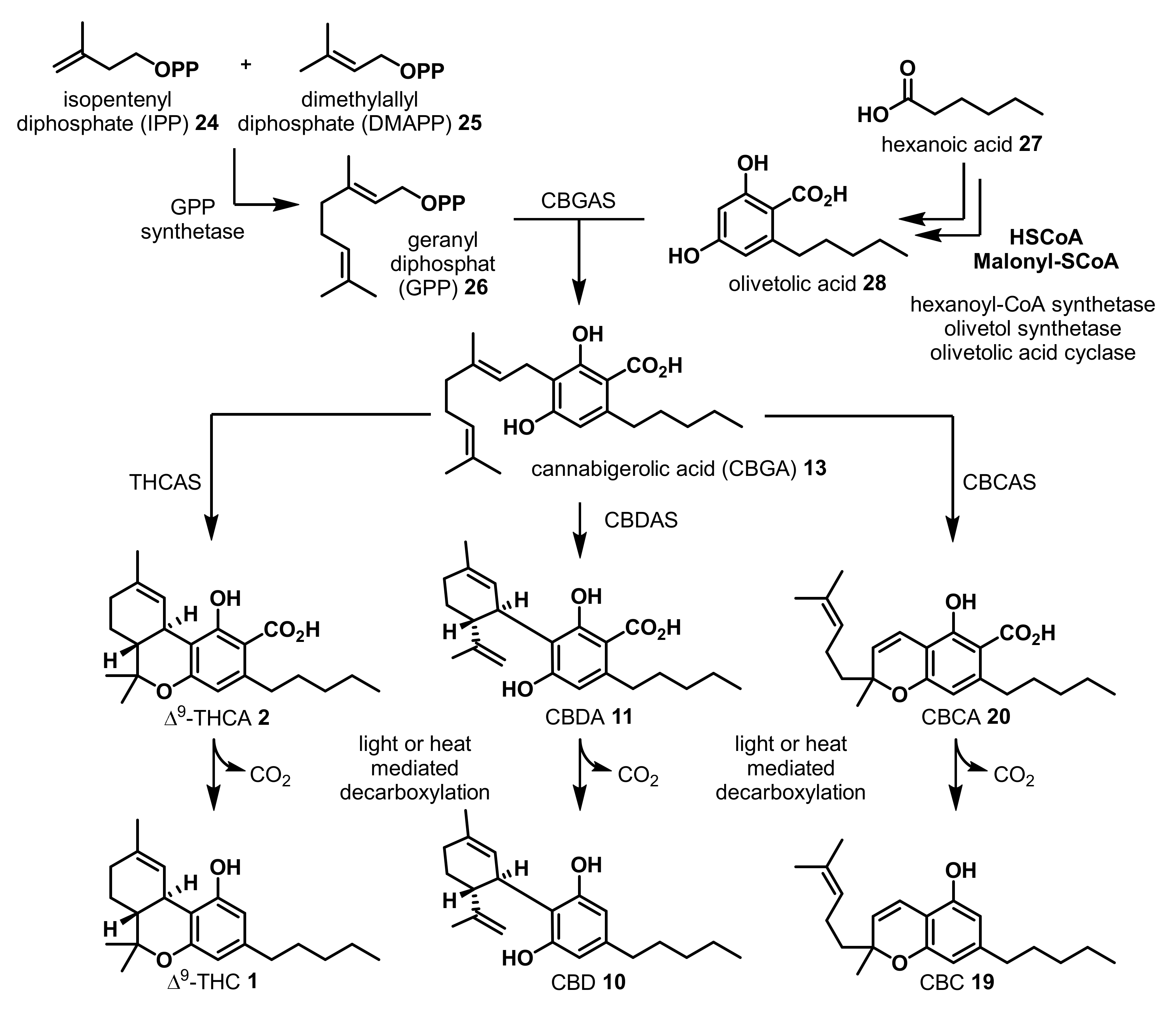
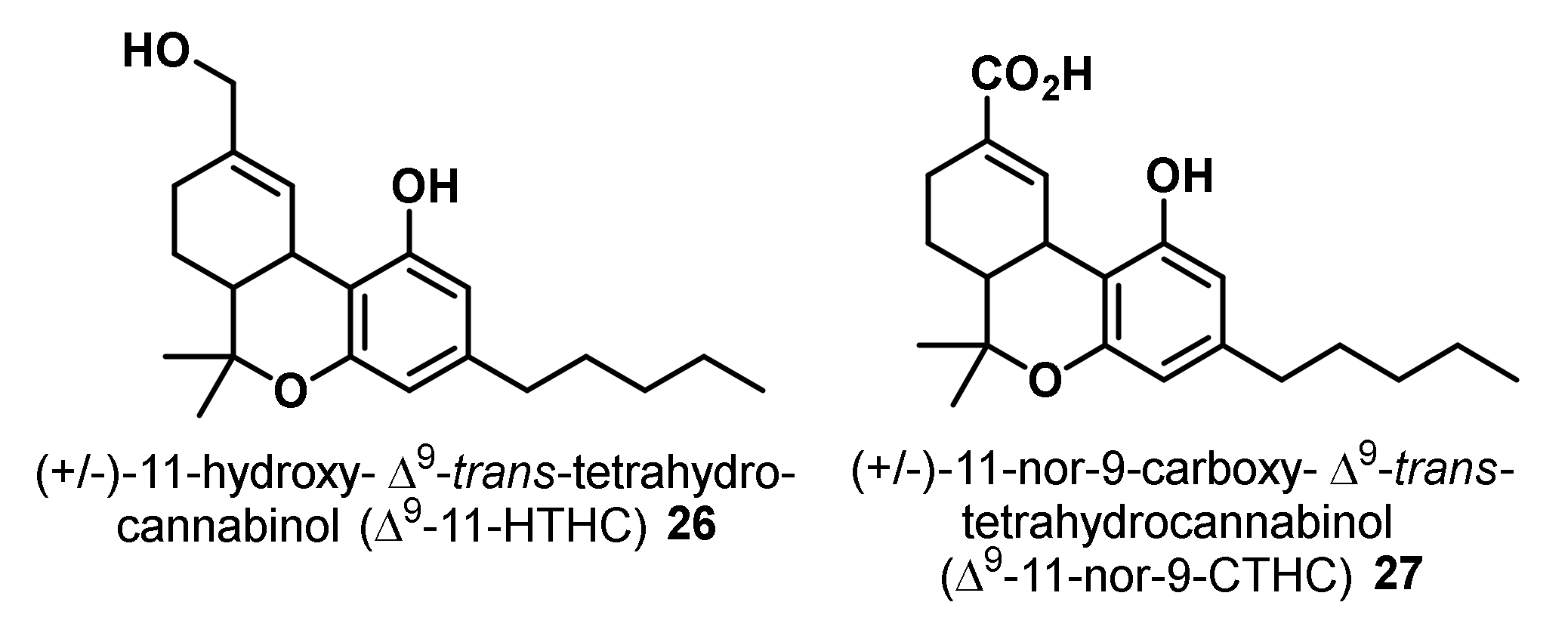
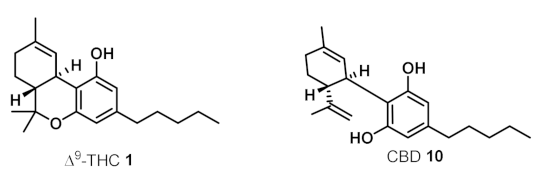
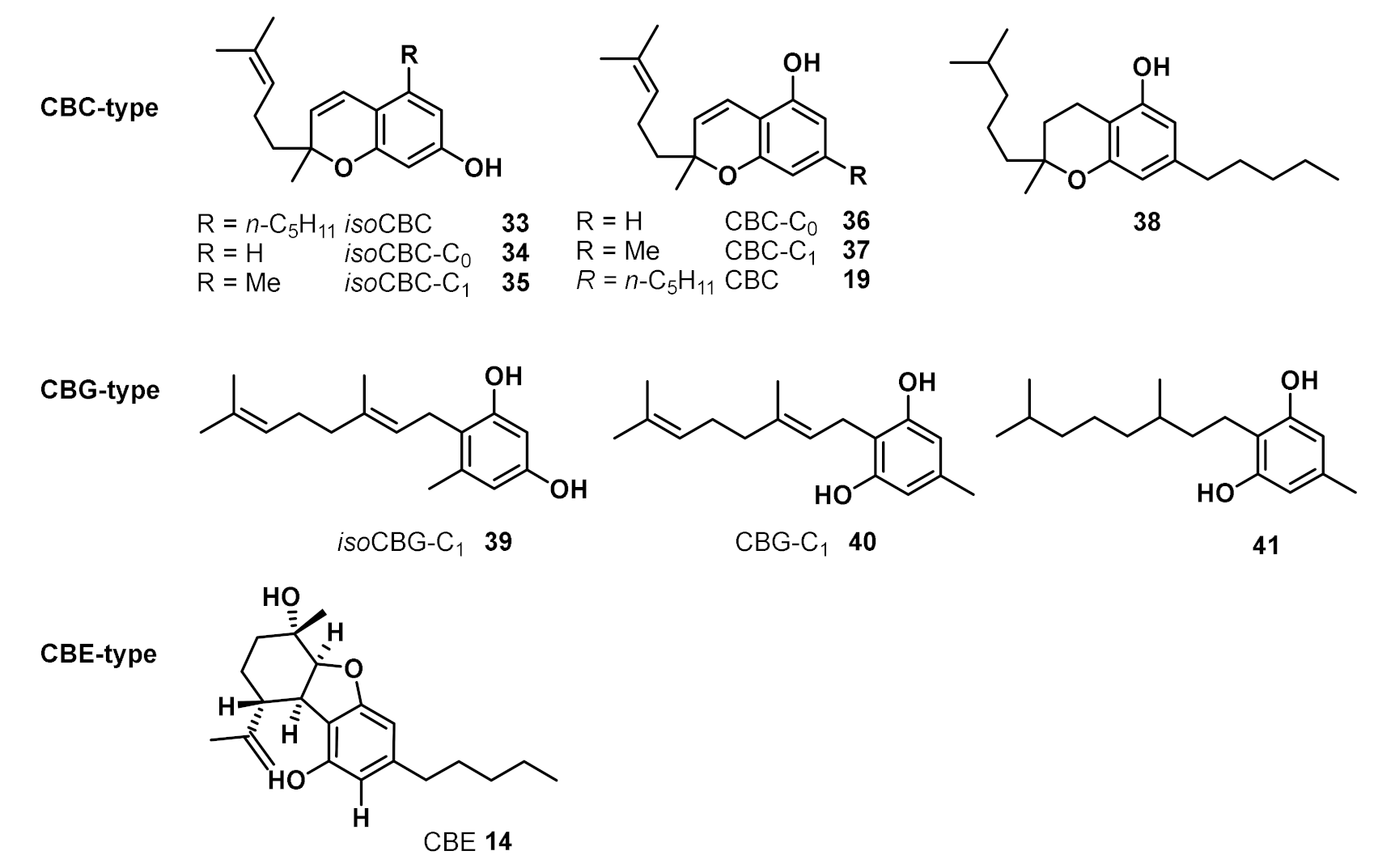
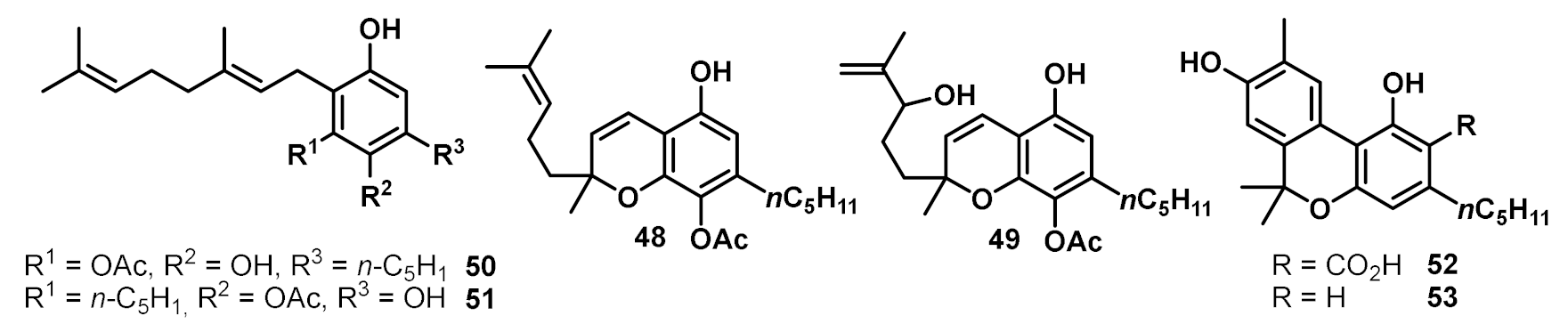
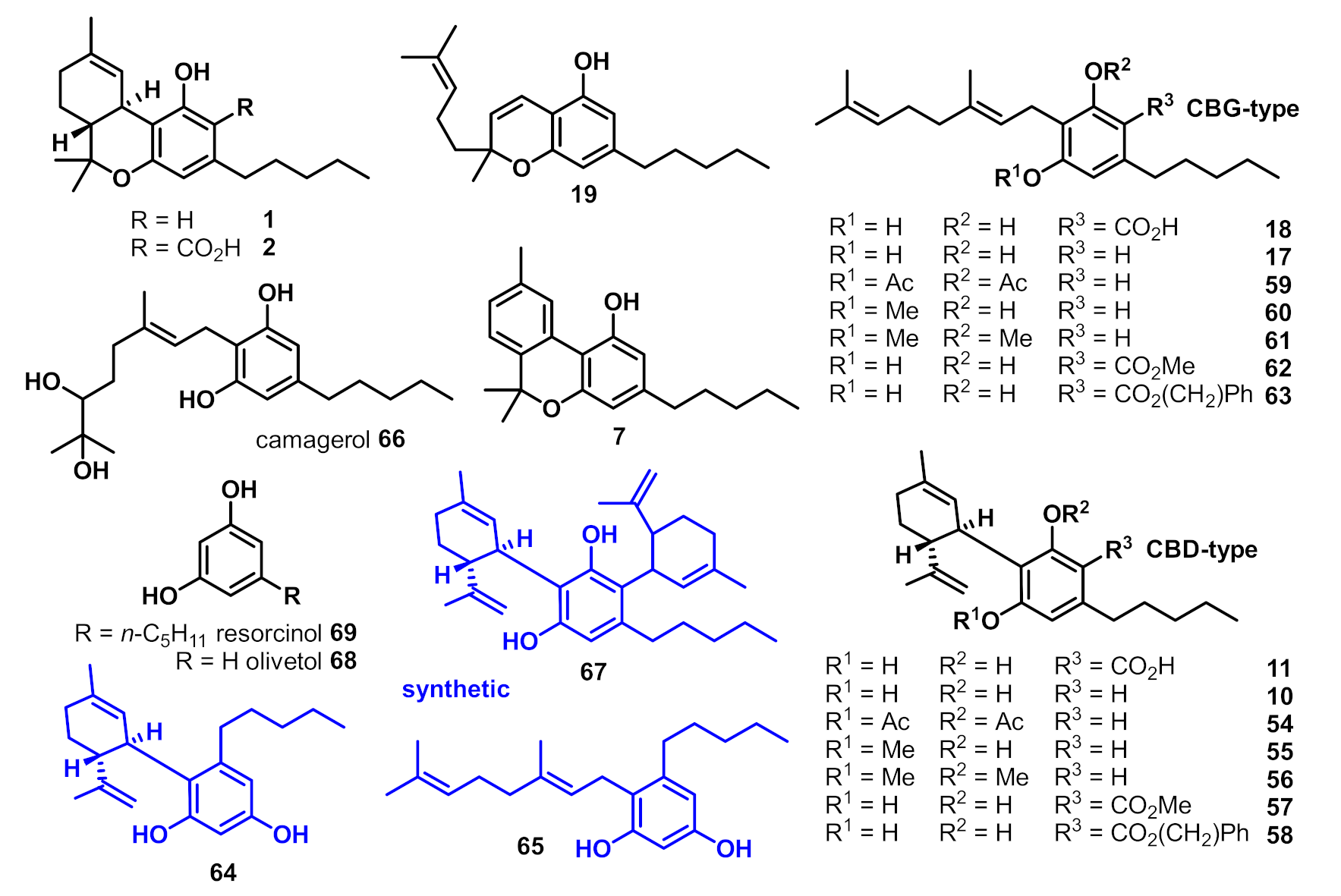
2. Structures, Abundance, and Biosynthetic Origin of Natural Cannabinoids from Cannabis sativa
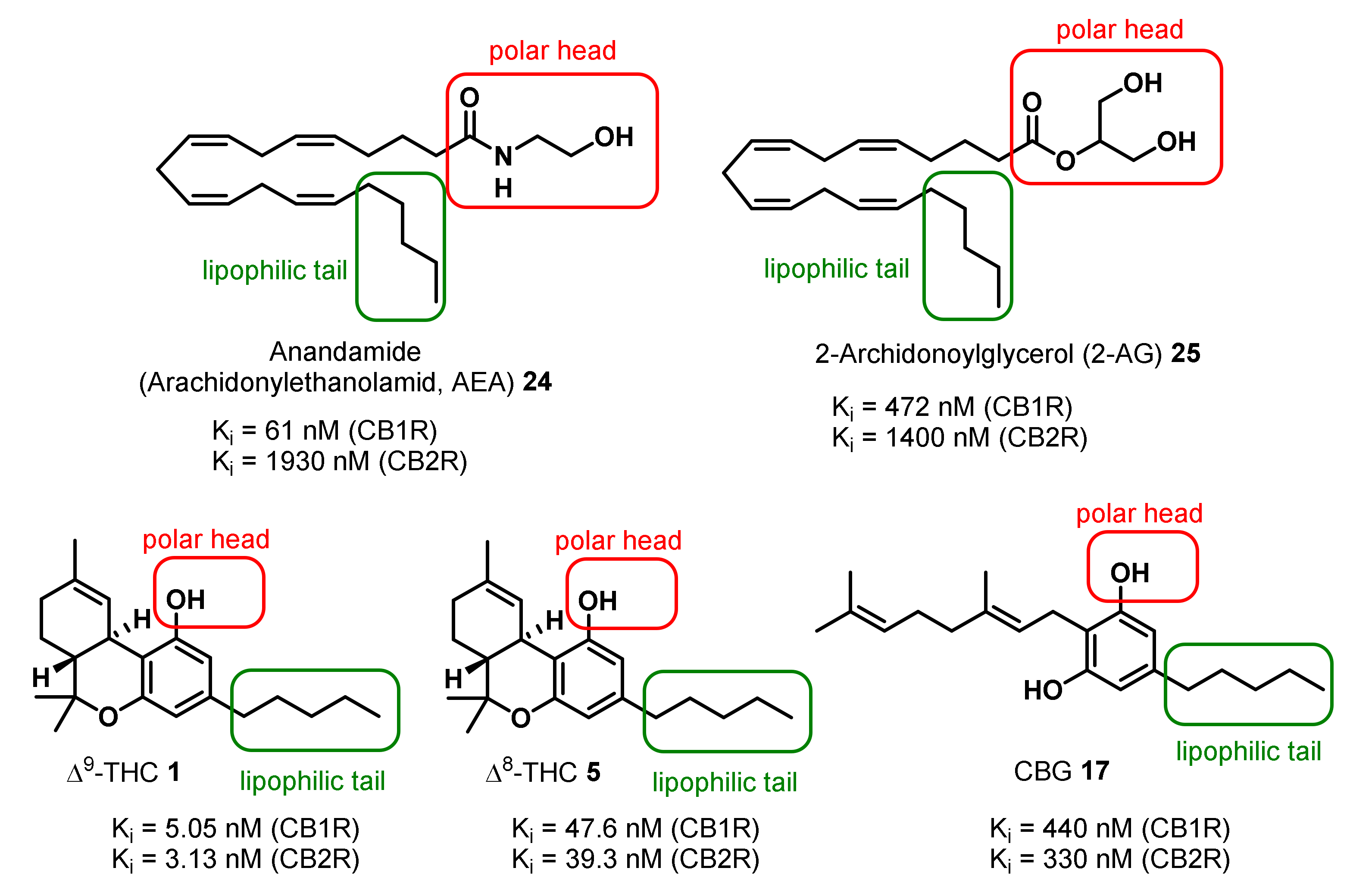
3. Bioactivities and Medical Uses of Phytocannabinoids from Cannabis Sativa and Synthetic Analogues
4. Antimicrobial Activities of Phytocannabinoids from Cannabis Sativa
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ’20 Progress—Development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- O’Neill, J. Securing New Drugs for Future Generations: The Pipeline of Antibiotics; Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2015. [Google Scholar]
- Klahn, P.; Brönstrup, M. New Structural Templates for Clinically Validated and Novel Targets in Antimicrobial Drug Research and Development. Curr. Top. Microbiol. Immunol. 2016, 389, 365–417. [Google Scholar] [CrossRef]
- Klahn, P.; Brönstrup, M. Bifunctional antimicrobial conjugates and hybrid antimicrobials. Nat. Prod. Rep. 2017, 34, 832–885. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017; p. 12. [Google Scholar]
- World Health Organization. Global Priority List of Antibiotic-resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; p. 12. [Google Scholar]
- Shoji, M.M.; Chen, A.F. Biofilms in Periprosthetic Joint Infections: A Review of Diagnostic Modalities, Current Treatments, and Future Directions. J. Knee Surg. 2020, 33, 119–131. [Google Scholar] [CrossRef]
- Gajula, B.; Munnamgi, S.; Basu, S. How bacterial bio fi lms affect chronic wound healing: A narrative review. Int. J. Surg. Glob. Heal. 2020, 3, e16. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Infections: Mechanisms of Biofilm Maturation and Detachment as Critical Determinants of Pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Solving the Antibiotic Crisis. ACS Infect. Dis. 2015, 1, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Gibbons, S. Anti-staphylococcal plant natural products. Nat. Prod. Rep. 2004, 21, 263–277. [Google Scholar] [CrossRef]
- Wright, G.D. Something old, something new: Revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef]
