Potential Anti-Tuberculosis Activity of the Extracts and Their Active Components of Anogeissus leiocarpa (DC.) Guill. and Perr. with Special Emphasis on Polyphenols
Abstract
1. Introduction
2. Results
2.1. Extracts and Their Antimycobacterial Effects
2.2. Phytochemistry and Antioxidant Effects
3. Discussion
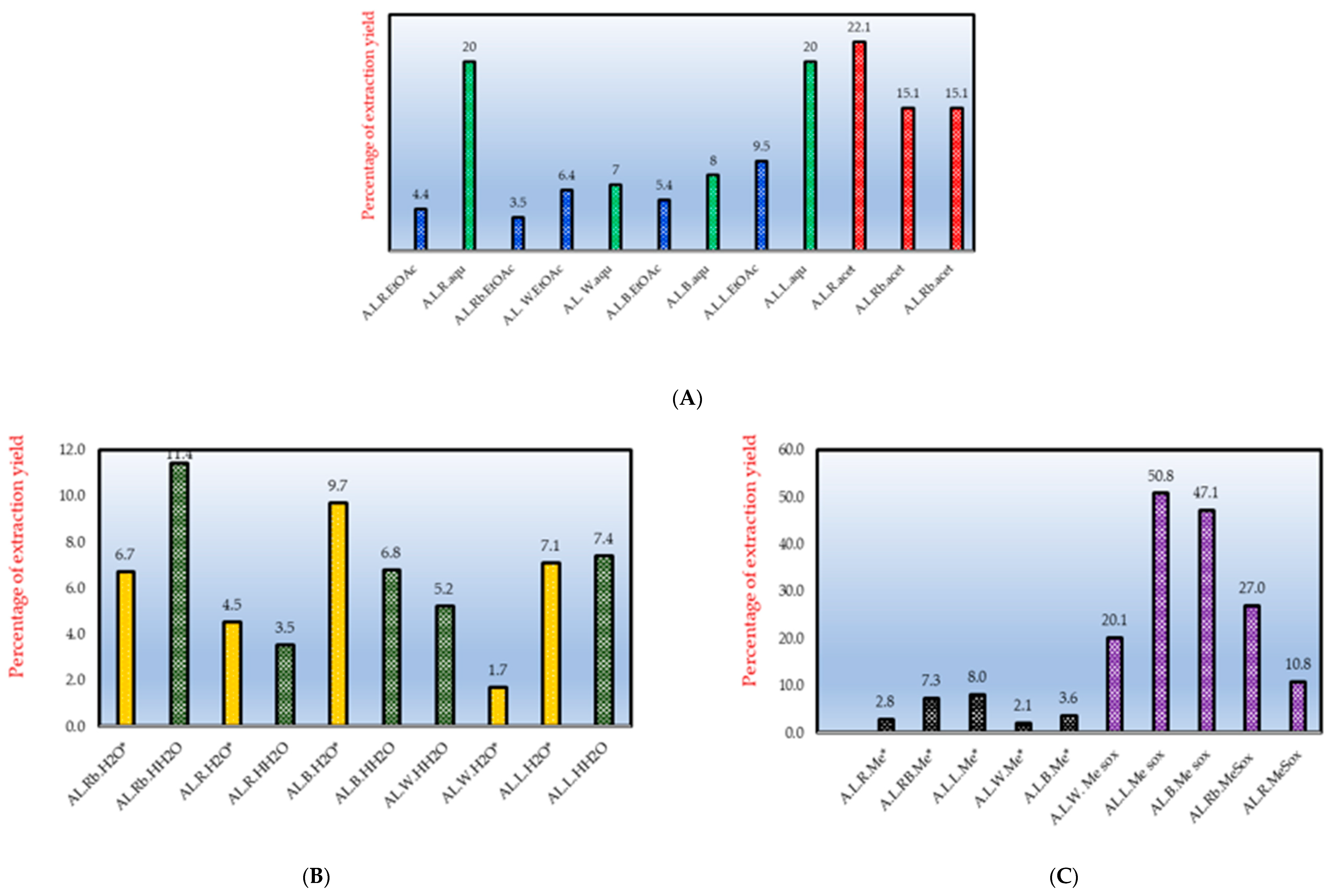
3.1. Extraction Yields, Total Antimycobacterial Activity and Antioxidative Effects
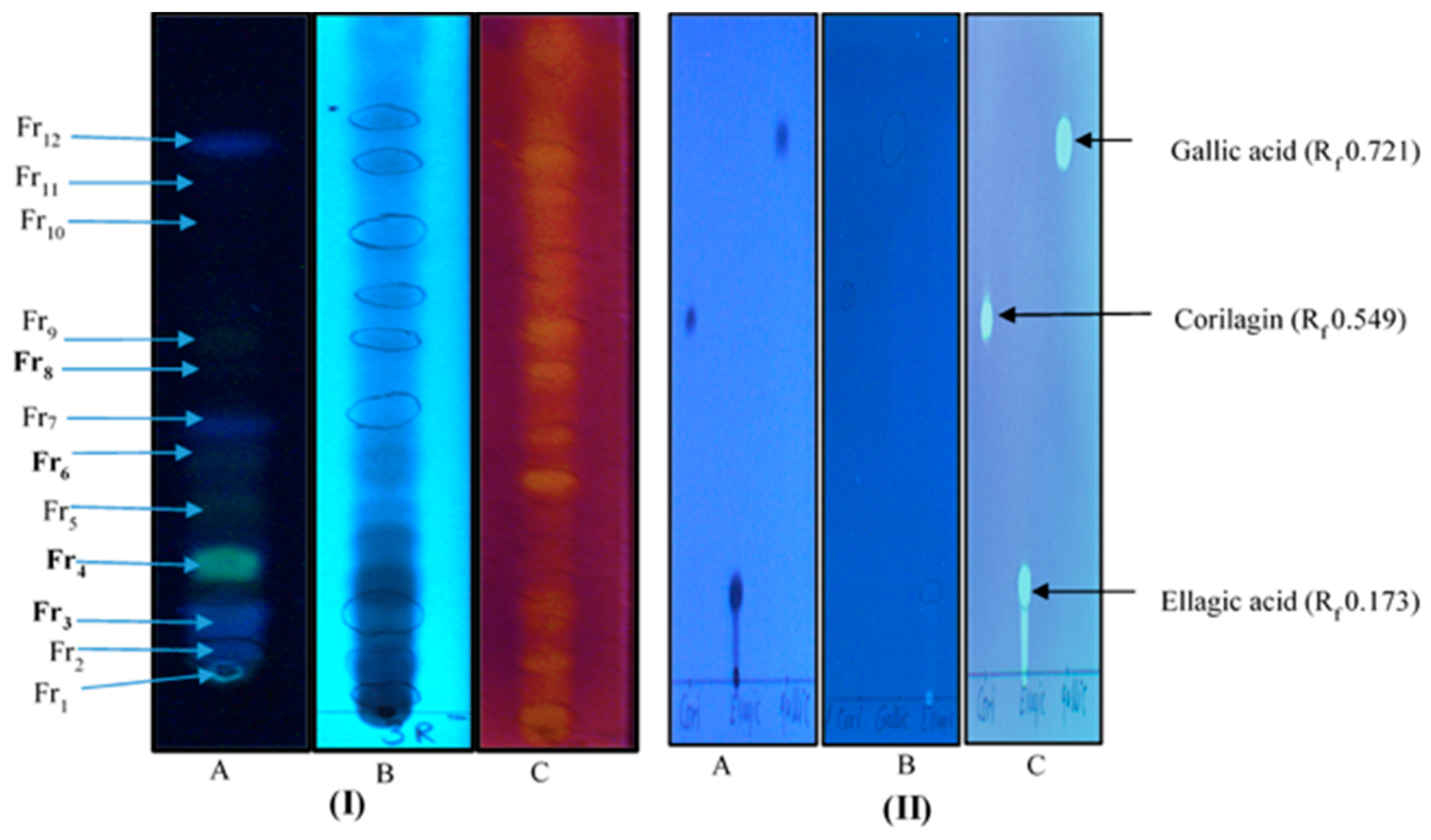
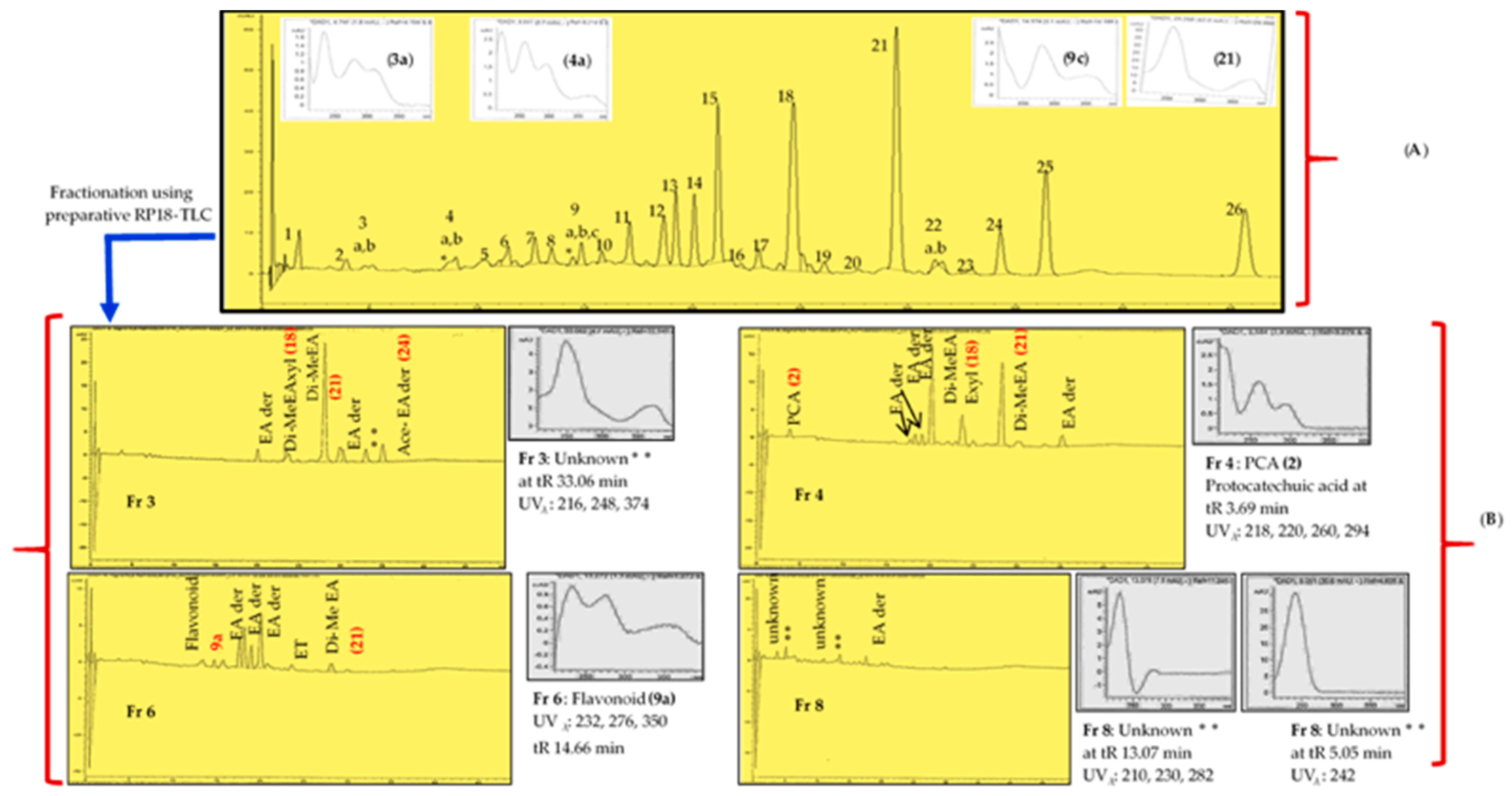
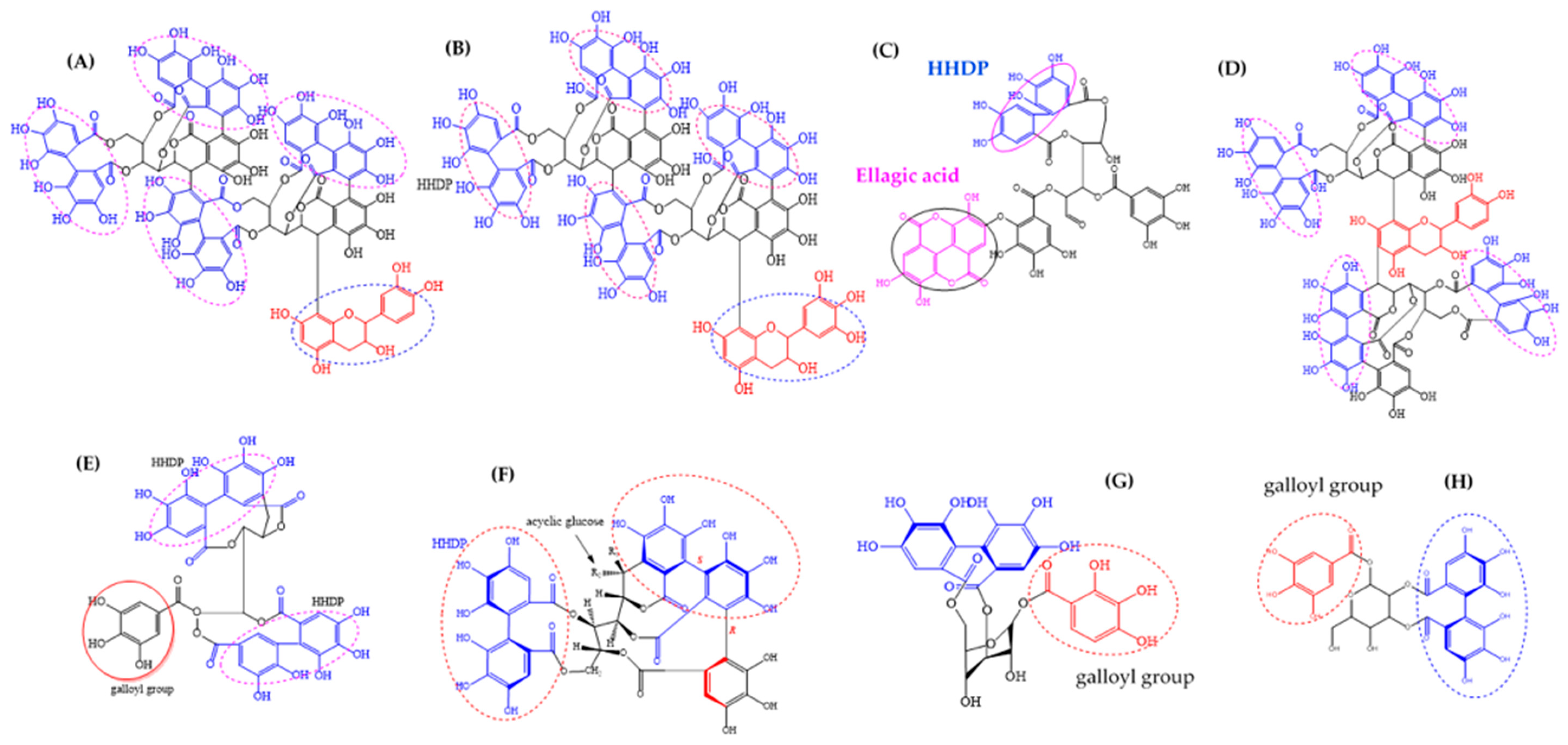
3.2. Ellagic Acid Derivatives and Ellagitannins in A. Leiocarpa and Their Suggested Impact on Its Antimycobacterial Effects
3.3. Flavonoids in A. Leiocarpa and Their Suggested Impact on Its Antimycobacterial Effects
4. Materials and Methods
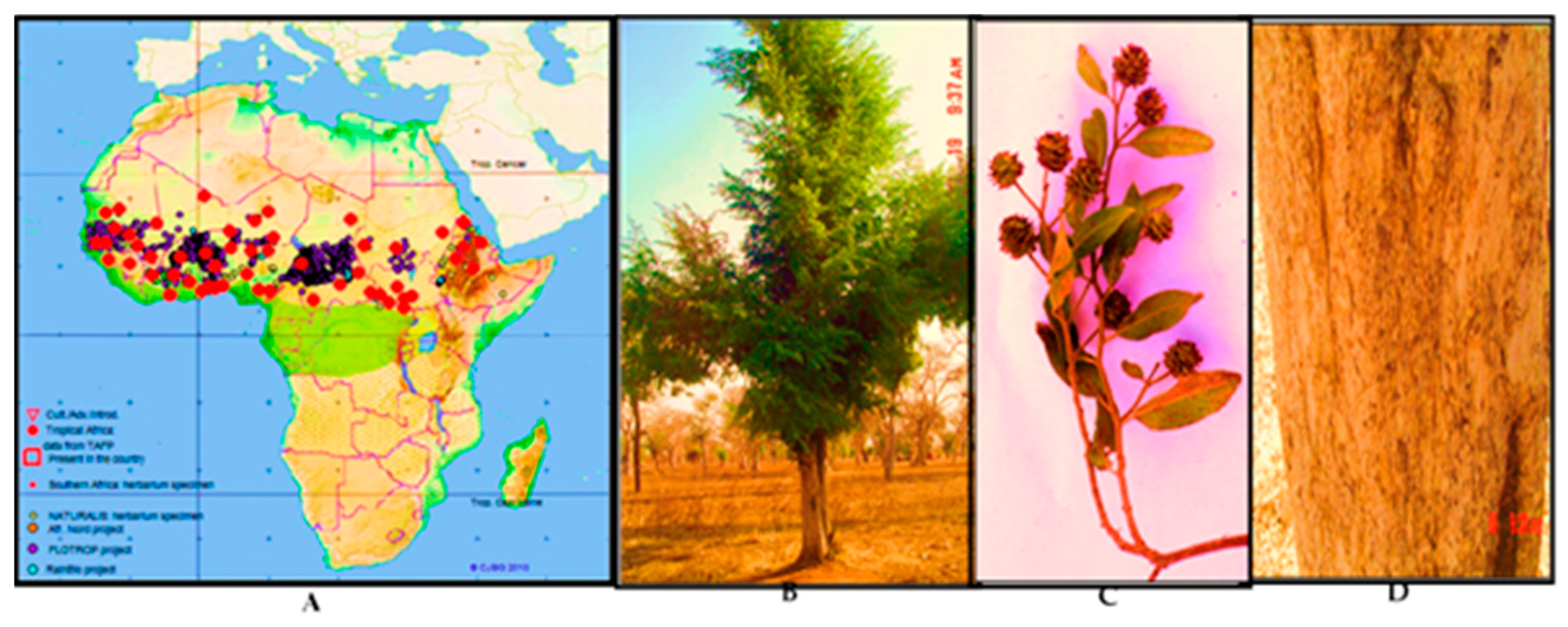
4.1. Plant Material
4.2. Extraction
4.2.1. Cold and Hot Methanol Extraction
4.2.2. Macerations and Hot Water Decoctions
4.2.3. Sequential Extraction and Solvent Partition
4.3. Phytochemical Analysis
