In Vitro and In Vivo Investigations into the Potential of Quinazoline and Quinoline Derivatives as NorA Efflux Pump Inhibitors Against Resistant Staphylococcus aureus Strains
Abstract
1. Introduction
2. Results
2.1. Impact of Compounds PQK4F and PQQ16P on CPX’s Minimum Inhibitory Concentration (MIC)
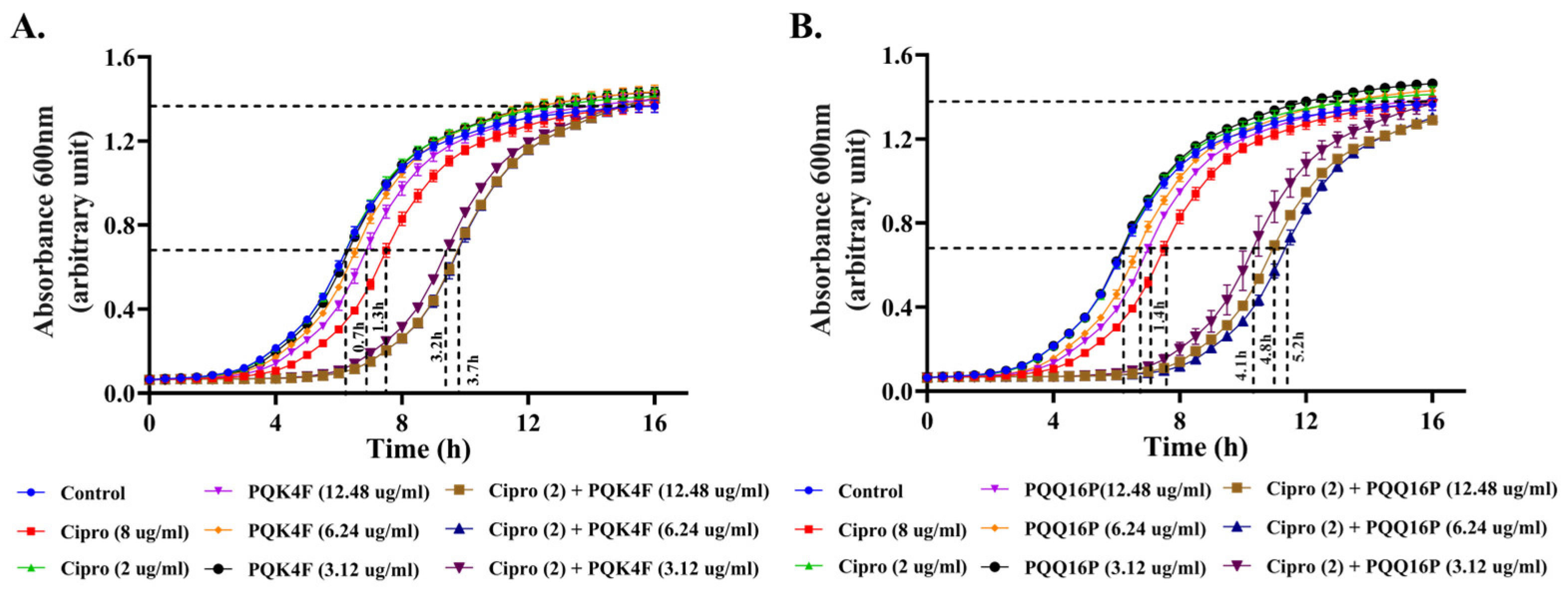
2.2. Effect of the Combination of CPX with Compounds PQK4F and PQQ16P on the Time–Kill Kinetics of S. aureus SA-1199B
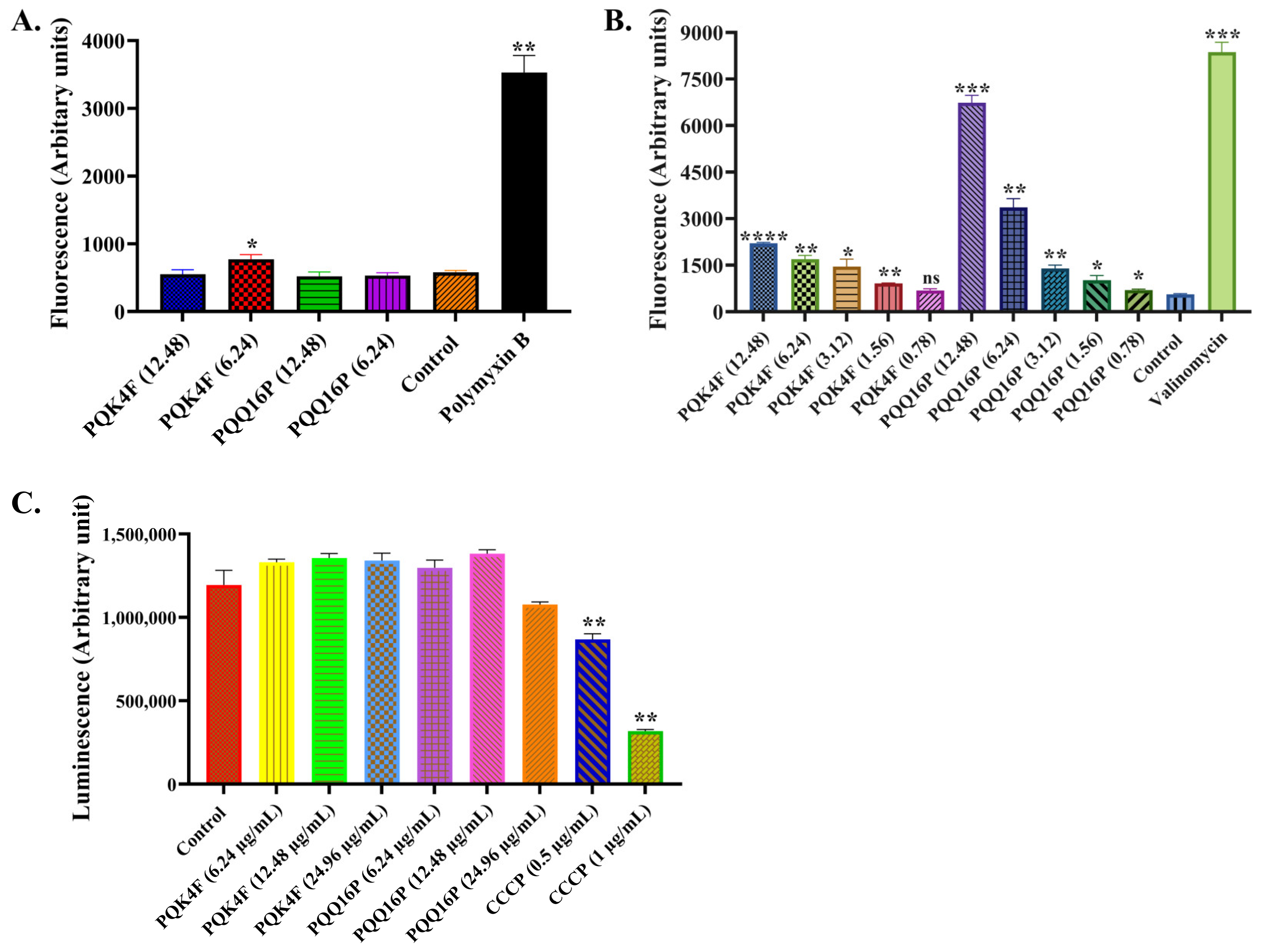
2.3. Membrane Permeabilization and Depolarization Activity of Compounds PQK4F and PQQ16P on S. aureus SA-1199B
2.4. Effect of Compounds PQK4F and PQQ16P on the Electron Transport Chain by ATP Bioluminescence Detection Assay
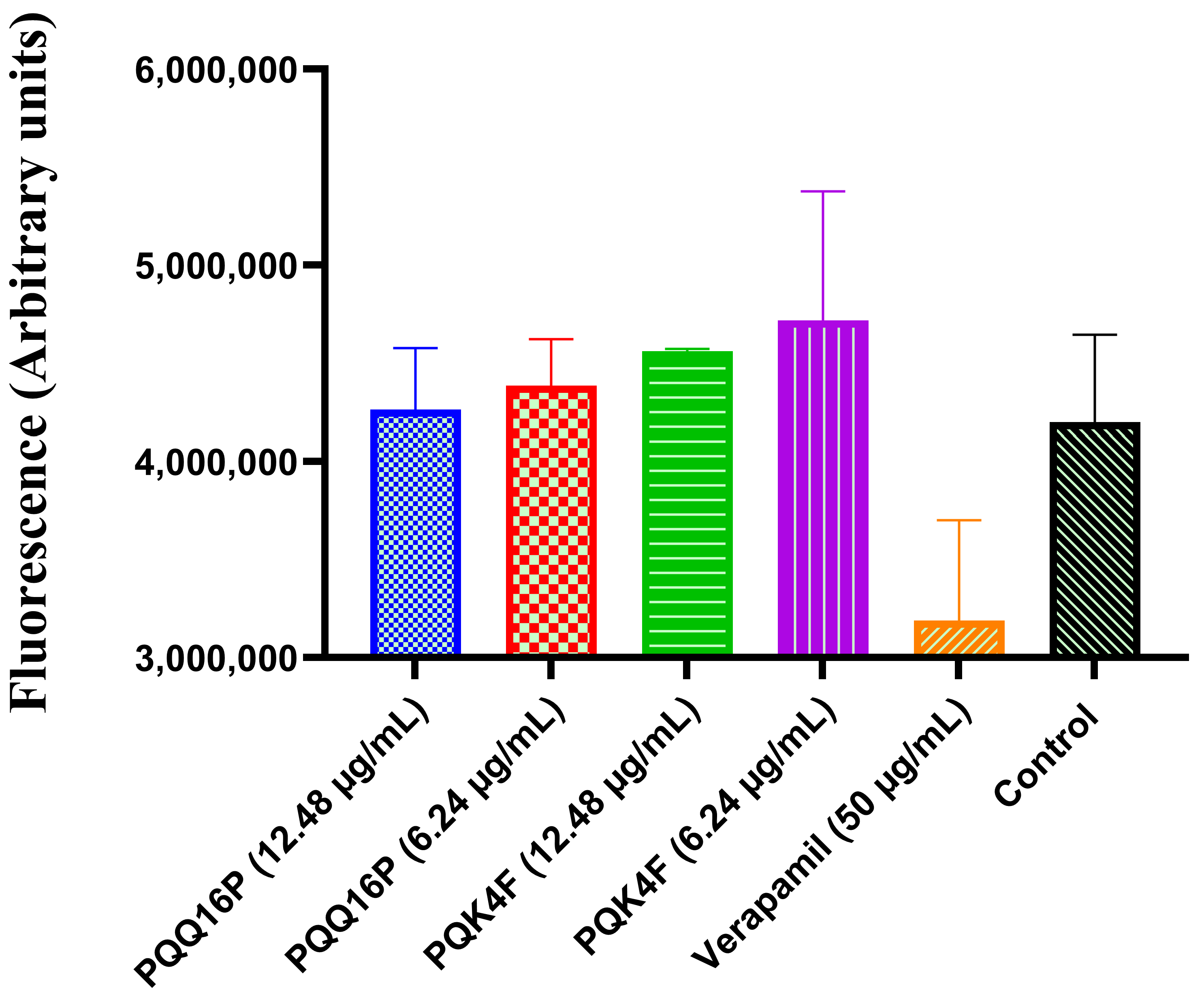
2.5. Effect of Compounds PQK4F and PQQ16P on Mammalian Ca2+ Channels
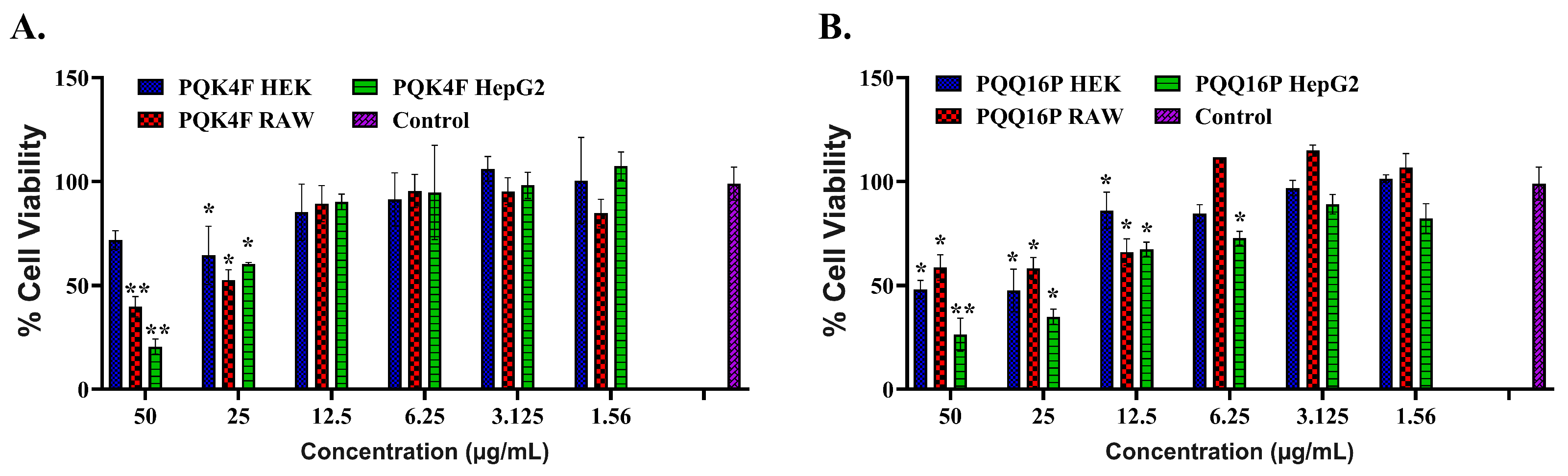
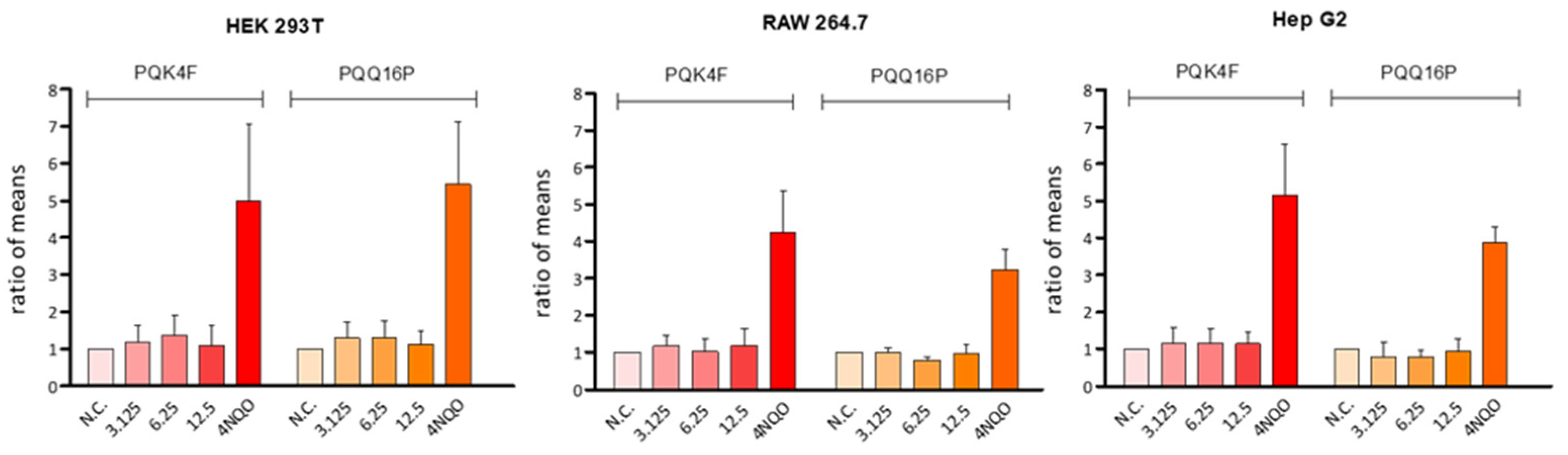
2.6. Toxicity Studies of Compounds PQK4F and PQQ16P Towards Eukaryotic Cells
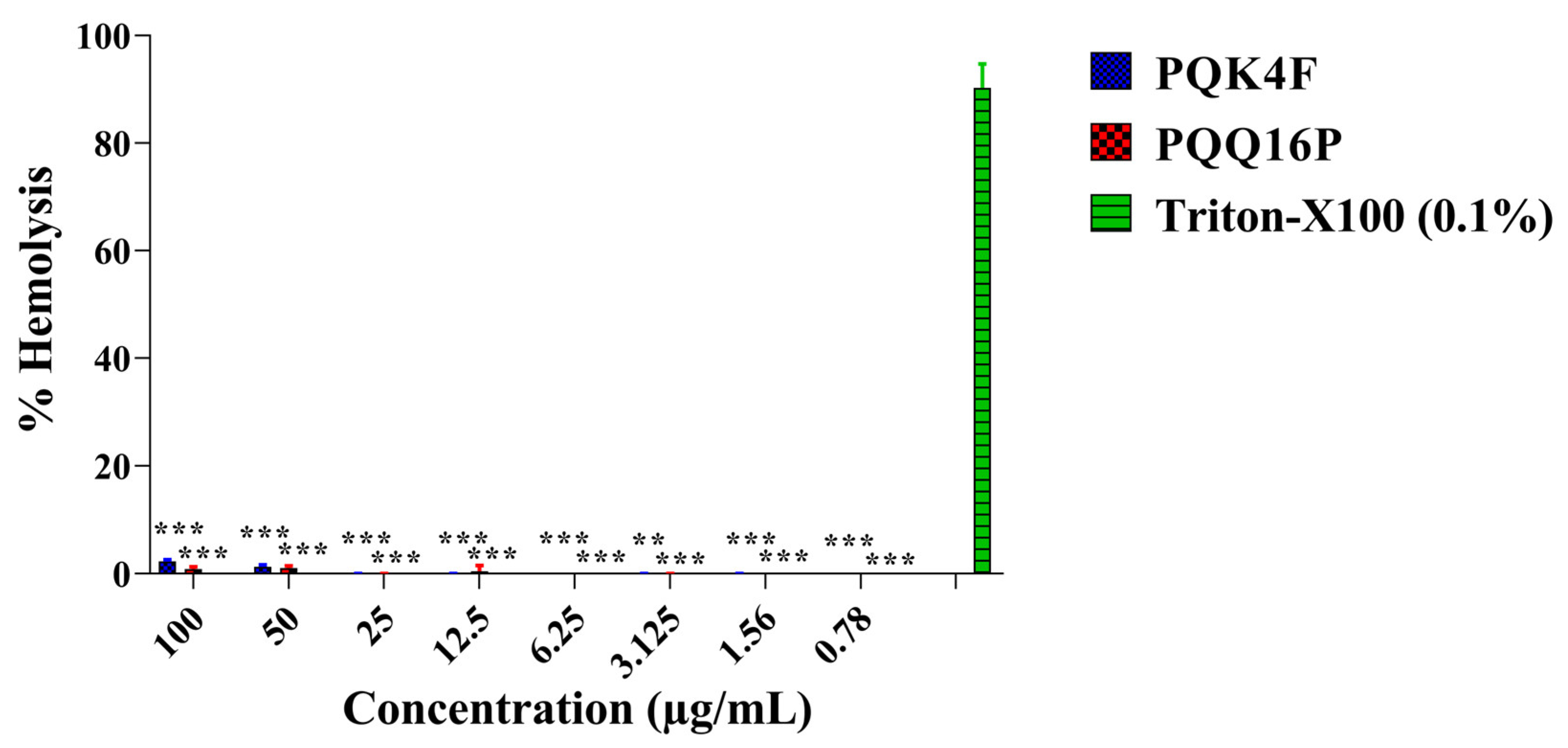
2.7. Hemolytic Activity Studies of the Compounds PQK4F and PQQ16P
2.8. Genotoxicity Assessment
2.9. Enhanced Post-Antibiotic Life of CPX in the Presence of Compounds PQK4F and PQQ16P
2.10. Mutation Frequency Analysis of Compounds PQK4F and PQQ16P to Check the Development of Its Resistant Mutants
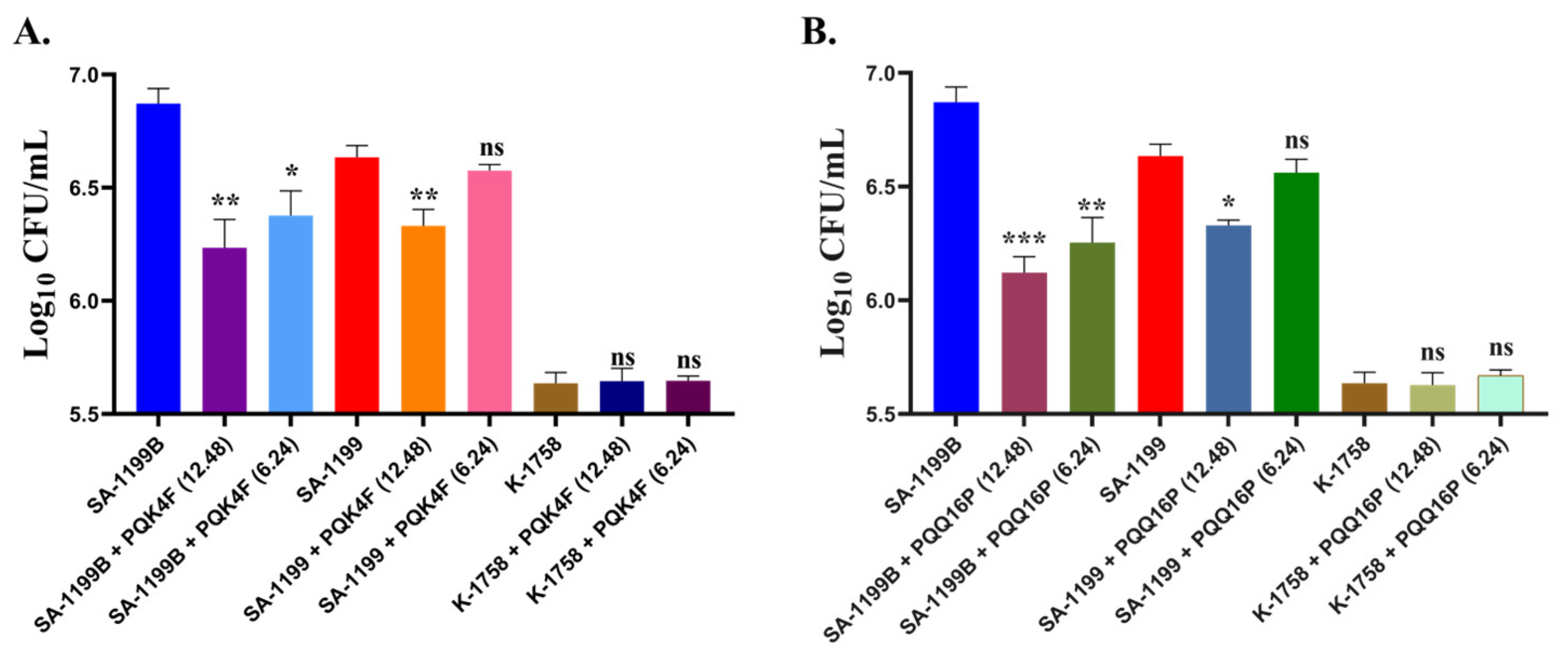
2.11. Effectiveness of PQK4F and PQQ16P in Mitigating Macrophage Invasion by norA Over-Expressing S. aureus
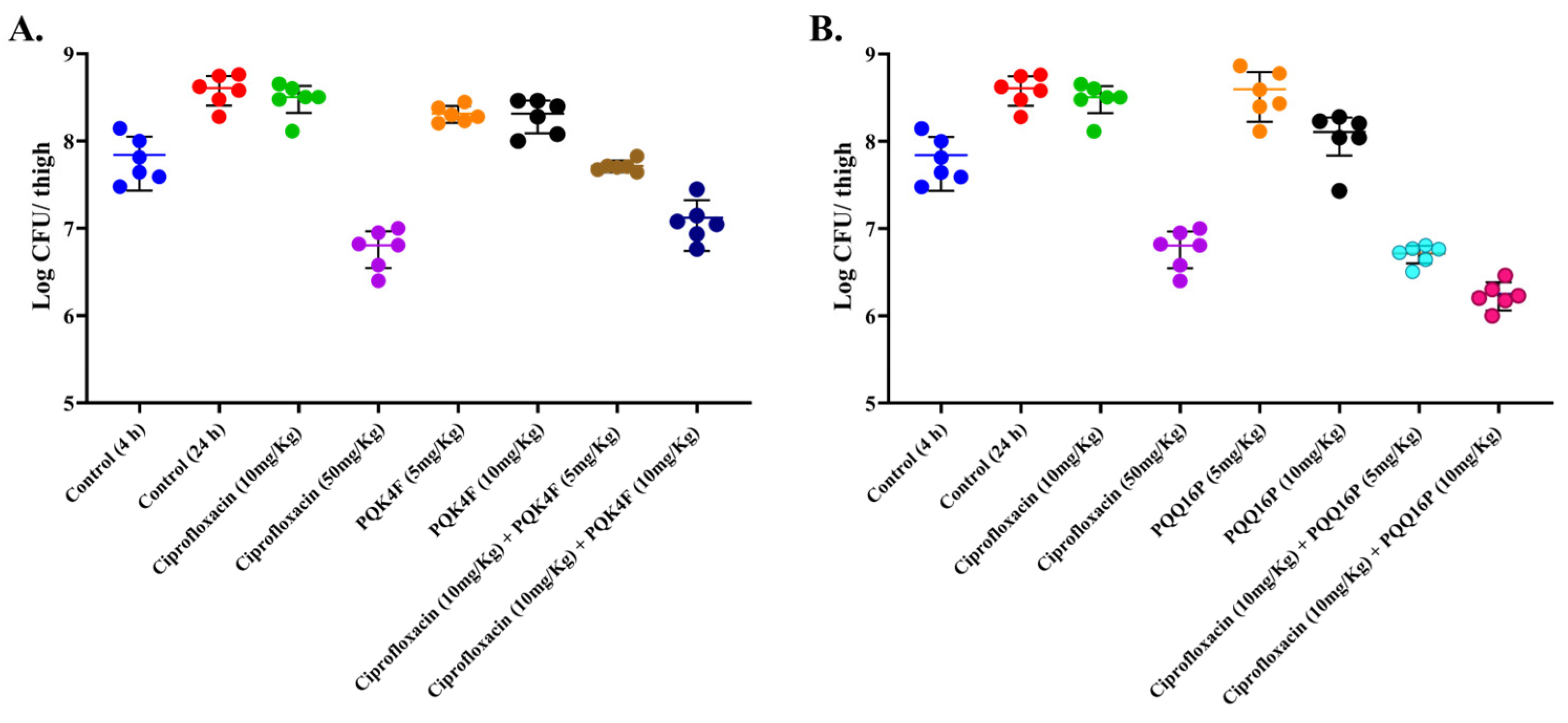
2.12. Mouse Thigh Infection Model In Vivo
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Cell Lines, and Growth Medium
4.2. MIC Determination and Synergy Assay
4.3. Membrane Potentiation Assay
4.4. Membrane Permeability Assay
4.5. Determination of Intracellular ATP Levels
4.6. Mammalian Ca2+ Channel Blocking Assay
4.7. Genotoxicity Test
4.7.1. Chemicals and Reagents
4.7.2. Alkaline Single-Cell Microgel Electrophoresis (Comet) Assay
4.8. Post-Antibiotic Assay
4.9. Mutation Prevention Concentration (MPC)
4.10. Time–Kill Kinetics Study
4.11. Hemolysis Assay
4.12. Mammalian Cytotoxicity
4.13. Macrophage Invasion Assay
4.14. In Vivo Thigh Infection Model
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gherardi, G. Staphylococcus aureus Infection: Pathogenesis and Antimicrobial Resistance. Int. J. Mol. Sci. 2023, 24, 8182. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus aureus. Virulence 2021, 12, 547. [Google Scholar] [CrossRef]
- Thomer, L.; Schneewind, O.; Missiakas, D. Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 343–364. Available online: https://www.annualreviews.org/content/journals/10.1146/annurev-pathol-012615-044351 (accessed on 12 March 2025). [CrossRef] [PubMed]
