Abstract
Background/Objectives: The propagation of antibiotic resistance genes (ARGs) poses a huge threat to environmental and human health. The ballast water from ships has been recognized as an important vector of ARGs. However, little is known about how ballast water from geographically isolated water affects ARGs in receiving waters. Methods: Herein, we investigated the changes in ARGs in receiving water by microcosm experiments simulating the discharge of ballast water. Results: The simulated discharge of ballast water increased the abundances of target ARGs, which were 1.3–5.6-fold higher in the mixture of ballast water and receiving water (microcosm M) than in receiving water at the end of the experiment. The enrichment of target ARGs was significantly associated with MGEs. Moreover, the discharge of ballast water changed the microbial communities in receiving water. Further network analysis identified potential ARG hosts, such as Pseudohongiellaa and Amphritea, with the abundance in microcosm M (0.23% and 0.036%) being higher than in receiving water (0.09% and 0.006%), the changes of which might be responsible for ARG variations. Conclusions: Overall, our findings suggest the discharge of ballast water might promote the spread of ARGs in different geographical waters and the corresponding ecological risks should not be ignored.
1. Introduction
Antibiotics are widely used in human medicine and aquaculture to treat bacterial infections and diseases [1]. Many residual antibiotics have entered the environment, resulting in the dissemination and enrichment of antibiotic resistance genes (ARGs) [2,3]. ARGs are now recognized as an emerging pollutant as they can spread rapidly in the environment through horizontal gene transfer mediated by mobile genetic elements (MGEs) [4,5]. The prevalence and dissemination of ARGs have become global problems for environmental systems and public health. At present, ARGs have been largely detected in aquatic environments, including rivers, lakes, and seawater. Several studies have reported that the discharge of wastewater and aquaculture waste could accelerate the enrichment of ARGs downstream or in the nearby waters [6,7]. Therefore, it was critical to understand the effect of the introduction or discharge of exogenous water on ARGs in the receiving water to control the development of antibiotic resistance.
Shipping is the main means of freight transport and accounts for more than 90% of the global freight volume. Ballast water is necessary for safe navigation as it maintains a ship’s balance [8]. Nearly 10 billion tons of ballast water are transferred annually worldwide [9]. Ballast water is a considerable pathway of biological invasion as it contains harmful algae, bacterial pathogens, and viruses [10,11,12,13,14]. Notably, the ballast water has become the hotpot of ARGs. In total, 26 types and 710 subtypes of ARGs were detected in ballast water from 13 ships from 11 countries and regions [15], which was more diverse than that in some natural water, such as the Pearl River [16] and sixteen estuaries in China [17]. The abundance of tetracycline ARGs in ballast water was even higher than that in ocean water [18]. Predictably, ballast water should be regarded as the potential hotpot for the transmission of ARGs across geographically isolated waters.
It was well known that the ecological microflora of the incoming ships’ ballast and receiving water bodies are obviously different as the departure and destination ports of foreign-going vessels are always far from each other [19,20,21]. Previous studies reported that the presence of ARGs was strongly linked to bacterial composition because of bacteria acting as important hosts of ARGs [22]. When the ballast water enters the receiving water, the species that are unable to adapt to the new environment may be eliminated while some species may become dominant as they are able to survive and grow [11,23]. As a result, it is largely unknown whether and how the bacterial composition and ARG hosts can be changed; thus, it is unknown how the inclusion of ballast water affects the ARGs in the receiving waters.
In this study, the discharge of ballast water was simulated by performing ballast water/receiving water microcosm experiments. The variation in ARGs/MGEs and bacterial communities in the microcosms was determined. The aims of this study were to (i) investigate the effects of ballast water discharge on the abundance of ARGs in receiving waters and (ii) explore the shift in MGEs and bacterial communities and their relationships with ARG variation in receiving waters. To our knowledge, this study provides the first evidence of the dissemination of ARGs in receiving waters that are affected by ballast water.
2. Results and Discussion
2.1. ARG Profiles in Initial Samples
A total of 41 and 25 subtypes of ARGs were annotated for the ballast and receiving water samples, respectively (Figure 1a, Table S3). The top 10 most abundant ARGs included bacA, mexW, mexF, sul1, fosX, acrB, vatE, aac(6″)-ib, ksgA, and aac(3″)-ia. These ARG subtypes belonged to the class of sulfonamides, tetracyclines, β-lactams, aminoglycosides, streptomycins, quinolones, multidrug, and other resistance genes, and their percentages were 13.0% and 10.5%; 8.4% and 7.1%; 10.7% and 26.6%; 5.9% and 11.4%; 1.5% and 7.0%; 0% and 0.3%; 46.8% and 17.8%; and 13.7% and 19.1% in the ballast and receiving waters, respectively (Figure 1b). These results indicated that diverse and abundant ARGs exist in these two samples, especially the ballast waters. It is well known that most of the ballast water comes from the inshore waters, which are susceptible to contamination by antibiotics from aquaculture and sewage [2,24]. Moreover, the profile of ARGs was obviously different between the ballast and receiving waters. The relative abundance of sulfonamides, tetracyclines, and multidrug resistance genes was higher in ballast water compared to receiving water, while the aminoglycosides, β-lactams, and streptomycins resistance genes showed the opposite trend. Those results suggested the potential risk of ARG dissemination through ballast water.
