A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery
Abstract
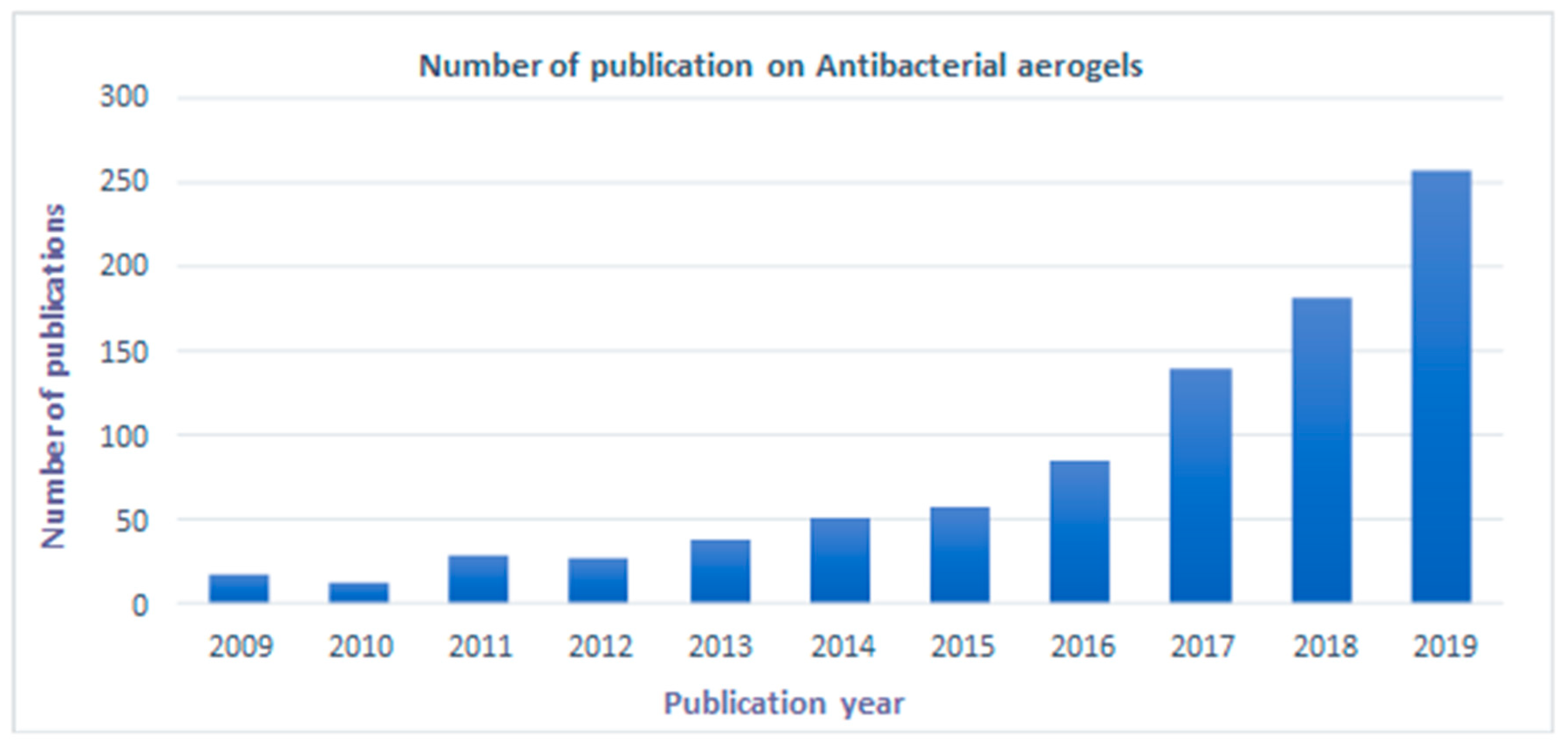
1. Introduction
2. Biopolymer-Based Aerogels
2.1. Chitosan-Based Aerogel
2.2. Cellulose-Based Aerogels
2.3. Alginate Based Aerogels
2.4. Other Biopolymer-Based Aerogels
2.5. Biomedical Applications of Biopolymer-Based Aerogels
3. Biopolymer-Based Aerogels for Antibacterial Delivery
3.1. Antibacterial Chitosan-Based Aerogels
3.2. Antibacterial Cellulose Based Aerogels
3.3. Antibacterial Alginates Based Aerogels
3.4. Other Antibacterial Biopolymer-Based Aerogels
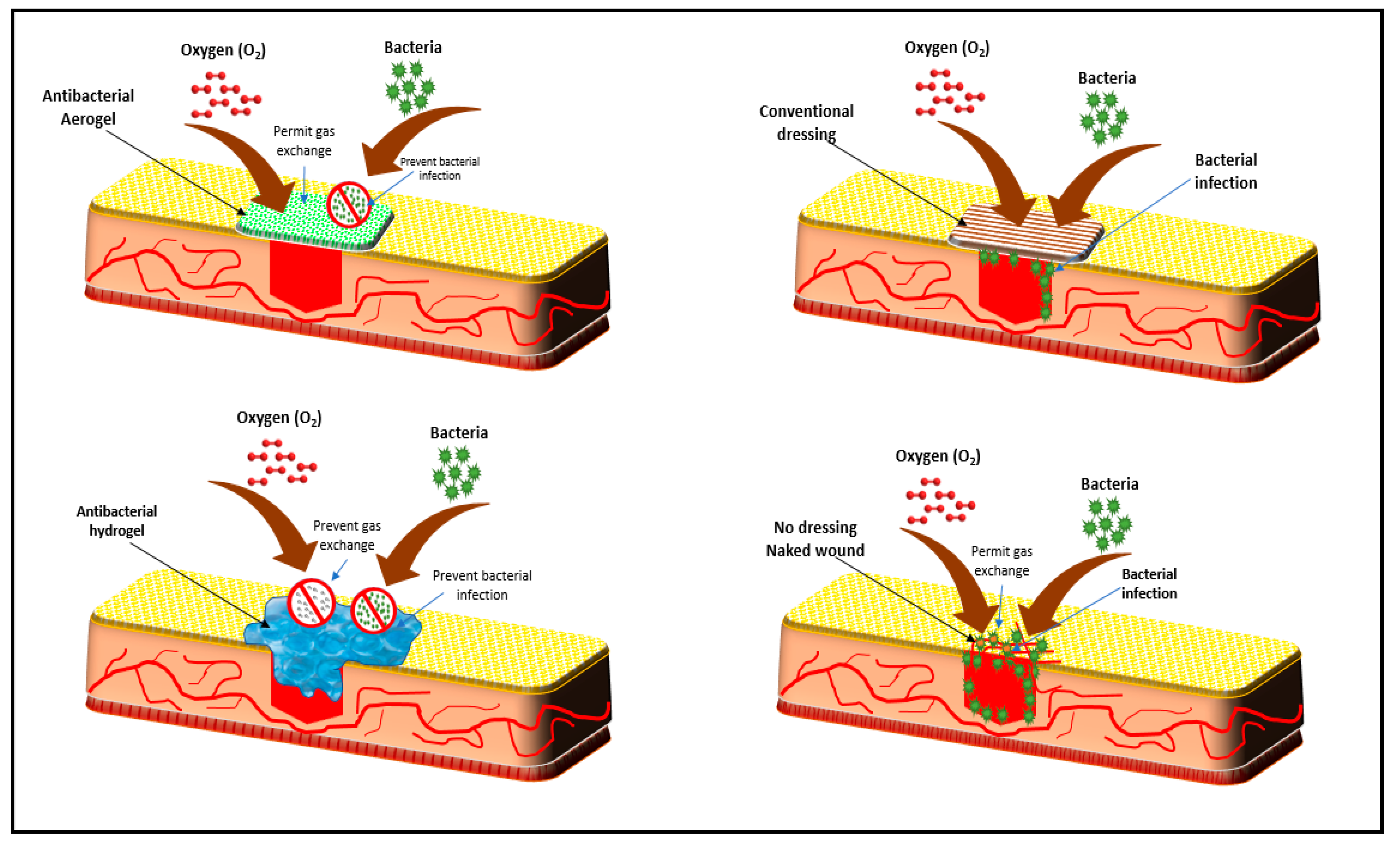
4. The Role of Biopolymer-Based Aerogel in Wound Healing Applications
5. Challenges and Propositions of Biopolymer-Based Antibacterial Aerogels
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.K.; Gaponik, N.; Eychmüller, A. Modern inorganic aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed]
- Surya, I.; Olaiya, N.; Rizal, S.; Zein, I.; Sri Aprilia, N.; Hasan, M.; Yahya, E.B.; Sadasivuni, K.; Abdul Khalil, H.P.S. Plasticizer enhancement on the miscibility and thermomechanical properties of polylactic acid-chitin-starch composites. Polymers 2020, 12, 115. [Google Scholar] [CrossRef]
- Tamer, T.M.; Collins, M.N.; Valachová, K.; Hassan, M.A.; Omer, A.M.; Mohy-Eldin, M.S.; Švík, K.; Jurčík, R.; Ondruška, Ľ.; Biró, C. MitoQ loaded chitosan-hyaluronan composite membranes for wound healing. Materials 2018, 11, 569. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Jummaat, F.; Yahya, E.B.; Olaiya, N.; Adnan, A.; Abdat, M.; NAM, N.; Halim, A.S.; Kumar, U.; Bairwan, R. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the road to biopolymer aerogels—Dealing with the solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Subrahmanyam, R.; Gurikov, P.; Meissner, I.; Smirnova, I. Preparation of biopolymer aerogels using green solvents. JoVE 2016, 113, e54116. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in medical implants: A brief review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Berardi, U.; Zaidi, S.M. Characterization of commercial aerogel-enhanced blankets obtained with supercritical drying and of a new ambient pressure drying blanket. Energy Build. 2019, 198, 542–552. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.; Chen, L.; Duan, G.; Mei, C.; Huang, C.; Han, J.; Jiang, S. Anisotropic nanocellulose aerogels with ordered structures fabricated by directional freeze-drying for fast liquid transport. Cellulose 2019, 26, 6653–6667. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Liu, Y.; Li, L.; Li, H.; Peng, X.-F.; Zhou, H. Highly durable superhydrophobic polymer foams fabricated by extrusion and supercritical CO2 foaming for selective oil absorption. ACS Appl. Mater. Interfaces 2019, 11, 7479–7487. [Google Scholar] [CrossRef]
- Pirzada, T.; Ashrafi, Z.; Xie, W.; Khan, S.A. Cellulose Silica Hybrid Nanofiber Aerogels: From Sol–Gel Electrospun Nanofibers to Multifunctional Aerogels. Adv. Funct. Mater. 2020, 30, 1907359. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Du, Y.; Liao, J.; Zhang, X. Phase-separation induced synthesis of superhydrophobic silica aerogel powders and granules. J. Solid State Chem. 2019, 279, 120971. [Google Scholar] [CrossRef]
- Yuan, B.; Zhou, S.-Y.; Chen, X.-S. Rapid prototyping technology and its application in bone tissue engineering. J. Zhejiang Univ. Sci. B 2017, 18, 303–315. [Google Scholar] [CrossRef]
- Tang, A.; Li, J.; Li, J.; Zhao, S.; Liu, W.; Liu, T.; Wang, J.; Liu, Y. Nanocellulose/PEGDA aerogel scaffolds with tunable modulus prepared by stereolithography for three-dimensional cell culture. J. Biomater. Sci. Polym. Ed. 2019, 30, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Qiu, J. 3D printing of glass by additive manufacturing techniques: A review. Front. Optoelectron. 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Saoud, K.M.; Saeed, S.; Bertino, M.F.; White, L.S. Fabrication of strong and ultra-lightweight silica-based aerogel materials with tailored properties. J. Porous Mater. 2018, 25, 511–520. [Google Scholar] [CrossRef]
- Kam, D.; Chasnitsky, M.; Nowogrodski, C.; Braslavsky, I.; Abitbol, T.; Magdassi, S.; Shoseyov, O. Direct cryo writing of aerogels via 3d printing of aligned cellulose nanocrystals inspired by the plant cell wall. Colloids Interfaces 2019, 3, 46. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Z.; Huang, T.; Liu, Y.; Guo, F.; Xi, J.; Gao, W.; Gao, C. Direct 3D printing of ultralight graphene oxide aerogel microlattices. Adv. Funct. Mater. 2018, 28, 1707024. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr. Polym. 2018, 180, 264–275. [Google Scholar] [CrossRef]
