Benzoxazole-Based Metal Complexes to Reverse Multidrug Resistance in Bacteria
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activity
2.2. Resistance Modulation Assay
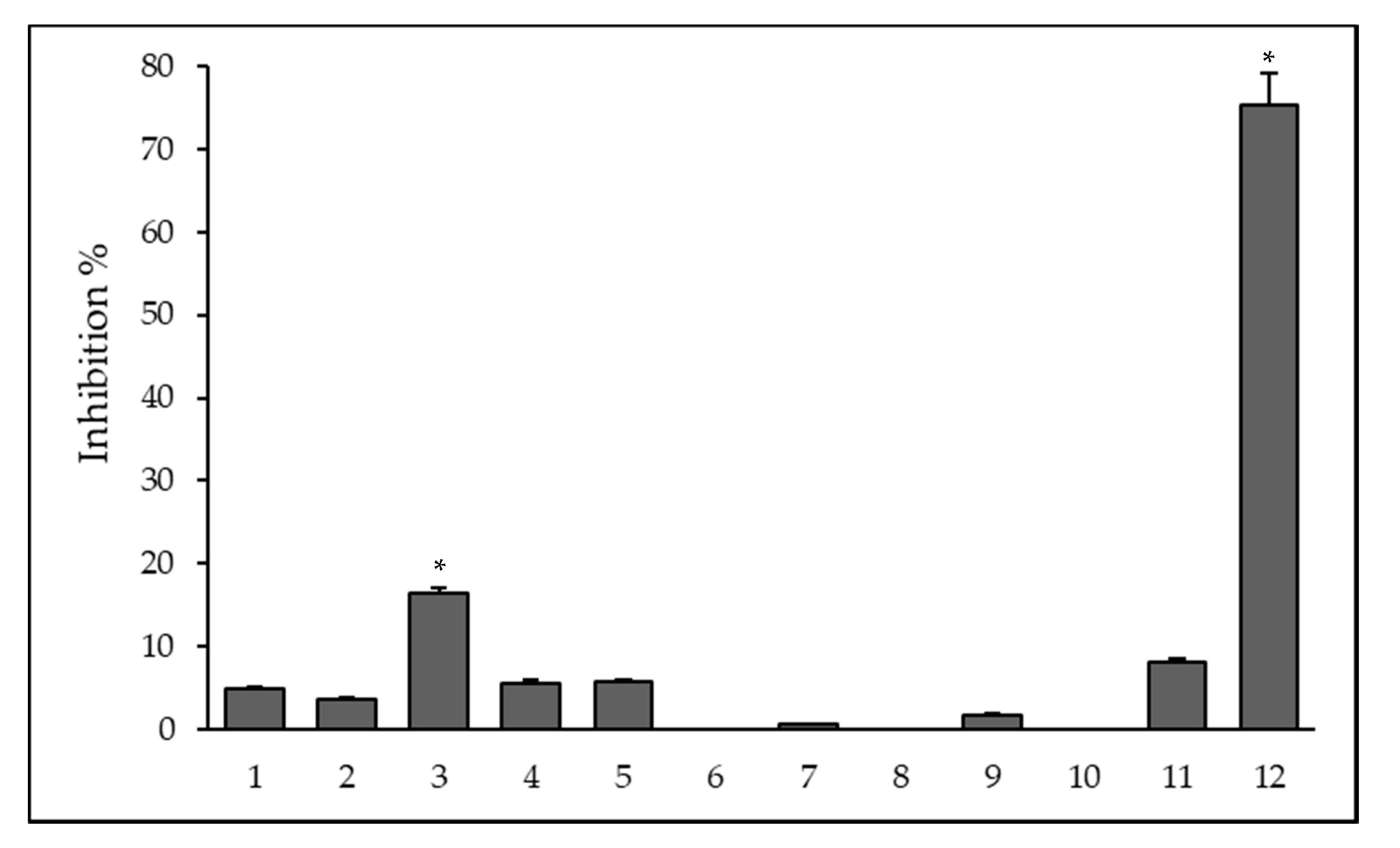
2.3. Efflux Pump Inhibiting Activity
2.4. Anti-Biofilm Activity
2.5. Inhibition of Quorum Sensing
2.6. Cytotoxic Effect on Human Cell Line
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Bacterial Strains
4.3. Cell Culture
4.4. Determination of Minimum Inhibitory Concentrations
4.5. Combination Effect
4.6. Real-Time Accumulation Assay
4.7. Anti-Biofilm Effect
4.8. Assay for Quorum Sensing Inhibition
4.9. Assay for Cytotoxic Effect
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019; pp. 1–29. [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Wang-Kan, X.; Blair, J.M.A.; Chirullo, B.; Betts, J.; La Ragione, R.M.; Ivens, A.; Ricci, V.; Opperman, T.J.; Piddock, L.J.V. Lack of AcrB efflux function confers loss of virulence on Salmonella enterica serovar Typhimurium. mBio 2017, 8, e00968-17. [Google Scholar] [CrossRef]
- Santos, A.L.S.D.; Galdino, A.C.M.; de Mello, T.P.; de Ramos, L.S.; Branquinha, M.H.; Bolognese, A.M.; Columbano Neto, J.; Roudbary, M. What are the advantages of living in a community? A microbial biofilm perspective! Mem. Inst. Oswaldo Cruz 2018, 113, e180212. [Google Scholar] [CrossRef] [PubMed]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef]
- Saxena, P.; Joshi, Y.; Rawat, K.; Bisht, R. Biofilms: Architecture, resistance, quorum sensing and control mechanisms. Indian J. Microbiol. 2019, 59, 3–12. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small organometallic compounds as antibacterial agents. Dalton Trans. 2012, 41, 6350–6358. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef]
- Imramovsky, A.; Kozic, J.; Pesko, M.; Stolarikova, J.; Vinsova, J.; Kralova, K.; Jampilek, J. Synthesis and antimycobacterial and photosynthesis-inhibiting evaluation of 2-[(E)-2-substituted-ethenyl]-1,3-benzoxazoles. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, J.; Macho, J.M.; Jiang, X.; Xie, D.; Jiang, F.; Liu, W.; Fu, L. Design, synthesis and antimicrobial evaluation of novel benzoxazole derivatives. Eur. J. Med. Chem. 2017, 126, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Berry, J.M.; Bradshaw, T.D.; Burger, A.M.; Seaton, A.; Wang, B.; Westwell, A.D.; Stevens, M.F.G. 4-substituted 4-hydroxycyclohexa-2,5-dien-1-ones with selective activities against colon and renal cancer cell lines. J. Med. Chem. 2003, 46, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Harada, H.; Saito, S.; Cui, J.; Tani, S. In vitro susceptibility of Helicobacter pylori to trifluoromethyl ketones. Bioorg. Med. Chem. Lett. 1999, 9, 193–194. [Google Scholar] [CrossRef]
- Kawase, M.; Motohashi, N.; Sakagami, H.; Kanamoto, T.; Nakashima, H.; Ferenczy, L.; Wolfard, K.; Miskolci, C.; Molnár, J. Antimicrobial activity of trifluoromethyl ketones and their synergism with promethazine. Int. J. Antimicrob. Agents 2001, 18, 161–165. [Google Scholar] [CrossRef]
- Zoltan, V.; Armada, A.; Cerca, P.; Amaral, L.; Savka, M.A.; Szegedi, E.; Kawase, M.; Motohashi, N.; Molnár, J. Inhibition of quorum sensing and efflux pump system by trifluoromethyl ketone proton pump inhibitors. In Vivo 2012, 26, 277–289. [Google Scholar]
- Watanabe, G.; Sekiya, H.; Tamai, E.; Saijo, R.; Uno, H.; Mori, S.; Tanaka, T.; Maki, J.; Kawase, M. Synthesis and antimicrobial activity of 2-trifluoroacetonylbenzoxazole ligands and their metal complexes. Chem. Pharm. Bull. 2018, 66, 732–740. [Google Scholar] [CrossRef]
- Spengler, G.; Kincses, A.; Rácz, B.; Varga, B.; Watanabe, G.; Saijo, R.; Sekiya, H.; Tamai, E.; Maki, J.; Molnár, J.; et al. Benzoxazole-based Zn(II) and Cu(II) complexes overcome multidrug-resistance in cancer. Anticancer Res. 2018, 38, 6181–6187. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef]
- Hunoor, R.S.; Patil, B.R.; Badiger, D.S.; Vadavi, R.S.; Gudasi, K.B. 2D HETCOR studies of 1,2-dihydroquinazolinone derivative: Synthesis, characterization and anti-microbial study of its transition metal complexes. Pharm. Chem. 2010, 2, 116–128. [Google Scholar]
- Alaudeen, M.; Sushama, P.G.; Dorothy, A.M. Synthesis and spectroscopic investigation of metal chelates of hydrazo pyrazolone derivative. Asian J. Chem. 2007, 19, 3403–3411. [Google Scholar]
- Gajdács, M. Multidrug Resistance Reversing Activity of Organoselenium Compounds. Ph.D Thesis, University of Szeged, Szeged, Hungary, 2018. [Google Scholar]