- Abouelhassan, Y.; Garrison, A.; Yang, H.; Chavez Riveros, A.; Burch, G.; Huigens III, R.W. Recent Progress in Natural Product-Inspired Programs Aimed at Addressing Antibiotic Resistance and Tolerance. J. Med. Chem. 2019, 62, 7618–7642. [Google Scholar] [CrossRef]
- Moloney, M.G. Natural Products as a Source for Novel Antibiotics. Trends Pharmacol. Sci. 2016, 37, 689–701. [Google Scholar] [CrossRef]
- Brown, D.G.; Lister, T.; May-Dracka, T.L. New natural products as new leads for antibacterial drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 413–418. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; Macnair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the Hidden Antibiotic Potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Elsohly, M.A. Biological Activity of Cannabichromene, its Homologs and Isomers. J. Clin. Pharm. 1981, 21, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Van Klingeren, B.; ten Ham, M. Antibacterial activity of Δ9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Schultz, O.E.; Haffner, G. Zur Kenntnis eines sedativen und antibakteriellen Wirkstoffes aus deutschem Faserhanf (Cannabis sativa). Z. Naturforschg. 1959, 14b, 98–100. [Google Scholar] [CrossRef]
- Krejci, Z. Hanf (Cannabis sativa) - Antibiotisches Heilmittelung 3. Mitteilung: Isolierung und Konstitution zweier aus Cannabis sativa gewonnener Säure. Pharmazie 1958, 12, 439–443. [Google Scholar]
- Elsohly, H.N.; Turner, C.E.; Clark, A.M.; Elsohly, M.A. Synthesis and Antimicrobial Activities of Certain Cannabichromene and Cannabigerol Related Compounds. J. Pharm. Sci. 1982, 71, 1319–1323. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids-Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Germany, 2017; pp. 1–36. [Google Scholar]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of cannabis sativa l. xvii. a review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef]
- Casajuana Kögel, C.; López-Pelayo, H.; Balcells-Olivero, M.M.; Colom, J.; Gual, A. Psychoactive constituents of cannabis and their clinical implications: A systematic review. Adicciones 2018, 30, 140–151. [Google Scholar] [CrossRef]
- Dalzell, H.C.; Uliss, D.B.; Richard Handrick, G.; Razdan, R.K. Hashish: Factors Influencing Double-Bond Stability in Cannabinoids. J. Org. Chem. 1981, 46, 949–953. [Google Scholar] [CrossRef]