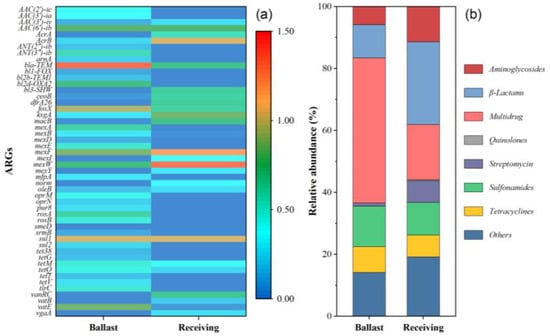
Figure 1.
Abundance of the ARG subtypes (a) and relative percentages of ARG types (b) in initial ballast and receiving water.
2.2. Changes in ARGs and MGEs in Microcosms
In order to further understand the effects of ballast water on ARGs in receiving water, several target ARGs that dominated in the four ARG classes (sulfonamides, tetracyclines, β-lactams, and aminoglycosides) were quantified in the initial ballast and receiving water, in addition to three microcosms by qPCR. The abundance of sul1, tetM, tetQ, and blaTEM was 1.7 × 10−4 and 1.2 × 10−5; 1.8 × 10−6 and 9.1 × 10−7; 3.3 × 10−6 and 2.1 × 10−6; and 3.6 × 10−7 and 1.2 × 10−7 copies/16S rRNA in the initial ballast and receiving water samples, respectively. The gene sul1 had the highest abundance among the detected ARGs, in accordance with previous reports of high abundances of sul1 in various types of water bodies, including aquaculture, lakes, groundwater, and seawater [25,26]. The genes tetM and tetQ widely exist in marine environments and can be transferred among bacteria [27,28]. Notably, the abundances of target genes, especially for sul1 and blaTEM, were obviously higher in the ballast water than in the receiving water (p < 0.05), which might be related to the heavy metal pollution in the ballast tank [29,30]. Heavy metal has been considered an important co-selection agent for ARGs [31]. Moreover, horizontal gene transfer is driven by MGEs, which could increase the spread of ARGs [22]. Therefore, MGEs comprising intI1 and intI2 were detected in the initial samples. The abundances of intI1 and intI2 were 1.3 × 10−5 and 3.0 × 10−6 copies/16S rRNA and 2.2 × 10−5 and 3.0 × 10−6 copies/16S rRNA in the initial ballast and receiving water samples, respectively.
All target ARGs exhibited similar trends in the microcosms, and their abundances in microcosm M were higher than that in microcosm R (Figure 2, Table S4). Specifically, the abundance of sul1 in microcosm M was 1.2 × 10−4, 4.9 × 10−4, and 1.6 × 10−3 copies/16S rRNA on days 1, 3, and 5, which was 2.6–8.7-fold higher relative to that in microcosm R (p < 0.05). It was worth noting that sul1 was even more abundant in microcosm M than in microcosm B on day 5. For tetM, the abundance in microcosm M was 1.7 × 10−7, 6.0 × 10−7, and 8.1 × 10−7 copies/16S rRNA on days 1, 3, and 5, which was 1.7-, 1.9-, and 2.9-fold higher relative to that in microcosm R and lower than that in microcosm B. The abundances of tetQ and blaTEM decreased in the order of microcosm B > M > R and were 1.3- and 1.8-fold higher in microcosm M than in microcosm R at the end of the experiment. These results indicated that the discharge of ballast water could contribute to the enrichment of ARGs in the receiving water. The findings were similar to those of previous studies that effluents from wastewater treatment plants could evidently elevate the ARG level in natural waters [6,32]. Among target ARGs, sul1 exhibited the greatest enrichment potential with the introduction of ballast water, which was probably associated with its high horizontal transfer potential. It has been frequently reported that sul1 can be carried by class 1 integron, which is a significant player in the horizontal transfer and global spread of ARGs [33,34]. Moreover, previous studies have also found that the wastewater discharge led to an increased abundance of tetM and tetQ in the receiving river [32,35].
