Novel Antiviral and Antibacterial Activities of Hibiscus schizopetalus
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity Assay
2.2. Antiviral Activity
2.3. Activity against Helicobacter Pylori
2.4. Anti-Mycobacterial Activity
2.5. Activity against Methicillin-Resistant Staphylococcus aureus (MRSA)
2.6. Antioxidant Activity
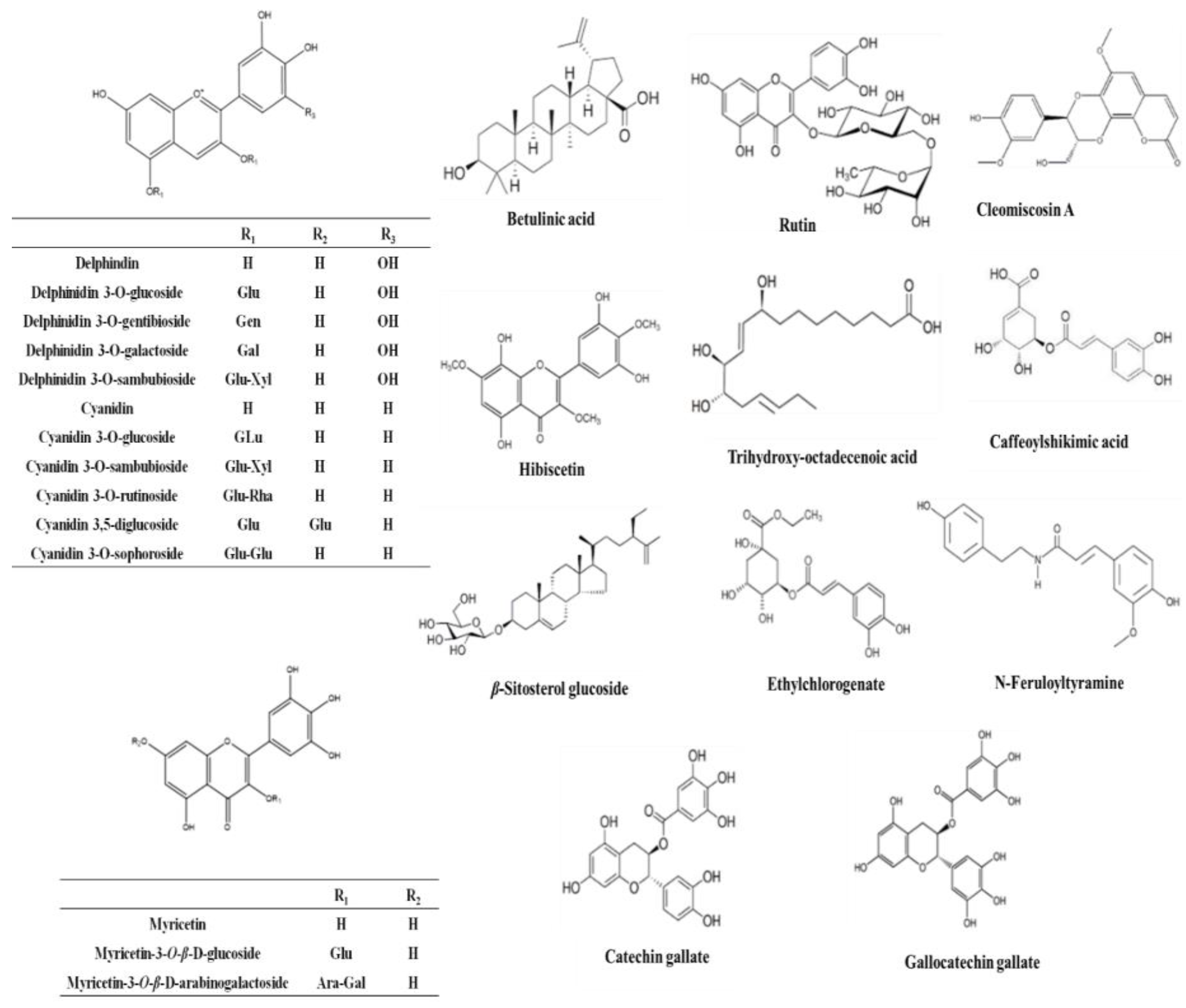
2.7. HPLC–HR–ESI–MS
3. Discussion
4. Materials and Methods
4.1. Plant Material and Chemicals
4.2. Extraction and Fractionation
4.3. Cytotoxicity Assay
4.4. Cell Culture and Virus
4.5. Antiviral Activity
4.6. Activity against Helicobacter pylori
4.7. Anti-Mycobacterial Activity
4.8. Activity against Methicillin-Resistant Staphylococcus aureus (MRSA)
4.9. Antioxidant Activity
- A.