4.3.1. Thin Layer Chromatography and Antioxidant Analysis Using The DPPH-Reagent
4.3.2. HPLC-UV/DAD Method
4.3.3. UHPLC/Q-TOF MS Method
4.4. Antimycobacterial Activity Tests
4.4.1. Agar Diffusion Method
4.4.2. Turbidimetric Microplate Method
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report; App Launched; World Health Organization (WHO): Geneva, Switzerland, 2020; Available online: https://www.who.int/tb/en/ (accessed on 20 February 2020).
- American Lung Association. Learn About Tuberculosis. 2018. Available online: https://www.lung.org/lung-health-and-diseases/lung-disease-lookup/tuberculosis/learn-about-tuberculosis.html (accessed on 25 December 2019).
- Askun, T. The significance of Flavonoids as Potential Anti-Tuberculosis compounds. RRJPTS 2015, 6–17. [Google Scholar]
- World Health Organization (WHO). Global Tuberculosis Report; World Health Organization (WHO): Geneva, Switzerland, 2013; Available online: https://apps.who.int/iris/handle/10665/91355 (accessed on 23 December 2019).
- World Health Organization (WHO). Global Tuberculosis Report; World Health Organization (WHO): Geneva, Switzerland, 2018; ISBN 978-92-4-156564-6. Available online: https://www.who.int/tb/publications/global_report/gtbr2018_main_text_28Feb2019.pdf?ua=1 (accessed on 25 December 2019).
- World Health Organization (WHO). Global Tuberculosis Report; World Health Organization (WHO): Geneva, Switzerland, 2019; Available online: https://www.who.int/tb/publications/factsheet_global.pdf (accessed on 25 February 2020).
- Asres, A.; Jerene, D.; Deressa, W. Delays to anti-tuberculosis treatment initiation among cases on directly observed treatment short course in districts of southwestern Ethiopia: A cross sectional study. BMC Infect. Dis. 2019, 19, 481. [Google Scholar] [CrossRef]
- The World Bank. Tuberculosis. Available online: https://www.worldbank.org/en/topic/health/brief/tuberculosis-control (accessed on 16 June 2019).
- Salih, E.Y.A. Ethnobotany, Phytochemistry and Antimicrobial Activity of Combretum, Terminalia and Anogeissus Species (Combretaceae) Growing Naturally in Sudan. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2019; pp. 1–193. [Google Scholar]
- Masoko, P.; Nxumalo, K.M. Validation of antimycobacterial plants used by traditional healers in three districts of the Limpopo province (South Africa). Evid. Based Complementary Altern. Med. 2013, 586247. [Google Scholar] [CrossRef] [PubMed]
- York, T.; Van Vuuren, S.F.; De Wet, H. An antimicrobial evaluation of plants used for the treatment of respiratory infections in rural Maputaland, KwaZulu-Natal, South Africa. J. Ethnopharmacol. 2012, 144, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Pires, D.; Aínsa, J.A.; Gracia, B.; Mulhovo, S.; Duarte, A.; Anes, E.; Ferreira, M.J.U. Antimycobacterial evaluation and preliminary phytochemical investigation of selected medicinal plants traditionally used in Mozambique. J. Ethnopharmacol. 2011, 137, 114–120. [Google Scholar] [CrossRef] [PubMed]
- The Plant List Database 2010. A Working List for All Plant Species. Available online: http://www.theplantlist.org/ (accessed on 25 March 2019).