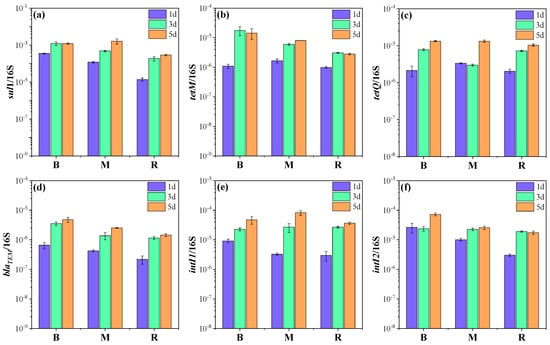
Figure 2.
The variation in ARG and MGE abundances with the culture time in each microcosm: (a) sul1; (b) tetM; (c) tetQ; (d) blaTEM; (e) intI1; (f) intI2. Microcosm B (ballast water), microcosm M (ballast water and receiving water at a ratio of 1:1), and microcosm R (receiving water).
In addition, the MGEs in microcosm M were more abundant than those in microcosm R during the experiment. The abundance of intI1 and intI2 in microcosm M was 8.2 × 10−5 and 2.6 × 10−5 copies/16S rRNA on day 5, which was 2.2- and 1.5-fold higher than that in microcosm R (Figure 2e,f). Interestingly, intI1 was 1.7-fold higher in microcosm M than in microcosm B. The results suggest that ballast water increased the level of MGEs in receiving water as well. It should be noted that MGEs can carry ARGs and can be transferred between homogeneous and even heterogeneous bacteria, thereby promoting the spread of ARGs [22]. Zhang et al. reported that intI1 could be used as an indicator of the potential of ARG dissemination [36]. As shown in Table 1, a significant positive correlation was found between intI1 and sul1, blaTEM. Moreover, intI2 had a positive relationship with tetM, tetQ, and blaTEM. Considering the more abundant ARGs and MGEs in ballast water than in receiving water and especially the higher abundance of sul1 and intI1 in microcosm M than in microcosm B, it is likely that horizontal transfer would occur in the receiving water. Therefore, the ecological risks associated with the higher abundance of ARGs in ballast water should not be ignored as ARGs may be propagated by horizontal gene transfer.

Table 1.
Pearson’s correlation coefficients between ARGs and MGEs.
2.3. Transformation of Bacterial Communities in Microcosms
The bacterial communities were analyzed in the initial ballast and receiving water, as well as three microcosms. The dominant phylum exhibited obvious differences in the initial ballast and receiving waters (Figure S1). Proteobacteria was the predominant phylum in ballast water, accounting for over 95% of the total community, while Proteobacteria (67.5%), Bacteroidetes (12.5%), Chloroflexi (4.9%), Actinobacteria (3.6%), and Patescibacteria (2.3%) were prevalent in the receiving water. The result was consistent with previous studies that the dominant phyla in seawater were Proteobacteria, Bacteroides, and Actinobacteria [2,13]. Moreover, clear differences were also observed in the bacterial community between the initial ballast and receiving waters at the genus level (Figure 3). Sulfitobacter (9.8%), OM43 clade (16.9%), Zhongshania (19.5%), and Amphritea (12.1%) were dominant genera in ballast water, while Thalassobaculum (5.6%), C1-B405 (10.1%), and Pseudohongiella (4.7%) occupied the majority of genera in the receiving water.
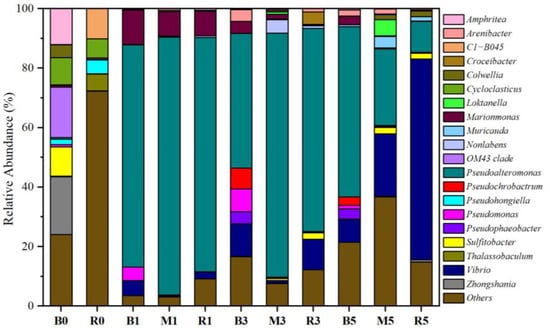
Figure 3.
The relative abundance variation in taxonomic genus with the culture time in each microcosm. Microcosm B (ballast water), microcosm M (ballast water and receiving water at a ratio of 1:1), and microcosm R (receiving water). B0 and R0 indicate the initial ballast and receiving water, respectively. B1, B3, and B5 indicate the microcosm B on days 1, 3, and 5, respectively. R1, R3, and R5 indicate the microcosm R on days 1, 3, and 5, respectively. M1, M3, and M5 indicate the microcosm M on days 1, 3, and 5, respectively.