- Total antioxidant capacity assay (TAC).
- B.
- 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical scavenging assay.
- C.
- 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging assay.
- D.
- Reducing power assay.
4.10. HPLC–HR–ESI–MS
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.; Wong, S.; Chan, H. A review on the phytochemistry and pharmacology of two Hibiscus species with spectacular flower colour change: H. tiliaceus and H. mutabilis. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1200–1208. [Google Scholar]
- Abdelhafez, O.H.; Othman, E.M.; Fahim, J.R.; Desoukey, S.Y.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Schirmeister, T.; Tawfike, A.; Abdelmohsen, U.R. Metabolomics analysis and biological investigation of three Malvaceae plants. Phytochem. Anal. 2020, 31, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Sarkar, J.; Bhattacharya, S.; Biswas, M. Thi layer chromatographic studies ad assessme t of a ti-i flammatory effect of Hibiscus schizopetalus leaf extracts in rats. Pharmacologyonline 2011, 2, 1431–1436. [Google Scholar]
- Wong, S.K.; Lim, Y.Y.; Chan, E.W.C. Antioxidant properties of Hibiscus: Species variation, altitudinal change, coastal influence and floral colour change. J. Trop. For. Sci. 2009, 21, 307–315. [Google Scholar]
- Zahid, H.; Rizwani, G.H. Antimicribial efficacy of Hibiscus schizopetalus (Mast) Hook. Hamdard Med. 2016, 59, 4. [Google Scholar]
- Wong, S.; Chan, E.W.; Chan, H. A review on the phytochemistry and pharmacology of two lesser-known Hibiscus species: H. taiwanensis and H. schizopetalus. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1341–1346. [Google Scholar]
- Jose, E.; Vijayan, K. New taraxerane esters from Hibiscus schizopetalus leaves. Indian J. Chem. 2006, 45B, 1328–1331. [Google Scholar] [CrossRef]
- Hayati, Z.; Yulia, W.; Karmil, T.F.; Azmy, A. Anti-bacterial activity of rosella flowers extract (Hibiscus sabdariffa linn) in inhibiting bacterial growth methicillin-resistant Staphylococcus aureus. In Proceedings of the Annual International Conference, Syiah Kuala University-Life Sciences & Engineering Chapter, Banda Aceh, Indonesia, 22–24 November 2012. [Google Scholar]
- Arullappan, S.; Zakaria, Z.; Basri, D.F. Preliminary screening of antibacterial activity using crude extracts of Hibiscus rosa sinensis. Trop. Life Sci. Res. 2009, 20, 109. [Google Scholar]
- Pour, P.M.; Fakhri, S.; Asgary, S.; Farzaei, M.H.; Echeverria, J. The signaling pathways, and therapeutic targets of antiviral agents: Focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Ito, T.; Masubuchi, M. Dereplication of microbial extracts and related analytical technologies. J. Antibiot. 2014, 67, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Michel, H.E.; Ezzat, M.I.; Okba, M.M.; L-Desoky, A.M.E.; Mohamed, S.O.; Ezzat, S.M. Optimization of an Extraction solvent for angiotensin-converting enzyme inhibitors from hibiscus sabdariffa l. based on its UPLC-MS/MS metabolic profiling. Molecules 2020, 25, 2307. [Google Scholar] [CrossRef] [PubMed]