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus Bloodstream Infections: Pathogenesis and Regulatory Mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, G.R.; Swetschinski, L.R.; Weaver, N.D.; Ikuta, K.S.; Mestrovic, T.; Gray, A.P.; Chung, E.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. The Burden of Antimicrobial Resistance in the Americas in 2019: A Cross-Country Systematic Analysis. Lancet Reg. Health-Am. 2023, 25, 100561. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Mestrovic, T.; Robles Aguilar, G.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Davis Weaver, N.; Han, C.; Wool, E.E.; Gershberg Hayoon, A.; Hay, S.I.; et al. The Burden of Bacterial Antimicrobial Resistance in the WHO European Region in 2019: A Cross-Country Systematic Analysis. Lancet Public Health 2022, 7, 897–913. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Miller, W.R.; Arias, C.A. ESKAPE Pathogens: Antimicrobial Resistance, Epidemiology, Clinical Impact and Therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641. [Google Scholar] [CrossRef]
- Mann, A.; Nehra, K.; Rana, J.S.; Dahiya, T. Antibiotic Resistance in Agriculture: Perspectives on Upcoming Strategies to Overcome Upsurge in Resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef]
- Singh, B.; Bhat, A.; Ravi, K. Antibiotics Misuse and Antimicrobial Resistance Development in Agriculture: A Global Challenge. Environ. Health 2024, 2, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Haaber, J.; Penadés, J.R.; Ingmer, H. Transfer of Antibiotic Resistance in Staphylococcus aureus. Trends Microbiol. 2017, 25, 893–905. Available online: https://www.sciencedirect.com/science/article/pii/S0966842X17301257?via%3Dihub (accessed on 12 March 2025). [CrossRef] [PubMed]
- Douglas, E.J.A.; Wulandari, S.W.; Lovell, S.D.; Laabei, M. Novel Antimicrobial Strategies to Treat Multidrug Resistant Staphylococcus aureus Infections. Microb. Biotechnol. 2023, 16, 1456. [Google Scholar] [CrossRef]
- Papkou, A.; Hedge, J.; Kapel, N.; Young, B.; MacLean, R.C. Efflux Pump Activity Potentiates the Evolution of Antibiotic Resistance across S. aureus Isolates. Nat. Commun. 2020, 11, 3970. [Google Scholar] [CrossRef]
- Rajabi, S.; Shivaee, A.; Khosravi, M.A.; Eshaghi, M.; Shahbazi, S.; Hosseini, F. Evaluation of Multidrug Efflux Pump Expression in Clinical Isolates of Staphylococcus aureus. Gene Rep. 2020, 18, 100537. [Google Scholar] [CrossRef]
- The Major Facilitator Superfamily, M.; Kolar, M.; Auxiliadora Dea-Ayuela, M.; Stephen, J.; Salam, F.; Lekshmi, M.; Kumar, S.H.; Varela, M.F. The Major Facilitator Superfamily and Antimicrobial Resistance Efflux Pumps of the ESKAPEE Pathogen Staphylococcus aureus. Antibiotics 2023, 12, 343. [Google Scholar] [CrossRef]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug Efflux Pumps in Staphylococcus aureus: An Update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Ganjloo, S.; Pourmand, M.R.; Mashhadi, R.; Ghazvini, K. Epidemiology of Efflux Pumps Genes Mediating Resistance among Staphylococcus aureus; a Systematic Review. Microb. Pathog. 2020, 139, 103850. [Google Scholar] [CrossRef]