Proteobacteria and Bacteroidetes were the dominant phyla and accounted for more than 99% of the total sequences in three microcosms (Figure S1). The relative abundance of Bacteroidetes gradually increased from 0.8% on day 1 to 11.7% at the end of microcosm B. By contrast, Bacteroidetes increased more in microcosm M, with the relative abundance being from 1.2% to 25.3%. Proteobacteria showed the opposite trends. The relative abundance of the two phyla had no obvious changes in microcosm R. Moreover, Actinobacteria, Chloroflexi, and Cyanobacteria could be ignored (<1%) with the experimental time. At the genus level, Marinomonas (8.2–11.7%) and Pseudoalteromonas (74.8–86.6%) were the dominant genera in the three microcosms on day 1, while their abundances varied among the different microcosms. As the experiment progressed, the abundance of Vibrio increased rapidly, while that of Pseudoalteromonas decreased continuously in three microcosms. On day 5, the dominant genera in microcosm B were Pseudoalteromonas (57.2%), followed by Vibrio (7.7%), Pseudophaeobacter (3.4%), Marinomonas (2.8%), and Pseudochrobactrum (2.8%), while the dominant genera in microcosm M belonged to Pseudoalteromonas (25.7%), Vibrio (20.9%), Loktanella (5.4%), and Muricauda (3.8%). The abundance of Pseudoalteromonas in microcosm M (25.7%) was obviously lower than that in microcosm B (57.2%) but higher than that in microcosm R (10.4%), and Vibrio exhibited the opposite trend. This indicates that the inclusion of ballast water could enrich Pseudoalteromonas and reduce Vibrio in the receiving water. In all, it is clear that ballast water could cause changes in the microbial community in receiving water.
2.4. Relationship Between Bacterial Communities, MGEs, and ARGs in Microcosms
Previous studies reported that the propagation or attenuation of ARGs was closely related to the changes in bacterial community and MGEs [37,38]. Network analysis was performed to investigate the co-occurrence patterns among ARGs, MGEs, and bacterial communities (genus level). As shown in Figure 4, Pseudohongiella and Thalassobaculum were significantly positively correlated with sul1, blaTEM, and intI1. Amphritea, Sulfitobacter, and Zhongshania were significantly positively related to tetM. This indicates that these bacteria were potential hosts of the target ARGs/MGEs. Pearson correlation analysis also supported that these bacteria were closely correlated with ARGs/MGEs (Figure 5). In addition, Amphritea, Colwellia, Sulfitobacter, Zhongshania, etc., were positively correlated with intI2, tetM, and tetQ. The co-occurrence of ARGs and MGEs in these bacteria implied that ARGs were likely to be enriched by horizontal transfer after the discharge of ballast water. On the other hand, the changes in the abundance of these potential bacterial hosts might be responsible for the variation in ARGs [27]. For example, the abundances of Amphritea were 0.4%, 0.036%, and 0.006% in microcosms B, M, and R on day 5, respectively, indicating that the introduction of ballast water increased its abundance and thus might lead to the enrichment of tetM. In conclusion, when ballast water was discharged into the receiving water, some ARGs were probably transmitted and enriched through horizontal gene transfer or the prevalence of bacterial hosts.
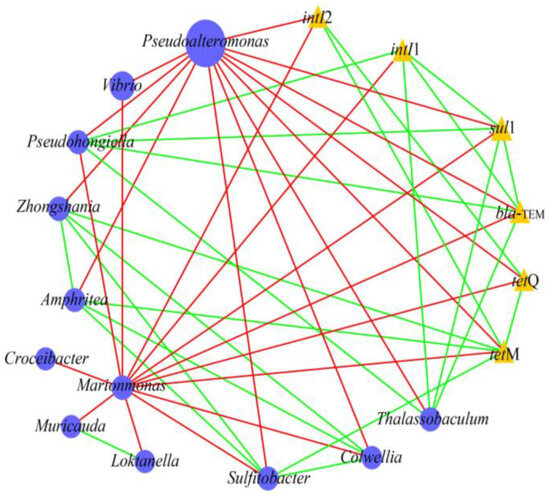
Figure 4.
Network analysis showing the potential bacterial hosts for ARGs and MGEs. The square and triangle nodes indicate bacteria and target ARGs/MGEs, respectively. The node size represents the abundance of bacteria/ARGs/MGEs. The edges correspond to significant positive (green) and negative (red) correlations between nodes.
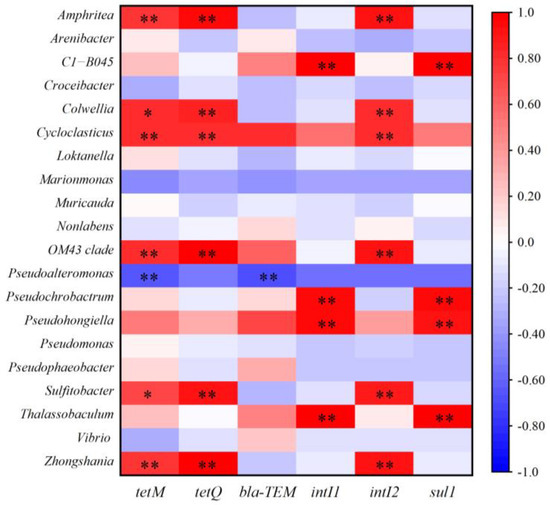
Figure 5.