- Bello, A.A.; Jimoh, A.A. Some physical and mechanical properties of African birch (Anogeissus leiocarpus) timber. J. Appl. Sci. Environ. Manag. 2018, 22, 79–84. [Google Scholar] [CrossRef]
- Hennenberg, K.J.; Goetze, D.; Minden, V.; Traoré, D.; Porembski, S. Size-class distribution of Anogeissus leiocarpus (Combretaceae) along forest–savanna ecotones in northern Ivory Coast. J. Trop. Ecol. 2005, 21, 273–281. [Google Scholar] [CrossRef]
- Arbab, A.H. Review on Anogeissus leiocarpus a potent African traditional drug. Int. J. Res. Pharm. Chem. 2014, 4, 496–500. [Google Scholar]
- El Ghazali, G.E.B.; Abdalla, W.E.; Khalidm, H.E.; Khalafalla, M.M.; Hamad, A.A. Medicinal Plants of the Sudan, part V. In Medicinal Plants of Ingassana Area. Sudan; National Centre for Research, Sudan Currency Printing Press: Khartoum, Sudan, 2003. [Google Scholar]
- Salih, E.Y.A.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Victor, Y.A. In-Vitro Assessment of Antioxidant and Antimicrobial Activities of Methanol Extracts of Six Wound Healing Medicinal Plants. J. Nat. Sci. Res. 2013, 3, 74–82. [Google Scholar]
- Mann, A.; Amupitan, J.O.; Oyewale, A.O.; Okogun, J.I.; Ibrahim, K.; Oladosu, P.; Lawson, L.; Olajide, I.; Nnamdi, A. Evaluation of in vitro antimycobacterial activity of Nigerian plants used for treatment of respiratory diseases. Afr. J. Biotechnol. 2008, 7. [Google Scholar] [CrossRef]
- Elegami, A.A.; El-Nima, E.I.; Tohami, M.E.; Muddathir, A.K. Antimicrobial activity of some species of the family Combretaceae. Phytother. Res. 2002, 16, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Gberikon, G.M.; Agada, J.O.; Yaji, M.E. Synergistic Effects of Anogeissus leiocarpus and Morinda lucida Leaves, Stems and Roots Extracts against Some Enteric Bacteria. IJSRP 2019, 9, 851–860. [Google Scholar]
- Garbi, M.I.; Kabbashi, A.S.; AbdelhafizElshikh, A. Bacteriostatic Effect of Anogeissus leicarpus Methanolic Leaves Extract. Res. Adv. Pharm. Life Sci. 2019, 2. [Google Scholar] [CrossRef]
- Mann, A.; Yusuf, A.; Daniyan, S. TLC analysis and bioactivity screening of the stem bark extract of Anogeissus leiocarpus against multi-resistant Staphylococcus aureus and quantification of its phytoconstituents. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 187–203. [Google Scholar]
- Muraina, I.A.; Adaudi, A.O.; Mamman, M.; Kazeem, H.M.; Picard, J.; McGaw, L.J.; Eloff, J.N. Antimycoplasmal activity of some plant species from northern Nigeria compared to the currently used therapeutic agent. Pharm. Biol. 2010, 48, 1103–1107. [Google Scholar] [CrossRef]
- Orlando, G.; Ferrante, C.; Zengin, G.; Sinan, K.I.; Bene, K.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Di Simone, S.; Recinella, L.; et al. Qualitative Chemical Characterization and Multidirectional Biological Investigation of Leaves and Bark Extracts of Anogeissus leiocarpus (DC.) Guill. & Perr. (Combretaceae). Antioxidants 2019, 8, 343. [Google Scholar]
- Eltayeb, I.M.; Muddathir, A.K.; Ali, H.A.R.; Ayoub, S.M.H. A comparative Study of In vitro Susceptibility of Madurella mycetomatis to Anogeissus leiocarpous Leaves, Roots and Stem Barks Extracts. Am. J. Phytomed. Clin. Ther. 2016, 4, 135–164. [Google Scholar]
- Ndjonka, D.; Abladam, E.D.; Djafsia, B.; Ajonina-Ekoti, I.; Achukwi, M.D.; Liebau, E. Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J. Helminthol. 2014, 88, 481–488. [Google Scholar] [CrossRef]
- Hubert, J.J.-M.N.; Sylvain, P.; Mahmoud, H.; Nicolas, B.; Romain, R.; Jean-Hugues, R. Identification of natural metabolites in mixture: A pattern recognition strategy based on 13C NMR. Anal. Chem. 2014, 86, 2955–2962. [Google Scholar] [CrossRef]
- Hamzaoui, M.; Renault, J.H.; Reynaud, R.; Hubert, J. Centrifugal partition extraction in the pH-zone-refining displacement mode: An efficient strategy for the screening and isolation of biologically active phenolic compounds. J. Chromatogr. B 2013, 937, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Shuaibu, M.N.; Pandey, K.; Wuyep, P.A.; Yanagi, T.; Hirayama, K.; Ichinose, A.; Tanaka, T.; Kouno, I. Castalagin from Anogeissus leiocarpus mediates the killing of Leishmania in vitro. Parasitol. Res. 2008, 103, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Attioua, B.; Lagnikab, L.; Yeoc, D.; Antheaumed, C.; Kaisere, M.; Wenigerf, B.; Vonthron-Sénécheauf, C. In vitro antiplasmodial and antileishmanial activities of flavonoids from Anogeissus leiocarpus (Combretaceae). Int. J. Pharmaceut. Rev. Res. 2011, 11, 1–6. [Google Scholar]
- Salih, E.; Julkunen-Tiitto, R.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Vuorela, H.; Fyhrquist, P. Characterization of antibacterial flavonoids and stilbenes of the root extract of Anogeissus leiocarpus by UHPLC-MS-QTOF. Planta Med. 2015, 81, PM_77. [Google Scholar] [CrossRef]