- Goani, Y.; Mechoulam, R. The Isomerization of Cannabidiol to Tetrahydrocannabinols. Tetrahedron 1966, 22, 1481–1488. [Google Scholar]
- Taylor, E.C.; Lenard, C.; Shvo, Y. Active Constituents of Hashish. Synthesis of dl/-Δ6–3,4-trans-Tetrahydrocannabinol. J. Am. Chem. Soc. 1966, 88, 367–369. [Google Scholar] [CrossRef]
- Hively, R.L.; Mosher, W.A.; Hoffmann, F.W. Isolation of trans-∆6-Tetrahydrocannabinol from Marijuana. J. Am. Chem. Soc. 1966, 88, 1832–1833. [Google Scholar] [CrossRef]
- Thomas, F.J.; Kayser, O. Minor Cannabinoids of Cannabis sativa L. J. Med. Sci. 2019, 88, 141–149. [Google Scholar] [CrossRef]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front. Pharmacol. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Küppers, F.J.E.M.; Bercht, C.A.L.; Salemink, C.A.; Terlouw, J.K.; Heerma, W. Pyrolysis of Cannabidiol. Structure Elucidation of four Pyrolytic Products. Tetrahedron 1975, 31, 1513–1516. [Google Scholar] [CrossRef]
- Shani, A.; Mechoulam, R. Cannabielsoic acids. Isolation and Synthesis by a Novel Oxidative Cyclization. Tetrahedron 1974, 30, 2437–2446. [Google Scholar]
- Shani, A.; Mechoulam, R. A new type of cannabinoid. Synthesis of cannabielsoic acid A by a novel photo-oxidative cyclisation. J. Chem. Soc. D Chem. Commun. 1970, 273–274. [Google Scholar] [CrossRef]
- Lousberg, R.J.J.C.; Brecht, C.A.L.; Van Ooyen, R.; Spronck, H.J.W. Cannabinodiol: Conclusive Identification and Synthesis of a New Cannabinoid from Cannabis Sativa. Phytochemistry 1977, 16, 595–597. [Google Scholar]
- Banister, S.D.; McConnor, M. The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonist New Psychoactive Substances: Evolution. In New Psychoactive Substances-Pharmacology, Clinical, Forensic and Analytical Toxicology; Maurer, H.H., Brandt, S.D., Eds.; Springer: Cham, Germany, 2018; pp. 191–226. [Google Scholar]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Elsohly, M.; Gul, W. Constituents of Cannabis sativa. In Handbook of Cannabis; Pertwee, R.G., Ed.; Oxford Press: Oxford, UK, 2016; pp. 3–22. Available online: https://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780199662685.001.0001/acprof-9780199662685-chapter-1 (accessed on 2 June 2020).
