The search for new plant-derived compounds with bioactive properties is crucial. Thus, and considering the potential effects of Ayahuasca, in this work, the biological activities of the plant materials used in the preparation of this beverage, namely antioxidant, anti-inflammatory and antimicrobial properties, were evaluated. In this sense, four decoctions of Ayahuasca were prepared (with two different plant materials, one source of DMT and another of β-carboline alkaloids). Besides, individual decoctions of each plant used in the preparation of Ayahuasca were also prepared. In addition, a commercial mixture was also purchased and evaluated.
2.1. Phytochemical Characterization and Phenolic Profile
Ayahuasca has been shown to have important beneficial health effects [
12,
17,
29,
30,
31,
32,
33,
34,
35]. Considering that there is a small number of phytochemical studies of the decoctions prepared from the plants
P. viridis,
B. caapi,
P. harmala and
M. hostilis, in the present study the determination of the content of flavonoids and total phenolics in these samples was performed. Additionally, the phenolic profile of the samples was determined by ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC/ESI-QTOF-MS).
Phenolic compounds are among the main secondary metabolites of plants, and it is possible to find some of them in all plants [
36,
37,
38]. These compounds have some interesting properties from a clinical point of view [
37,
39]. In the present study, the total phenolics were determined using the Folin-Ciocalteu colorimetric method (
Table 1). Although some studies describe limitations of the Folin-Ciocalteu colorimetric method, it remains widely used for the determination of total phenolics [
40]. All samples showed substantial concentrations of total phenolics, with
M. hostilis, commercial mixture and the mixture of
M. hostilis and
B. caapi showing the highest concentrations, and
P. harmala the lowest content of total phenolics. Recently, Hadadi et al. [
41], developed a study where determined the content of total phenols, using the same method, having also verified the presence of these compounds in extracts of
P. harmala.
Flavonoids have beneficial biological activities, namely anti-inflammatory, antimicrobial, antioxidant, cytotoxic and anti-tumor activities [
42,
43]. In the present study, flavonoids were determined by the aluminum chloride colorimetric method (
Table 1). All samples were found to have flavonoids in their composition, with
P. harmala and the mixture of
P. viridis and
P. harmala the samples having the highest flavonoid content. Contrariwise, the samples of
B. caapi and the mixture of
B. caapi and
M. hostilis showed the lowest levels of flavonoids.
The samples were then analyzed by liquid chromatography with high resolution mass spectrometric detection in order to identify the compounds and to further complement the initial phytochemical characterization. Thus, the identification of compounds was carried out by comparing their retention times and accurate mass spectra provided by the UHPLC-QTOF-MS with those of authentic standards when available. The phytochemical library of 48 standards (
Appendix A Figure A1) was used to characterize the metabolites present in the methanolic extracts. Thus, it was possible to verify that two groups of compounds were mainly present: hydroxybenzoic acids and flavonoids. The concentration of the identified compounds was estimated by comparing their peak areas in the chromatograms from the plant extracts with those of the corresponding standard solutions freshly prepared and analyzed by duplicate in the same batch as samples. The results are shown in
Table 2. In the sample of
P. viridis it was possible to quantify two hydroxybenzoic acids (protocatechuic acid, 4-hydroxybenzoic acid), two hydroxycinnamic acids (chlorogenic acid and neochlorogenic acid) and six flavonoids ((+)-catechin, (-)-epicatechin, quercetin-3-O-galactoside, quercetin-3-O-glucoside, quercetin-3-O-rutinoside and kaempferol-3-O-rutinoside). These results are similar to those obtained by Ma et al. [
44], where the phenolic compounds of a sample of
P. viridis were analyzed by liquid chromatography coupled to mass spectrometry, being (+)-catechin and (-)-epicatechin detected. Additionally, five other compounds were also detected (gallic acid, (+)-gallocatechin, dihydromyricetin, (+)-catechin-3-O-gallate and myricitrin) that are not part of the library used in this work [
44]. In the sample of
P. harmala it was only possible to quantify the hydroxybenzoic acids, protocatechuic, gentisic and salicylic acids. The remaining compounds were either not detected, or are below the limit of quantification. Regarding the sample of
M. hostilis, protocatechuic, 4-hydroxybenzoic and salicylic acids, and the flavonoids (+)-catechin and (-)-epicatechin were quantified. Regarding the
B. caapi sample, the hydroxybenzoic acids, protocatechuic and salicylic, and the flavonoids (+)-catechin, (-)-epicatechin, quercetin-3-O-glucoside, quercetin-3-O-rutinoside and phlorizin, were quantified. Finally, the commercial mixture was also analyzed by the same analytical method, allowing the quantification of three hydroxybenzoic acids (protocatechuic, 4-hydroxybenzoic and salicylic acids) and five flavonoids ((+)-catechin, (-)-epicatechin, quercetin-3-O-galactoside, quercetin-3-O-glucoside and quercetin-3-O-rutinoside). The chemical composition and the proportion of the identified compounds are variable in the different analyzed samples. It is difficult to compare the results obtained for the samples of
B. caapi,
M. hostilis,
P. harmala and for the commercial mixture, since most chromatographic studies focus on the detection of psychoactive compounds, such as DMT, or β-carboline alkaloids [
3,
45,
46,
47,
48,
49,
50]. Therefore, future research on these plant samples should be focused on the phytochemical characterization and potential bioactive effects associated with these compounds.