- Arenillas, A.; Menéndez, J.A.; Reichenauer, G.; Celzard, A.; Fierro, V.; Hodar, F.J.M.; Bailόn-Garcia, E.; Job, N. Properties of carbon aerogels and their organic precursors. In Organic and Carbon Gels; Springer: Berlin/Heidelberg, Germany, 2019; pp. 87–121. [Google Scholar]
- Hu, L.; He, R.; Lu, Z.; Zhang, K.; Bai, X. Step-freeze-drying method for carbon aerogels: A study of the effects on microstructure and mechanical property. RSC Adv. 2019, 9, 9931–9936. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A. Superabsorbent Polymers from the Cell Wall of Zygomycetes Fungi. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2010. [Google Scholar]
- Ambarish, C.N.; Sridhar, K.R. Isolation and characterization of chitin from exoskeleton of pill-millipedes. Trends Biomater. Artif. Organs 2015, 29, 155–159. [Google Scholar]
- Zhang, Z.; Sèbe, G.; Rentsch, D.; Zimmermann, T.; Tingaut, P. Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chem. Mater. 2014, 26, 2659–2668. [Google Scholar] [CrossRef]
- Nuryawan, A.; Abdullah, C.; Hazwan, C.M.; Olaiya, N.; Yahya, E.B.; Risnasari, I.; Masruchin, N.; Baharudin, M.; Khalid, H.; Abdul Khalil, H.P.S. Enhancement of oil palm waste nanoparticles on the properties and characterization of hybrid plywood biocomposites. Polymers 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Rinki, K.; Dutta, P.K.; Hunt, A.J.; Macquarrie, D.J.; Clark, J.H. Chitosan aerogels exhibiting high surface area for biomedical application: Preparation, characterization, and antibacterial study. Int. J. Polym. Mater. 2011, 60, 988–999. [Google Scholar] [CrossRef]
- Yi, L.; Yang, J.; Fang, X.; Xia, Y.; Zhao, L.; Wu, H.; Guo, S. Facile fabrication of wood-inspired aerogel from chitosan for efficient removal of oil from water. J. Hazard. Mater. 2020, 385, 121507. [Google Scholar] [CrossRef]
- Rubina, M.S.; Elmanovich, I.V.; Shulenina, A.V.; Peters, G.S.; Svetogorov, R.D.; Egorov, A.A.; Naumkin, A.V.; Vasil’kov, A.Y. Chitosan aerogel containing silver nanoparticles: From metal-chitosan powder to porous material. Polym. Test. 2020, 86, 106481. [Google Scholar] [CrossRef]
- Gómez, M.A.; Bonilla, J.M.; Coronel, M.A.; Martínez, J.; Morán-Trujillo, L.; Orellana, S.L.; Vidal, A.; Giacaman, A.; Morales, C.; Torres-Gallegos, C. Antibacterial activity against Staphylococcus aureus of chitosan/chondroitin sulfate nanocomplex aerogels alone and enriched with erythromycin and elephant garlic (Allium ampeloprasum L. var. ampeloprasum) extract. Pure Appl. Chem. 2018, 90, 885–900. [Google Scholar]
- Yazdanbakhsh, M.; Rashidi, A.; Rahimi, M.; Khajavi, R.; Shafaroodi, H. Alpha-cellulose extraction from wheat bran for preparing cellulose nanofibers. Am. J. Oil Chem. Technol. 2017, 5, 46–52. [Google Scholar]
- Manzato, L.; Takeno, M.L.; Pessoa-Junior, W.A.G.; Mariuba, L.A.M.; Simonsen, J. Optimization of cellulose extraction from jute fiber by Box-Behnken design. Fibers Polym. 2018, 19, 289–296. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, X.; Yu, W.; Zhang, Y. Kinetic analysis of cellulose extraction from banana pseudo-stem by liquefaction in polyhydric alcohols. Ind. Crops Prod. 2019, 137, 377–385. [Google Scholar] [CrossRef]
- Madsen, B.; Gamstedt, E.K. Wood versus plant fibers: Similarities and differences in composite applications. Adv. Mater. Sci. Eng. 2013, 2013, 564346. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Wang, X.; Liu, S.; Yao, Y. Characterization of the nano-cellulose aerogel from mixing CNF and CNC with different ratio. Mater. Lett. 2018, 229, 103–106. [Google Scholar] [CrossRef]
- Simón-Herrero, C.; Caminero-Huertas, S.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Effects of freeze-drying conditions on aerogel properties. J. Mater. Sci. 2016, 51, 8977–8985. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.; Xu, P.; Chen, B. Super-elastic and highly hydrophobic/superoleophilic sodium alginate/cellulose aerogel for oil/water separation. Cellulose 2018, 25, 3533–3544. [Google Scholar] [CrossRef]
- Zheng, T.; Li, A.; Li, Z.; Hu, W.; Shao, L.; Lu, L.; Cao, Y.; Chen, Y. Mechanical reinforcement of a cellulose aerogel with nanocrystalline cellulose as reinforcer. RSC Adv. 2017, 7, 34461–34465. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; He, Y.; Wang, H.; Wei, H.; Zhu, Q.; Zhang, T.; Qin, Y.; Du, A. Preparation, characterization, and in vitro evaluation of resveratrol-loaded cellulose aerogel. Materials 2020, 13, 1624. [Google Scholar] [CrossRef]