- Yeom, H.S.; Li, H.; Tang, Y.; Hsung, R.P. Total syntheses of cannabicyclol, clusiacyclol A and B, iso-eriobrucinol A and B, and eriobrucinol. Org. Lett. 2013, 15, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, F.; Stehle, F.; Kayser, O. The biosynthesis of cannabinoids. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 13–23. [Google Scholar]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Sirikantaramas, S.; Taura, F. Cannabinoids: Biosynthesis and Biotechnological Applications. In Cannabis sativa L.-Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M., Eds.; Springer: Cham, Germany, 2017; pp. 183–206. ISBN 9783319545646. [Google Scholar]
- Taura, F.; Tanaka, S.; Taguchi, C.; Fukamizu, T.; Tanaka, H.; Shoyama, Y.; Morimoto, S. Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett. 2009, 583, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811–12816. [Google Scholar] [CrossRef]
- Fellermeier, M.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Biosynthesis of cannabinoids - Incorporation experiments with 13C-labeled glucoses Monika. Eur. J. Biochem. 2001, 268, 1596–1604. [Google Scholar] [CrossRef]
- Page, J.E.; Boubakir, Z. Aromatic Prenyltransferase from Cannabis. U.S. Patent US2012/0144523 A1, 11 November 2012. [Google Scholar]
- Sirikantaramas, S.; Morimoto, S.; Shoyama, Y.; Ishikawa, Y.; Wada, Y.; Shoyama, Y.; Taura, F. The gene controlling marijuana psychoactivity. Molecular cloning and heterologous expression of Δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J. Biol. Chem. 2004, 279, 39767–39774. [Google Scholar] [CrossRef]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef]
- Morimoto, S.; Komatsu, K.; Taura, F.; Shoyama, Y. Enzymological evidence for cannabichromenic acid biosynthesis. J. Nat. Prod. 1997, 60, 854–867. [Google Scholar] [CrossRef]
- Morimoto, S.; Komatsu, K.; Taura, F.; Shoyama, Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998, 49, 1525–1529. [Google Scholar] [CrossRef]
- Moreno-Sanz, G. Can You Pass the Acid Test? Critical Review and Novel Therapeutic Perspectives of Δ9-Tetrahydrocannabinolic Acid, A. Cannabis Cannabinoid Res. 2016, 1, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, And Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Li, H. An Archaeological and Historical Account of Cannabis in China. Econ. Bot. 1974, 28, 437–448. [Google Scholar] [CrossRef]
- Touw, M. The religious and medicinal uses of Cannabis in China, India and Tibet. J. Psychoactive Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. In Recent Advances in Cannabinoid Physiology and Pathology. Advances in Experimental Medicine and Biology; Bukiya, A., Ed.; Springer: Cham, Germany, 2019; Volume 1162, pp. 1–12. [Google Scholar]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, J.B. Molecular Pharmacology of Phytocannabinoids. In Phytocannbinoids -Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Germany, 2017; pp. 61–102. [Google Scholar]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. In Phytocannabinoids-Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Germany, 2017; pp. 103–131. [Google Scholar]
- Appendino, G.; Chianese, G. Cannabinoids: Occurrence and Medicinal Chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. In Cannabinoids. Handbook of Experimental Pharmacology; Pertwee, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 168, pp. 299–325. [Google Scholar]
- Kano, M.; Ohno-shosaku, T.; Hashimotodani, Y.; Uchigashima, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: D 9 -tetrahydrocannabinol, cannabidiol and D 9 -tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Thakur, G.A.; Duclos, R.I.; Makriyannis, A. Natural cannabinoids: Templates for drug discovery. Life Sci. 2005, 78, 454–466. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, G.; Mabin, T.; Engelbrecht, A.M. Anti-inflammatory mechanisms of cannabinoids: An immunometabolic perspective. Inflammopharmacology 2019, 27, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ben Amar, M. Cannabinoids in medicine: A review of their therapeutic potential. J. Ethnopharmacol. 2006, 105, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; del Bel, E.A.; Guimarães, F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3364–3378. [Google Scholar] [CrossRef]
- Peters, M.; Murillo-rodriguez, E.; Hanus, L.O. Cannabidiol – Recent Advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar]
- Phillips, R.; Friend, A.J.; Gibson, F.; Houghton, E.; Gopaul, S.; Craig, J.V.; Pizer, B. Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood. Cochrane Database Syst. Rev. 2016, CD007786. [Google Scholar] [CrossRef]
- Novack, G.D. Cannabinoids for treatment of glaucoma. Curr. Opin. Ophthalmol. 2016, 27, 146–150. [Google Scholar] [CrossRef]
- Boychuk, D.G.; Goddard, G.; Mauro, G.; Orellana, M.F. The Effectiveness of Cannabinoids in the Management of Chronic Nonmalignant Neuropathic Pain: A Systematic Review. J. Oral Facial Pain 2015, 29, 7–14. [Google Scholar] [CrossRef]