- Schindler, B.D.; Kaatz, G.W. Multidrug Efflux Pumps of Gram-Positive Bacteria. Drug Resist. Updates 2016, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic Resistance Breakers: Current Approaches and Future Directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, G.; Acharya, Y.; Haldar, J. Antibiotic Adjuvants: A Versatile Approach to Combat Antibiotic Resistance. ACS Omega 2023, 8, 10757–10783. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yasmeen, N.; Pandey, A.; Ahmad Chaudhary, A.; Alawam, A.S.; Ahmad Rudayni, H.; Islam, A.; Lakhawat, S.S.; Sharma, P.K.; Shahid, M. Antibiotic Adjuvants: Synergistic Tool to Combat MultiDrug Resistant Pathogens. Front. Cell Infect. Microbiol. 2023, 13, 1293633. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic Adjuvants: Rescuing Antibiotics from Resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef]
- Martins, M.; Dastidar, S.G.; Fanning, S.; Kristiansen, J.E.; Molnar, J.; Pagès, J.-M.; Schelz, Z.; Spengler, G.; Viveiros, M.; Amaral, L. Potential Role of Non-Antibiotics (Helper Compounds) in the Treatment of Multidrug-Resistant Gram-Negative Infections: Mechanisms for Their Direct and Indirect Activities. Int. J. Antimicrob. Agents 2008, 31, 198–208. [Google Scholar] [CrossRef]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2019, 88, 26–40. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside. Indian. J. Med. Res. 2019, 149, 129. [Google Scholar] [CrossRef]
- Yoshida, H.; Bogaki, M.; Nakamura, S.; Ubukata, K.; Konno, M. Nucleotide Sequence and Characterization of the Staphylococcus aureus norA Gene, Which Confers Resistance to Quinolones. J. Bacteriol. 1990, 172, 6942–6949. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Sobkowiak, B.; Parreira, R.; Edgeworth, J.D.; Viveiros, M.; Clark, T.G.; Couto, I. Genetic Diversity of NorA, Coding for a Main Efflux Pump of Staphylococcus aureus. Front. Genet. 2019, 10, 421435. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Koide, A.; Kuang, H.; Torres, V.J.; Koide, S.; Wang, D.N.; Traaseth, N.J. Proton-Coupled Transport Mechanism of the Efflux Pump NorA. Nat. Commun. 2024, 15, 4494. [Google Scholar] [CrossRef] [PubMed]
- Lamut, A.; Peterlin Mašič, L.; Kikelj, D.; Tomašič, T. Efflux Pump Inhibitors of Clinically Relevant Multidrug Resistant Bacteria. Med. Res. Rev. 2019, 39, 2460–2504. [Google Scholar] [CrossRef]
- Brawley, D.N.; Sauer, D.B.; Li, J.; Zheng, X.; Koide, A.; Jedhe, G.S.; Suwatthee, T.; Song, J.; Liu, Z.; Arora, P.S.; et al. Structural Basis for Inhibition of the Drug Efflux Pump NorA from Staphylococcus aureus. Nat. Chem. Biol. 2022, 18, 706–712. [Google Scholar] [CrossRef]
- Lepri, S.; Buonerba, F.; Goracci, L.; Velilla, I.; Ruzziconi, R.; Schindler, B.D.; Seo, S.M.; Kaatz, G.W.; Cruciani, G. Indole Based Weapons to Fight Antibiotic Resistance: A Structure-Activity Relationship Study. J. Med. Chem. 2016, 59, 867–891. [Google Scholar] [CrossRef]