- Raman, S.; Keil, C.; Dieringer, P.; Hübner, C.; Bueno, A.; Gurikov, P.; Nissen, J.; Holtkamp, M.; Karst, U.; Haase, H. Alginate aerogels carrying calcium, zinc and silver cations for wound care: Fabrication and metal detection. J. Supercrit. Fluids 2019, 153, 104545. [Google Scholar] [CrossRef]
- Nawaz, M.; Moztahida, M.; Kim, J.; Shahzad, A.; Jang, J.; Miran, W.; Lee, D.S. Photodegradation of microcystin-LR using graphene-TiO2/sodium alginate aerogels. Carbohydr. Polym. 2018, 199, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Cardea, S.; Scognamiglio, M.; Reverchon, E. A new tool to produce alginate-based aerogels for medical applications, by supercritical gel drying. J. Supercrit. Fluids 2019, 146, 152–158. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Fan, M.; Zheng, P.; Zhuang, J.; Chen, L. A robust salt-tolerant superoleophobic alginate/graphene oxide aerogel for efficient oil/water separation in marine environments. Sci. Rep. 2017, 7, 46379. [Google Scholar] [CrossRef] [PubMed]
- Mallepally, R.R.; Bernard, I.; Marin, M.A.; Ward, K.R.; McHugh, M.A. Superabsorbent alginate aerogels. J. Supercrit. Fluids 2013, 79, 202–208. [Google Scholar] [CrossRef]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; Del Gaudio, P. Prilling and supercritical drying: A successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef]
- Robitzer, M.; David, L.; Rochas, C.; Di Renzo, F.; Quignard, F. Nanostructure of calcium alginate aerogels obtained from multistep solvent exchange route. Langmuir 2008, 24, 12547–12552. [Google Scholar] [CrossRef]
- Deze, E.G.; Papageorgiou, S.K.; Favvas, E.P.; Katsaros, F.K. Porous alginate aerogel beads for effective and rapid heavy metal sorption from aqueous solutions: Effect of porosity in Cu2+ and Cd2+ ion sorption. Chem. Eng. J. 2012, 209, 537–546. [Google Scholar] [CrossRef]
- Franco, P.; Pessolano, E.; Belvedere, R.; Petrella, A.; De Marco, I. Supercritical impregnation of mesoglycan into calcium alginate aerogel for wound healing. J. Supercrit. Fluids 2020, 157, 104711. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Obaidat, R.M.; Alnaief, M.; Mashaqbeh, H. Investigation of carrageenan aerogel microparticles as a potential drug carrier. Aaps Pharmscitech 2018, 19, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, S.; Zhu, W.; Huang, L.; Yang, C.; Li, S.; Liu, X.; Wang, R.; Hu, N.; Suo, Y. Agar aerogel containing small-sized zeolitic imidazolate framework loaded carbon nitride: A solar-triggered regenerable decontaminant for convenient and enhanced water purification. ACS Sustain. Chem. Eng. 2017, 5, 9347–9354. [Google Scholar] [CrossRef]
- Bilanovic, D.; Starosvetsky, J.; Armon, R.H. Preparation of biodegradable xanthan–glycerol hydrogel, foam, film, aerogel and xerogel at room temperature. Carbohydr. Polym. 2016, 148, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Dogenski, M.; Navarro-Díaz, H.J.; de Oliveira, J.V.; Ferreira, S.R.S. Properties of starch-based aerogels incorporated with agar or microcrystalline cellulose. Food Hydrocoll. 2020, 108, 106033. [Google Scholar] [CrossRef]
- Baudron, V.; Taboada, M.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Production of starch aerogel in form of monoliths and microparticles. Colloid Polym. Sci. 2020, 298, 477–494. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Du, Q.; Wang, Z.; Xia, Y.; Yedinak, E.; Lou, J.; Ci, L. High performance agar/graphene oxide composite aerogel for methylene blue removal. Carbohydr. Polym. 2017, 155, 345–353. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef]
- Čolić, M.; Tomić, S.; Bekić, M. Immunological aspects of nanocellulose. Immunol. Lett. 2020, 222, 80–89. [Google Scholar] [CrossRef]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Pircher, N.; Liebner, F.; Condon, B.D. Preparation, characterization and activity of a peptide-cellulosic aerogel protease sensor from cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef]
- Edwards, J.V.; Fontenot, K.; Liebner, F.; French, A.D.; Condon, B.D. Structure/function analysis of cotton-based peptide-cellulose conjugates: Spatiotemporal/kinetic assessment of protease aerogels compared to nanocrystalline and paper cellulose. Int. J. Mol. Sci. 2018, 19, 840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, M.; Chen, J.; Fan, S.; Liang, J.; Ding, L.; Chen, S. Flexible chitosan/carbon nanotubes aerogel, a robust matrix for in-situ growth and non-enzymatic biosensing applications. Sens. Actuators B Chem. 2016, 232, 750–757. [Google Scholar] [CrossRef]