- CLSI. Susceptibility Testing Process. In Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 10th ed.; Christopher, P.J., Polgar, E.P., Eds.; Clinical and Laboratory Standards Institute: Wayne, MI, USA, 2015; Volume 32, pp. 15–19. [Google Scholar]
- Viveiros, M.; Martins, A.; Paixão, L.; Rodrigues, L.; Martins, M.; Couto, I.; Fähnrich, E.; Kern, W.V.; Amaral, L. Demonstration of intrinsic efflux activity of E. coli K-12 AG100 by an automated ethidium bromide method. Int. J. Antimicrob. Agents 2008, 31, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.G.; Szabó, M.A.; Schelz, Z.; Szegedi, E.; Amaral, L.; Molnar, J. Quorum sensing inhibition by phenothiazines and related compounds. Lett. Drug Des. Discov. 2011, 8, 133–137. [Google Scholar] [CrossRef]
| Compound | MIC (µM) | |||||
|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | S. aureus 272123 (MRSA) | E. coli AG100 | E. coli AG100 A | C. violaceum 026 | E. cloacae 31298 | |
| 1 | 25 | 12.5 | 50 | 50 | >100 | >100 |
| 2 | 12.5 | 3.125 | 50 | 25 | >100 | >100 |
| 3 | 100 | 50 | 50 | 25 | >100 | >100 |
| 4 | 12.5 | 6.25 | 25 | 25 | >100 | >100 |
| 5 | 12.5 | 3.125 | 50 | 12.5 | >100 | >100 |
| 6 | 25 | 12.5 | 50 | 12.5 | >100 | >100 |
| 7 | 12.5 | 3.125 | >100 | 12.5 | >100 | >100 |
| 8 | 25 | 6.25 | 50 | 25 | >100 | >100 |
| 9 | 25 | 6.25 | 50 | 25 | >100 | >100 |
| 10 | >100 | >100 | >100 | >100 | >100 | >100 |
| 11 | 25 | 12.5 | 25 | 50 | >100 | >100 |
| 12 | 50 | 25 | >100 | 50 | >100 | >100 |
| Compound 1 | CIPMIC (µM) Combination with Compounds | |
|---|---|---|
| S. aureus MRSA | E. coli AG100 | |
| 3 | 6.25 | 0.016 |
| 5 | 6.25 | 0.016 |
| 7 | 6.25 | 0.016 |
| 8 | 6.25 | 0.008 |
| 9 | 6.25 | 0.008 |
| 12 | 6.25 | 0.016 |
| CIPMIC | 12.5 | 0.016 |
| Compound | RFI | |||
|---|---|---|---|---|
| S. aureus ATCC 25923 | S. aureus 272123 (MRSA) | E. coli AG100 | E. coli AG100 A | |
| 1 | −0.11 | −0.14 | −0.35 | 0 |
| 2 | −0.07 | −0.07 | 0.17 | −0.01 |
| 3 | −0.01 | 0.13 | −0.07 | −0.03 |
| 4 | −0.03 | −0.11 | −0.10 | −0.17 |
| 5 | 0.03 | −0.13 | 0.37 | −0.19 |
| 6 | 0.08 | −0.02 | 0.08 | −0.15 |
| 7 | 0.12 | −0.01 | −0.06 | 0.11 |
| 8 | −0.20 | −0.01 | 0.11 | −0.15 |
| 9 | −0.10 | −0.01 | −0.01 | −0.03 |
| 10 | 0 | 0 | −0.06 | 0 |
| 11 | 0.11 | −0.11 | 0.37 | 0.12 |