- Obouayeba, A.; Djyh, N.; Diabate, S.; Djaman, A.; N’guessan, J.; Kone, M.; Kouakou, T. Phytochemical and antioxidant activity of Roselle (Hibiscus sabdariffa L.) petal extracts. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1453–1465. [Google Scholar]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; el Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.-A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Sharma, M.C.; Smita, S.; Kohli, D.V. Phytochemical and anti-ulcer investigations of 95% ethanolic-benzene-chloroform leaf extract of Hibiscus tiliaceus Linn. in Albino rat model. Ann. Biol. Res. 2010, 1, 15–20. [Google Scholar]
- Barve, V.H.; Hiremath, S.; Pattan, S.; Pal, S. Phytochemical and pharmacological evaluation of Hibiscus mutabilis leaves. J. Chem. Pharm. Res. 2010, 2, 300–309. [Google Scholar]
- Yun, B.-S.; Lee, I.-K.; Ryoo, I.-J.; Yoo, I.-D. Coumarins with monoamine oxidase inhibitory activity and antioxidative coumarino-lignans from hibiscus s yriacus. J. Nat. Prod. 2001, 64, 1238–1240. [Google Scholar] [CrossRef]
- Salem, M.Z.; Olivares-Pérez, J.; Salem, A. Studies on biological activities and phytochemicals composition of Hibiscus species—A review. Life Sci. J. 2014, 11, 1–8. [Google Scholar]
- Torky, Z.A.; Hossain, M.M. Pharmacological evaluation of the hibiscus herbal extract against herpes simplex virus-type 1 as an antiviral drug in vitro. Int. J. Virol. 2017, 13, 68–79. [Google Scholar] [CrossRef]
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T.; Siristova, L.; Sigler, K. Sterols and triterpenoids with antiviral activity. Anti-Infect. Agents Med. Chem. (Former. Curr. Med. Chem.—Anti-Infect. Agents) 2009, 8, 193–210. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral activities of oleanolic acid and its analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef]
- Librán-Pérez, M.; Pereiro, P.; Figueras, A.; Novoa, B. Antiviral activity of palmitic acid via autophagic flux inhibition in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 95, 595–605. [Google Scholar] [CrossRef]
- Parvez, M.K.; Alam, P.; Arbab, A.H.; Al-Dosari, M.S.; Alhowiriny, T.A.; Alqasoumi, S.I. Analysis of antioxidative and antiviral biomarkers β-amyrin, β-sitosterol, lupeol, ursolic acid in Guiera senegalensis leaves extract by validated HPTLC methods. Saudi Pharm. J. 2018, 26, 685–693. [Google Scholar] [CrossRef]
