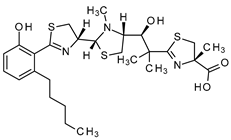
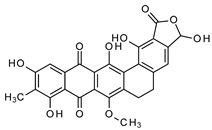
Abstract
Antimicrobial resistance (AMR) is considered one of the greatest threats to both human and animal health. Efforts to address AMR include implementing antimicrobial stewardship programs and introducing alternative treatment options. Nevertheless, effective treatment of infectious diseases caused by bacteria will still require the identification and development of new antimicrobial agents. Eight different natural products were tested for antimicrobial activity against seven pathogenic bacterial species (Brachyspira sp., Chlamydia sp., Clostridioides sp., Mannheimia sp., Mycobacterium sp., Mycoplasma sp., Pasteurella sp.). In a first pre-screening, most compounds (five out of eight) inhibited bacterial growth only at high concentrations, but three natural products (celastramycin A [CA], closthioamide [CT], maduranic acid [MA]) displayed activity at concentrations <2 µg/mL against Pasteurella sp. and two of them (CA and CT) also against Mannheimia sp. Those results were confirmed by testing a larger collection of isolates encompassing 64 Pasteurella and 56 Mannheimia field isolates originating from pigs or cattle, which yielded MIC90 values of 0.5, 0.5, and 2 µg/mL against Pasteurella and 0.5, 4, and >16 µg/mL against Mannheimia for CA, CT, and MA, respectively. CA, CT, and MA exhibited higher MIC50 and MIC90 values against Pasteurella isolates with a known AMR phenotype against commonly used therapeutic antimicrobial agents than against isolates with unknown AMR profiles. This study demonstrates the importance of whole-cell antibacterial screening of natural products to identify promising scaffolds with broad- or narrow-spectrum antimicrobial activity against important Gram-negative veterinary pathogens with zoonotic potential.
1. Introduction
Historically, natural products have played important roles as drugs in many therapeutic areas, including infectious diseases [1]. Following the discovery of antibiotics almost a century ago, several classes of antimicrobial agents of natural origin, such as β-lactams, tetracyclines, or aminoglycosides, were introduced as therapeutic agents. Antibiotic use, since then, has had an enormous impact on the treatment of infectious diseases in both human and veterinary medicine, but also on the ability to perform surgical procedures and immunosuppressing chemotherapy.
However, the ensuing frequent use of antimicrobial agents, including their misuse and overuse, has led to the concomitant emergence and dissemination of antimicrobial resistance (AMR) in bacteria [2,3,4,5,6], with the consequence that AMR is now considered one of the greatest threats to both human and animal health. In line with this, the Animal Health Law (Regulation (EU) 2016/429) states that “microorganisms that have developed resistance to antimicrobials should be treated as if they were transmissible diseases”. For 2019, predictive statistical models estimated a worldwide 4.95 million human deaths associated with bacterial AMR, thereof 1.27 million directly attributable to bacterial AMR [7]. Since antimicrobial administration to food animals and humans is a major driver of AMR and is projected to even increase in the future, bacterial AMR is anticipated to increase as well [8,9,10,11,12]. Thus, a continued presence of resistance determinants in commensal and pathogenic bacteria is the likely consequence of this uninterrupted selective pressure. Worldwide efforts have been and are being undertaken to address this threat [13,14]. In addition to antimicrobial stewardship programs and alternative novel and innovative treatment options [15], new antimicrobial agents will still be needed to combat AMR. Here, the role of natural products has declined in the current drug discovery process due to technical, economic, and intellectual property challenges [16], resulting in an antimicrobial agent pipeline that remains insufficient against priority pathogens [17,18]. The spectrum of chemical scaffolds used to develop novel antibacterial agents, thus, remains limited, so the discovery of new antimicrobial compound classes with novel modes of action is urgently needed [19].