- Fontenot, K.; Edwards, J.; Pircher, N.; Liebner, F.; Prevost, N. Peptide Derivatized Cellulosic Aerogel from Cotton As a Point of Care Diagnostic Protease Sensor; Amer Chemical Soc: Washington, DC, USA, 2016. [Google Scholar]
- Muñoz-Ruíz, A.; Escobar-García, D.M.; Quintana, M.; Pozos-Guillén, A.; Flores, H. Synthesis and characterization of a new collagen-alginate aerogel for tissue engineering. J. Nanomater. 2019, 2019, 2875375. [Google Scholar]
- Osorio, D.A.; Lee, B.E.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Kumari, R.; Dutta, P. Physicochemical and biological activity study of genipin-crosslinked chitosan scaffolds prepared by using supercritical carbon dioxide for tissue engineering applications. Int. J. Biol. Macromol. 2010, 46, 261–266. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; He, X.; Zhang, X.; Zhang, W.; Zhang, X. Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef]
- Gonçalves, V.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef]
- Mohammadian, M.; Kashi, T.S.J.; Erfan, M.; Soorbaghi, F.P. In-vitro study of Ketoprofen release from synthesized silica aerogels (as drug carriers) and evaluation of mathematical kinetic release models. Iran. J. Pharm. Res. IJPR 2018, 17, 818. [Google Scholar]
- Qin, L.; He, Y.; Zhao, X.; Zhang, T.; Qin, Y.; Du, A. Preparation, characterization, and in vitro sustained release profile of resveratrol-loaded silica aerogel. Molecules 2020, 25, 2752. [Google Scholar] [CrossRef]
- Wang, L.; Mu, R.-J.; Lin, L.; Chen, X.; Lin, S.; Ye, Q.; Pang, J. Bioinspired aerogel based on konjac glucomannan and functionalized carbon nanotube for controlled drug release. Int. J. Biol. Macromol. 2019, 133, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Castro, T.A.; Ibarra-Alonso, M.; Oliva, J.; Martínez-Luévanos, A. Porous aerogel and core/shell nanoparticles for controlled drug delivery: A review. Mater. Sci. Eng. C 2019, 96, 915–940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, W.; Zhang, X.; Meng, X.; Tong, G.; Deng, Y. Temperature-sensitive poly-NIPAm modified cellulose nanofibril cryogel microspheres for controlled drug release. Cellulose 2016, 23, 415–425. [Google Scholar] [CrossRef]
- Lovskaya, D.; Lebedev, A.; Menshutina, N. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Gao, M.; Zhao, Y.; Chen, Y. Sustained release of an essential oil by a hybrid cellulose nanofiber foam system. Cellulose 2020, 27, 2709–2721. [Google Scholar] [CrossRef]
- Han, W.; Han, D.W.; Chan, C.K.; Li, Y.; Chang, Q.; Yeung, K.L. silica alcogel containing essential oil for air and surface disinfections. Am. Chem. Soc. 2013, 245, 7–11. [Google Scholar]
- Uddin, K.M.; Orelma, H.; Mohammadi, P.; Borghei, M.; Laine, J.; Linder, M.; Rojas, O.J. Retention of lysozyme activity by physical immobilization in nanocellulose aerogels and antibacterial effects. Cellulose 2017, 24, 2837–2848. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Xuan, R.; Wang, Y.; Zou, C.; Zhang, Z.; Wan, Y.; Xu, Y. Flexible and monolithic zinc oxide bionanocomposite foams by a bacterial cellulose mediated approach for antibacterial applications. Dalton Trans. 2014, 43, 6762–6768. [Google Scholar] [CrossRef]
- Luong, N.D.; Lee, Y.; Nam, J.-D. Highly-loaded silver nanoparticles in ultrafine cellulose acetate nanofibrillar aerogel. Eur. Polym. J. 2008, 44, 3116–3121. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, D.; Wan, L.; Tan, H.; Fu, R. Adsorption and antibacterial activity of silver-dispersed carbon aerogels. J. Appl. Polym. Sci. 2006, 102, 1030–1037. [Google Scholar] [CrossRef]
- Bheekhun, N.; Talib, A.; Rahim, A.; Hassan, M.R. Aerogels in aerospace: An overview. Adv. Mater. Sci. Eng. 2013, 2013, 406065. [Google Scholar] [CrossRef]
- Pekala, R. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering strong silica aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.; Wang, Y.; Zhou, Z.; Zhang, X. Nanofibrillated cellulose as the support and reductant for the facile synthesis of Fe3O4/Ag nanocomposites with catalytic and antibacterial activity. J. Mater. Chem. A 2013, 1, 14910–14918. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.; Deng, H.; Hu, Y.; Li, B. Antibacterial multilayer films fabricated by layer-by-layer immobilizing lysozyme and gold nanoparticles on nanofibers. Colloids Surf. B Biointerfaces 2014, 116, 432–438. [Google Scholar] [CrossRef]
- Korehei, R.; Kadla, J.F. Encapsulation of T4 bacteriophage in electrospun poly (ethylene oxide)/cellulose diacetate fibers. Carbohydr. Polym. 2014, 100, 150–157. [Google Scholar] [CrossRef]
