Anti-Infectious Plants of the Thai Karen: A Meta-Analysis
Abstract
1. Introduction
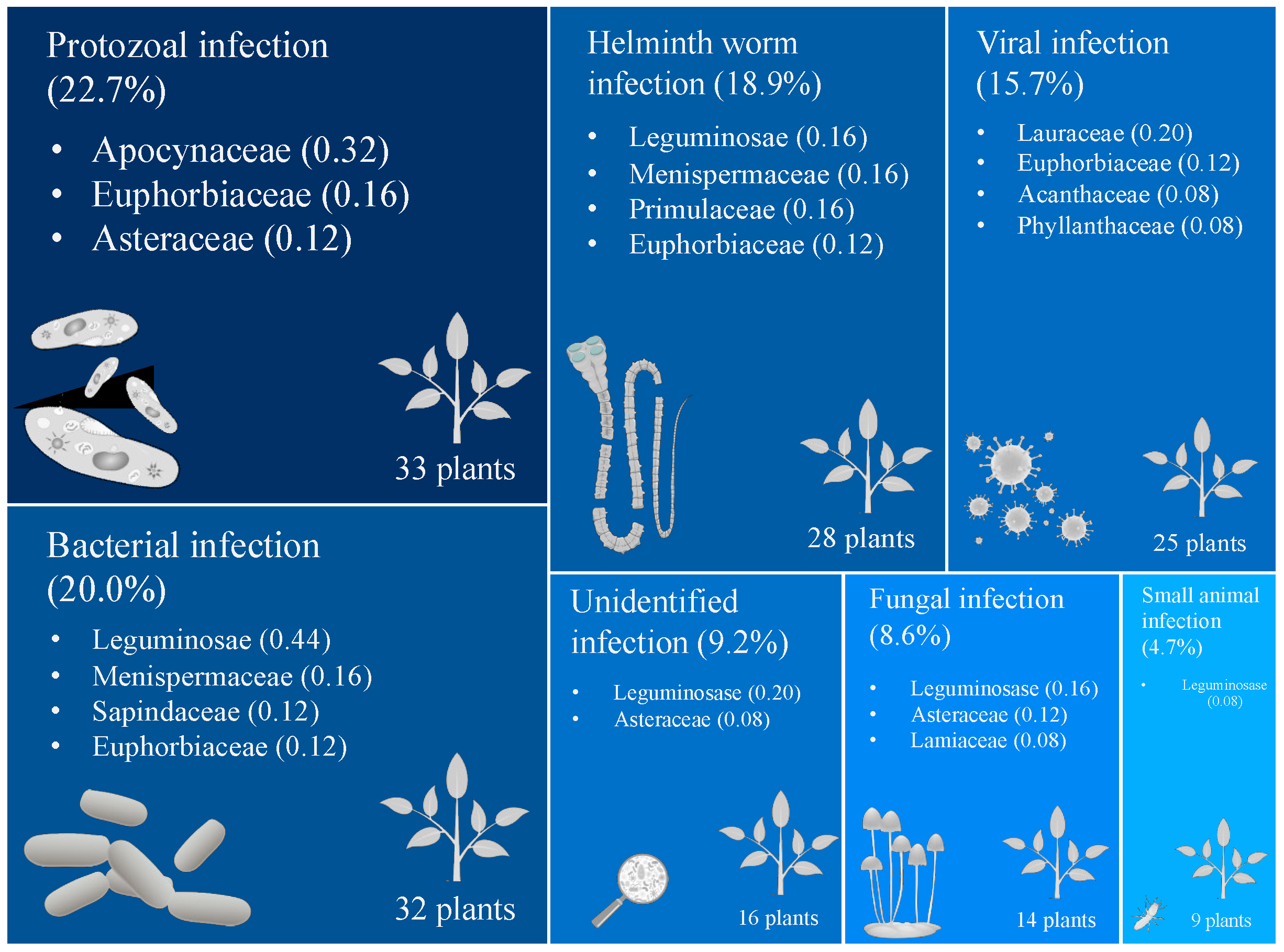
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acuna-Soto, R.; Stahle, D.W.; Cleaveland, M.K.; Therrell, M.D. Megadrought and megadeath in 16th century Mexico. Emerg. Infect. Dis. 2002, 8, 360–362. [Google Scholar] [CrossRef]
- Potter, C.W. A history of influenza. J. Appl. Microbiol. 2001, 91, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Black, S.; Rappuoli, R. Emerging infectious diseases: A proactive approach. Proc. Natl. Acad. Sci. USA 2017, 114, 4055–4059. [Google Scholar] [CrossRef] [PubMed]
- Edelman, A.; Gelding, A.; Konovalov, E.; McComiskie, R.; Penny, A.; Roberts, N.; Templeman, S.; Trewin, D.; Ziembicki, M.; Trewin, B. State of the Tropics 2014 Report; James Cook University: Cairns, Australia, 2014. [Google Scholar]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Norn, S.; Kruse, P.; Kruse, E. History of opium poppy and morphine. Dan. Med. Arb. 2005, 33, 171–184. [Google Scholar]
- Wolff, H.G.; Hardy, J.D.; Goodell, H. Studies on pain. Measurement of the effect of morphine, codeine, and other opiates on the pain threshold and an analysis of their relation to the pain experience. J. Clin. Investig. 1940, 19, 659–680. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef]
- WHO. WHO Model List of Essential Medicines: 16th List (Updated) March 2010; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- WHO. Guidelines for the Treatment of Malaria; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Pooma, R.; Suddee, S. Tem Smitinand’s Thai Plant Names, Revised; The Office of the Forest Herbarium, Department of National Parks, Wildlife and Plant Conservation: Bangkok, Thailand, 2014. [Google Scholar]
- Premsrirat, S. Ethnolinguistic Maps of Thailand; Mahidol University: Nakhon Pathom, Thailand, 2004. [Google Scholar]
- Phumthum, M.; Srithi, K.; Inta, A.; Junsongduang, A.; Tangjitman, K.; Pongamornkul, W.; Trisonthi, C.; Balslev, H. Ethnomedicinal plant diversity in Thailand. J. Ethnopharmacol. 2018, 214, 90–98. [Google Scholar] [CrossRef]
- Srithi, K.; Trisonthi, C.; Inta, A.; Balslev, H. Cross-cultural Comparison of Medicinal Plants Used to Treat Infections in Northern Thailand. Econ. Bot. 2019, 73, 86–95. [Google Scholar] [CrossRef]
- Srisawat, B. Hill Tribes in Thailand; Pickanes Printing Center: Bangkok, Thailand, 2002; Volume 2. [Google Scholar]
- Sorasak Sanoprai, K.M. Karen. Available online: https://www.sac.or.th/databases/ethnic-groups/ethnicGroups/79 (accessed on 19 March 2020).
- Tipraqsa, P.; Schreinemachers, P. Agricultural commercialization of Karen Hill tribes in northern Thailand. Agric. Econ. 2009, 40, 43–53. [Google Scholar] [CrossRef]