2.2. Antioxidant Activity
In this study, the antioxidant activity of the Ayahuasca decoctions was evaluated in order to identify new sources of antioxidants. Initially, the antioxidant activity of the extracts was evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and the results are presented in
Table 3. This colorimetric assay is widely used because it is quick and easy, consisting in the evaluation of the potential for free radical scavenging of the samples [
51]. Observing the results, it is possible to verify that the samples of
M. hostilis, the mixtures of
M. hostilis and
B. caapi and
M. hostilis and
P. harmala, and the commercial mixture showed a “very strong” antioxidant activity, because their antioxidant activity index (AAI) values were higher than 2.0 [
52]. Otherwise, the sample of
P. harmala presented values of AAI below 0.5, and, therefore, “poor” antioxidant activity [
52]. The remaining samples showed “strong” antioxidant activity [
52]. These results may be related with the phenolic compounds present in the samples, given that the ones with the better antioxidant activity (mixture of
M. hostilis and
B. caapi and a commercial mixture) are also those with higher concentration of total phenolics (
Table 1). To the best of our knowledge, there are no previous studies where the antioxidant activity of
P. viridis,
B. caapi and
M. hostilis was evaluated. Regarding the results obtained for
P. harmala, these are very similar to those obtained by other researchers, where the IC
50 values are always greater than 100, resulting in reduced antioxidant activity [
53,
54].
The comparison of the results of the antioxidant activity of a determined sample is not linear, since the mechanism of action is very complex and varies within matrices. Additionally, the measurement of this activity depends on the method employed [
27]. For these reasons, and in order to better understand the antioxidant mechanism of Ayahuasca decoctions, their antioxidant activity was also evaluated by the β-carotene bleaching test (
Table 3). This assay is widely used in the evaluation of the antioxidant activity of samples of natural origin, since it allows the evaluation of the ability of the samples to inhibit the lipid peroxidation [
40,
55]. In this test, the antioxidant activity evaluation is undertaken by comparing two competitive chemical reactions involving the potentially antioxidant compounds present in the samples and the antioxidant model β-carotene [
56]. As it was observed in the DPPH assay, the
P. harmala sample showed the highest IC
50 value and, consequently, the lowest antioxidant activity. Contrariwise, the samples with lower IC
50, and, therefore, greater antioxidant activity, were the commercial mixture and
P. viridis.
In general, the results obtained allowed concluding that the Ayahuasca is a good source of bioactive compounds with the ability to scavenge free radicals and to inhibit the lipid peroxidation.
2.4. Antimicrobial Activity
Currently, the resistance to conventional drugs by pathogenic microorganisms is a major concern, and the search for alternatives of natural origin has been growing [
62]. Thus, in this work, the potential antimicrobial activity of the Ayahuasca decoctions was evaluated against four Gram-positive and four Gram-negative bacteria. The tested strains were chosen because they are human infective, being some of them well known for their pathogenicity and resistance to antibiotics. Namely,
L. monocytogenes and
B. cereus, known foodborne pathogens, and
E. faecalis,
S. aureus,
E. coli,
A. baumannii,
P. aeruginosa and
S. Typhimurium, responsible for several health-related infections [
63,
64,
65,
66,
67,
68,
69,
70].
Initially, the disc diffusion assay was performed, with some samples presenting antibacterial activity (
Table 5). Analyzing the results, it was verified that six samples inhibited the bacterial growth in all strains. However, the commercial mixture was unable to inhibit the growth of
E. faecalis, and slightly inhibited the growth of
L. monocytogenes and
S. Typhimurium. Additionally, the mixture of
P. viridis and
B. caapi was not able to inhibit the growth of
L. monocytogenes,
S. Typhimurium,
E. coli and
E. faecalis. Regarding the mixture of
P. viridis and
P. harmala, there was also a reduced inhibition in the growth of
L. monocytogenes and absence of growth inhibition of
E. faecalis. The other samples showed remarkable antibacterial activity in all the tested strains. The strain that was less susceptible to the samples was
E. faecalis, with a range of inhibition diameters between 6.00 mm and 10.13 mm. These results can be explained by the ability of this microorganism to adapt to severe situations, namely environmental changes, salt concentrations, extreme alkaline pH, or even to the deprivation of nutrition [
71]. Otherwise, the most promising results were observed for
S. aureus and
A. baumannii, with inhibition diameters ranging between 20.39 mm and 13.27 mm and between 17.81 mm and 11.04 mm, respectively. It is also important to note that in this study it was possible to observe antimicrobial activity against Gram-negative bacteria, which usually have greater resistance to samples of natural origin [
72,
73].