A resurgence in natural product screening has been stimulated by recent progress in many different fields of research. These include not only bioinformatic analysis, genomics, and genome mining, as well as the exploitation of exotic ecological niches, but also involve improved understanding of AMR [20] and the development of new techniques to explore the chemistry of antimicrobial compounds [21]. Frequently identified with a specific research question in mind, most structures of interest are not routinely screened for activity against a broad panel of bacterial pathogens due to limited resources, especially in academia. Potential narrow-spectrum antimicrobials will, for example, almost certainly remain undiscovered this way. Examination of existing compound libraries is therefore still a promising strategy to identify new candidates for further development of novel antimicrobial drugs.
For the current study, we, therefore, selected eight substances out of six distinct chemotype classes from the natural product collection of the Leibniz-HKI based on, e.g., biotechnological accessibility, preliminary cell line toxicity, and primary bioactivity data. Particular emphasis was put on compounds with known anti-Gram-negative activity. All products selected were discovered in various ecological contexts, such as closthioamide, a unique polythioamide representing the first antibiotic found in an obligate anaerobic bacterium, or cervimycins from Streptomyces isolated from prehistoric cave wall paintings, which were made using bat dung [22,23].
The natural products selected were tested for antimicrobial activity against a diverse panel of bacterial species with importance as animal and/or zoonotic pathogens. For this purpose, we chose bacterial species that represent not only Gram-positive, Gram-negative, cell-wall-free, spore-forming, and acid-fast species, but also organisms with difficult-to-treat slow-growing or intracellular lifestyles. Additional criteria for choosing the bacterial species were (i) representing a relevant pathogen in human and/or veterinary medicine, (ii) the presence of frequently occurring AMR, an increasing problem for disease control [24], and (iii) the existence of established livestock animal models, which offers the possibility to evaluate the in vivo application of qualified substances at a later developmental stage (summarized in Table 1).

Table 1.
Background information on the bacterial species included in the current study concerning the diseases they cause in livestock, their most relevant AMR, and available infection models.
2. Results
The natural products were selected from the Leibniz-HKI natural compound collection, based on accessibility, data on preliminary activity, cytotoxicity, and individual properties. In total, eight natural products from six different compound classes exhibited promising characteristics. These products were screened following a two-stage process: First, in a pre-screening step, seven bacterial species were used to determine MIC (minimal inhibitory concentration) values. Based on the resulting activity profiles, we identified the most promising combinations of target species and test substances for secondary screening. Then, we determined the distribution of MIC values with a larger set of field isolates harboring varying resistance determinants.
The pre-screen results showed that the natural products inhibited growth in 5–78% of all isolates tested, with celastramycin A, closthioamide, and maduranic acid showing activity against several isolates at concentrations below 2 µg/mL (Figure 1). The remaining test compounds only inhibited growth in this concentration range in one control strain (cervimycin K1 in S. aureus) or, if at all, showed antibacterial activity at concentrations ≥2 µg/mL (Supplemental Table S1). In detail, micacocidin inhibited growth in two out of seven isolates of M. bovis at 16 µg/mL. Griseochelin and the cervimycin derivatives showed activity against two species, C. difficile and M. bovis, with MIC values of at least 4 µg/mL. Griseochelin was additionally active against B. hyodysenteriae at ≥2 µg/mL, while the cervimycin derivatives were additionally only active against one out of six isolates of the M. avium subspecies (8 µg/mL). In order to meet favorable pharmacokinetic/pharmacodynamic (PK/PD) relationships, low MIC values are preferred for further optimization in drug development. Closthioamide showed the broadest overall activity inhibiting the growth of isolates from six out of seven bacterial test species at relevant concentrations <2 µg/mL, followed by celastramycin A (three out of seven species), and maduranic acid (two out of seven species) (Figure 2). These compounds predominantly inhibited the growth of P. multocida field isolates, as well as the S. aureus control strain. In addition, celastramycin A, as well as closthioamide, inhibited M. haemolytica growth at concentrations below 1 µg/mL. Therefore, we selected the compounds celastramycin A, closthioamide, and maduranic acid for further screening.