- Muralidhar Reddy, P.; Maurya, N.; Velmurugan, B.K. Medicinal Use of Synthetic Cannabinoids—A Mini Review. Curr. Pharmacol. Rep. 2019, 5, 1–13. [Google Scholar] [CrossRef]
- Smith, L.A.; Azariah, F.; Lavender, V.T.C.; Stoner, N.S.; Bettinol, S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst. Rev. 2015, CD009464. [Google Scholar] [CrossRef] [PubMed]
- Pryce, G.; Baker, D. Emerging properties of cannabinoid medicines in management of multiple sclerosis. Trends Neurosci. 2005, 28, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Articles Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019, 0366, 1–16. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Tius, M.A. Synthesis of Classical/Nonclassical Hybrid Cannabinoids and Related Compounds. In Cutting-Edge Organic Synthesis and Chemical Biology of Bioactive Molecules; Kobayashi, Y., Ed.; Springer Nature: Singapore, 2019; pp. 247–289. ISBN 9789811362446. [Google Scholar]
- Schafroth, M.A.; Carreira, E.M. Synthesis of Phytocannabinoids. In Phytocannbinoids -Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Germany, 2017; pp. 37–60. [Google Scholar]
- Reekie, T.; Scott, M.; Kassiou, M. The evolving science of phytocannabinoids. Nat. Rev. Chem. 2018, 2, 0101. [Google Scholar] [CrossRef]
- Westphal, M.; Schafroth, M.A.; Sarott, R.; Imhof, M.; Bold, C.; Leippe, P.; Dhopeshwarkar, A.; Grandner, J.; Mackie, K.; Trauner, D.; et al. Synthesis of Photoswitchable Δ9-Tetrahydrocannabinol Derivatives Enables Optical Control of Cannabinoid Receptor 1 Signaling. J. Am. Chem. Soc. 2017, 139, 18206–18212. [Google Scholar] [CrossRef]
- Seely, K.A.; Lapoint, J.; Moran, J.H.; Fattore, L. Spice drugs are more than harmless herbal blends: A review of the pharmacology and toxicology of synthetic cannabinoids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 234–243. [Google Scholar] [CrossRef]
- Cottencin, O.; Rolland, B.; Karila, L. New Designer Drugs (Synthetic Cannabinoids and Synthetic Cathinones): Review of Literature. Curr. Pharm. Des. 2014, 20, 4106–4111. [Google Scholar] [CrossRef]
- Alipour, A.; Patel, P.B.; Shabbir, Z.; Gabrielson, S. Review of the many faces of synthetic cannabinoid toxicities. Ment. Heal. Clin. 2019, 9, 93–99. [Google Scholar] [CrossRef]
- FDA, FTC Warn Company Marketing Unapproved Cannabidiol Products with Unsubstantiated Claims to Treat Teething and Ear Pain in Infants, Autism, ADHD, Parkinson’s and Alzheimer’s Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-ftc-warn-company-marketing-unapproved-cannabidiol-products-unsubstantiated-claims-treat-teething (accessed on 3 May 2020).
- FDA Warns Company Marketing Unapproved Cannabidiol Products with Unsubstantiated Claims to Treat Cancer, Alzheimer’s Disease, Opioid Withdrawal, Pain and Pet Anxiety. Available online: https://www.fda.gov/news-events/press-announcements/fda-warns-company-marketing-unapproved-cannabidiol-products-unsubstantiated-claims-treat-cancer (accessed on 3 May 2020).
- Kabilek, J. Hemp as a medicament. Acta Univ. Palacki. Olomuc. Tom. VI 1955, 6, 1–81. Available online: http://www.chanvre-info.ch/info/de/IMG/pdf/Tschek_etude_kabelikEN.pdf (accessed on 2 June 2020).
- Kabelik, J. Hanf (Cannabis sativa)-Antibiotisches Heilmittel. 1. Mitteilung: Hanf in der Alt-und Volksmedizin. Pharmazie 1958, 12, 439–443. [Google Scholar]
- Krejci, Z. Hemp as a Medicament. Ph.D. Thesis, Faculty of Natural Sciences, Brno, Czech Republic, 1950. [Google Scholar]
- Krejci, Z. Hanf (Cannabis sativa) -Antibiotisches Heilmittel. 2. Mitteilung: Methodik und Ergebnisse der bakteriologischen Untersuchungen und vorläufige klinische Erfahrungen. Pharmazie 1959, 14, 155–166. Available online: https://bibliography.maps.org/bibliography/default/resource/11554 (accessed on 2 June 2020).
- Drobotko, V.G.; Rasba, E.I.; Ayzenman, B.E.; Zelepukha, S.I.; Novikova, S.I.; Kaganskaia, M.B. Fitoncidy ich rol’v prirode. Leningr. Izd. Univ. 1951, 143. [Google Scholar]
- Ferenczy, L. Antibacterial Substances in Seeds. Nature 1956, 178, 639–640. [Google Scholar] [CrossRef]
- Ferenczy, L.; Gracza, L.; Jakobey, I. An Antibacterial Preparatum from Hemp (Cannabis sativa L.). Naturwissenschaften 1958, 45, 188. [Google Scholar] [CrossRef]
- Wasim, K.; Haq, I.; Ashraf, M. Antimicrobial studies of the leaf of cannabis sativa L. Pak. J. Pharm. Sci. 1995, 8, 29–38. [Google Scholar]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Slavik, D.; Raskin, I. The composition of hemp (Cannabis sativa L.) seed oil and its potential as an important source of nutrition for man. Int. J. Chem. Biochem. Res. 2015, 3, 16. [Google Scholar] [CrossRef]
- Monika, K.N.; Kaur, M. Antimicrobial analysis of leaves of Cannabis sativa. J. Sci. 2014, 4, 123–127. [Google Scholar]
- Naveed, H.; Khan, T.A.; Ali, I.; Hassan, A.; Ali, H.; Din, Z.U.; Tabassum, S.; Majid, S.A.; Rehman, M.U. In vitro antibacterial activity of Cannabis sativa leaf extracts to some selective pathogenicbacterial strains. Int. J. Biosci. 2014, 4, 65–70. [Google Scholar] [CrossRef]