- Kaewsangsai, K. Ethnobotany of Karen in Khun Tuen Noi Village, Mea Tuen Subdistrict, Omkoi Distric, Chiang Mai Province; Chiang Mai University: Chiang Mai, Thailand, 2017. [Google Scholar]
- Kamwong, K. Ethnobotany of Karens at Ban Mai Sawan and Ban Huay Pu Ling, Ban Luang Sub-District, Chom Thong District, Chiang Mai Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2010. [Google Scholar]
- Phumthum, M.; Sadgrove, J.N. High-Value Plant Species Used for the Treatment of “Fever” by the Karen Hill Tribe People. Antibiotics 2020, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Phumthum, M. How far are we? Information from the three decades of ethnomedicinal studies in Thailand. Ethnobiol. Conserv. 2020, 9, 21. [Google Scholar] [CrossRef]
- Anderson, E.F. Plants and People of the Golden Triangle: Ethnobotany of the Hill Tribes of Northern Thailand; Dioscorides Press: Portland, OR, USA, 1993. [Google Scholar]
- Phumthum, M.; Balslev, H.; Barfod, A.S. Important Medicinal Plant Families in Thailand. Front. Pharmacol. 2019, 10, 1125. [Google Scholar] [CrossRef]
- Moerman, D.E. An analysis of the food plants and drug plants of native North America. J. Ethnopharmacol. 1996, 52, 1–22. [Google Scholar] [CrossRef]
- Bennett, B.C.; Husby, C.E. Patterns of medicinal plant use: An examination of the Ecuadorian Shuar medicinal flora using contingency table and binomial analyses. J. Ethnopharmacol. 2008, 116, 422–430. [Google Scholar] [CrossRef]
- Weckerle, C.S.; Cabras, S.; Castellanos, M.E.; Leonti, M. Quantitative methods in ethnobotany and ethnopharmacology: Considering the overall flora—Hypothesis testing for over- and underused plant families with the Bayesian approach. J. Ethnopharmacol. 2011, 137, 837–843. [Google Scholar] [CrossRef]
- Saslis-Lagoudakis, C.H.; Savolainen, V.; Williamson, E.M.; Forest, F.; Wagstaff, S.J.; Baral, S.R.; Watson, M.F.; Pendry, C.A.; Hawkins, J.A. Phylogenies reveal predictive power of traditional medicine in bioprospecting. Proc. Natl. Acad. Sci. USA 2012, 109, 15835. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Ferrús Pérez, M.A. Antimicrobial potential of legume extracts against foodborne pathogens: A review. Trends Food Sci. Technol. 2018, 72, 114–124. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Khadke, S.K.; Yamano, A.; Woo, J.-T.; Lee, J. Antimicrobial and antibiofilm activities of prenylated flavanones from Macaranga tanarius. Phytomedicine 2019, 63, 153033. [Google Scholar] [CrossRef] [PubMed]
- Chiavari-Frederico, M.O.; Barbosa, L.N.; Carvalho dos Santos, I.; Ratti da Silva, G.; Fernandes de Castro, A.; de Campos Bortolucci, W.; Barboza, L.N.; Campos, C.F.d.A.A.; Gonçalves, J.E.; Menetrier, J.V. Antimicrobial activity of Asteraceae species against bacterial pathogens isolated from postmenopausal women. PLoS ONE 2020, 15, e0227023. [Google Scholar] [CrossRef] [PubMed]