Figure 1.
Heat map of the pre-screening results. Shown are the absolute numbers of isolates at their individual minimal inhibitory concentrations (MICs) for all eight substances. The heat map shows in bold the number of isolates combined from all bacterial species with a specific MIC value for each compound. Except for C. suis (n = 2 biological replicates), all isolates were tested once. The color intensity increases with the absolute number of strains identified to have a specific MIC value for a natural product.

Figure 2.
Heat map of the pre-screening results showing the most highly active natural products with MIC values ≤2 µg/mL that were evaluated for activity against isolates of Gram-positive, as well as Gram-negative or cell-wall-free bacterial species. The heat map shows in bold the number of isolates (from an individual bacterial species with a specific MIC value for each compound. Except for C. suis (n = 2 biological replicates), all isolates were tested once. The color intensity increases with the absolute number of strains identified to have a specific MIC value for a natural product.
M. haemolytica and P. multocida play major roles as causative agents of severe infections in farm animals that are frequently subject to antimicrobial treatment [46,47,48,49]. Compounds specifically targeting both pathogens are of particular interest. Therefore, field isolates of P. multocida (n = 64) and M. haemolytica (n = 56) collected from various cattle and pig sources (see Supplemental Table S2) were tested to determine MIC values for celastramycin A, closthioamide, and maduranic acid. As already indicated by the pre-screening results (Figure 2), growth inhibition generally required higher concentrations of the natural products in M. haemolytica than in P. multocida (Figure 3A–C). When applying a cut-off for MIC values higher than 1 µg/mL, celastramycin A showed growth-inhibition rates of 100% for M. haemolytica, as well as for P. multocida. The MIC50 and MIC90 values of celastramycin A determined against M. haemolytica were both 0.5 µg/mL, while the corresponding values against P. multocida were 0.125 and 0.5 µg/mL, respectively. The percentage of isolates showing inhibition determined for closthioamide in general (i.e., no cut-off applied) were 60.7% and 100% for M. haemolytica and P. multocida, respectively. In line with this observation, closthioamide showed higher MIC50 and MIC90 values against M. haemolytica with 1 and 4 µg/mL, respectively, while the equivalent values against P. multocida were the same as for celastramycin A with 0.125 and 0.5 µg/mL, respectively. In line with the pre-screening results, maduranic acid showed no activity against M. haemolytica, yet inhibited growth at concentrations below 2 µg/mL in 79.7% of the P. multocida isolates tested. The resulting MIC50 and MIC90 values were both higher than 16 µg/mL against M. haemolytica, and 0.5 and 2 µg/mL against P. multocida, respectively. The distribution of the MIC values of celastramycin A, closthioamide, and maduranic acid against P. multocida displayed a bimodal curve. When respective isolates of P. multocida are grouped by their resistance status (known or unknown), those isolates with known antibiotic resistance displayed, in most cases, higher MICs for celastramycin A, closthioamide and maduranic acid than the total average (Figure 3D–F).

Figure 3.
MIC values of the highly active natural products (A,D) celastramycin A, (B,E) closthioamide, and (C,F) maduranic acid against (A–C) field isolates of P. multocida (n = 64) and M. haemolytica (n = 56), as well as against (D–F) P. multocida isolates with (n = 13) known resistances against commonly used chemotherapeutics (see Supplemental Table S2) and P. multocida isolates that were previously not tested against commonly used chemotherapeutics (“unknown status”, n = 51). Results of broth microdilution assays.
3. Discussion
The emergence, evolution, and spread of AMR threaten global health systems, so multifaceted strategies, such as the 2015 WHO Global Action Plan on AMR, have become key elements in the effort to successfully combat AMR [50]. These include the screening of compounds to detect novel antimicrobial agents.