- Islam, M.T.; Sarkar, C.; El-Kersh, D.M.; Jamaddar, S.; Uddin, S.J.; Shilpi, J.A.; Mubarak, M.S. Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother. Res. 2020, 34, 2471–2492. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Suttisri, R.; Pengsuparp, T.; Ruangrungsi, N. Chemical structure and antiviral activity of aerial part from Laggera pterodonta. J. Health Res. 2009, 23, 175–177. [Google Scholar]
- Masullo, M.; Pizza, C.; Piacente, S. Oleanane derivatives for pharmaceutical use: A patent review (2000–2016). Expert Opin. Ther. Pat. 2017, 27, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Kiyohara, H.; Munakata, K.; Shirahata, T.; Sunazuka, T.; Harigaya, Y.; Yamada, H. Pinellic acid from the tuber of Pinellia ternata Breitenbach as an effective oral adjuvant for nasal influenza vaccine. Int. Immunopharmacol. 2002, 2, 1183–1193. [Google Scholar] [CrossRef]
- Yadav, D.K.; Meena, A.; Srivastava, A.; Chanda, D.; Khan, F.; Chattopadhyay, S. Development of QSAR model for immunomodulatory activity of natural coumarinolignoids. Drug Des. Dev. Ther. 2010, 4, 173. [Google Scholar]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.; Berchová, K.; Majerová, M.; Pokorná, M.; Švajdlenka, E. In vitro synergistic effect of Hibiscus sabdariffa aqueous extract in combination with standard antibiotics against Helicobacter pylori clinical isolates. Pharm. Biol. 2016, 54, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Donkor, S.; Larbie, C.; Komlaga, G.; Emikpe, B.O. Phytochemical, antimicrobial, and antioxidant profiles of Duranta erecta L. parts. Biochem. Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.; Rizwani, G.H.; Shareef, H.; Ali, S.T. Antioxidant and urease inhibition activity of methanol extract of Hibiscus schizopetalus (Mast) Hook. J. Pharmacogn. Phytochem. 2014, 2, 7–11. [Google Scholar]
- François-Haugrin, F.; Monan, M.; Nossin, E.; Smith-Ravin, J.; Marcelin, O. Antioxidant activity of an isomer of gossypitrin (gossypetin-3’-O-glucoside) isolated in the petals of Talipariti elatum Sw., and determination of total phenolic content of the total flower. J. Pharmacogn. Phytochem. 2016, 5, 200. [Google Scholar]
- Masheta, D.Q.; Al-Azzawi, S.K. Antioxidant and Anti-Inflammatory Effects of Delphinidin on Glial Cells and Lack of Effect on Secretase Enzyme. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Elaissi, A.; Rouis, Z.; Salem, N.A.B.; Mabrouk, S.; ben Salem, Y.; Salah, K.B.H.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Chemical composition of 8 Eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. Med. 2012, 12, 81. [Google Scholar] [CrossRef]
- Takeuchi, H.; Baba, M.; Shigeta, S. An application of tetrazolium (MTT) colorimetric assay for the screening of anti-herpes simplex virus compounds. J. Virol. Methods 1991, 33, 61–71. [Google Scholar] [CrossRef]