- Liu, K.; Chen, L.; Huang, L.; Ni, Y.; Sun, B. Enhancing antibacterium and strength of cellulosic paper by coating triclosan-loaded nanofibrillated cellulose (NFC). Carbohydr. Polym. 2015, 117, 996–1001. [Google Scholar] [CrossRef]
- Henschen, J.; Illergård, J.; Larsson, P.A.; Ek, M.; Wågberg, L. Contact-active antibacterial aerogels from cellulose nanofibrils. Colloids Surf. B Biointerfaces 2016, 146, 415–422. [Google Scholar] [CrossRef]
- Ye, S.; He, S.; Su, C.; Jiang, L.; Wen, Y.; Zhu, Z.; Shao, W. Morphological, release and antibacterial performances of amoxicillin-loaded cellulose aerogels. Molecules 2018, 23, 2082. [Google Scholar] [CrossRef]
- Xiao, Y.; Rong, L.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Sui, X. A light-weight and high-efficacy antibacterial nanocellulose-based sponge via covalent immobilization of gentamicin. Carbohydr. Polym. 2018, 200, 595–601. [Google Scholar] [CrossRef] [PubMed]
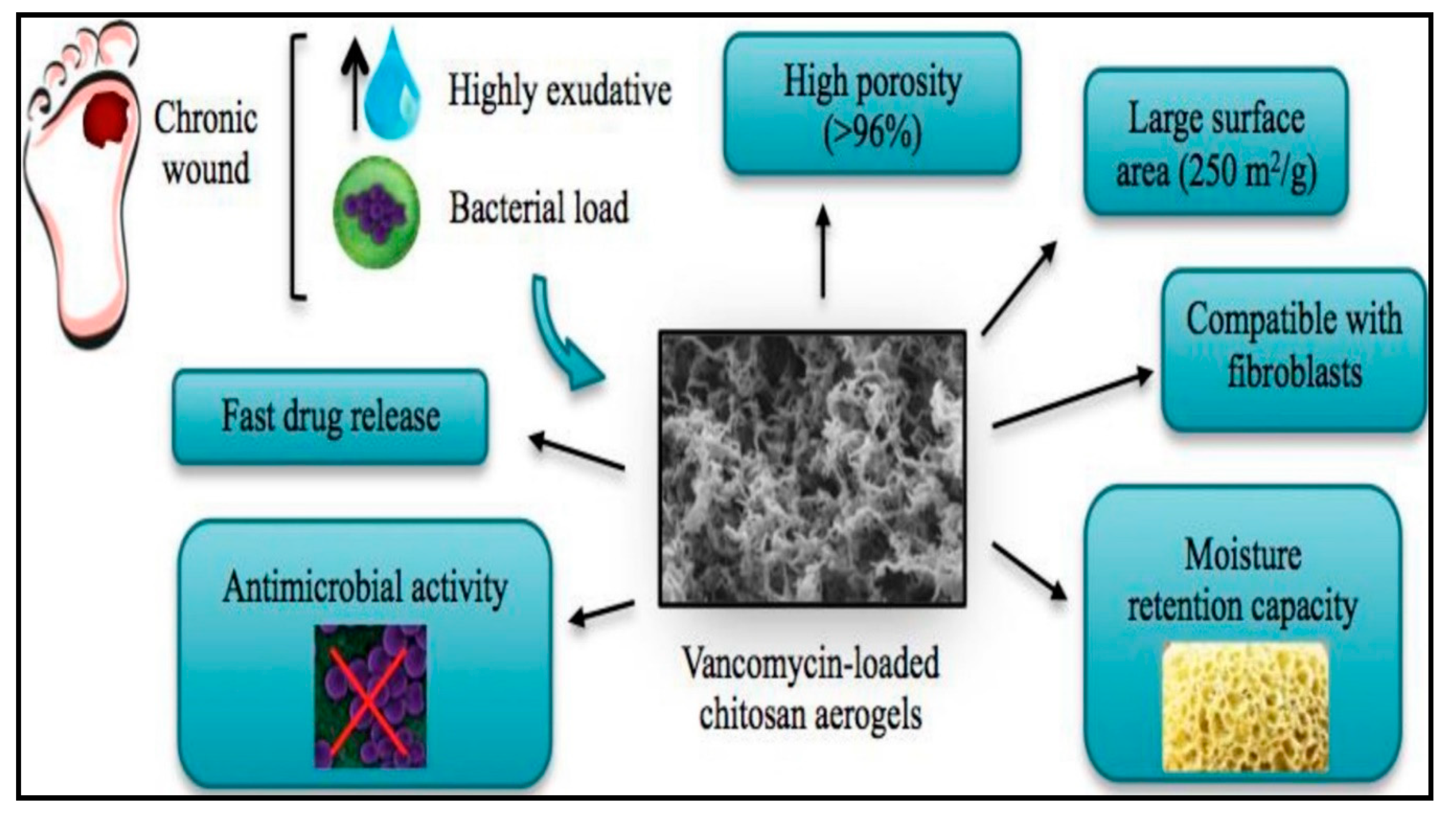
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Ren, X.; Huang, T.-S. N-halamine modified multiporous bacterial cellulose with enhanced antibacterial and hemostatic properties. Int. J. Biol. Macromol. 2020, 161, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Snorradóttir, B.S.; Hjálmarsdóttir, M.Á.; Sigurjónsson, Ó.E.; Másson, M. Experimental design for determining quantitative structure activity relationship for antibacterial chitosan derivatives. J. Mater. Chem. B 2016, 4, 4762–4770. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.d.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Wu, J.; Su, C.; Jiang, L.; Ye, S.; Liu, X.; Shao, W. Green and facile preparation of chitosan sponges as potential wound dressings. ACS Sustain. Chem. Eng. 2018, 6, 9145–9152. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Gurikov, P.; Monteiro, F.J.; Smirnova, I.; Alvarez-Lorenzo, C.; García-González, C.A. Jet cutting technique for the production of chitosan aerogel microparticles loaded with vancomycin. Polymers 2020, 12, 273. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, S.; Yan, J. Chitosan-Reinforced MFC/NFC Aerogel and Antibacterial Property. Adv. Polym. Technol. 2020, 2020, 7890215. [Google Scholar] [CrossRef]
- Wan, C.; Jiao, Y.; Sun, Q.; Li, J. Preparation, characterization, and antibacterial properties of silver nanoparticles embedded into cellulose aerogels. Polym. Compos. 2016, 37, 1137–1142. [Google Scholar] [CrossRef]
- Wasim, M.; Khan, M.R.; Mushtaq, M.; Naeem, A.; Han, M.; Wei, Q. Surface modification of bacterial cellulose by copper and zinc oxide sputter coating for uv-resistance/antistatic/antibacterial characteristics. Coatings 2020, 10, 364. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Dai, X.; Tao, T. Rapid Photocatalytic Degradation of pollutant from water under UV and sunlight via cellulose nanofiber aerogel wrapped by TiO2. J. Nanomater. 2018, 2018, 8752015. [Google Scholar] [CrossRef]
- Darpentigny, C.; Marcoux, P.R.; Menneteau, M.; Michel, B.; Ricoul, F.; Jean, B.; Bras, J.; Nonglaton, G. Antimicrobial cellulose nanofibril porous materials obtained by supercritical impregnation of thymol. ACS Appl. Bio Mater. 2020, 3, 2965–2975. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alhawari, S.M.; Amhimmid, K.; AbuAeshah, R.H.A.; Saada, A.O. Evaluation of in-vitroantibacterial activity of aqueous and alcoholic extracts of the peels punica granatum and olea europaea leaves. J. Sci. Technol. 2018, 2, 36–40. [Google Scholar]
- Navarro, J.R.; Rostami, J.; Ahlinder, A.; Mietner, J.B.; Bernin, D.; Saake, B.; Edlund, U. Surface-initiated controlled radical polymerization approach to in situ cross-link cellulose nanofibrils with inorganic nanoparticles. Biomacromolecules 2020, 21, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Han, G.; Wang, X.; Luo, J.; Sun, R. An ultra-light antibacterial bagasse–AgNP aerogel. J. Mater. Chem. B 2017, 5, 1155–1158. [Google Scholar] [CrossRef]
- Salomoni, R.; Léo, P.; Montemor, A.; Rinaldi, B.; Rodrigues, M. Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol. Sci. Appl. 2017, 10, 115. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Vijaya, J.J.; Jayaprakash, N.; Kombaiah, K.; Kaviyarasu, K.; Kennedy, L.J.; Ramalingam, R.J.; Al-Lohedan, H.A.; Mansoor-Ali, V.; Maaza, M. Bioreduction potentials of dried root of Zingiber officinale for a simple green synthesis of silver nanoparticles: Antibacterial studies. J. Photochem. Photobiol. B Biol. 2017, 177, 62–68. [Google Scholar] [CrossRef]
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vrček, I.V.; Tolić, S.; Štefanić, P.P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef]
