Antimicrobial and Antibiofilm Activities of Essential Oils against Escherichia coli O157:H7 and Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
1. Introduction
2. Results
2.1. Gas Chromatography-Mass Spectrometry (GC/MS) Analysis
2.2. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of EOs
2.3. Inhibition of Biofilm Formation by EOs
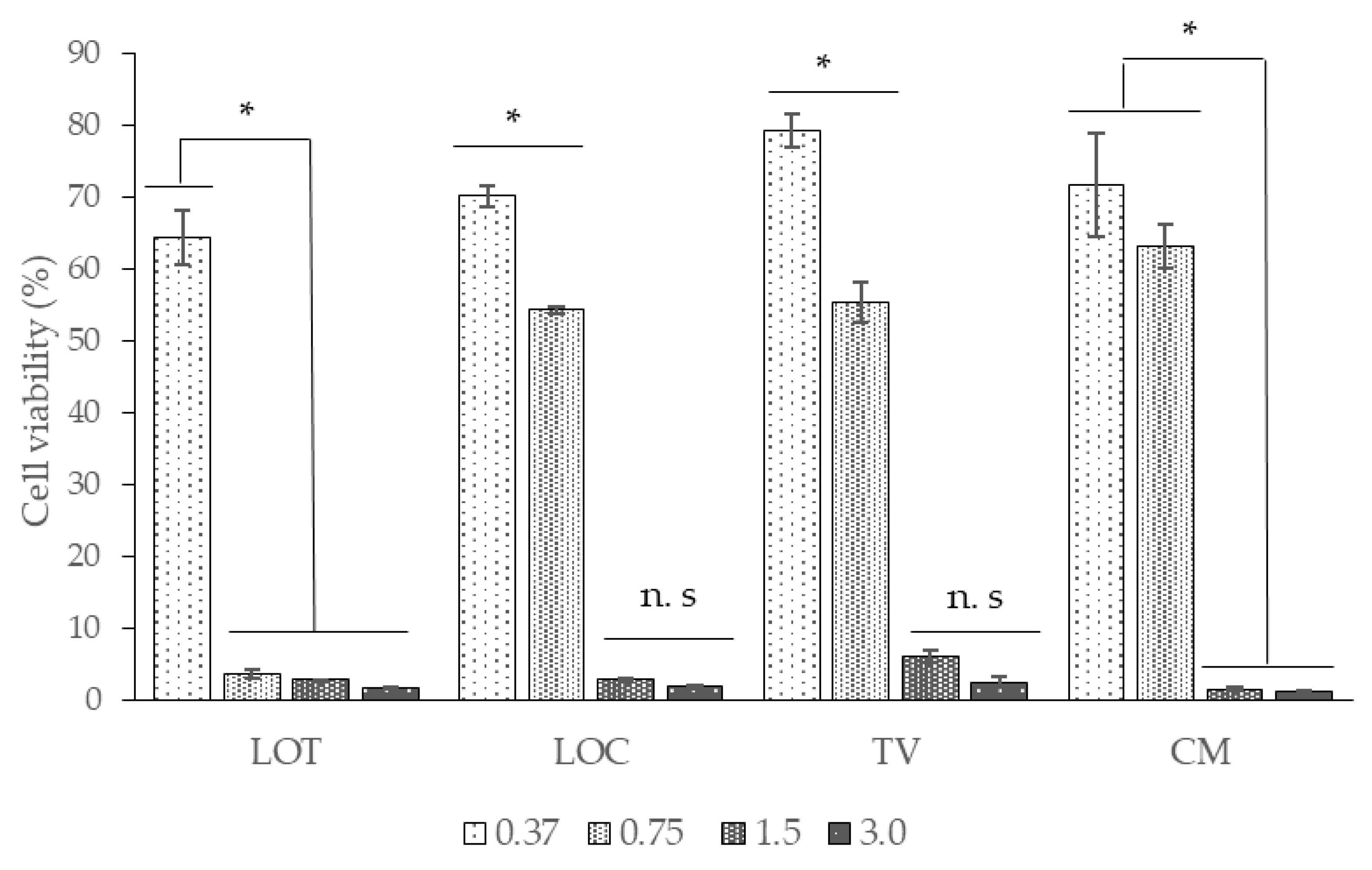
2.4. EOs Cytotoxicity Assay in the Vero Cell Line
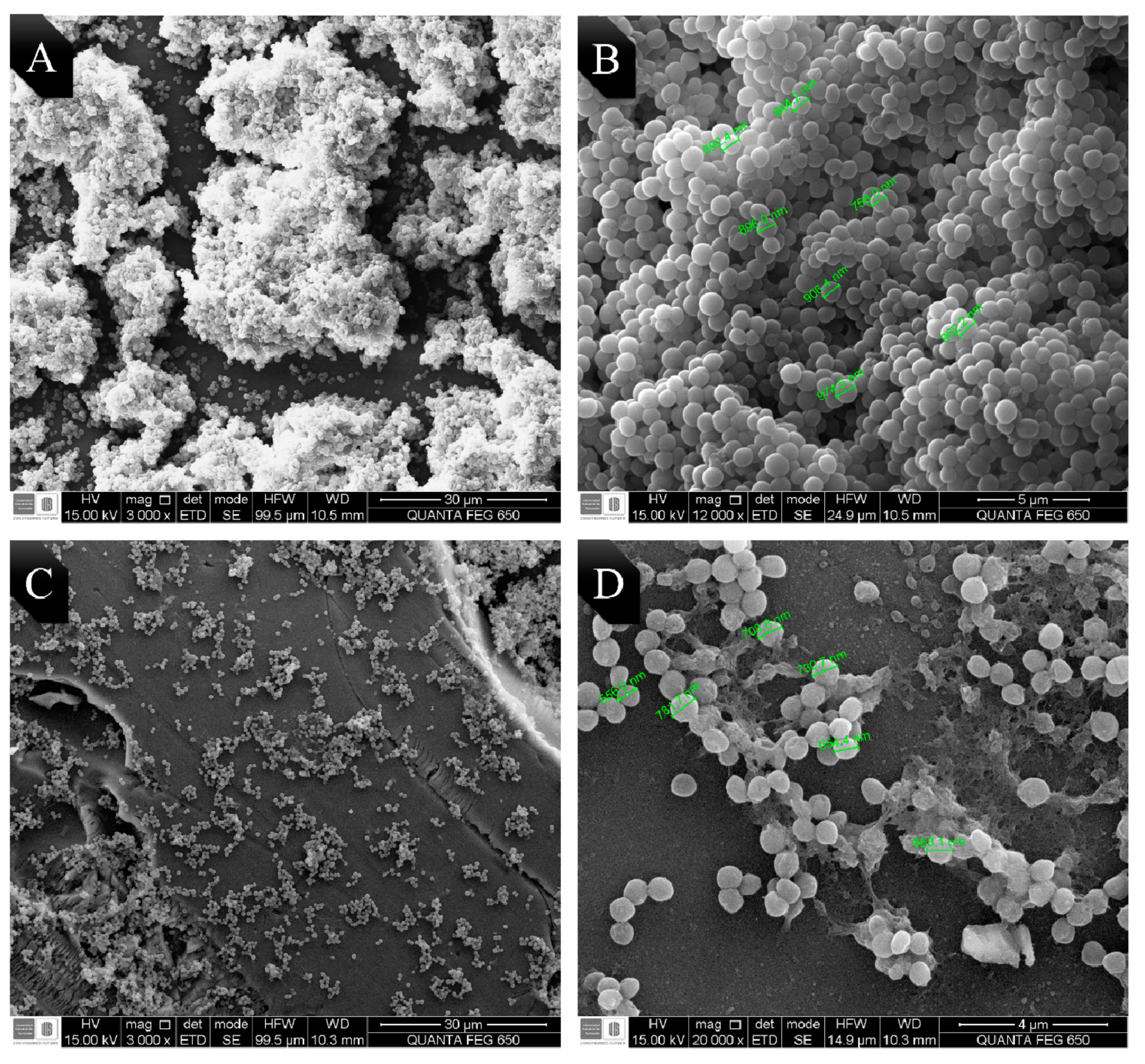
2.5. Visualization of the Morphological Alterations by Scanning Electron Microscopy (SEM)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material and Extraction
4.3. EOs Analysis by Gas Chromatography-Mass Spectrometry (GC/MS)
4.4. Bacterial Strains
4.5. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.6. Inhibition of Biofilm Formation
4.7. EO Cytotoxicity Assay in VERO Cell Line
4.8. Visualization of the Morphological Alterations by Scanning Electron Microscopy (SEM)
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Zhang, H.; Han, J.; Xing, H.; Wu, M.; Yang, T. Deadly Sins of Antibiotic Abuse in China. Infect. Control. Hosp. Epidemiol. 2017, 38, 758–759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. Antimicrobial drug use in food-producing animals and associated human health risks: What, and how strong, is the evidence? BMC Vet. Res. 2017, 13, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance Tomado de. 2014. Available online: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf (accessed on 14 September 2020).