- Sarmadyan, H.; Solhi, H.; Hajimir, T.; Najarian-Araghi, N.; Ghaznavi-Rad, E. Determination of the Antimicrobial Effects of Hydro-Alcoholic Extract of Cannabis Sativa on Multiple Drug Resistant Bacteria Isolated from Nosocomial Infections. Iran. J. Toxicol. 2014, 7, 967–972. [Google Scholar]
- Kaur, S.; Sharma, C.; Chaudhry, S.; Aman, R. Antimicrobial potential of three common weeds of kurukshetra: An in vitro study. Res. J. Microbiol. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Novak, J.; Zitterl-eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Singh, A.; Kitts, D.D.; Singh, A.P. Hemp (Cannabis sativa L.) Extract: Anti-Microbial Properties, Methods of Extraction, and Potential Oral Delivery. Food Rev. Int. 2019, 664–684. [Google Scholar] [CrossRef]
- Pal, G.K.; Kumar, B.; Shani, S.K. Antifungal Activity of Some Seed Extracts against Seed-borne. Int. J. Univers. Pharm. Life Sci. 2013, 3, 6–14. [Google Scholar]
- Chakraborty, S.; Afaq, N.; Singh, N.; Majumdar, S. Antimicrobial activity of Cannabis sativa, Thuja orientalis and Psidium guajava leaf extracts against methicillin-resistant Staphylococcus aureus. J. Integr. Med. 2018, 16, 350–357. [Google Scholar] [CrossRef]
- Wanas, A.S.; Radwan, M.M.; Mehmedic, Z.; Jacob, M.; Khan, I.A.; Elsohly, M.A. Antifungal activity of the volatiles of high potency Cannabis sativa L. Against Cryptococcus neoformans. Rec. Nat. Prod. 2015, 10, 214–220. [Google Scholar]
- Borchardt, J.R.; Wyse, D.L.; Sheaffer, C.C.; Kauppi, K.L.; Fulcher, R.G. Antimicrobial activity of native and naturalized plants of Minnesota and Wisconsin. J. Med. Plants Res. 2008, 2, 98–110. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Verma, S.K.; Chauhan, A.; Darokar, M.P. The essential oil of’ bhang’ (Cannabis sativa L.) for non-narcotic applications. Curr. Sci. 2014, 107, 645–650. [Google Scholar]
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; García-Gimeénez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) Seed Oil: Analytical and Phytochemical Characterization of the Unsaponi fiable Fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.; Azam, S.; Rehman, P.; Khadim, J. Evaluation of antimicrobial activity and ethnobotanical study of Cannabis sativa. Pure Appl. Biol. 2018, 7, 706–713. [Google Scholar] [CrossRef]
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Di, D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT Food Sci. Technol. 2020, 124, 109149. [Google Scholar] [CrossRef]
- Raina, S.; Thakur, A.; Sharma, A.; Pooja, D.; Minhas, A.P. Bactericidal activity of Cannabis sativa phytochemicals from leaf extract and their derived Carbon Dots and Ag@Carbon Dots. Mater. Lett. 2019, 262, 127122. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Mishra, P.C. Antibacterial analysis of crude extracts from the leaves of Tagetes erecta and Cannabis sativa. Int. J. Environ. Sci. 2012, 2, 1605–1609. [Google Scholar] [CrossRef]
- Ali, E.; Almagboul, A. Antimicrobial Activity of Cannabis sativa L. J. Chinese Med. 2012, 3, 61–64. [Google Scholar] [CrossRef]
- Lone, T.A.; Lone, R.A. Extraction of cannabinoids from cannabis sativa L plant and its potential antimicrobial activity. Univers. J. Med. Dent. 2012, 1, 51–055. [Google Scholar]
- Mkpenie, V.N.; Essien, E.E.; Udoh, I.I. Effect of extraction conditions on total polyphenol contents, antioxidant and antimicrobial activities of Cannabis sativa L. Electron. J. Environ. Agric. Food Chem. 2012, 11, 300–307. [Google Scholar]
- Kakar, S.A.; Tareen, R.B.; Azam Kakar, M.; Jabeen, H.; Kakar, S.U.R.; Al-Kahraman, Y.M.S.A.; Shafee, M. Screening of antibacterial activity of four medicinal plants of Balochistan-Pakistan. Pakistan J. Bot. 2012, 44, 245–250. [Google Scholar]
- Mathur, P.; Singh, A.; Srivastava, V.R.; Singh, D.; Mishra, Y. Antimicrobial activity of indigenous wildly growing plants: Potential source of green antibiotics. African J. Microbiol. Res. 2013, 7, 3807–3815. [Google Scholar] [CrossRef]
- Tandon, C.; Mathur, P. Antimicrobial Efficacy of Cannabis sativa L. (Bhang): A Comprehensive Review. Int. J. Pharm. Sci. Rev. Res. 2017, 44, 94–100. [Google Scholar]
- Głodowska, M.; Łyszcz, M. Cannabis sativa L. and its antimicrobial properties-A review. In Badania i Rozwój Młodych Naukowców w Polsce–Agronomia i Ochrona Roślin; Leśny, J., Chojnicki, B., Panfil, M., Nyćkowiak, J., Eds.; Młodzi Naukowcy: Poznań, Poland, 2017; pp. 77–82. [Google Scholar]
- Andre, C.M.; Hausman, J.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis Phenolics and their Bioactivities. Curr. Med. Chem. 2018, 1160–1185. [Google Scholar] [CrossRef]
- Do, J.M.M.; Russo, E.B.; Russo, E.B. Cannabis and Cannabis Extracts Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts ? J. Cannabis Ther. 2016, 1, 103–132. [Google Scholar] [CrossRef]