After the initial screening of the antimicrobial potential of the samples, the minimum inhibitory concentration (MIC) values were determined. For this, the resazurin microtiter assay was performed (
Table 6). The strains that presented the lowest MIC and, therefore, were more susceptible to the action of the samples, were
B. cereus and
A. baumannii, with values between 0.156 mg/mL and 5 mg/mL and between 0.625 mg/mL and 5 mg/mL, respectively. Similarly to what was verified in the disc diffusion assay, the mixtures of
P. viridis and
B. caapi and
P. viridis and
P. harmala showed the least promising results, with MIC values varying between 2.5 mg/mL and >10 mg/mL. However, these samples and the commercial mixture showed considerable MIC values against strains where no inhibition was observed in the disc diffusion assay. This result may be related to the poor diffusion of the extracts in the agar plates [
27]. The samples that, in general, showed better MIC values, and consequently greater antimicrobial activity, were the ones of
B. caapi and
P. harmala. In previous studies, the antimicrobial action of
P. harmala extracts against
E. coli and
S. Typhimurium was already reported [
74]; however, the MIC values obtained in that study (0.625 mg/mL) were lower than those obtained now (1.25 mg/mL for
E. coli and 2.5 mg/mL for
S. Typhimurium). Bussmann et al. [
75], also determined the MIC for
B. caapi against
S. aureus and
E. coli, but the values presented in that study (0.0625 mg/mL for
E. coli and 1 mg/mL for
S. aureus) are lower than those now determined. Nevertheless, these comparisons must be made with caution, since in the present study the samples consists of a decoction, whereas in the previous studies, methanolic [
74], and ethanolic [
75], extracts were used, which allows a better extraction of potential bioactive compounds. Furthermore, the differences in susceptibility of the used strains would also affect the results.
Considering the antimicrobial activity demonstrated by the samples, their anti-quorum sensing properties were also evaluated (
Table 7). For that, the biomonitor strain
Chromobacterium violaceum ATCC 12472 was used, which produces the pigment violacein and uses signal molecules of N-acyl homoserine lactone in order to monitor population density [
59]. Analyzing the results, it was observed that, with the exception of
M. hostilis, all samples were able to inhibit the production of violacein and, consequently, the quorum sensing. However, the samples of
B. caapi and
P. harmala stand out, as they produced a diameter of inhibition violacein production much higher than the other samples (13.26 mm and 13.16 mm, respectively). It should be noted that the inhibition diameters of these two samples were greater than that of resveratrol, used as a positive control.
Over the years, the antimicrobial properties of some phenolic compounds, namely protocatechuic acid [
76], gentisic acid [
77], catechin and epicatechin [
78], and other flavonoids [
36,
79,
80,
81,
82,
83] were reported. A high amount of these phenolic compounds in plants can lead to a more effective response in defense against pathogens [
36,
79,
80,
81,
82,
83]. Analyzing the
Table 1, it is possible to verify that
P. harmala presents the highest flavonoid content. Thus, and taking into account the antimicrobial activity previously described for flavonoids, the promising results obtained with this sample may be related to this group of compounds. Similarly, the results obtained for
B. caapi can also be related to its phytochemical composition. Observing the
Table 2, it is possible to verify that
B. caapi presents considerable values of protocatechuic acid, catechin and epicatechin. As previously mentioned, the antimicrobial activity of catechin and epicatechin has been described, and their action against pathogens is known.