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Ma, Z.; Bumunang, E.W.; Stanford, K.; Bie, X.; Niu, Y.D.; McAllister, T.A. Biofilm Formation by Shiga Toxin-Producing Escherichia coli on Stainless Steel Coupons as Affected by Temperature and Incubation Time. Microorganisms 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Prevention, (CDC). Antibiotic Resistance Threats in the United States, Tomado de. 2013. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 14 September 2020).
- Bauer, P.R.; Sampathkumar, P. Methicillin-Resistant Staphylococcus aureus Infection in ICU. Crit. Care Med. 2017, 45, 1413–1414. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control. Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Buelli, S.; Morigi, M. Shiga toxin-associated hemolytic uremic syndrome: Pathophysiology of endothelial dysfunction. Pediatr. Nephrol. 2010, 25, 2231–2240. [Google Scholar] [CrossRef]
- Caprioli, A.; Scavia, G.; Morabito, S. Public Health Microbiology of Shiga Toxin-Producing Escherichia coli. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chinese Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mayton, H.M.; Marcus, I.M.; Walker, S.L. Escherichia coli O157:H7 and Salmonella Typhimurium adhesion to spinach leaf surfaces: Sensitivity to water chemistry and nutrient availability. Food Microbiol. 2019, 78, 134–142. [Google Scholar] [CrossRef]
- Ueda, Y.; Mashima, K.; Miyazaki, M.; Hara, S.; Takata, T.; Kamimura, H.; Takagi, S.; Jimi, S. Inhibitory effects of polysorbate 80 on MRSA biofilm formed on different substrates including dermal tissue. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: An in vitro study. J. Med. Microbiol. 2009, 58, 1067–1073. [Google Scholar] [CrossRef]
- Cepas, V.; López, Y.; Muñoz, E.; Rolo, D.; Ardanuy, C.; Martí, S.; Xercavins, M.; Horcajada, J.P.; Bosch, J.; Soto, S.M. Relationship Between Biofilm Formation and Antimicrobial Resistance in Gram-Negative Bacteria. Microb. Drug Resist. 2019, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Fastenberg, J.H.; Hsueh, W.D.; Mustafa, A.; Akbar, N.A.; Abuzeid, W.M. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 219–229. [Google Scholar] [CrossRef]
- Potera, C. Forging a link between biofilms and disease. Science 1999, 283, 1837–1839. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; de Alencar, S.M.; de Sousa, R.L.M.; Moreno, A.M.; Da Gloria, E.M. Antimicrobial activity of several essential oils on pathogenic and beneficial bacteria. Ind. Crops Prod. 2017, 97, 128–136. [Google Scholar] [CrossRef]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Ruíz, C.A.; Arias, G.; Durán, C.; Salgar, W.; Cala, M. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J. Sep. Sci. 2010, 33, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.H.A.; Alves, C.N.; Guimarães, E.F.; Carreira, L.M.M.; Maia, J.G.S. Variability in essential oil composition of Piper dilatatum L.C. Rich. Biochem. Syst. Ecol. 2011, 39, 669–675. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef]
- Rajendrudu, G.; Rama Das, V.S. Interspecific differences in the constituents of essential oils of Cymbopogon. Chem. Lett. 1983, 92, 331–334. [Google Scholar]
- Kakaraparthi, P.S.; Srinivas, K.V.N.S.; Kumar, J.K.; Kumar, A.N.; Rajput, D.K.; Anubala, S. Changes in the essential oil content and composition of palmarosa (Cymbopogon martini) harvested at different stages and short intervals in two different seasons. Ind. Crops Prod. 2015, 69, 348–354. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Durán, D.C.; Córdoba, Y.; Caballero, D. Estudio comparativo de la composición química y la actividad antioxidante de los aceites esenciales de algunas plantas del género Lippia (Verbenaceae) cultivadas en Colombia. Rev. Acad. Colomb. Ciencias Exactas Físicas y Nat. 2015, 38, 89. [Google Scholar] [CrossRef]
- Paredes, D.; Ortiz, C.; Torres, R. Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Nanomed. 2014, 9, 1717–1729. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A. Métodos Estandarizados por el CLSI Para el Estudio de la Sensibilidad a Los Antifúngicos (Documentos M27-A3, M38-A y M44-A). Revista Iberoamericana de micología de España. 2004. Available online: http://www.guia.reviberoammicol.com/Capitulo15.pdf (accessed on 14 September 2020).