- Melchoulam, R.; Gaoni, Y. Hashish IV - The isolation and structure of cannabinolic, cannabidiolic and cannabigerolic acid. Tetrahedron 1965, 21, 1223–1229. [Google Scholar]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Zulfiqar, F.; ElSohly, M.A. Cannabinoid ester constituents from high-potency Cannabis sativa. J. Nat. Prod. 2008, 71, 536–542. [Google Scholar] [CrossRef]
- Radwan, M.M.; Ross, S.A.; Slade, D.; Ahmed, S.A.; Zulfiqar, F.; Elsohly, M.A. Isolation and characterization of new cannabis constituents from a high potency variety. Planta Med. 2008, 74, 267–272. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; Elsohly, M.A. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Structure determination and absolute configuration of cannabichromanone derivatives from high potency Cannabis sativa. Tetrahedron Lett. 2008, 49, 6050–6053. [Google Scholar] [CrossRef]
- Radwan, M.M.; ElSohly, M.A.; Slade, D.; Ahmed, S.A.; Khan, I.A.; Ross, S.A. Biologically active cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2009, 72, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.F. Characterization a strain of methicillin-resistant Staphylococcus aureus (EMRSA-15) by conventional and molecular methods. J. Hosp. Infect. 1993, 25, 45–52. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M.; Ruble, C.A. Efflux-Mediated Fluoroquinolone Resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1993, 37, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.L.; Farrell, A.M.; Eady, E.A.; Cove, J.H.; Cunlifiv, W.J. Characterisation and molecular cloning of the novel macrolide- streptogramin B resistance determinant from Staphylococcus epidernddis. J. Antimicrob. Chemother. 1989, 24, 851–862. [Google Scholar] [CrossRef]
- Gibbons, S.; Udo, E.E. The Effect of Reserpine, a Modulator of Multidrug Efflux Pumps, on the in vitro Activity of Tetracycline Against Clinical Isolates of Methicillin Resistant Staphylococcus aureus ( MRSA ) Possessing the tet (K) Determinant. Phyther. Res. 2000, 140, 139–140. [Google Scholar] [CrossRef]
- Allison, K.R.; Brynildsen, M.P.; Collins, J.J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 2011, 473, 216–220. [Google Scholar] [CrossRef]
- Davies, J. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1. [Google Scholar] [CrossRef]
- Conlon, B.P. Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells: An investigation of persister cells, their formation and their role in S. aureus disease. BioEssays 2014, 36, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behav. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; de Medina, V.S.; Rivas-Santisteban, R.; Callado, C.S.C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB1 and CB2 Receptors and at CB1–CB2 Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Marzo, V. Di Correspondence Effects of cannabinoids and Cannabis extracts on TRP channels and endocannabinoid. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Mcpartland, J.M.; Guy, G.W.; Marzo, V. Di Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System. PLoS ONE 2014, 9, e89566. [Google Scholar] [CrossRef]
- Fride, E. The endocannabinoid-CB receptor system: Importance for development and in pediatric disease. Neuroendocrinol. Lett. 2004, 25, 24–30. [Google Scholar]
- Fride, E. The endocannabinoid-CB 1 receptor system in pre- and postnatal life. Eur. J. Pharmacol. 2004, 500, 289–297. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef]
- Sharma, P.; Murthy, P.; Bharath, M.M.S. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149–156. [Google Scholar] [PubMed]
- Schwilke, E.W.; Schwope, D.M.; Karschner, E.L.; Lowe, R.H.; Darwin, W.D.; Kelly, D.L.; Goodwin, R.S.; Gorelick, D.A.; Huestis, M.A. Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin. Chem. 2009, 55, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Acid, C.; Cannabidiolic, T.O. Purification and Characterization of Cannabidiolic-acid Synthase from Cannabis sativa L. J. Biol. Chem. 1996, 271, 17411–17416. [Google Scholar]
- Poovelikunnel, T.; Gethin, G.; Humphreys, H. Mupirocin resistance: Clinical implications and potential alternatives for the eradication of MRSA. J. Antimicrob. Chemother. 2015, 70, 2681–2692. [Google Scholar] [CrossRef] [PubMed]
- Simor, A.E.; Stuart, T.L.; Louie, L.; Watt, C.; Ofner-agostini, M.; Gravel, D.; Mulvey, M.; Loeb, M.; Mcgeer, A.; Bryce, E.; et al. Mupirocin-Resistant, Methicillin-Resistant Staphylococcus aureusStrains in Canadian Hospitals. Antimicrob. Agents Chemother. 2007, 51, 3880–3886. [Google Scholar] [CrossRef]
Not available. |
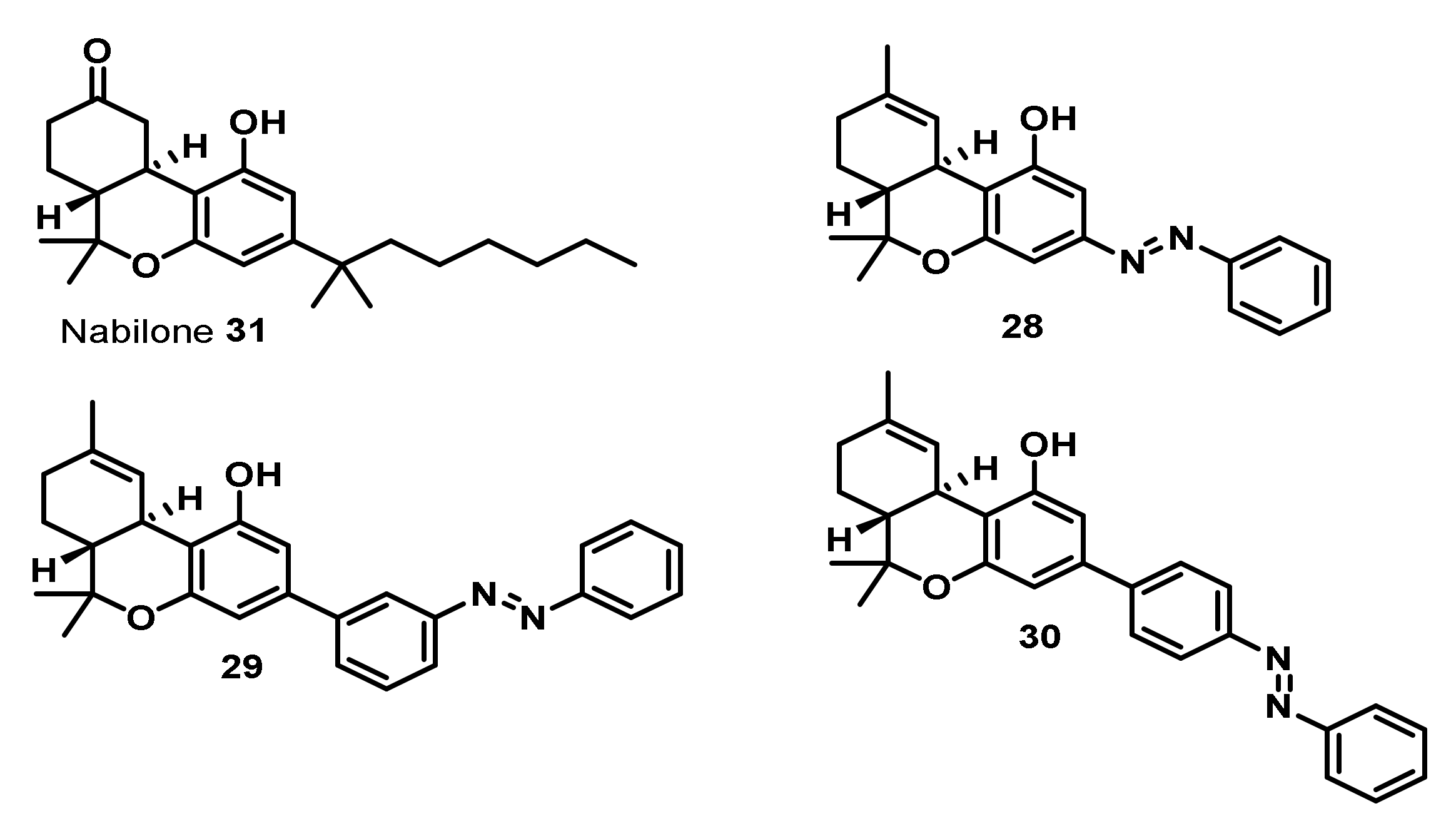
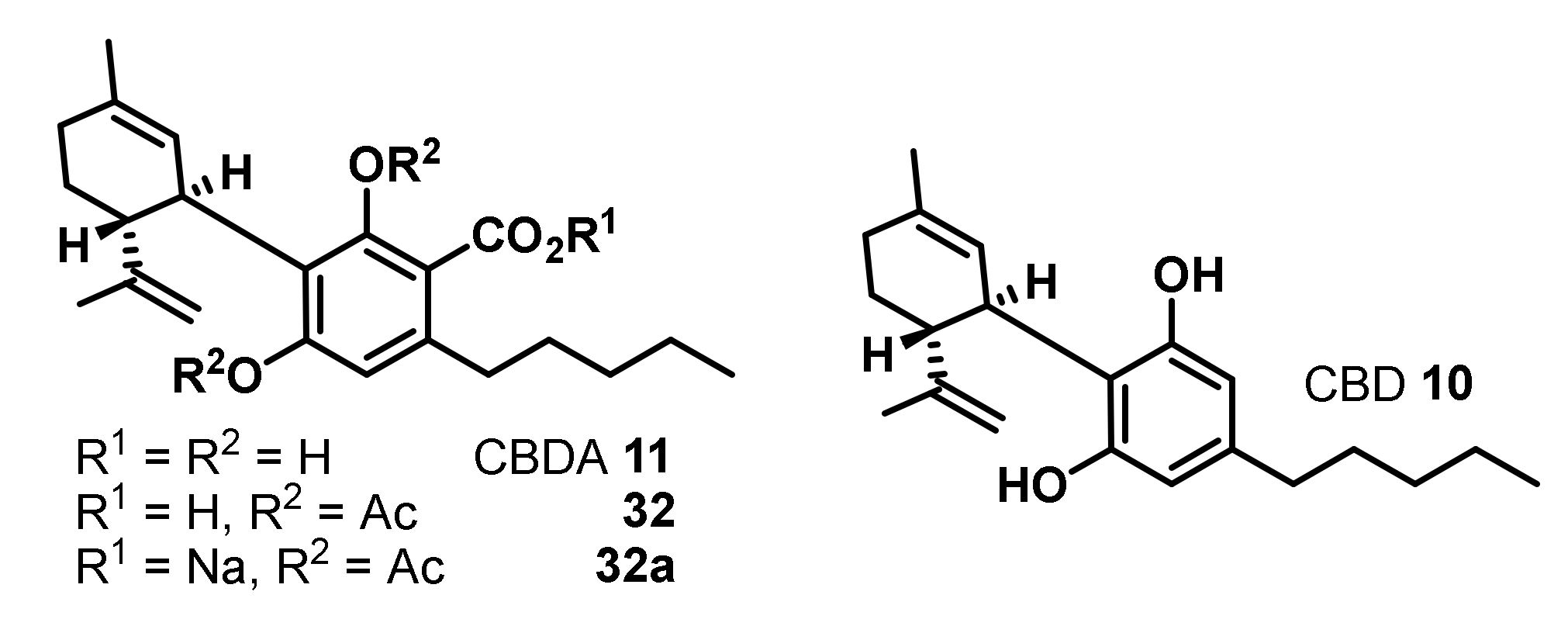

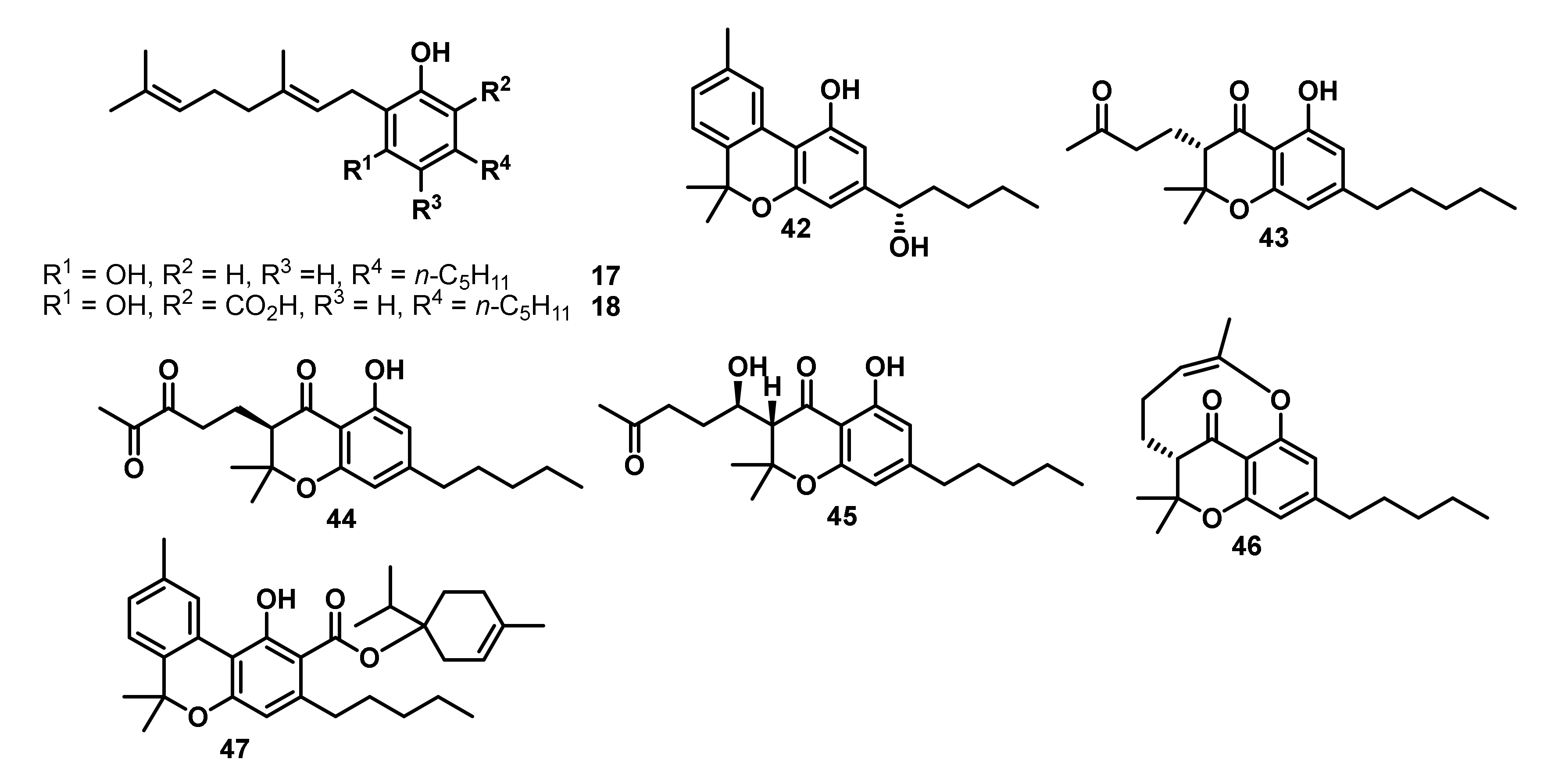
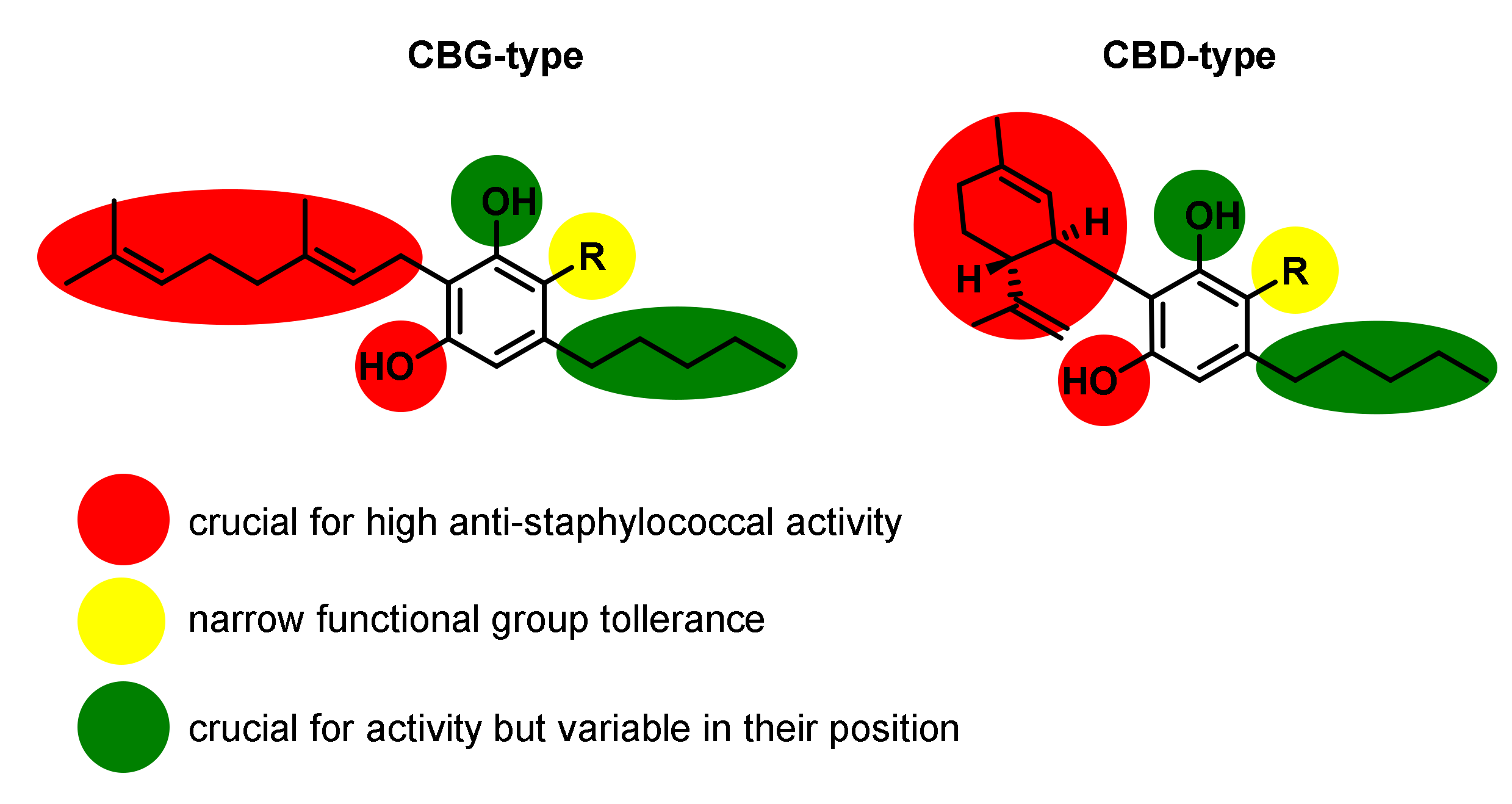
| Entry | Compounds | Antibacterial Activity MIC [µg/mL] | ||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus a | S. pyogenes | S. milleri | S. faecalis | E. coli | S. typhi | P. vulgaris | ||
| 1 | Δ9-THC (1) | 2–5 a | 5 | 2 | 5 | >100 | >100 | >100 |
| 2 | CBD (10) | 1–5 a | 2 | 1 | 5 | >100 | >100 | >100 |
 | ||||||||
| Entry | Compounds | Antibacterial Activity MIC [µg/mL] | Antimycotic Activity MIC [µg/mL] | ||||