- Kuspradini, H.; Wulandari, I.; Putri, A.S.; Tiya, S.Y.; Kusuma, I.W. Phytochemical, antioxidant and antimicrobial properties of Litsea angulata extracts. F1000Research 2018, 7, 1839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hua, Z.; Song, Y.; Feng, C. Monoterpenoid indole alkaloids from Alstonia rupestris with cytotoxic, antibacterial and antifungal activities. Fitoterapia 2014, 97, 142–147. [Google Scholar] [CrossRef]
- Kot, B.; Wierzchowska, K.; Piechota, M.; Czerniewicz, P.; Chrzanowski, G. Antimicrobial activity of five essential oils from lamiaceae against multidrug-resistant Staphylococcus aureus. Nat. Prod. Res. 2019, 33, 3587–3591. [Google Scholar] [CrossRef]
- Sultana, S.; Asif, H.M.; Akhtar, N.; Ahmad, K. Medicinal plants with potential antipyretic activity: A review. Asian Pac. J. Trop. Dis. 2015, 5, S202–S208. [Google Scholar] [CrossRef]
- Ahmad, W.; Jantan, I.; Bukhari, S.N.A. Tinospora crispa (L.) Hook. f. & Thomson: A Review of Its Ethnobotanical, Phytochemical, and Pharmacological Aspects. Front. Pharmacol. 2016, 7, 205. [Google Scholar] [CrossRef]
- Sulaiman, F.A.; Fuad, N.; Rahman, F.; Iqbal, A.; Darnis, D.S. Antioxidant and antimicrobial properties of Tinospora crispa (putarwali) Stems methanolic extract. J. Teknol. 2016, 78, 78. [Google Scholar] [CrossRef][Green Version]
- Mohammed, A.I.C.; Manish, G.; Dinesh, C.K. Antimicrobial Activity of Tinospora Crispa Root Extracts. Int. J. Res. Ayurveda Pharm. 2012, 3, 41–49. [Google Scholar]
- Sanseera, D.; Niwatananun, W.; Liawruangrath, B.; Liawruangrath, S.; Baramee, A.; Pyne, S.G. Chemical Composition and Biological Activities of the Essential Oil from Leaves of Cleidion javanicum Bl. J. Essent. Oil Bear. Plants 2012, 15, 186–194. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P. Review on the chemical composition of Litsea cubeba essential oils and the bioactivity of its major constituents citral and limonene. J. Essent. Oil Res. 2019, 31, 361–378. [Google Scholar] [CrossRef]
- Sowmiya, K.; Prakash, J.T.J. Biosysnthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from Leaf Extract of Acmella Oleracea. Int. J. Scientific Tech. Res. 2019, 8, 1003–1013. [Google Scholar]
- Fred-Jaiyesimi, A.A.; Abo, K.A. Phytochemical and Antimicrobial analysis of the crude extract, petroleum ether and chloroform fractions of Euphorbia heterophylla Linn Whole Plant. Pharmacogn. J. 2010, 2, 1–4. [Google Scholar] [CrossRef]
- Alves, N.S.F.; Setzer, W.N.; da Silva, J.K.R. The chemistry and biological activities of Peperomia pellucida (Piperaceae): A critical review. J. Ethnopharmacol. 2019, 232, 90–102. [Google Scholar] [CrossRef]