A. baumannii is a pathogen responsible for multidrug-resistant nosocomial infections [
84]. This microorganism is often associated with bloodstream infections and pneumonia associated with ventilation, which can even be fatal [
70,
84]. Additionally,
A. baumannii has a plastic genome, which allows adaptation to adverse and stressful environments [
70]. The virulence factors of this pathogen are known, namely the ability to form biofilms [
59]. Biofilms produced by bacteria are highly resistant, as they are protected by the extracellular matrix [
85]. In addition, the lower susceptibility of Gram-negative bacteria to inhibition by plant extracts has been described [
72,
73]. Given the promising results of
B. caapi and
P. harmala, concerning the antibacterial and anti-quorum sensing activities, their anti-biofilm activity against
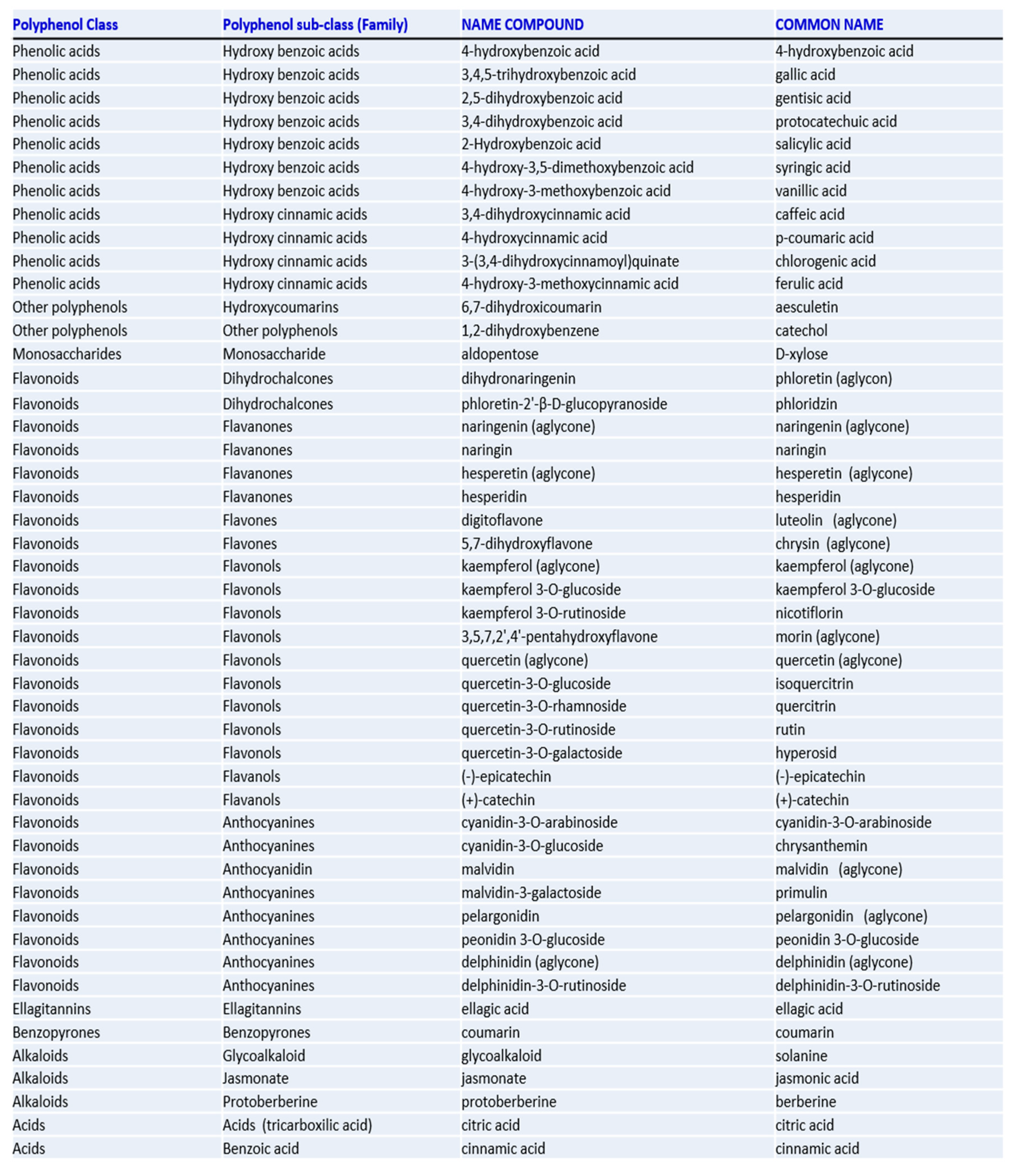
A. baumannii was further evaluated. For that, biofilms formed in polystyrene coupons in the presence of the samples were observed by SEM (
Figure 1). Analyzing the
Figure 1a it is possible to verify that the biofilm of
A. baumannii presents a three-dimensional structure with several layers of cells connected to each other. In contrast, when the biofilm was formed in the presence of
B. caapi, the
A. baumannii cells appear in small number, with no connection between them and without the three-dimensional structure (
Figure 1b). Observing
Figure 1c, it appears that the small number of
A. baumannii cells are partially destroyed when the biofilm grew in the presence of
P. harmala. These results suggest that
P. harmala and
B. caapi present anti-biofilm activity against
A. baumannii. Together with the results obtained in the anti-quorum sensing test, these data allow to infer that these samples are able to inhibit biofilm formation, not only by inhibiting the bacterial growth, but also by inhibiting the cells adhesion to the surface [
86].