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Olasupo, N.A.; Fitzgerald, D.J.; Gasson, M.J.; Narbad, A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Typhimurium. Lett. Appl. Microbiol. 2003, 37, 448–451. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control. 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Kim, J.M.; Marshall, M.R.; Cornell, J.A.; Preston, J.F., III; Wei, C.I. Antibacterial Activity of Carvacrol, Citral, and Geraniol against Salmonella typhimurium in Culture Medium and on Fish Cubes. J. Food Sci. 1995, 60, 1364–1368. [Google Scholar] [CrossRef]
- Pattnaik, S.; Subramanyam, V.R.; Kole, C. Antibacterial and antifungal activity of ten essential oils in vitro. Microbios 1996, 86, 237–246. [Google Scholar] [PubMed]
- Pandey, M.C.; Sharma, J.R.; Dikshit, A. Antifungal Evaluation of the Essential Oil of Cymbopogon pendulus (Nees ex Steud.) Wats. cv. Praman. Flavour Fragr. J. 1996, 11, 257–260. [Google Scholar] [CrossRef]
- Delespaul, Q.; de Billerbeck, V.G.; Roques, C.G.; Michel, G.; Marquier-Viñuales, C.; Bessière, J.-M. The Antifungal Activity of Essential Oils as Determined by Different Screening Methods. J. Essent. Oil Res. 2000, 12, 256–266. [Google Scholar] [CrossRef]
- Pedroso, R.; Ueda-Nakamura, T.; Prado Dias Filho, B.; Aparicio Garcia Cortez, D.; Elaine Ranieri Cortez, L.; Andres Morgado-Diaz, J.; Nakamura, C. Biological activities of essential oil obtained from Cymbopogon citratus on Crithidia deanei. Acta Protozool. 2006, 45, 231–240. [Google Scholar]
- Simic, A.; Rančic, A.; Sokovic, M.D.; Ristic, M.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Essential Oil Composition of Cymbopogon winterianus and Carum carvi and Their Antimicrobial Activities. Pharm. Biol. 2008, 46, 437–441. [Google Scholar] [CrossRef]
- Kakarla, S.; Ganjewala, D. Antimicrobial Activity of Essential Oils of Four Lemongrass (Cymbopogon flexuosus Steud) Varieties. Med. Aromat. Plant. Sci. Biotechnol. 2009, 3, 107–109. [Google Scholar]
- Gitaari, N.; Kareru, P.; Githua, M. Antimicrobial Potential of Pelargonium citrosum and Rosmarinus officinalis Essential Oils. Int. Res. J. Pure Appl. Chem. 2019, 18, 1–5. [Google Scholar] [CrossRef]
- Dhar, P.; Chan, P.; Cohen, D.T.; Khawam, F.; Gibbons, S.; Snyder-Leiby, T.; Dickstein, E.; Rai, P.K.; Watal, G. Synthesis, Antimicrobial Evaluation, and Structure–Activity Relationship of α-Pinene Derivatives. J. Agric. Food Chem. 2014, 62, 3548–3552. [Google Scholar] [CrossRef]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Ultee, A.; Gorris, L.G.; Smid, E.J. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J. Appl. Microbiol. 1998, 85, 211–218. [Google Scholar] [CrossRef]
- Kumar, A.; Kamal, A.; Singh, S.; Padalia, R.C.; Tandon, S.; Chauhan, A.; Saikia, D.; Verma, R.S. Chemical composition, antimicrobial activity, kinetics and mechanism of action of Himalayan-thyme (Thymus linearis Benth.). J. Essent. Oil Res. 2019, 1–10. [Google Scholar] [CrossRef]
- Martínez-Graciá, C.; González-Bermúdez, C.A.; Cabellero-Valcárcel, A.M.; Santaella-Pascual, M.; Frontela-Saseta, C. Use of herbs and spices for food preservation: Advantages and limitations. Curr. Opin. Food Sci. 2015, 6, 38–43. [Google Scholar] [CrossRef]