Pearson correlation between ARGs and bacteria genus (* p < 0.05, ** p < 0.01).
3. Materials and Methods
3.1. Sample Collection and Processing
Ballast water sample was collected from a cargo ship in Yangshan Port (30°37′ N, 122°04′ E) in Shanghai, China. The ballast water was originally pumped in the Arabian Sea (20°22′ N, 72°61′ E), and no exchange was conducted during 28 days of sailing. Sample was collected from the manhole using sterile plastic buckets. Seawater from the East China Sea was collected as the receiving water. The two locations are more than 5000 nautical miles apart and geographically separated by the Indian Ocean and Pacific Ocean, so the water exchange between them is limited. The samples were transported to the laboratory by an ice box within 4 h. The physicochemical characteristics of water samples are given in Table S1.
3.2. Metagenomic Analysis of ARGs in the Initial Samples
The ARG profiles in the initial ballast and receiving water samples were determined by Metagenomics. Briefly, 2.0 L of ballast and receiving water samples was filtered by 0.22 μm membrane (Millipore, Billerica, MA, USA), and the DNA in filters was extracted using PowerSoil DNA Isolation Kit (Qiagen, Hilden, Germany). After fragmentation and paired-end fragment library construction, the adaptor-appended fragments were sequenced using the Illumina HiSeq 2000 platform (San Diego, CA, USA). The size of the raw data for each sample was approximately 10 Gb. The low-quality reads that contained ambiguous nucleotides or had a quality score < 20 were removed. Clean reads were assembled into scaftig fragments using MEGAHIT (v1.2.9) assembler [39]. Only scaftig fragments > 500 bp were retained for further analysis. The retained scaftig fragments were used for ORF prediction using MetaGeneMark (v3.38) with default parameters [40]. Non-redundant genes were obtained by CD-HIT (v4.8.1), and SOAPaligner (v2.21) was used to compare the clean reads with the gene pool in order to calculate the number of matches [41]. The unigene was obtained by removing gene classes containing less than three mapped reads. The local BLAST program (2.13.0+) was used to compare the unigene with antibiotic resistance genes database (ARDB) at e-value of <10−5 to annotate the ARGs [41].
3.3. Microcosm Setup and DNA Extraction
The ballast and receiving water samples were supplemented with 2% (v:v) 2216E medium before the experiment [27]. Experiments were conducted in 2 L glass jars with a working volume of 1.5 L. Three microcosms were set up: the mixture of ballast water and receiving water at a ratio of 1:1 (microcosm M) to simulate the discharge of ballast water into the receiving water, and ballast water (microcosm B) and receiving water (microcosm R) as the control. Each microcosm was performed in triplicate with a total of nine glass jars. The microcosms were maintained for 5 days at 28 °C in a shaker. Samples (100 mL) were periodically collected from each glass jar (days 1, 3, and 5) on an ultra-clean worktable (AIRTECH, Tianjin, China). The collected samples (number = 27) were filtered through a sterile 0.22 μm membrane (Millipore, Billerica, MA, USA) for genomic DNA extraction using PowerSoil DNA Isolation Kit (Qiagen, Germany).
3.4. Quantification of ARGs
The concentration and purity of DNA were evaluated using a microspectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The qualified DNA was used in the next analysis. qPCR assays were conducted using the CFX96 Connect System (BioRad, Hercules, CA, USA). The 16S rRNA gene was quantified using a 20 μL volume system, which included 1 μL of template DNA, 1 μL of each primer (10 μM), 7 μL of nuclease-free ddH2O, and 10 μL of SYBR Green SuperMix (Zoman, Beijing, China). Four ARGs (sul1, tetM, tetQ, and blaTEM) and two MGEs (intI1 and intI2) were determined in 10 μL reaction system, which contained 1 μL of template DNA, 0.5 μL of each primer (10 μM), 3 μL of nuclease-free ddH2O, and 5 μL of SYBR Green Mix (Zoman, China). Detailed information regarding the primers is given in Table S2. Ten-fold serially diluted standard plasmid with known quantities was used for the construction of calibration curves. The R2 values of calibration curves for all target genes were over 0.99, and the PCR efficiencies were in the range of 0.95~1.15. The abundances of ARGs and MGEs were expressed as copy numbers per bacterial 16S rRNA gene.