- Phumthum, M.; Balslev, H. Use of Medicinal Plants Among Thai Ethnic Groups: A Comparison. Econ. Bot. 2019, 73, 64–75. [Google Scholar] [CrossRef]
- Cook, F. Economic Botany Data Collection Standard Prepared for the International Working Group on Taxonomic Databases for Plant Sciences (TDWG); Royal Botanic Gardens: Kew, London, UK, 1995. [Google Scholar]
- Tardío, J.; Pardo-de-Santayana, M. Cultural Importance Indices: A Comparative Analysis Based on the Useful Wild Plants of Southern Cantabria (Northern Spain)1. Econ. Bot. 2008, 62, 24–39. [Google Scholar] [CrossRef]
- Phillips, O.; Gentry, A. The useful plants of Tambopata, Peru: I Statistical hypotheses tests with a new quantitative technique. Econ. Bot. 1993, 47, 15–32. [Google Scholar] [CrossRef]
| Species | Bacterial infection | Fungal infection | Helminth worm infection | Protozoal infection | Small animal infection | Unidentified infection | Viral infection | Total CI value |
|---|---|---|---|---|---|---|---|---|
| Acacia caesia (L.) Willd. | 0.04 | - | 0.04 | - | - | - | 0.04 | 0.12 |
| Acacia concinna (Willd.) DC. | 0.04 | 0.04 | - | - | - | 0.04 | - | 0.12 |
| Acalypha spiciflora Burm.f. | - | - | - | 0.04 | - | - | - | 0.04 |
| Achyranthes aspera L. | - | - | - | - | - | - | 0.04 | 0.04 |
| Acmella oleracea (L.) R.K.Jansen | - | - | 0.04 | - | - | - | - | 0.04 |
| Acmella paniculata (Wall. ex DC.) R.K.Jansen | - | - | 0.04 | - | - | - | - | 0.04 |
| Acorus calamus L. | 0.04 | - | - | - | - | - | - | 0.04 |
| Aesculus assamica Griff. | 0.08 | - | - | - | - | - | 0.04 | 0.12 |
| Ageratina adenophora (Spreng.) R.M.King & H.Rob. | - | - | - | - | - | 0.04 | - | 0.04 |
| Aloe vera (L.) Burm.f. | - | - | - | - | - | - | 0.04 | 0.04 |
| Alstonia rostrata C. E. C. Fisch. | - | - | - | 0.04 | - | - | - | 0.04 |
| Alstonia scholaris (L.) R. Br. | - | - | - | 0.08 | - | - | - | 0.08 |
| Amphineurion marginatum (Roxb.) D.J.Middleton | - | - | 0.04 | - | - | - | - | 0.04 |
| Angiopteris evecta (G. Forst.) Hoffm. | 0.04 | - | - | - | - | - | - | 0.04 |
| Annona squamosa L. | - | - | - | - | 0.04 | - | - | 0.04 |
| Archidendron jiringa (Jack) I.C.Nielsen | - | - | - | - | - | 0.08 | - | 0.08 |
| Arisaema auriculatum Buchet | 0.04 | - | - | - | - | - | - | 0.04 |
| Aristolochia tagala Cham. | - | - | 0.04 | - | - | 0.04 | 0.04 | 0.12 |
| Artemisia atrovirens Hand.-Mazz. | - | - | - | - | - | - | 0.04 | 0.04 |
| Artocarpus heterophyllus Lam. | - | - | 0.04 | - | - | - | - | 0.04 |
| Betula alnoides Buch.-Ham. ex D.Don | - | - | - | - | - | - | 0.04 | 0.04 |
| Blumea balsamifera (L.) DC. | - | - | - | 0.04 | - | - | - | 0.04 |
| Brucea javanica (L.) Merr. | - | - | - | 0.04 | - | - | - | 0.04 |
| Buddleja asiatica Lour. | - | - | - | - | - | 0.04 | - | 0.04 |