- Goodger, J.Q.; Woodrow, I.E. α, β-Unsaturated monoterpene acid glucose esters: Structural diversity, bioactivities and functional roles. Phytochemistry 2011, 72, 2259–2266. [Google Scholar] [CrossRef]
- Gong, E.Y. (Ed.) Antiviral Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ocazionez, R.E.; Meneses, R.; Torres, F.Á.; Stashenko, E. Virucidal activity of Colombian Lippia essential oils on dengue virus replication in vitro. Memórias Inst. Oswaldo Cruz 2010, 105, 304–309. [Google Scholar] [CrossRef]
- Gescher, K.; Kühn, J.; Hafezi, W.; Louis, A.; Derksen, A.; Deters, A.; Lorentzen, E.; Hensel, A. Inhibition of viral adsorption and penetration by an aqueous extract from Rhododendron ferrugineum L. as antiviral principle against herpes simplex virus type-1. Fitoterapia 2011, 82, 408–413. [Google Scholar] [CrossRef]
- Okba, M.M.; el Gedaily, R.A.; Ashour, R.M. UPLC–PDA–ESI–qTOF-MS profiling and potent anti-HSV-II activity of Eucalyptus sideroxylon leaves. J. Chromatogr. B 2017, 1068, 335–342. [Google Scholar] [CrossRef]
- Bonacorsi, C.; Raddi, M.S.G.; Carlos, I.Z.; Sannomiya, M.; Vilegas, W. Anti-Helicobacter pylori activity and immunostimulatory effect of extracts from Byrsonima crassa Nied (Malpighiaceae). BMC Complement. Altern. Med. 2009, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zheng, M.; Wang, B.; Fu, L.; Zhao, W.; Li, P.; Xu, J.; Zhu, H.; Jin, H.; Yin, D. Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation. Antimicrob. Agents Chemother. 2011, 55, 5185–5193. [Google Scholar] [CrossRef] [PubMed]
- Tunney, M.M.; Ramage, G.; Field, T.R.; Moriarty, T.F.; Storey, D.G. Rapid colorimetric assay for antimicrobial susceptibility testing of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2004, 48, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharmacother. 2020, 128, 110303. [Google Scholar] [CrossRef]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Atik-Bekkara, F. Evaluation of antioxidant activity of hydromethanolic extracts of some medicinal species from South Algeria. J. Chin. Med. Assoc. 2014, 77, 302–307. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef]
- Gamaleldin, N.M.; Bakeer, W.; Sayed, A.M.; Shamikh, Y.I.; El-Gendy, A.O.; Hassan, H.M.; Horn, H.; Abdelmohsen, U.R.; Hozzein, W.N. Exploration of chemical diversity and antitrypanosomal activity of some red sea-derived actinomycetes using the OSMAC approach supported by LC-MS-based metabolomics and molecular modelling. Antibiotics 2020, 9, 629. [Google Scholar] [CrossRef]
- Tawfike, A.; Attia, E.Z.; Desoukey, S.Y.; Hajjar, D.; Makki, A.A.; Schupp, P.J.; Edrada-Ebel, R.; Abdelmohsen, U.R. New bioactive metabolites from the elicited marine sponge-derived bacterium Actinokineospora spheciospongiae sp. nov. AMB Express 2019, 9, 12. [Google Scholar] [CrossRef]