|---|---|---|---|---|---|---|---|
| S. aureus a | B. subtilis a | M. smegmatis a | C. albicans b | S. cerevisiae b | T. mentagrophytes b | ||
| 1 | CBC (19) | 1.56 | 0.39 | 12.5 | nt | 25 | 25 |
| 2 | isoCBC (33) | nt | 0.78 | 25.0 | 50 | nt | nt |
| 3 | CBC-C0 (36) | 12.5 | 6.25 | 12.5 | 50 | 25 | 25 |
| 4 | CBC-C1 (37) | 3.12 | 3.12 | 3.12 | nt | 6.25 | 6.25 |
| 5 | isoCBC-C0 (34) | 12.5 | 6.25 | 12.5 | 12.5 | nt | nt |
| 6 | isoCBC-C1 (35) | nt | 0.78 | 25.0 | 50.0 | nt | nt |
| 7 | 38 | 0.78 | 1.56 | 3.12 | nt | 12.5 | 50.0 |
| 8 | isoCBG-C1 (39) | 3.12 | 1.56 | 6.25 | 25.0 | 12.5 | 6.25 |
| 9 | CBG-C1 (40) | 1.56 | 1.56 | 6.25 | 25.0 | 6.25 | 6.25 |
| 10 | 41 | 1.56 | 0.78 | 3.12 | 12.5 | 6.25 | 25.0 |
| 11 | CBE (14) | 50 | 25 | 50 | 50.0 | 50.0 | 25.0 |
| 12 | Streptomycin | 6.25 | 3.12 | 1.56 | |||
| 13 | Amphotericin B | 1.56 | 0.19 | 12.5 | |||
 | |||||||
| Entry | Compounds | Antibacterial Activity IC50 [µM] | Antimycotic Activity MIC IC50 [µM] | Antileishmanial Activity MIC IC50 [µM] | Antiprotozoal Activity IC50 [µM] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRSA | S. aureus | E. coli | M. intracellulare | C. albicans | C. krusei | L. donovani | P. falciparum | |||
| D6 | W2 | |||||||||
| 1 | 48 | nt | nt | nt | nt | nt | nt | 40.3 | 7.2 | 4.0 |
| 2 | 49 | 24.4 | 29.6 | na | na | 60.5 | 60.5 | 57.5 | na | na |
| 3 | 50 | 53.4 | na | na | na | na | nt | 10.7 | 7.2 | 6.7 |
| 4 | 51 | 6.7 | 12.2 | na | na | na | 53.4 | 42.7 | na | na |
| 5 | 52 | nt | nt | na | 30.6 | 4.6 | nt | nt | nt | nt |
| 6 | 53 | nt | 3.5 | 54.0 | na | na | 54.0 | nt | nt | nt |
| 7 | Ciprofloxacin | 0.4 | 0.4 | 0.1 | 1.5 | |||||
| 8 | Amphotericin B | 0.3 | 0.7 | |||||||
| 9 | Pentamidine | 3.8 | ||||||||
| 10 | Chloroquine | 0.1 | 0.5 | |||||||
 | ||||||||||
| Entry | Compound | Antibacterial Activity MIC [µg/mL] | |||||
|---|---|---|---|---|---|---|---|
| SA-1199B a | RN-4220 b | XU212 c | ATCC25923 d | EMRSA-15 e | EMRSA-16 e | ||
| 1 | CBD 10 | 2 | 2 | 2 | 2 | 2 | 2 |
| 2 | CBDA 11 | 1 | 1 | 1 | 0.5 | 1 | 1 |
| 3 | 54–58 | >100 | >100 | >100 | >100 | >100 | >100 |
| 4 | CBC 19 | 2 | 2 | 1 | 2 | 2 | 2 |
| 5 | CBG 17 | 4 | 2 | 4 | 4 | 2 | 1 |
| 6 | CBGA 18 | 1 | 1 | 1 | 1 | 2 | 1 |
| 7 | 59–61 | >100 | >100 | >100 | >100 | >100 | >100 |
| 8 | 62 | 64 | nt | 64 | nt | nt | nt |
| 9 | 63 | >100 | >100 | >100 | >100 | >100 | >100 |
| 10 | Δ9-THC 1 | 8 | 4 | 8 | 4 | 8 | 4 |
| 11 | Δ9-THCA 2 | 2 | 1 | 1 | 1 | 2 | 0.5 |
| 12 | CBN 7 | 1 | 1 | 1 | 1 | 1 | nt |
| 13 | 64 | 1 | 1 | 1 | 1 | 1 | 1 |
| 14 | 65 | 2 | 1 | 0.5 | 1 | 2 | nt |
| 15 | 66 | 32 | 32 | 16 | 16 | 16 | 32 |
| 16 | 67 | >100 | >100 | >100 | >100 | >100 | >100 |
| 17 | 68 | 64 | 64 | 64 | 128 | 64 | 64 |
| 18 | 69 | >100 | >100 | >100 | >100 | >100 | >100 |
| 19 | norfloxacin | 32 | 1 | 4 | 1 | 0.5 | 128 |
| 20 | erythromycin | 0.25 | 64 | >128 | 0.25 | >128 | >128 |
| 21 | tetracycline | 0.25 | 0.25 | 128 | 0.25 | 0.125 | 0.125 |
| 22 | oxacillin | 0.25 | 0.25 | 128 | 0.125 | 32 | >128 |
 | |||||||
| Entry | Compound/Structure | Antibacterial Activity against MRSA USA300 a MIC [µg/mL] | Anti-Biofilm Activity [µg/mL] 100% Inhibition | Anti-Persister Cell Activity |
|---|---|---|---|---|
| 1 | 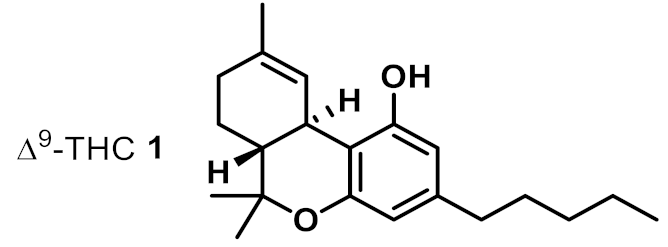 | 2 | 4 | ++ |
| 2 | 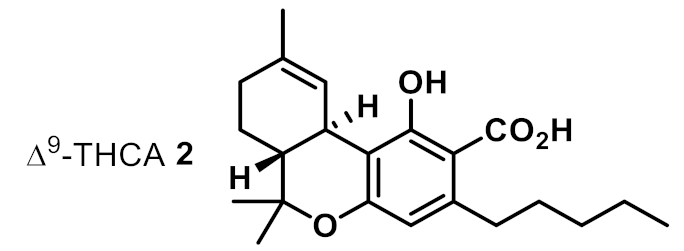 | 4 | 4 | ++ |
| 3 | 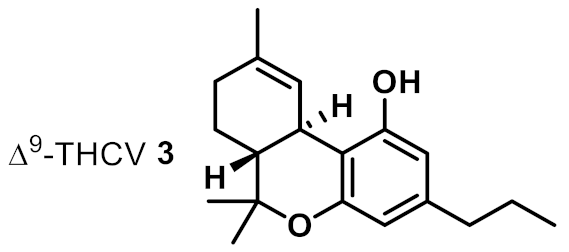 | 4 | 8 | ++ |
| 4 | 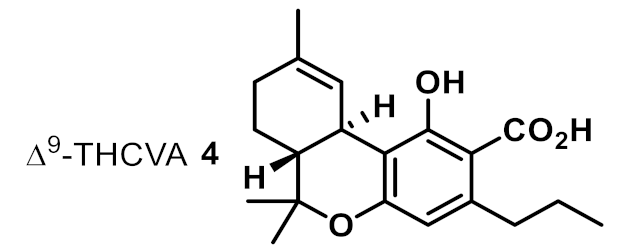 | 16 | >16 | na |
| 5 | 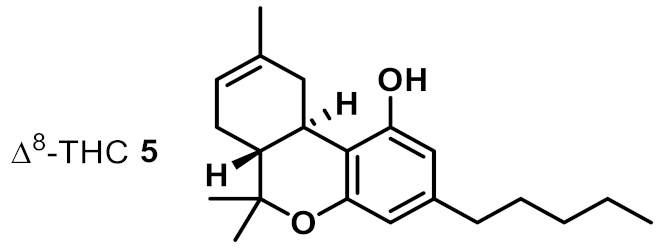 | 2 | 2 | ++ |
| 6 | 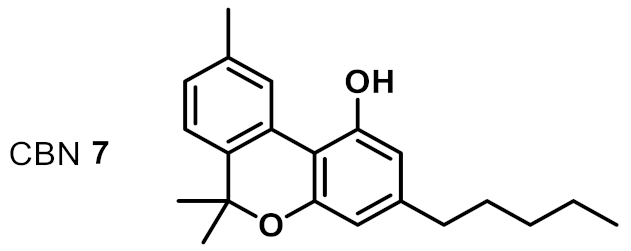 | 2 | 4 | ++ |
| 7 | 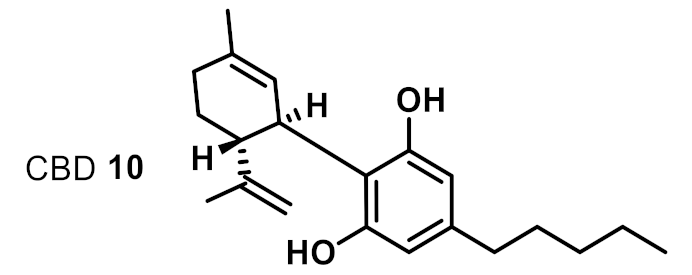 | 2 | 4 | +++ |
| 8 | 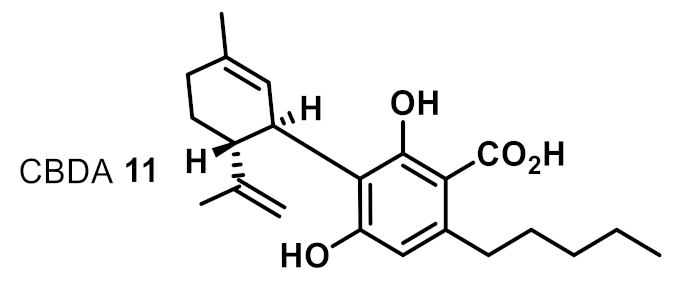 | 16 | 8 | + |
| 9 | 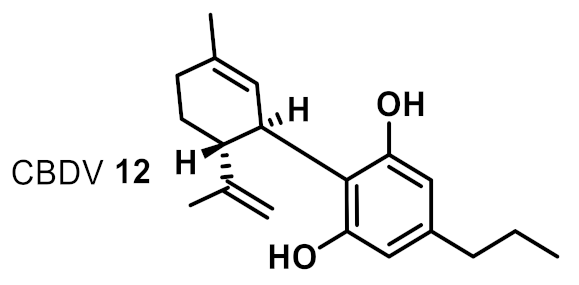 | 8 | 8 | ++ |
| 10 | 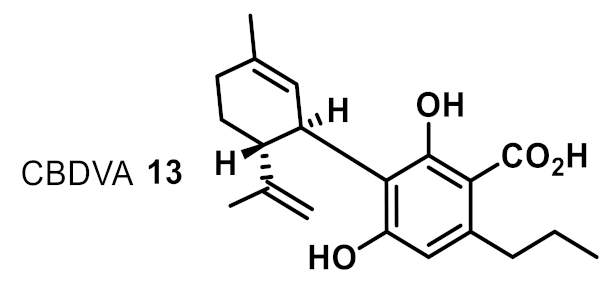 | 32 | na | na |
| 11 | 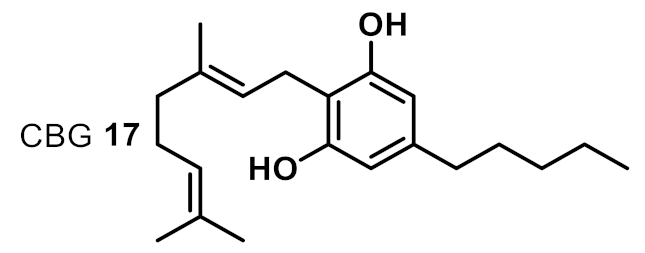 | 2 | 2 | ++++ |
| 12 | 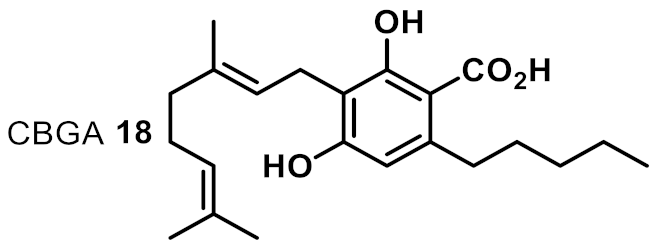 | 4 | 4 | ++++ |
| 13 | 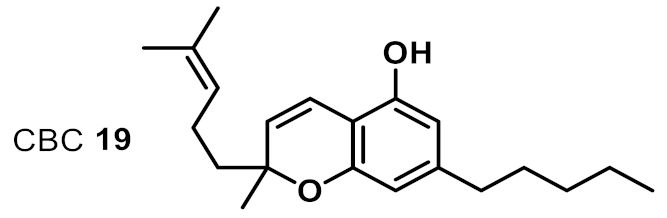 | 8 | 8 | ++ |
| 14 | 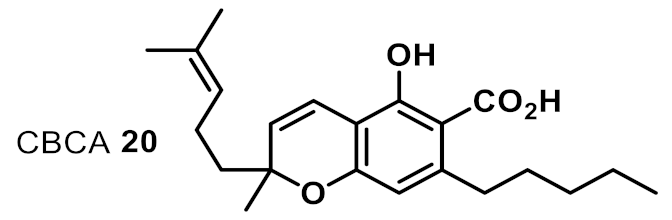 | 2 | 4 | +++ |
| 15 | 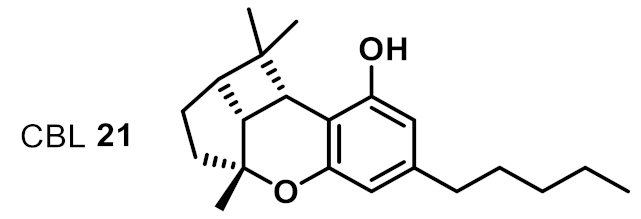 | >32 | na | na |
| 16 | 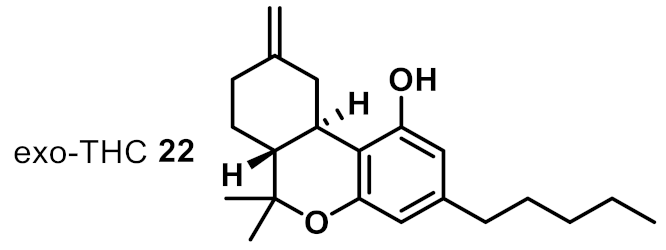 | 2 | 2 | ++ |
| 17 | 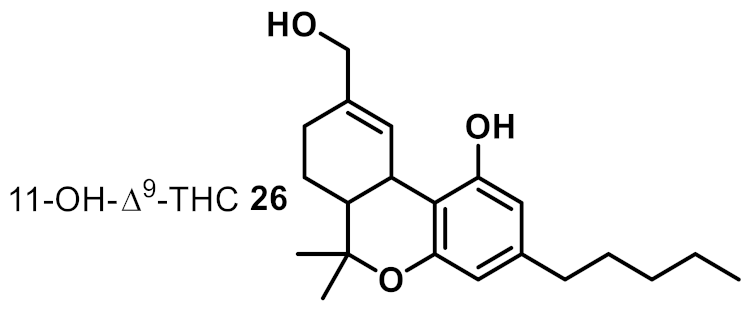 | >32 | ~8 | na |
| 18 | 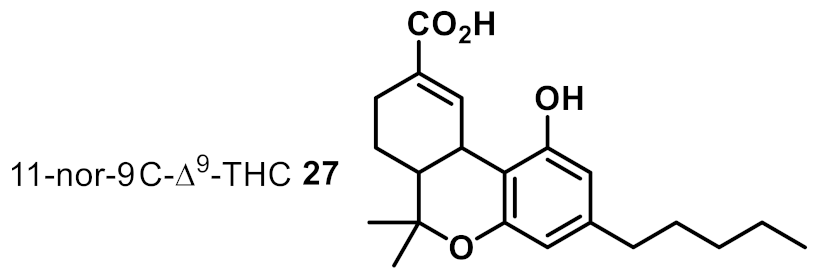 | >32 | na | na |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klahn, P. Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits? Antibiotics 2020, 9, 297. https://doi.org/10.3390/antibiotics9060297
Klahn P. Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits? Antibiotics. 2020; 9(6):297. https://doi.org/10.3390/antibiotics9060297
Chicago/Turabian StyleKlahn, Philipp. 2020. "Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits?" Antibiotics 9, no. 6: 297. https://doi.org/10.3390/antibiotics9060297
APA StyleKlahn, P. (2020). Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits? Antibiotics, 9(6), 297. https://doi.org/10.3390/antibiotics9060297