3.5. Bacterial Community
DNA extracts were subjected to 16S rRNA gene high-throughput sequencing in order to determine the variation in bacterial communities during the experiments. The hypervariable regions (V3–V4) of 16S rRNA gene were amplified with the primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) containing specific barcodes. PCR was performed in 50 μL reaction system, which consisted of 25 μL of Taq DNA polymerase (5 U/μL), 0.2 μL of each primer (0.5 μmol/L), 4 μL of template DNA, and 20.6 μL of ddH2O. The PCR temperature program was initiated with denaturation for 5 min at 95 °C, followed by 30 cycles of 40 s at 95 °C, 40 s at 58 °C, 60 s at 72 °C, and a final elongation for 5 min at 72 °C. The PCR products were sequenced by an Illumina Miseq-PE250 platform. The quantitative insights into microbial ecology (QIIME, v1.8.0) pipeline was used to filter out the low-quality sequences. After chimera detection, the sequences in each library were binned into OTUs at an identity threshold ≥ 97% using USEARCH (v11.0.667). Taxonomic identification of OTUs was annotated against the Greengenes database using the BLAST [42].
3.6. Statistical Analysis
A t-test was used to assess the significance of the differences in ARGs between microcosms using SPSS 22.0 (p < 0.05). Pearson correlation among ARGs/MGEs was also conducted by SPSS 22.0. Network analysis was performed on the basis of Spearman correlation (r > 0.8, p < 0.01) between ARGs/MGEs and the dominant genera and then visualized using Cytoscape 3.7.1.
4. Conclusions
The spread of ARGs as emerging pollutants in different waters has received widespread attention. The discharge of ballast water leads to the spread of marine organisms and ARGs between geographically separated waters. The main conclusions of this study were as follows: (i) The studied ballast water increased the abundance of ARGs in the receiving water. (ii) MGEs were obviously correlated with ARGs, which might promote the spread of ARGs through horizontal gene transfer. (iii) The bacterial community structure in the receiving water was affected by ballast water. (iv) The discharge of ballast water may affect the potential bacterial hosts of ARGs and ultimately affect the ARGs in receiving waters. In all, this study indicates that shipping can potentially transfer ARGs globally to areas that are relatively unaffected by human activities, thereby leading to the invasion of ARGs. Further research is needed to reveal the fate of ARGs that are introduced by ballast water, especially their spread to the indigenous microbial flora via horizontal gene transfer.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics14040340/s1, Table S1: The physicochemical characteristics of water samples; Table S2: Primers and PCR conditions for ARG analyses; Table S3: Abundance of the ARG subtypes in initial ballast and receiving water; Table S4: The variation in ARG and MGE abundances with the culture time in each microcosm; Figure S1: The relative abundance variation in taxonomic phylum in each microcosm; References [43,44,45,46] were cited by Table S2.
Author Contributions
Conceptualization, R.W. and C.J.; methodology, J.S.; software, C.S.; validation, J.S., B.L. and R.W.; formal analysis, C.S.; investigation, B.L.; resources, B.L.; data curation, C.J.; writing—original draft preparation, J.S.; writing—review and editing, J.S. and B.L.; visualization, C.J. and C.S.; supervision, R.W. and B.L.; project administration, B.L.; funding acquisition, J.S. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation of China (42307503, 42477445) and the Shanghai Sailing Program (23YF1415900).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
Author Rui Wang was employed by the company CCCC National Engineering Research Center of Dredging and Equipment Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lu, J.; Wu, J.; Zhang, C.; Zhang, Y.X.; Lin, Y.C.; Luo, Y.M. Occurrence, distribution, and ecological-health risks of selected antibiotics in coastal waters along the coastline of China. Sci. Total Environ. 2018, 644, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Y.X.; Wu, J. Continental-scale spatio-temporal distribution of antibiotic resistance genes in coastal waters along coastline of China. Chemosphere 2020, 247, 125908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Lu, J.; Wu, J.; Wang, J.H.; Luo, Y.M. Potential risks of microplastics combined with superbugs: Enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicol. Environ. Saf. 2020, 187, 109852. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in Northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Graham, D.W.; Olivares-Rieumont, S.; Knapp, C.W.; Lima, L.; Werner, D.; Bowen, E. Antibiotic resistance gene abundances associated with waste discharges to the Almendares River near Havana, Cuba. Environ. Sci. Technol. 2010, 45, 418–424. [Google Scholar] [CrossRef]