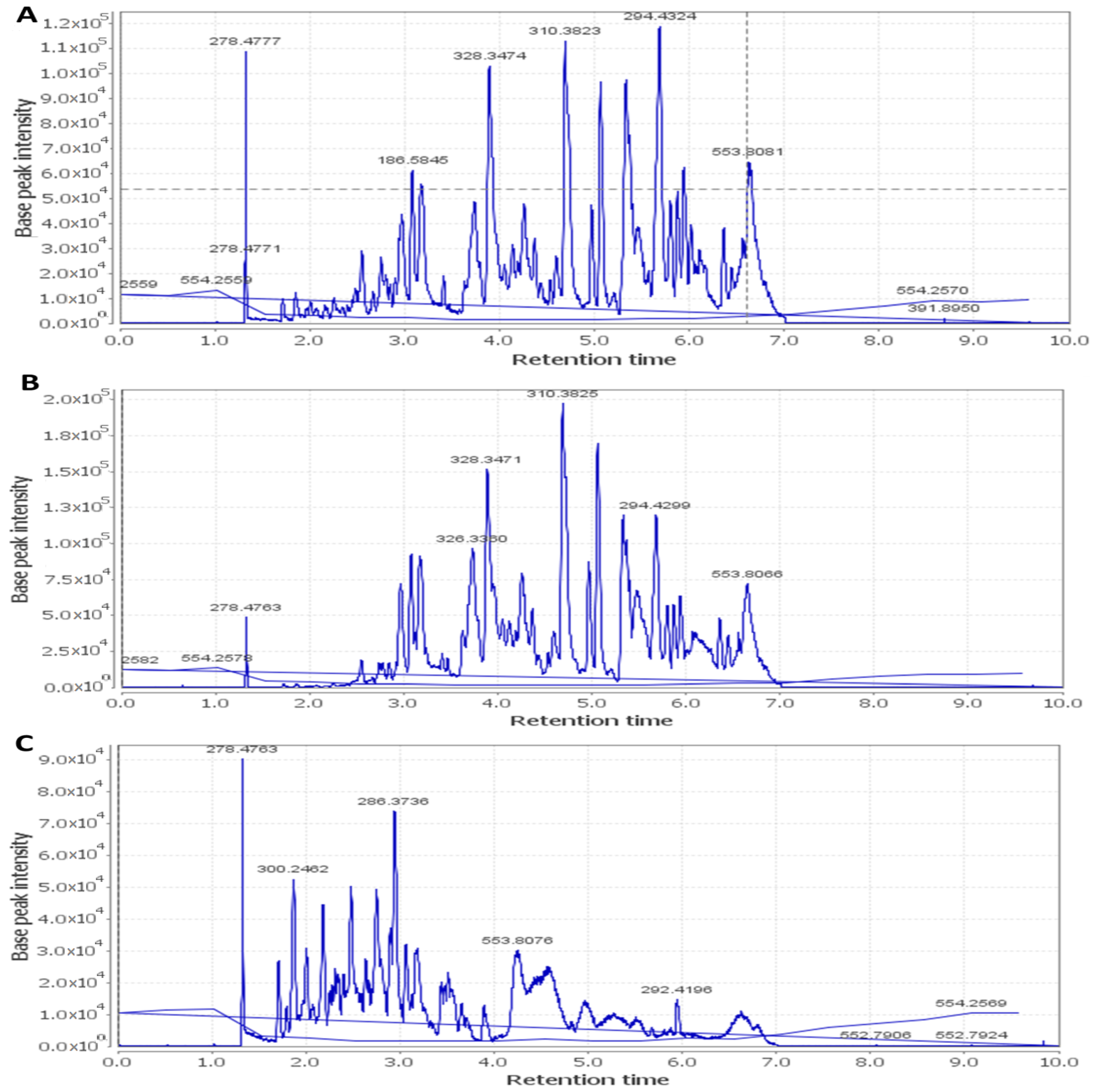
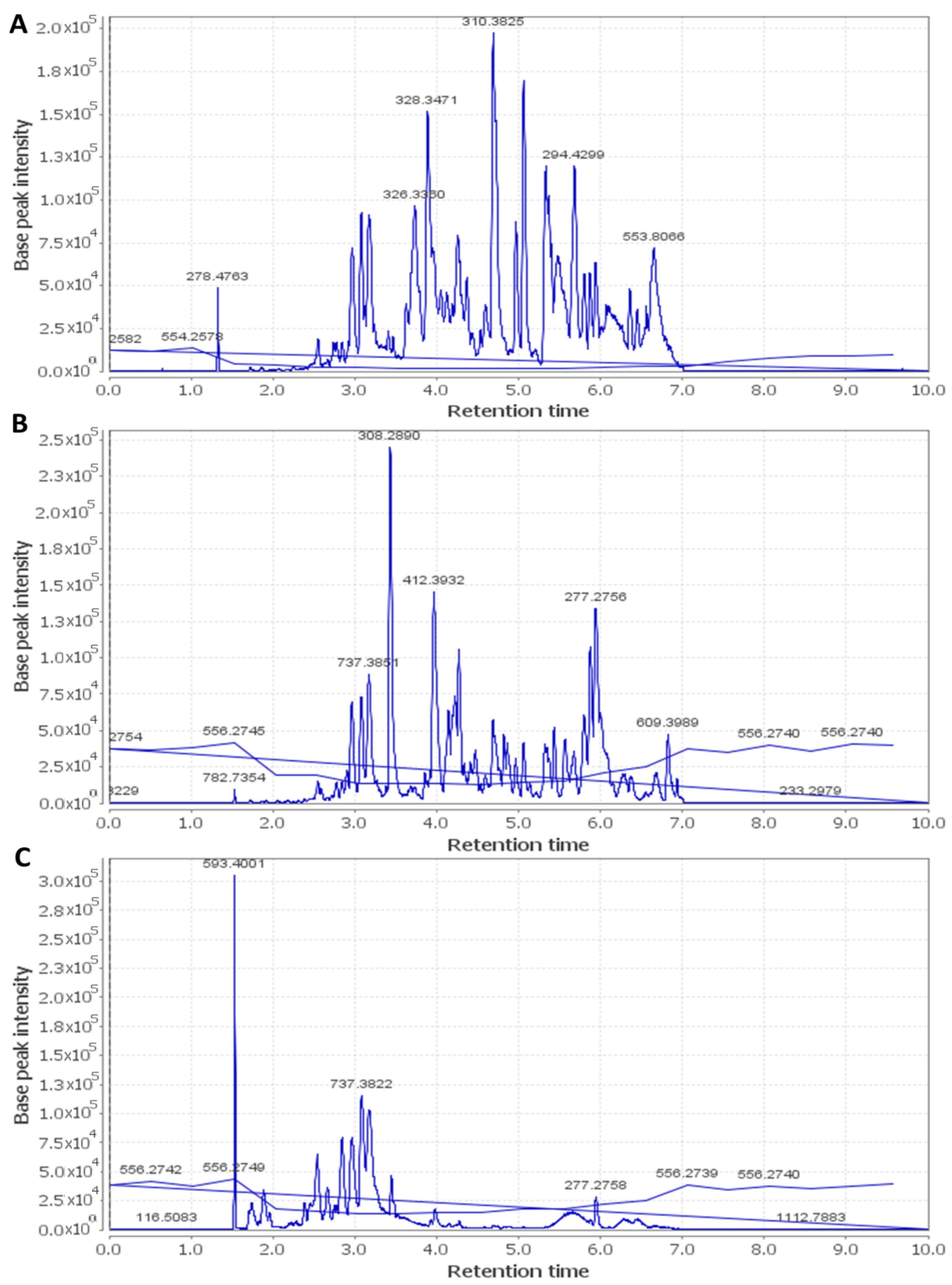
| Name of Virus | Protocol A | Protocol B | Protocol C | |||
|---|---|---|---|---|---|---|
| Adenovirus | Et-E | 39.66 | Et-E | 58.40 | Et-E | 72.94 c |
| DCM-F | 78.78 d | |||||
| Bu-F | 33.41 a | |||||
| Acyclovir | 41.95 | Acyclovir | 59.90 | Acyclovir | 61.35 b | |
| CoxB4 | Et-E | 33.25 | Et-E | 22.50 | Et-E | 54.24 b |
| DCM-F | 60.90 c | |||||
| Bu-F | 33.96 a | |||||
| Acyclovir | 41.50 | Acyclovir | 36.32 | Acyclovir | 53.90 b | |
| HSV I | Et-E | 47.57 | Et-E | 25.90 | Et-E | 62.67 a |
| DCM-F | 91.80 c | |||||
| Bu-F | 80.79 b | |||||
| Acyclovir | 75.20 | Acyclovir | 42.33 | Acyclovir | 95.48 c | |
| HSV II | Et-E | 9.49 | Et-E | 27.79 | Et-E | 30.84 b |
| DCM-F | 51.75 c | |||||
| Bu-F | 1.78 a | |||||
| Acyclovir | 23.05 | Acyclovir | 46.44 | Acyclovir | 55.69 d | |
| HAV | Et-E | 0.55 | Et-E | 0 | Et-E | 12.10 b |
| DCM-F | 18.28 c | |||||
| Bu-F | 5.60 a | |||||
| Acyclovir | 21.60 | Acyclovir | 0 | Acyclovir | 30.47 d | |
| Virus | Parameter | Sample | Standard | ||
|---|---|---|---|---|---|
| Et-E | DCM-F | Bu-F | Acyclovir | ||
| Adenovirus | IC50 | 58.89 b | 54.88 a | 108.31 d | 91.92 c |
| SI | 3.8 | 5.93 | 2.66 | 8.34 | |
| CoxB4 | IC50 | 72.0 b | 64.13 a | 107.30 c | 72.79 b |
| SI | 3.20 | 5.08 | 2.68 | 10.53 | |
| HSV I | IC50 | 66.86 d | 29.85 b | 50.94 c | 26.99 a |
| SI | 3.45 | 10.91 | 5.66 | 28.42 | |
| HSVII | IC50 | 126.65 c | 74.17 b | - | 68.60 a |
| SI | 1.82 | 4.39 | 11.18 | ||
| HAV | IC50 | - | 168.67 b | - | 95.40 a |
| SI | 1.93 | 8.04 | |||
| Sample/Standard | Helicobacter pylori | Mycobacterium tuberculosis | MRSA | |||
|---|---|---|---|---|---|---|
| MIC90 | MIC | MIC90 | MIC | MIC90 | MIC | |
| Et-E | 22.7 c | 62.5 c | 98.6 d | 125 d | 17.56 b | 31.25 c |
| DCM-F | 2.9 a | 3.9 a | 12.3 c | 15.63 c | 44.4 c | 62.5 d |
| Bu-F | 13.7 b | 15.63 b | 5.6 b | 7.81 b | 3.7 a | 7.81 b |
| Clarithromycin | 0.7 a | 1.95 a | - | - | - | - |
| Isoniazid | - | - | 0.04 a | 0.24 a | - | - |
| Vancomycin | - | - | - | - | 2.23 a | 3.9 a |
| Sample/Standard | TAC% | ABTS | DPPH | Iron Reducing Power |
|---|---|---|---|---|
| (AAE) | IC50 (µg/mL) | EC50 (µg/mL) | ||
| Et-E | 1850.83 b | 710.51 c | 473.29 c | 391.45 c |
| DCM-F | 800.16 c | 819.51 d | 1118.92 d | 528.0 d |
| Bu-F | 2050.50 a | 151.48 b | 271.68 b | 114.47 b |
| Ascorbic acid | - | 57.76 a | 19.50 a | 22 a |
| Metabolite | Molecular Formula | Exact Mass | Et-E | DCM-F | Bu-F |
|---|---|---|---|---|---|
| Anthocyanins | |||||
| Cyanidin | C15H10O6 | 286.177914 | + | ++ | ++ |
| Cyanidin O-rhamnoside | C21H20O10 | 432.385735 | + | +++ | ++ |
| Cyanidin O-galactoside/Cyanidin O-glucoside | C21H20O11 | 448.956000 | +++ | ++ | ++ |