- Tarrés, Q.; Oliver-Ortega, H.; Alcalà, M.; Espinach, F.X.; Mutjé, P.; Delgado-Aguilar, M. Research on the Strengthening Advantages on Using Cellulose Nanofibers as Polyvinyl Alcohol Reinforcement. Polymers 2020, 12, 974. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials—Binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Vignolini, S. Controlling the photonic properties of cholesteric cellulose nanocrystal films with magnets. Adv. Mater. 2017, 29, 1701469. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.W.; Wei, H.; Gauthier, A.C.; Song, J.; Jin, Y.; Xiao, H. Superhydrophobic modification of cellulose and cotton textiles: Methodologies and applications. J. Bioresour. Bioprod. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Kumari, P.; Pathak, G.; Gupta, R.; Sharma, D.; Meena, A. Cellulose nanofibers from lignocellulosic biomass of lemongrass using enzymatic hydrolysis: Characterization and cytotoxicity assessment. DARU J. Pharm. Sci. 2019, 27, 683–693. [Google Scholar] [CrossRef]
- Lee, I.; Kim, S.-H.; Rethinasabapathy, M.; Haldorai, Y.; Lee, G.-W.; Choe, S.R.; Jang, S.-C.; Kang, S.-M.; Han, Y.-K.; Roh, C. Porous 3D Prussian blue/cellulose aerogel as a decorporation agent for removal of ingested cesium from the gastrointestinal tract. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Souza, S.F.; Mariano, M.; Reis, D.; Lombello, C.B.; Ferreira, M.; Sain, M. Cell interactions and cytotoxic studies of cellulose nanofibers from Curauá natural fibers. Carbohydr. Polym. 2018, 201, 87–95. [Google Scholar] [CrossRef]
- Nordli, H.R.; Chinga-Carrasco, G.; Rokstad, A.M.; Pukstad, B. Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr. Polym. 2016, 150, 65–73. [Google Scholar] [CrossRef]
- Batista, M.; Gonçalves, V.S.; Gaspar, F.; Nogueira, I.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef]
- Trucillo, P.; Cardea, S.; Baldino, L.; Reverchon, E. Production of liposomes loaded alginate aerogels using two supercritical CO2 assisted techniques. J. CO2 Util. 2020, 39, 101161. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Huang, Z.; Liu, S.; Yu, H.; Teng, C. Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol as potential wound dressing: In vitro and in vivo evaluation. Compos. Part B Eng. 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; El-Naggar, M.E.; Elsherbiny, D.A.; Ali, S.; Abdel-Aziz, M.S.; Abdel-Monem, Y.K. Antibacterial carrageenan/cellulose nanocrystal system loaded with silver nanoparticles, prepared via solid-state technique. J. Environ. Chem. Eng. 2020, 8, 104276. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Schreml, S.; Meier, R.J.; Kirschbaum, M.; Kong, S.C.; Gehmert, S.; Felthaus, O.; Küchler, S.; Sharpe, J.R.; Wöltje, K.; Weiß, K.T. Luminescent dual sensors reveal extracellular pH-gradients and hypoxia on chronic wounds that disrupt epidermal repair. Theranostics 2014, 4, 721. [Google Scholar] [CrossRef]
- Kur-Piotrowska, A.; Bukowska, J.; Kopcewicz, M.M.; Dietrich, M.; Nynca, J.; Slowinska, M.; Gawronska-Kozak, B. Foxn1 expression in keratinocytes is stimulated by hypoxia: Further evidence of its role in skin wound healing. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- He, H.; Xia, D.L.; Chen, Y.P.; Li, X.D.; Chen, C.; Wang, Y.F.; Shen, L.; Hu, Y.L.; Gu, H.Y. Evaluation of a two-stage antibacterial hydrogel dressing for healing in an infected diabetic wound. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wang, C. A facile restructuring of 3D high water absorption aerogels from methoxy polyethylene glycol-polycaprolactone (mPEG-PCL) nanofibers. Mater. Sci. Eng. C 2019, 94, 965–975. [Google Scholar] [CrossRef]
- Sreekumar, P.; Thomas, S.P.; marc Saiter, J.; Joseph, K.; Unnikrishnan, G.; Thomas, S. Effect of fiber surface modification on the mechanical and water absorption characteristics of sisal/polyester composites fabricated by resin transfer molding. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1777–1784. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Ko, E.; Kim, H. Preparation of chitosan aerogel crosslinked in chemical and ionical ways by non-acid condition for wound dressing. Int. J. Biol. Macromol. 2020, 164, 2177–2185. [Google Scholar] [CrossRef]
- Lu, H.; Yuan, L.; Yu, X.; Wu, C.; He, D.; Deng, J. Recent advances of on-demand dissolution of hydrogel dressings. Burn. Trauma 2018, 6, 35. [Google Scholar] [CrossRef]
- Deuber, F.; Adlhart, C. Tailoring the microstructure of ultra-light nanofiber aerogels by solid templating and their application as wound dressing materials. À Jour SVC Chem. Life Sci. Biotechnol. 2018, 18, 17–19. [Google Scholar]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef]