- Li, J.N.; Cheng, W.X.; Xu, L.K.; Jiao, Y.N.; Baig, S.A.; Chen, H. Occurrence and removal of antibiotics and the corresponding resistance genes in wastewater treatment plants: Effluents’ influence to downstream water environment. Environ. Sci. Pollut. Res. 2016, 23, 6826–6835. [Google Scholar] [CrossRef]
- Ruiz, G.M.; Rawlings, T.K.; Dobbs, F.C.; Drake, L.A.; Mullady, T.; Huq, A.; Colwell, R.R. Global spread of microorganisms by ships. Nature 2000, 408, 49–50. [Google Scholar] [CrossRef]
- Miller, A.W.; Minton, M.S.; Ruiz, G.M. Geographic limitations and regional differences in ships’ ballast water management to reduce marine invasions in the contiguous United States. BioScience 2011, 61, 880–887. [Google Scholar] [CrossRef]
- Altug, G.; Gurun, S.; Cardak, M.; Ciftci, P.S.; Kalkan, S. The occurrence of pathogenic bacteria in some ships’ ballast water incoming from various marine regions to the Sea of Marmara, Turkey. Mar. Environ. Res. 2012, 81, 35–42. [Google Scholar] [CrossRef]
- Brinkmeyer, R. Diversity of bacteria in ships ballast water as revealed by next generation DNA sequencing. Mar. Pollut. Bull. 2016, 107, 277–285. [Google Scholar] [PubMed]
- Kim, Y.; Aw, T.G.; Teal, T.K.; Rose, J.B. Metagenomic investigation of viral communities in ballast water. Environ. Sci. Technol. 2015, 49, 8396–8407. [Google Scholar] [PubMed]
- Wu, H.X.; Chen, C.; Wang, Q.; Lin, J.D.; Xue, J.Z. The biological content of ballast water in China: A review. Aquac. Fish. 2017, 2, 241–246. [Google Scholar]
- Lv, B.Y.; Cui, Y.X.; Tian, W.; Li, J.; Xie, B.; Yin, F. Abundances and profiles of antibiotic resistance genes as well as co-occurrences with human bacterial pathogens in ship ballast tank sediments from a shipyard in Jiangsu Province, China. Ecotoxicol. Environ. Saf. 2018, 157, 169–175. [Google Scholar]
- Lv, B.; Jiang, C.; Han, Y.; Wu, D.; Jin, L. Diverse bacterial hosts and potential risk of antibiotic resistomes in ship ballast water revealed by metagenomic binning. Environ. Res. 2024, 253, 119056. [Google Scholar]
- Zhou, L.; Xu, P.; Gong, J.; Huang, S.; Chen, W.; Fu, B.; Huang, X. Metagenomic profiles of the resistome in subtropical estuaries: Co-occurrence patterns, indicative genes, and driving factors. Sci. Total Environ. 2022, 810, 152263. [Google Scholar] [CrossRef]
- Zheng, D.; Yin, G.; Liu, M.; Hou, L.; Yang, Y.; Liu, X.; Wu, H. Metagenomics highlights the impact of climate and human activities on antibiotic resistance genes in China’s estuaries. Environ. Pollut. 2022, 301, 119015. [Google Scholar] [CrossRef]
- Gerhard, W.A.; Gunsch, C.K. Higher normalized concentrations of tetracycline resistance found in ballast and harbor water compared to ocean water. Mar. Pollut. Bull. 2019, 151, 110796. [Google Scholar]
- Darling, J.A.; Martinson, J.; Gong, Y.G.; Okum, S.; Pilgrim, E.; Lohan, K.M.P.; Carney, K.J.; Ruiz, G.M. Ballast water exchange and invasion risk posed by intracoastal vessel traffic: An evaluation using high throughput sequencing. Environ. Sci. Technol. 2018, 52, 9926–9936. [Google Scholar]
- Hess-Erga, O.; Moreno-Andrés, J.; Enger, Ø.; Vadstein, O. Microorganisms in ballast water: Disinfection, community dynamics, and implications for management. Sci. Total Environ. 2019, 657, 704–716. [Google Scholar]
- Zaiko, A.; Martinez, J.L.; Ardura, A.; Clusa, L.; Borrell, Y.J.; Samuiloviene, A.; Roca, A.; Garcia-Vazquez, E. Detecting nuisance species using NGST: Methodology shortcomings and possible application in ballast water monitoring. Mar. Environ. Res. 2015, 112, 64–72. [Google Scholar]
- Li, B.; Yang, Y.; Ma, L.P.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [PubMed]
- Nogales, B.; Lanfranconi, M.P.; Piña-Villalonga, J.M.; Bosch, R. Anthropogenic perturbations in marine microbial communities. FEMS Microbiol. Rev. 2011, 35, 275–298. [Google Scholar] [PubMed]
- Ying, C.W.; Chang, M.J.; Hu, C.H.; Chang, Y.T.; Chao, W.L.; Yeh, S.L.; Chang, S.J.; Hsu, J.T. The effects of marine farm-scale sequentially integrated multi-trophic aquaculture systems on microbial community composition, prevalence of sulfonamide-resistant bacteria and sulfonamide resistance gene sul1. Environ. Sci. Technol. 2018, 643, 681–691. [Google Scholar]