| Caesalpinia sappan L. | 0.04 | - | - | - | - | - | - | 0.04 |
| Cajanus cajan (L.) Millsp. | 0.04 | - | - | - | 0.04 | 0.04 | - | 0.12 |
| Cassytha filiformis L. | - | - | - | - | - | - | 0.04 | 0.04 |
| Celtis tetrandra Roxb. | - | - | - | - | - | - | 0.04 | 0.04 |
| Centella asiatica (L.) Urb. | - | - | - | - | - | - | 0.04 | 0.04 |
| Cheilocostus speciosus (J.Koenig) C.D.Specht | - | - | - | - | - | 0.04 | - | 0.04 |
| Chromolaena odorata (L.) R.M.King & H.Rob. | - | 0.04 | - | 0.04 | - | - | - | 0.08 |
| Chrozophora tinctoria (L.) A.Juss. | - | - | 0.04 | - | - | - | - | 0.04 |
| Cissampelos hispida Forman | - | - | 0.04 | - | - | - | - | 0.04 |
| Cissus javana DC. | 0.04 | - | - | - | - | - | - | 0.04 |
| Clausena excavata Burm.f. | - | - | - | - | 0.04 | 0.04 | - | 0.08 |
| Cleidion javanicum Blume | 0.08 | - | - | 0.16 | - | - | 0.08 | 0.32 |
| Codiaeum variegatum (L.) Rumph. ex A.Juss. | 0.04 | - | - | - | - | - | - | 0.04 |
| Combretum indicum (L.) DeFilipps | - | - | 0.08 | - | - | - | - | 0.08 |
| Croton sepalinus Airy Shaw | - | - | - | 0.04 | - | - | - | 0.04 |
| Cyclea barbata Miers | 0.04 | - | - | - | - | - | - | 0.04 |
| Cyclocodon lancifolius subsp. Celebicus (Blume) K.E.Morris & Lammers | - | - | - | - | - | - | 0.04 | 0.04 |
| Dactylicapnos scandens (D.Don) Hutch. | - | - | - | - | - | 0.04 | - | 0.04 |
| Dendrocalamus hamiltonii Nees & Arn. ex Munro | - | 0.04 | - | - | - | - | - | 0.04 |
| Derris elliptica (Wall.) Benth. | - | - | - | - | 0.04 | - | - | 0.04 |
| Dianella ensifolia (L.) DC. | - | - | - | 0.04 | - | - | - | 0.04 |
| Diospyros mollis Griff. | - | - | 0.04 | - | - | - | - | 0.04 |
| Drymaria cordata (L.) Willd. ex Schult. | - | - | - | - | - | 0.04 | - | 0.04 |
| Eichhornia crassipes (Mart.) Solms | 0.04 | - | - | - | - | - | - | 0.04 |
| Elephantopus scaber L. | - | - | - | 0.04 | - | - | - | 0.04 |
| Eleusine indica (L.) Gaertn. | - | - | - | 0.04 | - | - | - | 0.04 |
| Embelia sessiliflora Kurz | - | - | 0.16 | - | - | - | - | 0.16 |
| Entada rheedii Spreng. | 0.04 | - | - | - | - | - | - | 0.04 |
| Erythrina subumbrans (Hassk.) Merr. | 0.04 | - | - | - | - | - | - | 0.04 |
| Euphorbia heterophylla L. | - | - | 0.04 | - | - | - | - | 0.04 |
| Ficus fistulosa Reinw. ex Blume | 0.04 | - | - | - | - | - | - | 0.04 |
| Flemingia lineata (L.) Aiton | 0.04 | - | - | - | - | - | - | 0.04 |
| Flueggea leucopyrus Willd. | - | - | - | - | - | - | 0.08 | 0.08 |
| Glinus herniarioides (Gagnep.) Tardieu | - | - | - | - | - | - | 0.04 | 0.04 |
| Glochidion sphaerogynum (MÃ_ll.Arg.) Kurz | - | - | 0.04 | - | - | - | - | 0.04 |
| Gmelina arborea Roxb. | - | 0.04 | - | - | - | - | - | 0.04 |
| Grewia nervosa (Lour.) Panigrahi | - | - | 0.04 | - | - | - | - | 0.04 |