- Buonerba, F.; Lepri, S.; Goracci, L.; Schindler, B.D.; Seo, S.M.; Kaatz, G.W.; Cruciani, G. Improved Potency of Indole-Based NorA Efflux Pump Inhibitors: From Serendipity toward Rational Design and Development. J. Med. Chem. 2017, 60, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hequet, A.; Burchak, O.N.; Jeanty, M.; Guinchard, X.; Le Pihive, E.; Maigre, L.; Bouhours, P.; Schneider, D.; Maurin, M.; Paris, J.M.; et al. 1-(1H-Indol-3-Yl)Ethanamine Derivatives as Potent Staphylococcus aureus NorA Efflux Pump Inhibitors. ChemMedChem 2014, 9, 1534–1545. [Google Scholar] [CrossRef]
- Chandal, N.; Tambat, R.; Kalia, R.; Kumar, G.; Mahey, N.; Jachak, S.; Nandanwar, H. Efflux Pump Inhibitory Potential of Indole Derivatives as an Arsenal against norA over-Expressing Staphylococcus aureus. Microbiol. Spectr. 2023, 11, e0487622. [Google Scholar] [CrossRef]
- Felicetti, T.; Mangiaterra, G.; Cannalire, R.; Cedraro, N.; Pietrella, D.; Astolfi, A.; Massari, S.; Tabarrini, O.; Manfroni, G.; Barreca, M.L.; et al. C-2 Phenyl Replacements to Obtain Potent Quinoline-Based Staphylococcus aureus NorA Inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 584–597. [Google Scholar] [CrossRef]
- Felicetti, T.; Cedraro, N.; Astolfi, A.; Cernicchi, G.; Mangiaterra, G.; Vaiasicca, S.; Massari, S.; Manfroni, G.; Barreca, M.L.; Tabarrini, O.; et al. New C-6 Functionalized Quinoline NorA Inhibitors Strongly Synergize with Ciprofloxacin against Planktonic and Biofilm Growing Resistant Staphylococcus aureus Strains. Eur. J. Med. Chem. 2022, 241, 114656. [Google Scholar] [CrossRef] [PubMed]
- Cedraro, N.; Cannalire, R.; Astolfi, A.; Mangiaterra, G.; Felicetti, T.; Vaiasicca, S.; Cernicchi, G.; Massari, S.; Manfroni, G.; Tabarrini, O.; et al. From Quinoline to Quinazoline-Based S. aureus NorA Efflux Pump Inhibitors by Coupling a Focused Scaffold Hopping Approach and a Pharmacophore Search. ChemMedChem 2021, 16, 3044–3059. [Google Scholar] [CrossRef]
- Fontaine, F.; Hequet, A.; Voisin-Chiret, A.S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. First Identification of Boronic Species as Novel Potential Inhibitors of the Staphylococcus aureus NorA Efflux Pump. J. Med. Chem. 2014, 57, 2536–2548. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.; Héquet, A.; Voisin-Chiret, A.S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. Boronic Species as Promising Inhibitors of the Staphylococcus aureus NorA Efflux Pump: Study of 6-Substituted Pyridine-3-Boronic Acid Derivatives. Eur. J. Med. Chem. 2015, 95, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.G.; Slotved, H.C.; Molgaard, P.; Olsen, C.E.; Christensen, S.B. Chalcone Inhibitors of the NorA Efflux Pump in Staphylococcus aureus Whole Cells and Enriched Everted Membrane Vesicles. Bioorg Med. Chem. 2012, 20, 4514–4521. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, I.A.; Koul, S.; Koul, J.L.; Taneja, S.C.; Ali, I.; Ali, F.; Sharma, S.; Mirza, Z.M.; Kumar, M.; et al. Novel Structural Analogues of Piperine as Inhibitors of the NorA Efflux Pump of Staphylococcus aureus. J. Antimicrob. Chemother. 2008, 61, 1270–1276. [Google Scholar] [CrossRef]