- Lin, H.; Zhang, J.; Chen, H.J.; Wang, J.M.; Sun, W.C.; Zhang, X.; Yang, Y.Y.; Wang, Q.; Ma, J.W. Effect of temperature on sulfonamide antibiotics degradation, and on antibiotic resistance determinants and hosts in animal manures. Sci. Total Environ. 2017, 607, 725–732. [Google Scholar]
- Niu, Z.G.; Zhang, K.; Zhang, Y. Occurrence and distribution of antibiotic resistance genes in the coastal area of the Bohai Bay, China. Mar. Pollut. Bull. 2016, 107, 245–250. [Google Scholar]
- Zhang, K.; Zhang, Y.; Xin, R.; Zhang, Y.P.; Niu, Z.G. Variation pattern of terrestrial antibiotic resistances and bacterial communities in seawater/freshwater mixed microcosms. Chemosphere 2018, 200, 201–208. [Google Scholar]
- Dönhöfer, A.; Franckenberg, S.; Wickles, S.; Berninghausen, O.; Beckmann, R.; Wilson, D.N. Structural basis for tetM-mediated tetracycline resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 16900–16905. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Chen, J.; Xue, J.; Wu, H. Comparison of heavy metal pollution and ecological risk assessment in ballast tank sediments based on two applicable reference standards. Mar. Pollut. Bull. 2023, 196, 115543. [Google Scholar]
- Feng, D.; Chen, X.; Tian, W.; Qian, Q.; Shen, H.; Liao, D.; Lv, B. Pollution characteristics and ecological risk of heavy metals in ballast tank sediment. Environ. Sci. Pollut. Res. Int. 2017, 24, 3951–3958. [Google Scholar]
- Li, L.G.; Xia, Y.; Zhang, T. Co-occurrence of antibiotic and metal resistance genes revealed in complete genome collectio. ISME J. 2017, 11, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Zhang, X.X.; Miao, Y.; Zhao, Y.; Ye, L.; Li, B.; Zhang, T. Fate of antibiotic resistance genes and their associations with bacterial community in livestock breeding wastewater and its receiving river water. Water Res. 2017, 124, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kashif, J.; Buriro, R.; Memon, J.; Yaqoob, M.; Soomro, J.; Dongxue, D.; Jinhu, H.; Liping, W. Detection of class 1 and 2 integrons, β-lactamase genes and molecular characterization of sulfonamide resistance in escherichia coli isolates recovered from poultry in China. Pak. Vet. J. 2013, 33, 321–324. [Google Scholar]
- Nigro, S.J.; Farrugia, D.N.; Paulsen, I.T.; Hall, R.M. A novel family of genomic resistance islands, abgri2, contributing to aminoglycoside resistance in Acinetobacter baumannii isolates belonging to global clone 2. J. Antimicrob. Chemother. 2013, 68, 554–557. [Google Scholar] [CrossRef]
- Proia, L.; von Schiller, D.; Sanchez-Melsio, A.; Sabater, S.; Borrego, C.M.; Rodríguez-Mozaz, S.; Balcazar, J.L. Occurrence and persistence of antibiotic resistance genes in river biofilms after wastewater inputs in small rivers. Environ. Pollut. 2016, 210, 121–128. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Liu, R.; Zhang, T.; Li, M.; Zhang, Z.R.; Qu, Z.T.; Yuan, Z.T.; Yu, H.C. Influence of reclaimed water discharge on the dissemination and relationships of sulfonamide, sulfonamide resistance genes along the Chaobai River, Beijing. Front. Environ. Sci. Eng. 2019, 13, 87–98. [Google Scholar] [CrossRef]
- Narciso-da-Rocha, C.; Manaia, C.M. The influence of the autochthonous wastewater microbiota and gene host on the fate of invasive antibiotic resistance genes. Sci. Total Environ. 2017, 575, 932–940. [Google Scholar] [CrossRef]
- Su, H.C.; Liu, S.; Hu, X.J.; Xu, X.R.; Xu, W.J.; Xu, Y.; Li, Z.J.; Wen, G.L.; Liu, Y.S.; Cao, Y.C. Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Sci. Total Environ. 2017, 607–608, 357–366. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J. Alteration of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar]
- Zhu, W.H.; Lomsadze, A.; Borodovsky, M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010, 38, e132. [Google Scholar]
- Guo, J.H.; Li, J.; Chen, H.; Philip, L.B.; Yuan, Z.G. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar]
- Luo, Y.; Mao, D.Q.; Rysz, M.; Zhou, Q.X.; Zhang, H.J.; Xu, L.; Alvarez, P.J.J. Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 44, 7220–7225. [Google Scholar] [CrossRef] [PubMed]
- De Gheldre, Y.; Avesani, V.; Berhin, C.; Delmée, M.; Glupczynski, Y. Evaluation of Oxoid combination discs for detection of extended-spectrum β-lactamases. J. Antimicrob. Chemother. 2003, 52, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, Z.T.; Yang, K.; Graham, D.; Xie, B. Relationships between antibiotics and antibiotic resistance gene levels in municipal solid waste leachates in Shanghai, China. Environ. Sci. Technol. 2015, 49, 4122–4128. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).