- Uzun, M. Developments in nonwovens for wound dressings. In Advances in Technical Nonwovens; Elsevier: Amsterdam, The Netherlands, 2016; pp. 443–472. [Google Scholar]
- Gould, L.J.; Abadir, P.M.; White-Chu, E.F. Age, frailty, and impaired wound healing. Princ. Pract. Geriatr. Surg. 2020, 2020, 465–482. [Google Scholar]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
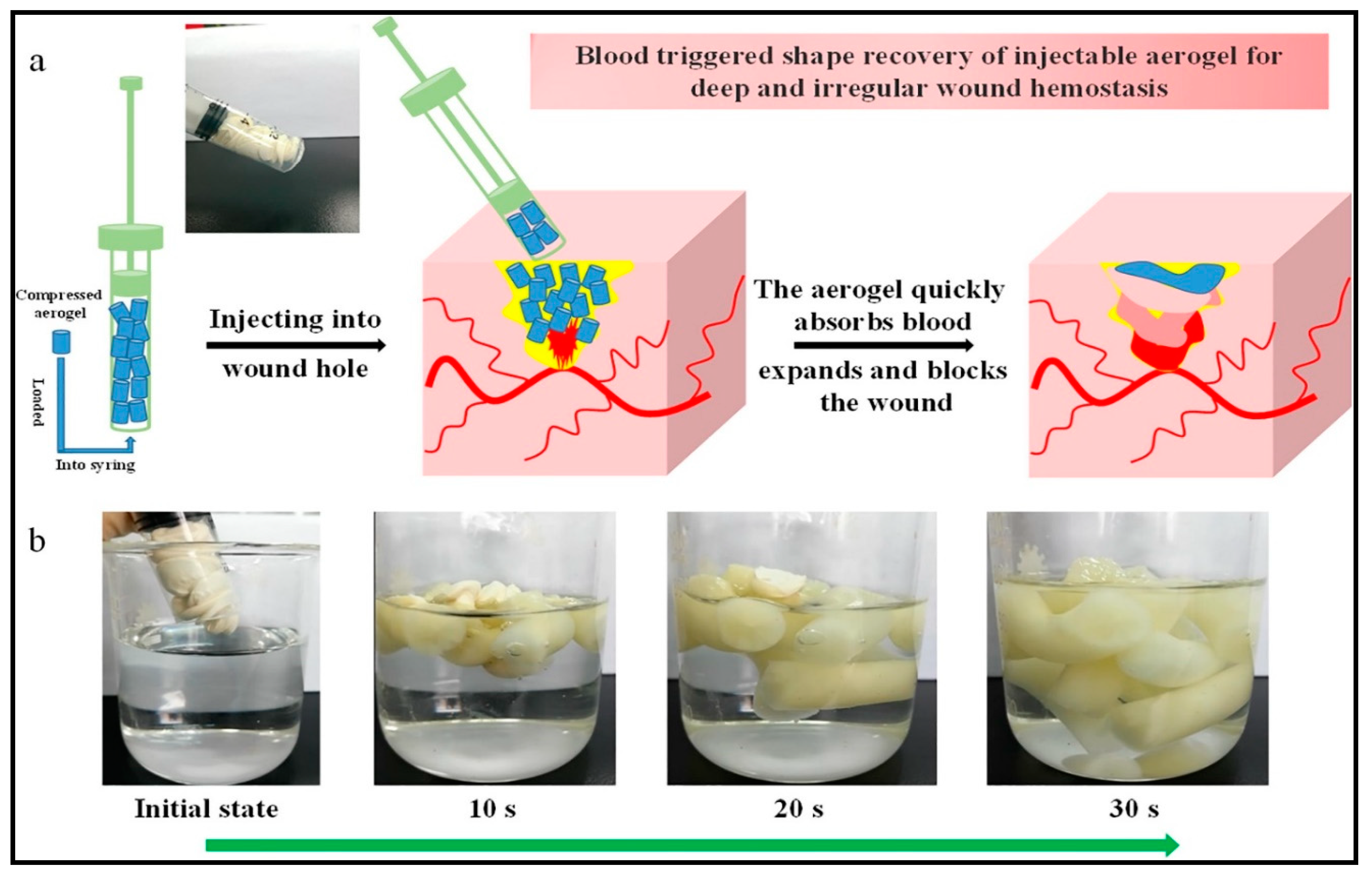
- Fan, X.; Li, Y.; Li, X.; Wu, Y.; Tang, K.; Liu, J.; Zheng, X.; Wan, G. Injectable antibacterial cellulose nanofiber/chitosan aerogel with rapid shape recovery for noncompressible hemorrhage. Int. J. Biol. Macromol. 2020, 154, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Uzun, M. Testing dressings and wound management materials. In Advanced Textiles for Wound Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–54. [Google Scholar]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing from Nature: Biopolymers and biocomposites as smart wound care materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; James, R.; Kumbar, S.G.; Laurencin, C.T. Chitosan as a biomaterial: Structure, properties, and applications in tissue engineering and drug delivery. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 91–113. [Google Scholar]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crop. Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Bangde, P.; Dandekar, P.; Adivarekar, R.V. Cytotoxicity and hemostatic activity of chitosan/carrageenan composite wound healing dressing for traumatic hemorrhage. Carbohydr. Polym. 2020, 239, 116106. [Google Scholar] [CrossRef]
- Choudhary, P.; Ramalingam, B.; Das, S.K. Fabrication of Chitosan Reinforced Multifunctional Graphene Nanocomposite as Antibacterial Scaffolds for Hemorrhage Control and wound Healing Application. ACS Biomater. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Li, N.; Wan, G.; Ali, M.A.; Tang, K. Rapid hemostatic chitosan/cellulose composite sponge by alkali/urea method for massive haemorrhage. Int. J. Biol. Macromol. 2020, 146, 2769–2778. [Google Scholar] [CrossRef]
- Teixeira, M.L.; Marcussi, S.; de CS Rezende, D.A.; Magalhães, M.L.; Nelson, D.L.; das G Cardoso, M. Essential oil from lippia origanoides (verbenaceae): Haemostasis and enzymes activity alterations. Med. Chem. 2019, 15, 207–214. [Google Scholar] [CrossRef]
- Moghimi, R.; Aliahmadi, A.; Rafati, H. Antibacterial hydroxypropyl methyl cellulose edible films containing nanoemulsions of Thymus daenensis essential oil for food packaging. Carbohydr. Polym. 2017, 175, 241–248. [Google Scholar] [CrossRef]
- Pereira, S.G.; Moura, J.; Carvalho, E.; Empadinhas, N. Microbiota of chronic diabetic wounds: Ecology, impact, and potential for innovative treatment strategies. Front. Microbiol. 2017, 8, 1791. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Endes, C.; Mueller, S.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D.; Foster, E.J. Elucidating the potential biological impact of cellulose nanocrystals. Fibers 2016, 4, 21. [Google Scholar] [CrossRef]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef]
- Endes, C.; Camarero-Espinosa, S.; Mueller, S.; Foster, E.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M. A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnology 2016, 14, 78. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alfallous, K.A.; Wali, A.; Hameid, S.; Zwaid, H. Growth Rate and Antibiotic Sensitivity Effect of Some Natural and Petroleum Based Materials on Staphylococcus aureus. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 7–11. [Google Scholar]
- Pan, X.; Redding, J.E.; Wiley, P.A.; Wen, L.; McConnell, J.S.; Zhang, B. Mutagenicity evaluation of metal oxide nanoparticles by the bacterial reverse mutation assay. Chemosphere 2010, 79, 113–116. [Google Scholar] [CrossRef]


| Biopolymer | Biological Properties | Molecular Structure | References |
|---|---|---|---|
| Chitosan | - Hemostatic agent due to positive charges that can bind to negative charges on red blood cells. - Antibacterial and anti-fungal. - Mucoadhesive properties - Wound healing acceleration and immune system stimulation. | 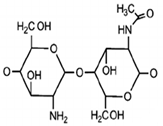 | [29] |
| Cellulose | - High water absorption and holding capacities. - Good wound exudates drainage capacity. - Support and enhance the growth and proliferation of cells. | 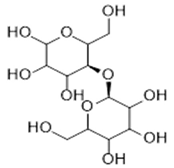 | [30] |