| Harrisonia perforata (Blanco) Merr. | - | 0.04 | - | - | - | - | - | 0.04 |
| Hedyotis ampliflora Hance | - | 0.04 | - | - | - | - | - | 0.04 |
| Helicteres elongata Wall. ex Bojer | - | - | - | 0.04 | - | - | - | 0.04 |
| Heliotropium indicum L. | - | - | - | - | - | - | 0.04 | 0.04 |
| Hydnocarpus ilicifolia King | - | - | 0.04 | - | - | - | - | 0.04 |
| Ixora henryi H.Lév. | - | - | - | 0.04 | - | - | - | 0.04 |
| Justicia gendarussa Burm.f. | - | - | - | - | - | - | 0.04 | 0.04 |
| Lepisanthes senegalensis (Poir.) Leenh. | - | - | - | 0.04 | - | - | - | 0.04 |
| Leucaena leucocephala (Lam.) de Wit | - | - | 0.04 | - | - | - | - | 0.04 |
| Litsea cubeba (Lour.) Pers. | 0.04 | 0.04 | - | 0.08 | - | - | 0.12 | 0.28 |
| Litsea glutinosa (Lour.) C.B.Rob. | - | - | - | - | 0.04 | - | - | 0.04 |
| Luffa cylindrica (L.) M.Roem. | - | - | - | - | 0.04 | - | - | 0.04 |
| Mallotus philippensis (Lam.) Müll.Arg. | - | - | 0.04 | - | - | - | - | 0.04 |
| Mangifera indica L. | 0.04 | - | - | - | - | - | - | 0.04 |
| Melia azedarach L. | - | - | 0.04 | - | - | - | - | 0.04 |
| Melicope glomerata (W. G. Craib) T.G. Hartley | - | - | - | 0.08 | - | - | - | 0.08 |
| Microcos paniculata L. | - | - | 0.04 | - | - | - | - | 0.04 |
| Millingtonia hortensis L.f. | - | - | - | 0.04 | - | - | - | 0.04 |
| Momordica charantia L. | - | - | - | - | - | - | 0.04 | 0.04 |
| Morus macroura Miq. | 0.04 | - | - | - | - | - | - | 0.04 |
| Mucuna macrocarpa Wall. | 0.04 | - | - | - | - | - | - | 0.04 |
| Mucuna pruriens (L.) DC. | - | 0.04 | - | - | - | - | - | 0.04 |
| Mussaenda sanderiana Ridl. | - | - | - | - | - | - | 0.04 | 0.04 |
| Ocotea lancifolia (Schott) Mez | - | - | - | - | - | - | 0.04 | 0.04 |
| Oroxylum indicum (L.) Kurz | 0.04 | - | - | - | - | - | 0.04 | 0.08 |
| Oxalis acetosella L. | 0.04 | - | - | - | - | - | - | 0.04 |
| Passiflora foetida L. | - | - | 0.04 | - | - | - | - | 0.04 |
| Peliosanthes macrophylla Wall. ex Baker | - | - | 0.04 | - | - | - | - | 0.04 |
| Peperomia pellucida (L.) Kunth | - | - | 0.04 | - | - | - | - | 0.04 |
| Persicaria barbata (L.) H.Hara | - | - | - | 0.04 | - | - | - | 0.04 |
| Phlogacanthus curviflorus (Wall.) Nees | - | - | - | - | 0.04 | - | - | 0.04 |
| Phymatopteris cruciformis (Ching) Pic. Serm. | - | - | - | - | 0.04 | - | - | 0.04 |
| Picrasma javanica Blume | - | - | - | 0.04 | - | - | - | 0.04 |
| Plectranthus scutellarioides (L.) R.Br. | - | - | - | - | 0.04 | - | - | 0.04 |
| Plumbago indica L. | - | 0.04 | - | - | - | - | - | 0.04 |
| Pothos chinensis (Raf.) Merr. | 0.04 | - | - | - | - | - | - | 0.04 |
| Psidium guajava L. | - | - | - | 0.04 | - | - | - | 0.04 |
| Pteridium aquilinum Kuhn var. wightianum Tryon | 0.04 | - | - | - | - | - | - | 0.04 |