- Fyhrquist, P.; Salih, E.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R. Ellagitannins, ellagic acid derivatives and ampelopsin in antimicrobial root and stem bark extracts of some selected African species of Terminalia and Anogeissus leiocarpus. Planta Med. 2014, 80, P1L10. [Google Scholar] [CrossRef]
- Tyagi, J.S.; Sharma, D. Mycobacterium smegmatis and tuberculosis. Trends Microbiol. 2002, 2, 68–69. [Google Scholar] [CrossRef]
- Panas, M.W.; Jain, P.; Yang, H.; Mitra, S.; Biswas, D.; Wattam, A.R.; Jacobs, W.R. Noncanonical SMC protein in Mycobacterium smegmatis restricts maintenance of Mycobacterium fortuitum plasmids. Proc. Natl. Acad. Sci. USA 2014, 111, 13264–13271. [Google Scholar] [CrossRef]
- Eloff, J.N. A proposal on expressing the antibacterial activity of plant extracts - a small first step in applying scientific knowledge to rural primary health care in South Africa. S. Afr. J. Sci. 2000, 96, 116–118. [Google Scholar]
- Heftmann, E. Chromatography: Fundamentals and Applications of Chromatographic and Electrophoretic Methods. Part A: Fundamentals and Techniques; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 2011; pp. 423–425. [Google Scholar]
- Kuete, V.; Tabopda, T.K.; Ngameni, B.; Nana, F.; Tshikalange, T.E.; Ngadjui, B.T. Antimycobacterial, antibacterial and antifungal activities of Terminalia superba (Combretaceae). S. Afr. J. Bot. 2010, 76, 125–131. [Google Scholar] [CrossRef]
- Patil, U.H.; Gaikwad, D.K. Ethno-pharmacological review of a herbal drug: Anogeissus latifolia. Int. J. Pharma. Sci. Res. 2011, 2, 41–43. [Google Scholar]
- Kaneko, E.; Kaneko, M. Sugar Derivatives and Application of Same. U.S. Patent 8,350,024 B2, 8 January 2013. [Google Scholar]
- Adigun, J.O.; Amupitan, J.O.; Kelly, D.R. Isolation and investigation of antimicrobial effect of 3, 4, 3’-tri-O-methylflavellagic acid and its glucoside from Anogeissus leocarpus. Bull. Chem. Soc. Ethiop. 2000, 14, 169–174. [Google Scholar] [CrossRef]
- Kondo, Y.; Toida, T.; Kusano, G.; Imai, J. Specific inhibition of formation of acid-fastness in mycobacteria by 3, 3′-di-O-methylellagic acid. Experientia 1979, 35, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Shilpi, J.A.; Ali, M.T.; Saha, S.; Hasan, S.; Gray, A.I.; Seidel, V. Molecular docking studies on InhA, MabA and PanK enzymes from Mycobacterium tuberculosis of ellagic acid derivatives from Ludwigia adscendens and Trewia nudiflora. Silico Pharmacol. 2015, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Asres, K.; Bucar, F.; Edelsbrunner, S.; Kartnig, T.; Höger, G.; Thiel, W. Investigations on antimycobacterial activity of some Ethiopian medicinal plants. Phytother. Res. 2001, 15, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Diop, E.A.; Jacquat, J.; Drouin, N.; Queiroz, E.F.; Wolfender, J.L.; Diop, T.; Rudaz, S. Quantitative CE analysis of punicalagin in Combretum aculeatum extracts traditionally used in Senegal for the treatment of tuberculosis. Electrophoresis 2019, 40, 2820–2827. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: The evidence so far. Evid. Based Complement. Alternat. Med. 2013, 1–15. [Google Scholar] [CrossRef]
- Villalba, K.J.O.; Barka, F.V.; Pasos, C.V.; Rodríguez, P.E. Food Ellagitannins: Structure, Metabolomic Fate, and Biological Properties. In Tannins-Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2019. [Google Scholar]
- phane Quideau, S. Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; World Scientific: Singapore, 2009. [Google Scholar]
- Puupponen-Pimiä, R.; Liisa, N.; Hanna-Leena, A.; Kirsi-Marja, O.-C. The action of berry phenolics against human intestinal pathogens. Biofactors 2005, 23, 243–251. [Google Scholar] [CrossRef]
- Mohan, C.G. Structural Bioinformatics: Applications in Preclinical Drug Discovery Process; Springer: Berlin/Heidelberg, Germany, 2019; Volume 27. [Google Scholar]
- Davis, C.K.; Nasla, K.; Anjana, A.K.; Rajanikant, G.K. Taxifolin as dual inhibitor of Mtb DNA gyrase and isoleucyl-tRNA synthetase: In silico molecular docking, dynamics simulation and in vitro assays. Silico Pharmacol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Jeon, D.; Jeong, M.C.; Lee, E.; Jin, B.; Ryoo, S.; Kim, Y. Antituberculosis activity of a naturally occurring flavonoid, isorhamnetin. J. Nat. Prod. 2016, 79, 961–969. [Google Scholar] [CrossRef]
- Safwat, N.A.; Kashef, M.T.; Aziz, R.K.; Amer, K.F.; Ramadan, M.A. Quercetin 3-O-glucoside recovered from the wild Egyptian Sahara plant, Euphorbia paralias L., inhibits glutamine synthetase and has antimycobacterial activity. Tuberculosis 2018, 108, 106–113. [Google Scholar] [CrossRef]