| Alginates | - Preserving a solid-like attribute at acidic conditions. - Hemostatic properties, which are useful for bleeding wounds. - Good mucoadhesive properties. - Barrier protects immobilized material toward physical stress. | 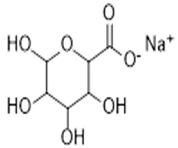 | [31] |
| Year | Type of Aerogels | Remark/Incorporated Antibacterial Material | Reference |
|---|---|---|---|
| 1931 | Silica aerogel | The first invention of aerogel. | [14] |
| 1968 | Metal oxide and silica | Development of sol-gel route for aerogel fabrication. | [95] |
| 1989 | Polymer aerogels | Organic and carbon aerogel using sol-gel route. | [96] |
| 1997 | Polymer aerogels | Ultralight aerogels using cross-linking techniques. | [97] |
| 2006 | Carbon-based aerogels | Silver as antibacterial material and direct immersion of organic aerogels in aqueous AgNO3 solutions. | [94] |
| 2008 | Nanocellulose-based aerogel | The first use of silver nanoparticles for antibacterial properties in nanocellulose aerogel. | [93] |
| 2011 | Chitosan aerogels | Antibacterial mesoporous pure chitosan aerogels. | [37] |
| 2013 | Nanofibrillated cellulose aerogel | Iron oxide and silver nanoparticles dispersed in nanofibrillated cellulose aerogel. | [98] |
| 2013 | Silica alcogel | Essential oils of medicinal plants used as antibacterial materials for air and surface disinfection. | [90] |
| 2014 | Cellulose-based aerogel | Lysozyme, Zinc oxide and gold nanoparticles immobilized in the cellulose network as antibacterial agents. | [92,99] |
| 2014 | Cellulose diacetate fibers | Bacteriophage used as an antibacterial agent, which encapsulate within the cellulose fibers. | [100] |
| 2015 | Cellulosic-based paper | Triclosan used as antibacterial material, in addition to improving the strength. | [101] |
| 2016 | Cellulose aerogels | Layer-by-layer surface-modified cellulose aerogel was able to adhere to bacterial cells from aquatic solutions. | [102] |
| 2017 | Nanocellulose aerogels | Lysozyme enzyme immobilized inside the cellulose aerogel as antibacterial material. | [91] |
| 2018 | Cellulose-based aerogels | Amoxicillin and gentamicin antibiotics loaded in the microcrystalline cellulose network. | [103,104] |
| 2019 | Chitosan aerogel | Vancomycin antibiotic-loaded inside the chitosan aerogel. | [105] |
| 2020 | Bacterial cellulose aerogel | Modified cellulose with N-isopropyl acrylamide, which used as an antibacterial agent after enduring chlorination. | [106] |
| 2020 | Nanocellulose aerogel | Essential oils as a natural antibacterial agent in the aerogel. | [89] |
| Functionality | Hydrogels | Aerogels | Reference |
|---|---|---|---|
| Fluids absorption in wet wounds | Does not absorb fluids and cannot be used in wet wounds. | Able to absorb large amounts of fluids in wet wounds. | [145,146] |
| Stop hemorrhage | Not applicable. | Can be used to absorb fluid and expand to fill the wound. | [147] |
| Mechanical stability | Poor mechanical strength and liable to tearing easily. | Excellent mechanical strength and easy to handle. | [148] |
| Wounds aeration | Does not provide aeration to wounds. | The high porosity of aerogels provides suitable aeration for wounds. | [149] |
| Potential cytotoxicity | Relatively higher | Lower cytotoxicity. | [150] |
| Reliability for patients | Difficult for patients to change their dressings. | Easy for patients to change their dressings. | [151] |
| Cost of production | Lower cost of production. | Higher cost of production. | [152] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, E.B.; Jummaat, F.; Amirul, A.A.; Adnan, A.S.; Olaiya, N.G.; Abdullah, C.K.; Rizal, S.; Mohamad Haafiz, M.K.; Khalil, H.P.S.A. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics 2020, 9, 648. https://doi.org/10.3390/antibiotics9100648
Yahya EB, Jummaat F, Amirul AA, Adnan AS, Olaiya NG, Abdullah CK, Rizal S, Mohamad Haafiz MK, Khalil HPSA. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics. 2020; 9(10):648. https://doi.org/10.3390/antibiotics9100648
Chicago/Turabian StyleYahya, Esam Bashir, Fauziah Jummaat, A. A. Amirul, A. S. Adnan, N. G. Olaiya, C. K. Abdullah, Samsul Rizal, M. K. Mohamad Haafiz, and H. P. S. Abdul Khalil. 2020. "A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery" Antibiotics 9, no. 10: 648. https://doi.org/10.3390/antibiotics9100648
APA StyleYahya, E. B., Jummaat, F., Amirul, A. A., Adnan, A. S., Olaiya, N. G., Abdullah, C. K., Rizal, S., Mohamad Haafiz, M. K., & Khalil, H. P. S. A. (2020). A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics, 9(10), 648. https://doi.org/10.3390/antibiotics9100648