- Sangwan, P.L.; Koul, J.L.; Koul, S.; Reddy, M.V.; Thota, N.; Khan, I.A.; Kumar, A.; Kalia, N.P.; Qazi, G.N. Piperine Analogs as Potent Staphylococcus aureus NorA Efflux Pump Inhibitors. Bioorg Med. Chem. 2008, 16, 9847–9857. [Google Scholar] [CrossRef]
- dos Santos, J.F.S.; Tintino, S.R.; da Silva, A.R.P.; Cristina, C.R.; Scherf, J.R.; de S. Silveira, Z.; de Freitas, T.S.; de Lacerda Neto, L.J.; Barros, L.M.; Irwin, I.R.; et al. Enhancement of the Antibiotic Activity by Quercetin against Staphylococcus aureus Efflux Pumps. J. Bioenerg. Biomembr. 2021, 53, 157–167. [Google Scholar] [CrossRef]
- Muniz, D.F.; dos Santos Barbosa, C.R.; de Menezes, I.R.A.; de Sousa, E.O.; Pereira, R.L.S.; Júnior, J.T.C.; Pereira, P.S.; de Matos, Y.M.L.S.; da Costa, R.H.S.; de Morais Oliveira-Tintino, C.D.; et al. In Vitro and in Silico Inhibitory Effects of Synthetic and Natural Eugenol Derivatives against the NorA Efflux Pump in Staphylococcus aureus. Food Chem. 2021, 337, 127776. [Google Scholar] [CrossRef]
- Espinoza, J.; Urzúa, A.; Sanhueza, L.; Walter, M.; Fincheira, P.; Muñoz, P.; Mendoza, L.; Wilkens, M. Essential Oil, Extracts, and Sesquiterpenes Obtained From the Heartwood of Pilgerodendron uviferum Act as Potential Inhibitors of the Staphylococcus aureus NorA Multidrug Efflux Pump. Front. Microbiol. 2019, 10, 337. [Google Scholar] [CrossRef]
- Kalia, N.P.; Mahajan, P.; Mehra, R.; Nargotra, A.; Sharma, J.P.; Koul, S.; Khan, I.A. Capsaicin, a Novel Inhibitor of the NorA Efflux Pump, Reduces the Intracellular Invasion of Staphylococcus aureus. J. Antimicrob. Chemother. 2012, 67, 2401–2408. [Google Scholar] [CrossRef]
- Diniz-Silva, H.T.; Magnani, M.; de Siqueira, S.; de Souza, E.L.; de Siqueira-Júnior, J.P. Fruit Flavonoids as Modulators of Norfloxacin Resistance in Staphylococcus aureus That Overexpresses norA. LWT-Food Sci. Technol. 2017, 85, 324–326. [Google Scholar] [CrossRef]
- Singh, S.; Kalia, N.P.; Joshi, P.; Kumar, A.; Sharma, P.R.; Kumar, A.; Bharate, S.B.; Khan, I.A. Boeravinone B, A Novel Dual Inhibitor of NorA Bacterial Efflux Pump of Staphylococcus aureus and Human P-Glycoprotein, Reduces the Biofilm Formation and Intracellular Invasion of Bacteria. Front. Microbiol. 2017, 8, 1868. [Google Scholar] [CrossRef]
- de Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Limaverde, P.W.; Figueredo, F.G.; Campina, F.F.; da Cunha, F.A.B.; da Costa, R.H.S.; Pereira, P.S.; Lima, L.F.; de Matos, Y.M.L.S.; et al. Inhibition of the Essential Oil from Chenopodium ambrosioides L. and α-Terpinene on the NorA Efflux-Pump of Staphylococcus aureus. Food Chem. 2018, 262, 72–77. [Google Scholar] [CrossRef]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella Sativa Essential Oil and Its Bioactive Compounds as Resistance Modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.N.; de Oliveira, A.B.M.; Ferreira, A.K.; Silva, E.; de Sousa, L.M.S.; França Rocha, M.C.; De, J.P.; Júnior, S.; William Kaatz, G.; da Silva Almeida, J.R.G.; et al. Modulation of the Resistance to Norfloxacin in Staphylococcus aureus by Bauhinia Forficata Link. Nat. Prod. Res. 2021, 35, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Cernicchi, G.; Felicetti, T.; Sabatini, S. Microbial Efflux Pump Inhibitors: A Journey around Quinoline and Indole Derivatives. Molecules 2021, 26, 6996. [Google Scholar] [CrossRef]