- Ouhayoun, J.-P. Penetrating the plaque biofilm: Impact of essential oil mouthwash. J. Clin. Periodontol. 2003, 30 (Suppl. 5), 10–12. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.R.; Roller, S.; Murray, D.B.; Naidu, A.S. Antimicrobial Action of Carvacrol at Different Stages of Dual-Species Biofilm Development by Staphylococcus aureus and Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 797–803. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Linton, M.; Ward, P.; Campbell, M.; Kelly, C.; Pinkerton, L.; Stef, L.; Pet, I.; Stef, D.; Iancu, T.; et al. The Antimicrobial Effect of a Commercial Mixture of Natural Antimicrobials Against Escherichia coli O157:H7. Foodborne Pathog. Dis. 2019, 16, 119–129. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; McLandsborough, L.; Weiss, J. Inhibition and inactivation of Listeria monocytogenes and Escherichia coli O157:H7 colony biofilms by micellar-encapsulated eugenol and carvacrol. J. Food Prot. 2006, 69, 2947–2954. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.H.; Kim, S.I.; Cho, M.H.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef]
- Caiazza, N.C.; Toole, G.A.O. Alpha-Toxin Is Required for Biofilm Formation by Staphylococcus aureus. J. Bacteriol. 2003, 185, 3214–3217. [Google Scholar] [CrossRef]
- Manabe, A.; Nakayama, S.; Sakamoto, K. Effects of essential oils on erythrocytes and hepatocytes from rats and dipalmitoyl phosphatidylcholine-liposomes. Jpn. J. Pharmacol. 1987, 44, 77–84. [Google Scholar] [CrossRef]
- Suzuki, Y.; Furuta, H. Stimulation of guinea pig neutrophil superoxide anion-producing system with thymol. Inflammation 1988, 12, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nakamura, S.; Sugiyama, K.; Furuta, H. Differences of superoxide production in blood leukocytes stimulated with thymol between human and non-human primates. Life Sci. 1987, 41, 1659–1664. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Montiel-Ramos, J.; Zapata, B.; Durán, C.; Betancur-Galvis, L.; Stashenko, E. Citral and carvone chemotypes from the essential oils of Colombian Lippia alba (Mill.) N.E. Brown: Composition, cytotoxicity and antifungal activity. Mem. Inst. Oswaldo Cruz 2009, 104, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Vasconcellos, F.; Lenardão, E.; do Amaral, R.; Jacob, R.; Leivas Leite, F. Evaluation of potential use of Cymbopogon sp. essential oils, (R)-citronellal and N-citronellylamine in cancer chemotherapy. Int. J. Appl. Res. Nat. Prod. 2013, 6, 11–15. [Google Scholar]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Adetunji, V.O.; Odetokun, I.A. Assessment of Biofilm in E. coli O157:H7 and Salmonella Strains: Influence of Cultural Conditions. Am. J. Food Technol. 2012, 7, 582–595. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI: Wayne, NI, USA, 2017. [Google Scholar]