| Cyanidin 3-sambubioside | C26H28O15 | 580.761473 | + | +++ | + |
| Cyanidin O-rutinoside | C27H30O15 | 594.399238 | + | + | +++ |
| Cyanidin di-O-glucoside | C27H30O16 | 610.308112 | + | ++ | + |
| Cyanidin 3-(digalloylglucoside) | C35H28O19 | 752.646798 | + | ++ | + |
| Cyanidin 3-(acetylgalloylgalactoside) | C30H26O16 | 642.403723 | + | +++ | + |
| Gossypetin | C15H10O8 | 318.287501 | + | +++ | + |
| Cyanidin 3-(O-succinoyl-glucopyranoside) | C25H24O14 | 548.577150 | + | ++ | + |
| Cyanidin 3-(oxalylglucoside) | C23H20O14 | 520.445023 | + | +++ | + |
| Cyanidin 3-(cinnamoylglucoside) | C30H26O12 | 578.566536 | + | +++ | + |
| Cyanidin 3-(malonylxyloside) | C23H20O13 | 504.916039 | + | +++ | + |
| Cyanidin 3-(xylosylarabinoside) | C25H26O14 | 550.464496 | + | +++ | + |
| Gossypetin glucoside (Gossypitrin)/Myricetin-O-glucoside | C21H20O13 | 480.861466 | + | + | +++ |
| Gossypetin glucuronide | C21H18O14 | 494.876248 | + | + | +++ |
| Delphinidin | C15H10O7 | 302.192080 | + | + | +++ |
| Delphinidin galactoside/Delphinidin glucoside | C21H20O12 | 464.370561 | + | + | +++ |
| Delphinidin 3-sambubioside (Hibiscin) | C26H28O16 | 596.714621 | ++ | ++ | +++ |
| Delphinidin neohesperidoside/Delphinidin rutinoside | C27H30O16 | 610.371790 | + | + | +++ |
| Dimethyl-delphinidin-glucosyl acetate | C25H26O13 | 534.494206 | + | +++ | + |
| Delphinidin 3-gentiobioside | C27H29O17 | 626.404530 | + | +++ | + |
| Hibicuslide C | C13H12O4 | 232.493625 | + | + | + |
| Hibiscetin | C15H10O9 | 334.283647 | + | ++ | + |
| Hibiscetin glucoside | C21H20O14 | 496.438349 | + | +++ | + |
| Hibiscus lactone (Hydroxycitric acid lactone) | C6H6O7 | 190.177052 | + | +++ | + |
| Hibiscus acid hydroxyethyldimethylether | C10H16O8 | 264.266440 | + | + | +++ |
| Hibiscus acid hydroxyethylether | C8H12O8 | 236.156126 | + | + | +++ |
| Malvidin | C17H14O7 | 330.249624 | + | +++ | + |
| Malvidin O-(coumaroylglucoside) | C32H30O14 | 638.416871 | + | + | +++ |
| Petunidin | C16H12O7 | 316.269633 | + | +++ | + |
| Pelargonidin | C15H10O5 | 270.221390 | + | ++ | + |
| Pelargonidin glucoside | C21H20O10 | 432.385735 | + | +++ | + |
| Flavonoids/Phenolic acids | |||||
| Kaempferol | C15H10O6 | 286.194597 | + | + | + |
| Kaempferol rhamnoside | C22H22O10 | 446.215495 | + | - | +++ |
| Kaempferol O-glucuronide | C21H18O12 | 462.214878 | + | - | +++ |
| Kaempferol-3-O-glucuronic acid methyl ether | C18H18O14 | 476.012800 | + | + | + |
| Kaempferol O-rutinoside | C27H30O15 | 594.284269 | + | - | +++ |
| Kaempferitrin | C27H30O14 | 578.749072 | + | - | +++ |
| Scutellarein rhamnopyranoside glucopyranoside | C27H30O15 | 594.284027 | + | - | +++ |
| Myricetin | C15H10O8 | 318.287992 | + | +++ | + |