- Felicetti, T.; Cannalire, R.; Pietrella, D.; Latacz, G.; Lubelska, A.; Manfroni, G.; Barreca, M.L.; Massari, S.; Tabarrini, O.; Kieć-Kononowicz, K.; et al. 2-Phenylquinoline S. aureus NorA Efflux Pump Inhibitors: Evaluation of the Importance of Methoxy Group Introduction. J. Med. Chem. 2018, 61, 7827–7848. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M. Mechanisms of Fluoroquinolone Resistance in Genetically Related Strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 1997, 41, 2733–2737. [Google Scholar] [CrossRef]
- Uribe, E.G. ATP Synthesis Driven by a K+-Valinomycin-Induced Charge Imbalance across Chloroplast Grana Membranes. FEBS Lett. 1973, 36, 143–147. [Google Scholar] [CrossRef]
- Koh, J.Y.; Cotman, C.W. Programmed Cell Death: Its Possible Contribution to Neurotoxicity Mediated by Calcium Channel Antagonists. Brain Res. 1992, 587, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Barnett, P.; Perlmutter, J.; Dunman, P.M. Identification of Acinetobacter baumannii Serum-Associated Antibiotic Efflux Pump Inhibitors. Antimicrob. Agents Chemother. 2014, 58, 6360. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Fleet, J.L.; Bailey, D.G.; McArthur, E.; Wald, R.; Rehman, F.; Garg, A.X. Calcium-Channel Blocker–Clarithromycin Drug Interactions and Acute Kidney Injury. JAMA 2013, 310, 2544–2553. [Google Scholar] [CrossRef]
- AlMatar, M.; Albarri, O.; Makky, E.A.; Köksal, F. Efflux Pump Inhibitors: New Updates. Pharmacol. Rep. 2020, 73, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pidwill, G.R.; Gibson, J.F.; Cole, J.; Renshaw, S.A.; Foster, S.J. The Role of Macrophages in Staphylococcus aureus Infection. Front. Immunol. 2020, 11, 620339. [Google Scholar] [CrossRef]
- Palazzotti, D.; Felicetti, T.; Sabatini, S.; Moro, S.; Barreca, M.L.; Sturlese, M.; Astolfi, A. Fighting Antimicrobial Resistance: Insights on How the Staphylococcus aureus NorA Efflux Pump Recognizes 2-Phenylquinoline Inhibitors by Supervised Molecular Dynamics (SuMD) and Molecular Docking Simulations. J. Chem. Inf. Model. 2023, 63, 4875–4887. [Google Scholar] [CrossRef]
- Kumawat, M.; Nabi, B.; Daswani, M.; Viquar, I.; Pal, N.; Sharma, P.; Tiwari, S.; Sarma, D.K.; Shubham, S.; Kumar, M. Role of Bacterial Efflux Pump Proteins in Antibiotic Resistance across Microbial Species. Microb. Pathog. 2023, 181, 106182. [Google Scholar] [CrossRef]
- Wertheim, H.F.L.; Vos, M.C.; Ott, A.; Van Belkum, A.; Voss, A.; Kluytmans, J.A.J.W.; Van Keulen, P.H.J.; Vandenbroucke-Grauls, C.M.J.E.; Meester, M.H.M.; Verbrugh, H.A. Risk and Outcome of Nosocomial Staphylococcus aureus Bacteraemia in Nasal Carriers versus Non-Carriers. Lancet 2004, 364, 703–705. [Google Scholar] [CrossRef]