- Owuama, C. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afr. J. Microbiol. Res. 2018. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- García Rico, R.O.; Herrera Arias, F.C. Evaluación de la inhibición del crecimiento de cinco cepas bacterianas patógenas por extractos acuosos de Allium sativum, Allium fistulosum y Allium cepa: Estudio preliminar in vitro. Bistua Rev. La Fac. Ciencias Básicas 2007, 5, 68–79. [Google Scholar]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. In Current Protocols in Microbiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 1–29. ISBN 9780471729259. [Google Scholar]
- Kifer, D.; Mu, V.; Maja, Š. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1, 8-cineole against Staphylococcus aureus planktonic and bio fi lm growth. J. Antibiot. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Arizza, V.; Dayton, T.; Camarda, L.; Di Stefano, V. In vitro anti-biofilm activity of Boswellia spp. oleogum resin essential oils. Lett. Appl. Microbiol. 2008, 47, 433–438. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology; International Union of Pure and Applied Chemistry: Zurich, Switzerland, 1997; ISBN 0-9678550-9-8. [Google Scholar]
- Hennebelle, T.; Sahpaz, S.; Joseph, H.; Bailleul, F. Ethnopharmacology of Lippia alba. J. Ethnopharmacol. 2008, 116, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, R.; Tandon, S.; Pandey, R. Anti-biofilm and anti-virulence potential of 3,7-dimethyloct-6-enal derived from Citrus hystrix against bacterial blight of rice caused by Xanthomonas oryzae pv. oryzae. Microb. Pathog. 2018, 115, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Atshan, S.S.; Shamsudin, M.N.; Than, L.; Lung, T.; Sekawi, Z.; Ghaznavi-rad, E.; Pei, C.P. Comparative Characterisation of Genotypically Different Clones of MRSA in the Production of Biofilms. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Singh, V.K.; Mishra, A.; Jha, B. Anti-quorum Sensing and Anti-biofilm Activity of Delftia tsuruhatensis Extract by Attenuating the Quorum Sensing-Controlled Virulence Factor Production in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2017, 7, 1–16. [Google Scholar] [CrossRef]

| Compound | Type | CF | CM | CN | CO | LACA | LACI | LOC | LOF | LOT | RO | SG | SO | SV | TL | TV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-Isopropenyl-4-methyl-1-cyclohexane | OC | - | - | - | - | - | - | - | - | - | - | - | - | 24.4 | - | - |
| 2,6-dimethyl-2,6-octadiene | OC | - | - | 6.1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Benzyl acetate | OC | - | - | - | 10.3 | - | - | - | - | - | - | - | - | - | - | - |
| Geranyl acetate | OC | 0.5 | 1.3 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Thymyl acetate | OC | - | - | - | - | - | - | - | - | 3.9 | - | - | - | - | - | - |
| Carvacrol | OC | - | - | - | - | - | - | 18.8 | - | 10.7 | - | - | - | - | - | - |
| Thymyl methyl eter | OC | - | - | - | - | - | - | - | - | 4.6 | - | - | - | - | - | - |
| Thymol | OC | - | - | - | - | - | - | 32.7 | - | 22.1 | - | - | - | - | - | 23.0 |
| Benzyl benzoate | OC | - | - | - | 20.8 | - | - | - | - | - | - | - | - | - | - | - |
| Methyl benzoate | OC | - | - | - | 3.7 | - | - | - | - | - | - | - | - | - | - | - |
| Cis Cinnamile acetate | OC | - | - | - | 5.4 | - | - | - | - | - | - | - | - | - | - | - |
| Camphene | MH | - | - | - | - | - | - | - | 2.6 | - | 7.7 | - | - | - | - | - |