| 12 | −0.02 | −0.02 | 0.04 | 0.09 |
| CPZ 1 | 0.17 | - | 0.38 | 0.48 |
| VER 2 | - | 0.14 | - | - |
| Compound | Inhibition Zone in mm | SD 1 (±) |
|---|---|---|
| 3 | 56 | 3.3 |
| 4 | 47 | 2.1 |
| 5 | 16 | 0.9 |
| 11 | 53 | 1.4 |
| PMZ | 16 | 1.8 |
| Compounds | MRC-5 | |
|---|---|---|
| IC50 1 (µM) | SD 2 (±) | |
| 1 | >100 | - |
| 2 | >100 | - |
| 3 | 44.45 | 2.72 |
| 4 | 17.47 | 4.82 |
| 5 | 24.57 | 1.42 |
| 6 | 4.72 | 0.01 |
| 7 | 76.10 | 0.89 |
| 8 | 12.24 | 0.62 |
| 9 | >100 | - |
| 10 | 89.11 | 6.02 |
| 11 | 65.89 | 3.8 |
| 12 | >100 | - |

| Compound | R | M | Structure 1 | Molecular Weight |
|---|---|---|---|---|
| 1 | CF3 | − | − | 229 |
| 2 | CF2Cl | − | − | 245.6 |
| 3 | CF2H | − | − | 211 |
| 4 | CF3 | Zn | L2Zn | 522 |
| 5 | CF2Cl | Zn | L2Zn | 554.6 |
| 6 | CF2H | Zn | L2Zn | 486 |
| 7 | CF3 | Cu | L2Cu | 520 |
| 8 | CF3 | Ni | L2Ni | 551 (apical 2H2O) |
| 9 | CF3 | Mg | L2Mg | 481 |
| 10 | CF3 | Pd | L2Pd | 563 |
| 11 | CF3 | Ag | L2Ag2 | 672 |
| 12 | CF3 | Fe | L3Fe | 740 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kincses, A.; Szabó, S.; Rácz, B.; Szemerédi, N.; Watanabe, G.; Saijo, R.; Sekiya, H.; Tamai, E.; Molnár, J.; Kawase, M.; et al. Benzoxazole-Based Metal Complexes to Reverse Multidrug Resistance in Bacteria. Antibiotics 2020, 9, 649. https://doi.org/10.3390/antibiotics9100649
Kincses A, Szabó S, Rácz B, Szemerédi N, Watanabe G, Saijo R, Sekiya H, Tamai E, Molnár J, Kawase M, et al. Benzoxazole-Based Metal Complexes to Reverse Multidrug Resistance in Bacteria. Antibiotics. 2020; 9(10):649. https://doi.org/10.3390/antibiotics9100649
Chicago/Turabian StyleKincses, Annamária, Stefánia Szabó, Bálint Rácz, Nikoletta Szemerédi, Genki Watanabe, Ryosuke Saijo, Hiroshi Sekiya, Eiji Tamai, Joseph Molnár, Masami Kawase, and et al. 2020. "Benzoxazole-Based Metal Complexes to Reverse Multidrug Resistance in Bacteria" Antibiotics 9, no. 10: 649. https://doi.org/10.3390/antibiotics9100649
APA StyleKincses, A., Szabó, S., Rácz, B., Szemerédi, N., Watanabe, G., Saijo, R., Sekiya, H., Tamai, E., Molnár, J., Kawase, M., & Spengler, G. (2020). Benzoxazole-Based Metal Complexes to Reverse Multidrug Resistance in Bacteria. Antibiotics, 9(10), 649. https://doi.org/10.3390/antibiotics9100649