- Abbey, T.C.; Deak, E. What’s New from the CLSI Subcommittee on Antimicrobial Susceptibility Testing M100, 29th Edition. Clin. Microbiol. Newsl. 2019, 41, 203–209. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between Natural Products and Antibiotics against Infectious Diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Zimmermann, S.; Klinger-Strobel, M.; Bohnert, J.A.; Wendler, S.; Rödel, J.; Pletz, M.W.; Löffler, B.; Tuchscherr, L. Clinically Approved Drugs Inhibit the Staphylococcus aureus Multidrug NorA Efflux Pump and Reduce Biofilm Formation. Front. Microbiol. 2019, 10, 492781. Available online: https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2019.02762 (accessed on 12 March 2025). [CrossRef] [PubMed]
- Wu, M.; Maier, E.; Benz, R.; Hancock, R.E.W. Mechanism of Interaction of Different Classes of Cationic Antimicrobial Peptides with Planar Bilayers and with the Cytoplasmic Membrane of Escherichia coli. Biochemistry 1999, 38, 7235–7242. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Lanneluc, I.; Connil, N.; Cottenceau, M.; Pons, A.M.; Sablé, S. Mechanism of Bactericidal Activity of Microcin L in Escherichia coli and Salmonella enterica. Antimicrob. Agents Chemother. 2011, 55, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Huang, J.X.; Ramu, S.; Butler, M.S.; Cooper, M.A. Ramoplanin at Bactericidal Concentrations Induces Bacterial Membrane Depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 6819. [Google Scholar] [CrossRef]
- French, S.; Farha, M.; Ellis, M.J.; Sameer, Z.; Côté, J.P.; Cotroneo, N.; Lister, T.; Rubio, A.; Brown, E.D. Potentiation of Antibiotics against Gram-Negative Bacteria by Polymyxin B Analogue SPR741 from Unique Perturbation of the Outer Membrane. ACS Infect. Dis. 2020, 6, 1405–1412. [Google Scholar] [CrossRef]
- Tambat, R.; Jangra, M.; Mahey, N.; Chandal, N.; Kaur, M.; Chaudhary, S.; Verma, D.K.; Thakur, K.G.; Raje, M.; Jachak, S.; et al. Microbe-Derived Indole Metabolite Demonstrates Potent Multidrug Efflux Pump Inhibition in Staphylococcus aureus. Front. Microbiol. 2019, 10, 2153. [Google Scholar] [CrossRef]
- OECD. Overview of the Set of OECD Genetic Toxicology Test Guidelines and Updates Performed in 2014-2015, 2nd ed.; OECD Series on Testing and Assessment, No. 238; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- di Vito, R.; Levorato, S.; Fatigoni, C.; Acito, M.; Sancineto, L.; Traina, G.; Villarini, M.; Santi, C.; Moretti, M. In Vitro Toxicological Assessment of PhSeZnCl in Human Liver Cells. Toxicol. Res. 2023, 39, 105. [Google Scholar] [CrossRef]
- Rondini, T.; Branciari, R.; Franceschini, E.; Acito, M.; Fatigoni, C.; Roila, R.; Ranucci, D.; Villarini, M.; Galarini, R.; Moretti, M. Olive Mill Wastewater Extract: In Vitro Genotoxicity/Antigenotoxicity Assessment on HepaRG Cells. Int. J. Environ. Res. Public Health 2024, 21, 1050. [Google Scholar] [CrossRef]