| Limonene | MH | - | - | - | - | 29.0 | 3.9 | - | 7.2 | - | - | - | - | - | - | - |
| p-Cymene | MH | - | - | - | - | - | - | 1.1 | 11.2 | 3.7 | - | - | - | - | - | 20.0 |
| trans-β-Ocimene | MH | - | 1.9 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| α-Phellandrene | MH | - | - | - | - | - | - | - | 9.9 | - | - | - | - | - | - | - |
| α-Pinene | MH | - | - | - | - | - | - | - | 2.3 | - | 12.7 | 2.6 | - | - | - | - |
| β-Myrcene | MH | - | - | - | - | - | - | - | 1.5 | - | - | - | - | - | 0.9 | - |
| γ-Terpinene | MH | - | - | - | - | - | - | 5.2 | - | - | - | - | - | - | - | 9.5 |
| cis-Pulegol | MH | - | - | - | - | - | - | - | - | - | - | - | - | 7.1 | - | - |
| 1,8-Cineol | OM | - | - | - | - | - | - | - | 11.6 | - | 17.5 | 36.6 | 5.3 | - | - | - |
| Camphor | OM | - | - | - | - | - | - | - | - | - | 14.8 | - | 8.5 | - | - | - |
| Carvone | OM | - | - | - | - | 31.3 | - | - | - | - | - | - | - | - | - | - |
| Citronellal | OM | - | - | 11.6 | - | - | - | - | - | - | - | - | - | - | - | - |
| Geranial | OM | 33.0 | - | - | - | - | 27.0 | - | - | - | - | - | - | - | - | - |
| Geraniol | OM | 7.9 | 38.7 | 17.8 | - | - | 6.1 | - | - | - | - | - | - | - | - | - |
| Linalool | OM | - | 3.2 | - | 11.7 | - | - | - | - | - | - | - | - | - | - | 4.7 |
| Neral | OM | 24.5 | - | - | - | - | 15.4 | - | - | - | - | - | - | - | - | - |
| Piperitone | OM | - | - | - | - | 1.5 | - | - | - | - | - | - | - | - | - | - |
| β-Citronellol | OM | - | - | 16.9 | - | - | - | - | - | - | - | - | - | - | - | - |
| cis-Thujone | OM | - | - | - | - | - | - | - | - | - | - | - | 5.8 | - | - | - |
| Estragole | OM | - | - | - | - | - | - | - | - | - | - | - | - | - | 79.9 | - |
| Pulegone | OM | - | - | - | - | - | - | - | - | - | - | - | - | 11.1 | - | - |
| trans-Thujone | OM | - | - | - | - | - | - | - | - | - | - | - | 20.4 | - | - | - |
| Germacrene B | SH | - | - | - | - | - | - | - | - | - | - | 10.8 | - | - | - | - |
| Germacrene D | SH | - | - | - | - | 12.2 | - | - | - | - | - | 15.4 | - | - | - | - |
| trans-β-Caryophyllene | SH | - | - | - | - | - | 11.8 | 6.4 | 11.3 | 7.9 | 7.8 | - | - | 11.8 | - | 9.5 |
| α-Humulene | SH | - | - | - | - | - | - | - | 6.2 | - | - | - | 9.8 | - | - | - |
| β-Bourbonene | SH | - | - | - | - | 2.4 | - | - | - | - | - | - | - | - | - | - |
| trans-Nerolidol | OS | - | - | - | - | - | - | - | - | - | - | 24.0 | - | - | - | - |
| Monoterpenic hydrocarbons (MH) | - | 1.9 | - | - | 29.0 | 3.9 | 6.3 | 34.7 | 3.7 | 20.4 | 2.6 | - | 7.1 | 0.9 | 29.5 | |
| Oxygenated monoterpenes (OM) | 65.4 | 41.9 | 46.3 | 11.7 | 32.8 | 48.5 | - | 11.6 | - | 32.3 | 36.6 | 40.0 | 11.1 | 79.9 | 4.7 | |
| Oxygenated Compounds (OC) | 0.5 | 1.3 | 6.1 | 40.2 | - | - | 51.5 | - | 41.3 | - | - | - | 24.4 | - | 23.0 | |
| Sesquiterpene hydrocarbons (SH) | - | - | - | - | 14.6 | 11.8 | 6.4 | 17.5 | 7.9 | 7.8 | 26.2 | 9.8 | 11.8 | - | 9.5 | |
| Oxygenated sesquiterpenes (OS) | - | - | - | - | - | - | - | - | - | - | 24.0 | - | - | - | - | |
| TOTAL MAJOR COMPONENTS IDENTIFIED (%) | 65.9 | 45.1 | 52.4 | 51.9 | 76.4 | 64.2 | 64.2 | 63.8 | 52.9 | 60.5 | 89.4 | 49.8 | 54.4 | 80.8 | 66.7 | |