| Myricetin O-arabinogalactoside | C26H28O17 | 612.594615 | + | + | +++ |
| Quercetin | C15H10O7 | 302.289996 | + | +++ | + |
| Quercitrin/Kaempferol glucoside | C21H20O11 | 448.956137 | + | ++ | +++ |
| Rutin | C27H30O16 | 610.658348 | + | - | +++ |
| Apigenin | C15H10O5 | 270.221390 | + | +++ | + |
| Apigenin-O-acetylxyloside | C23H22O11 | 474.215366 | + | - | +++ |
| O-Caffeoyl-hydroxycitric acid | C15H14O11 | 370.137822 | + | - | +++ |
| O-Caffeoylshikimic acid | C16H16O8 | 336.317691 | + | +++ | + |
| Catechin gallate | C22H18O10 | 442.440382 | + | +++ | + |
| Gallocatechin gallate | C22H18O11 | 458.434848 | + | +++ | + |
| Ethylchlorogenate | C18H22O9 | 382.143465 | + | +++ | + |
| Trimethylhydroxycitric acid | C9H14O8 | 250.149487 | + | - | +++ |
| Coumaroylquinic acid | C16H18O8 | 338.281091 | + | - | +++ |
| N-Feruloyltyramine | C18H19NO4 | 312.252784 | + | + | +++ |
| Cleomiscosin A | C20H18O8 | 386.267194 | + | +++ | - |
| Cleomiscosin C/D | C21H20O9 | 416.424465 | + | +++ | - |
| Sterols/Terpenes/Fatty acids | |||||
| β-Sitosterol | C29H50O | 414.405049 | + | +++ | - |
| β-Sitosterol glucoside | C35H60O6 | 576.740355 | + | +++ | - |
| Hydroxy taraxeryl acetate | C32H52O3 | 484.950167 | + | +++ | - |
| Betulinic acid/Oleanolic acid | C30H48O3 | 456.920845 | + | +++ | - |
| Mansonone H | C15H14O4 | 258.253920 | + | +++ | - |
| Palmitic acid | C16H32O2 | 256.1772030 | + | +++ | - |
| Linolenic acid | C18H30O2 | 278.474709 | + | +++ | - |
| Linoleic acid | C18H32O2 | 280.3010786 | + | +++ | - |
| Trihydroxy-octadecenoic acid | C18H34O5 | 330.3540819 | + | +++ | - |
| Stearic acid | C18H36O2 | 284.3896759 | + | +++ | - |
| Docosanoic acid | C22H44O2 | 340.35180228 | + | +++ | - |
| Docosanedione | C22H42O2 | 338.31881164 | + | +++ | - |
| Methyldocosadienoic acid | C23H42O2 | 350.31891388 | + | +++ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shiekh, R.A.; Abdelmohsen, U.R.; Ashour, H.M.; Ashour, R.M. Novel Antiviral and Antibacterial Activities of Hibiscus schizopetalus. Antibiotics 2020, 9, 756. https://doi.org/10.3390/antibiotics9110756
El-Shiekh RA, Abdelmohsen UR, Ashour HM, Ashour RM. Novel Antiviral and Antibacterial Activities of Hibiscus schizopetalus. Antibiotics. 2020; 9(11):756. https://doi.org/10.3390/antibiotics9110756
Chicago/Turabian StyleEl-Shiekh, Riham A., Usama Ramadan Abdelmohsen, Hossam M. Ashour, and Rehab M. Ashour. 2020. "Novel Antiviral and Antibacterial Activities of Hibiscus schizopetalus" Antibiotics 9, no. 11: 756. https://doi.org/10.3390/antibiotics9110756
APA StyleEl-Shiekh, R. A., Abdelmohsen, U. R., Ashour, H. M., & Ashour, R. M. (2020). Novel Antiviral and Antibacterial Activities of Hibiscus schizopetalus. Antibiotics, 9(11), 756. https://doi.org/10.3390/antibiotics9110756