| Rauvolfia serpentina (L.) Benth. ex Kurz | - | - | - | 0.08 | - | - | - | 0.08 |
| Rauvolfia verticillata (Lour.) Baill. | - | - | - | 0.12 | - | - | - | 0.12 |
| Rhinacanthus nasutus (L.) Kurz | - | - | - | - | - | - | 0.04 | 0.04 |
| Rotheca serrata (L.) Steane & Mabb. | - | 0.04 | 0.04 | 0.04 | - | 0.04 | - | 0.16 |
| Rubia cordifolia L. | - | - | - | 0.04 | - | - | - | 0.04 |
| Saccharum officinarum L. | - | - | 0.04 | - | - | - | - | 0.04 |
| Sambucus javanica Blume | - | - | - | 0.04 | - | - | - | 0.04 |
| Sapindus rarak DC. | 0.04 | - | - | - | - | - | - | 0.04 |
| Sarcandra glabra (Thunb.) Nakai | - | - | 0.04 | - | - | - | - | 0.04 |
| Scoparia dulcis L. | 0.04 | 0.04 | - | 0.04 | - | - | - | 0.12 |
| Senna alata (L.) Roxb. | - | 0.08 | - | - | - | 0.04 | - | 0.12 |
| Senna occidentalis (L.) Link | - | - | - | 0.04 | - | - | - | 0.04 |
| Smilax corbularia Kunth | - | - | - | - | - | 0.04 | 0.04 | 0.08 |
| Solanum indicum L. | 0.04 | - | - | - | - | - | - | 0.04 |
| Strobilanthes cusia (Nees) Kuntze | 0.04 | - | - | - | - | - | - | 0.04 |
| Styrax benzoides W. G. Craib | - | - | - | 0.04 | - | - | - | 0.04 |
| Syzygium fruticosum DC. | - | - | - | - | - | 0.04 | - | 0.04 |
| Tabernaemontana pandacaqui Lam. | - | 0.04 | - | - | - | - | - | 0.04 |
| Tadehagi triquetrum (L.) H.Ohashi | 0.08 | - | 0.08 | - | - | - | - | 0.16 |
| Tamarindus indica L. | 0.04 | - | - | - | - | - | - | 0.04 |
| Tectona grandis L.f. | - | - | - | 0.04 | - | - | - | 0.04 |
| Tinospora crispa (L.) Hook. f. & Thomson | 0.12 | - | 0.12 | 0.04 | - | 0.04 | - | 0.32 |
| Tithonia diversifolia (Hemsl.) A.Gray | - | 0.08 | - | - | - | 0.04 | - | 0.12 |
| Trevesia palmata (Roxb. ex Lindl.) Vis. | - | - | 0.04 | - | - | - | - | 0.04 |
| Triadica cochinchinensis Lour. | - | - | - | 0.04 | - | - | 0.04 | 0.08 |
| Trichosanthes tricuspidata Lour. | - | - | - | 0.04 | - | - | - | 0.04 |
| Vitex trifolia L. | - | - | - | - | - | - | 0.04 | 0.04 |
| Xylia xylocarpa (Roxb.) Taub. | - | - | - | 0.04 | - | - | - | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phumthum, M.; Balslev, H. Anti-Infectious Plants of the Thai Karen: A Meta-Analysis. Antibiotics 2020, 9, 298. https://doi.org/10.3390/antibiotics9060298
Phumthum M, Balslev H. Anti-Infectious Plants of the Thai Karen: A Meta-Analysis. Antibiotics. 2020; 9(6):298. https://doi.org/10.3390/antibiotics9060298
Chicago/Turabian StylePhumthum, Methee, and Henrik Balslev. 2020. "Anti-Infectious Plants of the Thai Karen: A Meta-Analysis" Antibiotics 9, no. 6: 298. https://doi.org/10.3390/antibiotics9060298
APA StylePhumthum, M., & Balslev, H. (2020). Anti-Infectious Plants of the Thai Karen: A Meta-Analysis. Antibiotics, 9(6), 298. https://doi.org/10.3390/antibiotics9060298