- Lovell, D.P.; Omori, T. Statistical Issues in the Use of the Comet Assay. Mutagenesis 2008, 23, 171–182. [Google Scholar] [CrossRef]
- Rázquin-Olazarán, I.; Shahrour, H.; Martínez-De-Tejada, G. A Synthetic Peptide Sensitizes Multi-Drug Resistant Pseudomonas aeruginosa to Antibiotics for More than Two Hours and Permeabilizes Its Envelope for Twenty Hours. J. Biomed. Sci. 2020, 27, 85. Available online: https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-020-00678-3 (accessed on 12 March 2025). [CrossRef]
- Drugeon, H.B.; Juvin, M.E.; Bryskier, A. Relative Potential for Selection of Fluoroquinolone-Resistant Streptococcus pneumoniae Strains by Levofloxacin: Comparison with Ciprofloxacin, Sparfloxacin and Ofloxacin. J. Antimicrob. Chemother. 1999, 43, 55–59. Available online: https://academic.oup.com/jac/article/43/suppl_3/55/2488486? (accessed on 12 March 2025). [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]

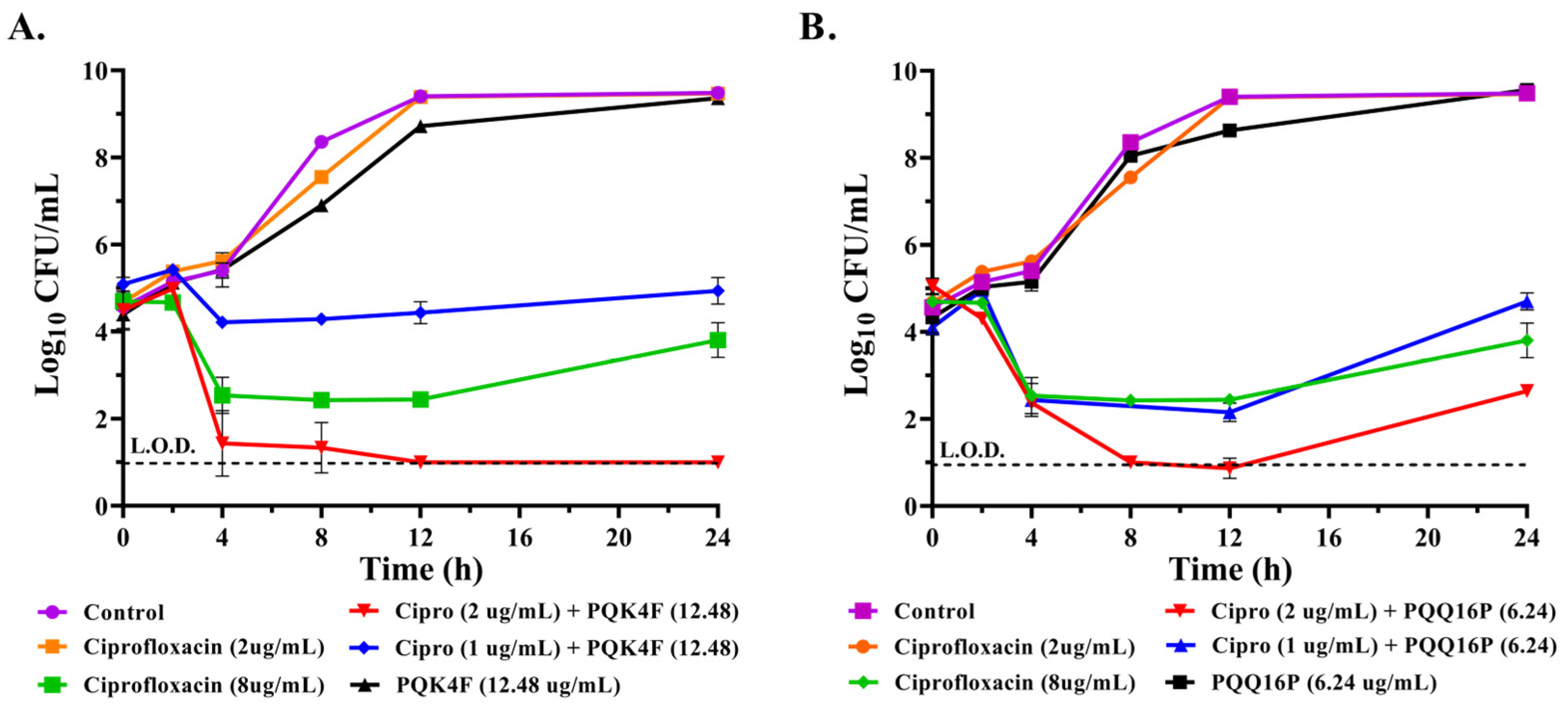
| CPX Conc. | 0.25 × MIC | 0.5 × MIC | 1 × MIC | 2 × MIC | 4 × MIC | 8 × MIC |
|---|---|---|---|---|---|---|
| CFU Count | ||||||
| CPX alone | UC | UC | UC | 2.75 × 10−9 | 2.3 × 10−8 | <10−9 |
| +PQK4F (6.24 µg/mL) | UC | 1 × 10−9 | <10−9 | <10−9 | <10−9 | <10−9 |
| +PQK4F (12.48 µg/mL) | 5.26 × 10−9 | 3 × 10−7 | <10−9 | <10−9 | <10−9 | <10−9 |
| +PQQ16P (3.12 µg/mL) | UC | 1.67 × 10−9 | <10−9 | <10−9 | <10−9 | <10−9 |
| +PQQ16P (6.24 µg/mL) | UC | 1 × 10−7 | <10−9 | <10−9 | <10−9 | <10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandal, N.; Sharma, N.; Cernicchi, G.; Felicetti, T.; Rondini, T.; Acito, M.; Nandanwar, H.; Sabatini, S. In Vitro and In Vivo Investigations into the Potential of Quinazoline and Quinoline Derivatives as NorA Efflux Pump Inhibitors Against Resistant Staphylococcus aureus Strains. Antibiotics 2025, 14, 339. https://doi.org/10.3390/antibiotics14040339
Chandal N, Sharma N, Cernicchi G, Felicetti T, Rondini T, Acito M, Nandanwar H, Sabatini S. In Vitro and In Vivo Investigations into the Potential of Quinazoline and Quinoline Derivatives as NorA Efflux Pump Inhibitors Against Resistant Staphylococcus aureus Strains. Antibiotics. 2025; 14(4):339. https://doi.org/10.3390/antibiotics14040339
Chicago/Turabian StyleChandal, Nishtha, Nidhi Sharma, Giada Cernicchi, Tommaso Felicetti, Tommaso Rondini, Mattia Acito, Hemraj Nandanwar, and Stefano Sabatini. 2025. "In Vitro and In Vivo Investigations into the Potential of Quinazoline and Quinoline Derivatives as NorA Efflux Pump Inhibitors Against Resistant Staphylococcus aureus Strains" Antibiotics 14, no. 4: 339. https://doi.org/10.3390/antibiotics14040339
APA StyleChandal, N., Sharma, N., Cernicchi, G., Felicetti, T., Rondini, T., Acito, M., Nandanwar, H., & Sabatini, S. (2025). In Vitro and In Vivo Investigations into the Potential of Quinazoline and Quinoline Derivatives as NorA Efflux Pump Inhibitors Against Resistant Staphylococcus aureus Strains. Antibiotics, 14(4), 339. https://doi.org/10.3390/antibiotics14040339