- Sasikumar, K.; Ghosh, A.R.; Dusthackeer, A. Antimycobacterial potentials of quercetin and rutin against Mycobacterium tuberculosis H37Rv. 3 Biotech 2018, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, A.; Chotipong, A.; Suavansri, T.; Boongird, S.; Timsuksai, P.; Vimuttipong, S.; Chuaynugul, A. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch. Pharm. Res. 2004, 27, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.A.; Agertt, V.; Janovik, V.; Cruz, R.C.; Campos, M.; Guillaume, D.; dos Santos, A.R. Antimycobacterial activity of the fractions and compounds from Scutia buxifolia. Rev. Bras. Farmacogn. 2012, 22, 45–52. [Google Scholar] [CrossRef]
- Mativandlela, S.P.; Muthivhi, T.; Kikuchi, H.; Oshima, Y.; Hamilton, C.; Hussein, A.A.; Lall, N. Antimycobacterial flavonoids from the leaf extract of Galenia africana. J. Nat. Prod. 2009, 72, 2169–2171. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Ngameni, B.; Mbaveng, A.T.; Ngadjui, B.; Meyer, J.M.; Lall, N. Evaluation of flavonoids from Dorstenia barteri for their antimycobacterial, antigonorrheal and anti-reverse transcriptase activities. Acta Trop. 2010, 116, 100–104. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, X.; Gao, F.; Song, J.; Sun, J.; Wang, L.; Sun, X.; Lu, Z.; Zhang, H. Identification of plant-derived natural products as potential inhibitors of the Mycobacterium tuberculosis proteasome. BMC Complement. Altern. Med. 2014, 14, 400. [Google Scholar] [CrossRef]
- Pawar, A.; Jha, P.; Chopra, M.; Chaudhry, U.; Saluja, D. Screening of natural compounds that targets glutamate racemase of Mycobacterium tuberculosis reveals the anti-tubercular potential of flavonoids. Sci. Rep. 2020, 10, 949. [Google Scholar] [CrossRef]
- Mdluli, K.; Ma, Z. Mycobacterium tuberculosis DNA gyrase as a target for drug discovery. Infect. Disord. Drug Targets Former. Curr. Drug Targets Infect. Disord. 2007, 7, 159–168. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013. [Google Scholar] [CrossRef]
- Salih, E.Y.; Fyhrquist, P.; Abdalla, A.; Abdelgadir, A.Y.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Fahmi, M.K.; Elamin, M.H.; Ali, H.A. LC-MS/MS tandem mass spectrometry for analysis of phenolic compounds and pentacyclic triterpenes in antifungal extracts of Terminalia brownii (Fresen). J. Antibiot. 2017, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, B.; Sajadian, S.A.; Khorram, M.; Samimi, A. Mathematical modeling of supercritical fluid extraction of oil from canola and sesame seeds. Braz. J. Chem. Eng. 2013, 30, 159–166. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.; Vogler, B.; Reeb, S.; Klaiber, I.; Papajewski, S.; Roos, G.; Vasquez, E.; Setzer, M.C.; Kraus, W. Isoterchebulin and 4, 6-O-Isoterchebuloyl-d-glucose, Novel Hydrolyzable Tannins from Terminalia macroptera. J. Nat. Prod. 2001, 64, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Taulavuori, K.; Julkunen-Tiitto, R.; Hyöky, V.; Taulavuori, E. Blue mood for superfood. J. Nat. Prod. Commun. 2013, 8, 1–2. [Google Scholar] [CrossRef]
- Fyhrquist, P.; Laakso, I.; Marco, S.G.; Julkunen-Tiitto, R.; Hiltunen, R. Antimycobacterial activity of ellagitannin and ellagic acid derivate rich crude extracts and fractions of five selected species of Terminalia used for treatment of infectious diseases in African traditional medicine. S. Afr. J. Bot. 2014, 90, 1–16. [Google Scholar] [CrossRef]
- Salih, E.Y.; Julkunen-Tiitto, R.; Lampi, A.M.; Kanninen, M.; Luukkanen, O.; Sipi, M.; Lehtonen, M.; Vuorela, H.; Fyhrquist, P. Terminalia laxiflora and Terminalia brownii contain a broad spectrum of antimycobacterial compounds including ellagitannins, ellagic acid derivatives, triterpenes, fatty acids and fatty alcohols. J. Ethnopharmacol. 2018, 227, 82–96. [Google Scholar] [CrossRef]
- Matthew, A.W.; Franklin, R.C.; William, A.C.; Micheal, N.D.; George, M.E.; Janet, F.H.; Fred, C.T. Clinical Laboratory Standards Institute. Method for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In CLSI document M7-A7, 7th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013; pp. 14–34. [Google Scholar]


| Extracts of Anogeissus leiocarpa | IZD | SEM | AI |
|---|---|---|---|
| R. MeSox | 21.33 | 0.33 | 0.45 |
| R. acetone | 15.45 | 0.33 | 0.32 |
| R. hex | NA | ||
| R. EtOAc | 28.50 | 0.29 | 0.60 |
| R. aqu | 18.67 | 0.17 | 0.39 |
| R. Dic | 16.00 | 0.00 | 0.34 |
| R. H2O* | 18.67 | 0.17 | 0.39 |
| R. Me* | 21.33 | 0.17 | 0.45 |
| R. HH2O | 13.30 | 0.17 | 0.28 |
| Rb. acetone | 18.33 | 0.17 | 0.39 |
| Rb. Me* | 17.67 | 0.17 | 0.37 |
| Rb. Dic | 16.33 | 0.17 | 0.34 |
| Rb. H2O* | 18.67 | 0.17 | 0.39 |
| Rb. hex | NA | ||
| W. MeSox | 20.17 | 0.33 | 0.42 |
| W. hex | NA | ||
| W. H2O* | NA | ||
| W. CHCl3 | 13.67 | 0.17 | 0.29 |
| W. HH2O | 15.00 | 0.00 | 0.32 |
| W. aqu | 14.00 | 0.00 | 0.29 |
| W. EtOAc | 25.17 | 0.17 | 0.53 |
| W. Me* | 20.33 | 0.33 | 0.40 |
| B. EtOAc | 28.67 | 0.17 | 0.60 |
| B. aqu | 25.00 | 0.00 | 0.53 |
| B. MeSox | 14.83 | 0.17 | 0.31 |
| B. hex | NA | ||
| B. CHCl3 | NA | ||
| B. H2O* | 17.67 | 0.17 | 0.37 |
| B. Me* | 18.67 | 0.17 | 0.39 |
| B. HH2O | 20.67 | 0.17 | 0.44 |
| L. EtOAc | 30.50 | 0.29 | 0.64 |