| Abbrev. | Species | E. coli O157:H7 | MRSA | ||
|---|---|---|---|---|---|
| MIC50 | MBC | MIC50 | MBC | ||
| LACA | L. alba (carvona) | >3 | >3 | >3 | >3 |
| LACI | L. alba (citral) | >3 | >3 | >3 | >3 |
| CN | C. nardus | >3 | >3 | >3 | >3 |
| CM | C. martini | 1.4 | >3 | >3 | >3 |
| CF | C. flexuosos | >3 | >3 | 2.4 | 3 |
| LOT | L. origanoides (thymol) | 0.9 | 1.5 | 1.6 | 3 |
| LOC | L. origanoides (carvacrol) | 0.4 | 0.7 | 0.6 | 1.5 |
| LOF | L. origanoides (phellandrene) | >3 | >3 | >3 | >3 |
| RO | Rosmarinus offiicinalis | >3 | >3 | >3 | >3 |
| SO | Salvia officinalis | >3 | >3 | >3 | >3 |
| SG | Swinglea glutinosa | >3 | >3 | >3 | >3 |
| TL | Tagetes lucida | >3 | >3 | >3 | >3 |
| TV | Thymus vulgaris | 0.9 | 1.5 | >3 | >3 |
| SV | Satureja viminea | >3 | >3 | >3 | >3 |
| CO | Cananga odorata | >3 | >3 | >3 | >3 |
| Species | E. coli O157:H7 | MRSA | ||
|---|---|---|---|---|
| MIBC50 (mg/mL) | Inhibition (%) | MIBC50 (mg/mL) | Inhibition (%) | |
| L. alba (carvone) | >3 | 16.9 | >3 | 0 |
| L. alba (citral) | >3 | 12.9 | >3 | 36.9 |
| C. nardus | >3 | 21.0 | >3 | 4.1 |
| C. martini | 1.12 | 55.0 | >3 | 25.8 |
| C. flexuosos | >3 | 36.2 | 1.83 | 78.71 |
| L. origanoides (thymol) | 0.45 | 70.3 | 1.2 | 82.61 |
| L. origanoides (carvacrol) | 0.19 | 73.9 | 0.07 | 81.09 |
| L. origanoides (phellandrene) | >3 | 32.3 | >3 | 38.15 |
| Rosmarinus offiicinalis | >3 | 34.7 | >3 | 25.15 |
| Salvia officinalis | >3 | 29.3 | >3 | 35.9 |
| Swinglea glutinosa | >3 | 21.0 | >3 | 6 |
| Tagetes lucida | >3 | 49.4 | >3 | 49.2 |
| Thymus vulgaris | 0.45 | 70.9 | >3 | 39.3 |
| Satureja viminea | >3 | 23.5 | >3 | 54.5 |
| Cananga odorata | >3 | 16.9 | 0,57 | 83.0 |
| SPECIES | CC50 | SI | |
|---|---|---|---|
| EC | SA | ||
| C. martini | 0.72 | - | 0.51 |
| L. origanoides (thymol) | 0.46 | 0.53 | 0.37 |
| L. origanoides (carvacrol) | 0.65 | 2.05 | 1.00 |
| Thymus vulgaris | 0.73 | 0.74 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Sequeda, N.; Cáceres, M.; Stashenko, E.E.; Hidalgo, W.; Ortiz, C. Antimicrobial and Antibiofilm Activities of Essential Oils against Escherichia coli O157:H7 and Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 730. https://doi.org/10.3390/antibiotics9110730
Gómez-Sequeda N, Cáceres M, Stashenko EE, Hidalgo W, Ortiz C. Antimicrobial and Antibiofilm Activities of Essential Oils against Escherichia coli O157:H7 and Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics. 2020; 9(11):730. https://doi.org/10.3390/antibiotics9110730
Chicago/Turabian StyleGómez-Sequeda, Nicolás, Marlon Cáceres, Elena E. Stashenko, William Hidalgo, and Claudia Ortiz. 2020. "Antimicrobial and Antibiofilm Activities of Essential Oils against Escherichia coli O157:H7 and Methicillin-Resistant Staphylococcus aureus (MRSA)" Antibiotics 9, no. 11: 730. https://doi.org/10.3390/antibiotics9110730
APA StyleGómez-Sequeda, N., Cáceres, M., Stashenko, E. E., Hidalgo, W., & Ortiz, C. (2020). Antimicrobial and Antibiofilm Activities of Essential Oils against Escherichia coli O157:H7 and Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics, 9(11), 730. https://doi.org/10.3390/antibiotics9110730