| L. aqu | 24.67 | 0.17 | 0.52 |
| L. H2O* | 26.67 | 0.17 | 0.56 |
| L. hex | NA | ||
| L. Me* | 20.00 | 0.29 | 0.42 |
| L. acetone | 19.94 | 0.29 | 0.42 |
| L. Dic | NT | ||
| L. MeSox | 24.17 | 0.17 | 0.51 |
| L. HH2O | 20.33 | 0.33 | 0.43 |
| Rifampicin | 47.5 | 0.29 | 1.00 |
| Methanol | NA | ||
| Hexane | NA |
| A. leiocarpa Crude Extracts and Fractions | Total Activity (in mL/g) | MIC (in µg/mL) |
|---|---|---|
| W. MeSox | 40.2 | 5000 ** |
| W. Me* | 4.2 | 5000 |
| B. EtOAc | 21.6 | 2500 ** |
| B. aqu | 16 | 5000 |
| B. HH2O | 13.6 | 5000 |
| R. MeSox and its preparativereversed phase 18(RP18)-thin layer chromatography(TLC) fractions (Fr3-Fr8) | 43.2 | 2500 |
| Fr 3 (Rf 0.095) | 1500 (IC 89) ** | |
| Fr 4 (Rf 0.159) | 1000 (IC 89) ** | |
| Fr 6 (Rf 0.276) | 2000 (IC 91) ** | |
| Fr 8 (Rf 0.457) | 3000 (IC 90) ** | |
| R. Me* | 5.6 | 5000 |
| R. EtOAc | 7.04 | 625 ** |
| L. MeSox | 101.6 | 5000 |
| L. HH2O | 14.8 | 5000 |
| L. H2O* | 28.4 | 2500 |
| L. EtOAc | 38 | 2500 |
| Pure compounds known to be present in A. leiocarpa | ||
| Gallic acid | 500 (IC 98) ** | |
| Quercetin | 250 (IC 94) ** | |
| Apigenin | 250 (IC 97) ** | |
| Corilagin | 1000 (IC 94) ** | |
| Ellagic acid | 500 (IC 98) ** | |
| Rifampicin | 39.06 µg/mL (3.90 µg/mL, IC 98) ** | |
| RP18-Thin Layer Chromatography Fractions and Their Compounds | Molecular Formula | Rt HPLC-DAD | Rt LC-MS | [M-H]− | Exact Calculated MW | UVλ max (HPLC-DAD) | Peak Area % | Distance Moved in cm on TLC Plate | RP18 TLC Rf Value | DPPH Reactive |
|---|---|---|---|---|---|---|---|---|---|---|
| Fr3 MeSox | 1.2 | 0.0945 | Yes | |||||||
| Ellagic acid derivative | 20.04 | 254, 366 | 2.71 | |||||||
| Di-methyl ellagic acid xyloside (18) | C21H18O12 | 23.47 | 9.65 | 461.0739 | 462.0739 | 246, 376 | 4.93 | |||
| Di-methyl-ellagic acid (21) | C16H10O8 | 28.09 | 11.25 | 329.0318 | 330.0318 | 246, 380 | 38.43 | |||
| Ellagic acid derivative | 29.94 | 248, 374 | 8.05 | |||||||
| Unknown | 33.06 | 216, 248, 374 | 4.31 | |||||||
| Acetylated ellagic acid derivative (24) | 35.08 | 12.80 | 343.0477 | 344.0477 | 222, 246, 370 | 5.99 | ||||
| Fr4 MeSox | 1.9 | 0.1496 | Yes | |||||||
| Protocatechuic acid (2) | C7H6O4 | 3.69 | 1.34 | 153.0196 | 154.0196 | 218, 220, 260, 294 | 1.5 | |||
| Ellagic acid derivative | 18.12 | 246, 370 | 1.63 | |||||||
| Ellagic acid derivative | 18.90 | 210, 254, 362 | 1.7 | |||||||
| Ellagic acid derivative | 19.98 | 210, 254, 368 | 19.12 | |||||||
| Di-methyl ellagic acid xyloside (18) | C21H18O12 | 23.52 | 246, 376 | 9.09 | ||||||
| Di-methyl ellagic acid (21) | C16H10O8 | 28.07 | 11.25 | 329.0318 | 330.0318 | 246, 380 | 21.92 | |||
| Ellagic acid derivative | 35.08 | 246, 382 | 3.18 | |||||||
| Fr6 MeSox | 3.5 | 0.2756 | Yes | |||||||
| Unknown flavonoid (9a) | 14.66 | 232, 276, 350 | 2.31 | |||||||
| Ellagic acid derivative | 17.54 | 254, 378 | 9.84 | |||||||
| Ellagic acid derivative | 18.12 | 246, 370 | 13.41 | |||||||
| Unknown | 18.93 | 254, 360 | 7.77 | |||||||
| Ellagic acid derivatives | 19.99 | 254, 362 | 22.09 | |||||||
| ellagitannin (unknown) | 23.64 | 220, 248, 378 | 2.92 | |||||||
| Di-methyl ellagic acid (21) | C16H10O8 | 29.01 | 11.25 | 329.0318 | 330.0318 | 248, 376 | 3.99 | |||
| Fr8 MeSox | 5.8 | 0.4567 | Yes | |||||||
| Unknown | 5.057 | 242 | 3.65 | |||||||
| Unknown | 13.07 | 210, 230, 282 | 3.99 | |||||||
| Ellagic acid derivative | 17.52 | 254, 384 | 3.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, E.Y.A.; Julkunen-Tiitto, R.; Luukkanen, O.; Sipi, M.; Fahmi, M.K.M.; Fyhrquist, P.J. Potential Anti-Tuberculosis Activity of the Extracts and Their Active Components of Anogeissus leiocarpa (DC.) Guill. and Perr. with Special Emphasis on Polyphenols. Antibiotics 2020, 9, 364. https://doi.org/10.3390/antibiotics9070364
Salih EYA, Julkunen-Tiitto R, Luukkanen O, Sipi M, Fahmi MKM, Fyhrquist PJ. Potential Anti-Tuberculosis Activity of the Extracts and Their Active Components of Anogeissus leiocarpa (DC.) Guill. and Perr. with Special Emphasis on Polyphenols. Antibiotics. 2020; 9(7):364. https://doi.org/10.3390/antibiotics9070364
Chicago/Turabian StyleSalih, Enass Y. A., Riitta Julkunen-Tiitto, Olavi Luukkanen, Marketta Sipi, Mustafa K. M. Fahmi, and Pia Johanna Fyhrquist. 2020. "Potential Anti-Tuberculosis Activity of the Extracts and Their Active Components of Anogeissus leiocarpa (DC.) Guill. and Perr. with Special Emphasis on Polyphenols" Antibiotics 9, no. 7: 364. https://doi.org/10.3390/antibiotics9070364
APA StyleSalih, E. Y. A., Julkunen-Tiitto, R., Luukkanen, O., Sipi, M., Fahmi, M. K. M., & Fyhrquist, P. J. (2020). Potential Anti-Tuberculosis Activity of the Extracts and Their Active Components of Anogeissus leiocarpa (DC.) Guill. and Perr. with Special Emphasis on Polyphenols. Antibiotics, 9(7), 364. https://doi.org/10.3390/antibiotics9070364