Of the eight compounds tested in the pre-screen, only celastramycin A, closthioamide, and maduranic acid showed promising antimicrobial activities at concentrations ≤2 µg/mL, with closthioamide inhibiting growth in most species (five out of seven). Closthioamide is, thus, the sole compound identified in this study with characteristics pointing towards a broad-spectrum antibiotic that is active against both Gram-negative and Gram-positive bacteria. In a previous study, closthioamide demonstrated activity against a broad spectrum of Gram-positive bacteria via the inhibition of DNA gyrase [51]. Celastramycin A and maduranic acid were active against fewer species at concentrations below 2 µg/mL. Two species, i.e., M. haemolytica and P. multocida, were inhibited by closthioamide and celastramycin A, while maduranic acid only inhibited growth in P. multocida. Both species frequently co-occur in respiratory diseases of farm animals [52,53]. While P. multocida has a broader host-range that also includes humans [54], M. haemolytica is predominantly pathogenic for ruminants [37]. Economic losses due to treatment costs and reduced weight gain in beef production [55,56], as well as decreased milk yield and other negative effects on dairy cow health [57,58,59], highlight the importance of treatment options for infections caused by these two pathogens; especially, since multidrug-resistant (MDR) isolates are increasingly isolated from cattle with bovine respiratory disease (BRD). In Germany, the rates of MDR isolates have increased more than six-fold in P. multocida within a five-year period (2015–2020) [60]. That study reported MDR rates of 13.9% and 5.1%, while rates of pan-susceptibility were only 15.1% and 12.1% in isolates of P. multocida and M. haemolytica, respectively [60]. Another recent study identified a novel high-level macrolide resistance determinant in M. haemolytica, further limiting treatment options for BRD [61]. New antimicrobial agents that target P. multocida and M. haemolytica would consequently be highly advantageous. Therefore, we enlarged the panel of bacterial isolates from these species by testing field isolates that originated from cattle and pigs (P. multocida) or only from cattle (M. haemolytica). Comparing the distribution of MIC values for the three compounds tested, isolates of M. haemolytica overall showed higher MIC values than those of P. multocida. As both genera are rather closely related, we have, so far, no physiological or mechanistic explanation for this observation. As an example, M. haemolytica isolates had higher MIC values than P. multocida isolates with respect to closthioamide and maduranic acid. The latter did not inhibit growth in M. haemolytica, yet reached a MIC90 of 2 µg/mL for P. multocida. As coinfections of M. haemolytica and P. multocida are frequently present in BRD, effective treatment requires agents targeting both species. Therefore, maduranic acid could be interesting for further investigation as a narrower-spectrum treatment option for infections with P. multocida other than BRD, especially due to its reported activity against Gram-positive bacteria [62]. Closthioamide showed activity against both species; however, the MIC90 of 4 µg/mL against M. haemolytica leaves only a narrow therapeutic window. The broad spectrum of bacterial species inhibited in growth by this compound (six out of seven) yet motivates further tests also against AMR-isolates of other relevant pathogens. Predestined for further testing would be, e.g., Histophilus somni and M. bovis, as in the context of BRD, both are the major bacterial agents of the syndrome besides P. multocida and M. haemolytica [52]. Further testing of closthioamide should therefore include field isolates of M. bovis, as closthioamide also displayed low MIC values against this species in the pre-screening, as well as isolates of H. somni, which was not part of this investigation, to cover the four most important bacterial pathogens from the BRD complex. Celastramycin A achieved the best results with MIC90 values for both panels of P. multocida and M. haemolytica field isolates. Moreover, celastramycin A has a modulatory effect on the immune system [63], like several other antibiotic classes (e.g., macrolides) [64,65,66], making this substance a promising candidate for further research.
While the MIC values for all three substances formed a single defined peak in all M. haemolytica isolates tested, isolates of P. multocida, in contrast, showed a bimodal MIC distribution. This observation partly reflects the classification of the isolates into two groups—with or without known AMR phenotype—and is most pronounced for maduranic acid. There, isolates with known resistance exclusively belong to the peak of MIC values ≥0.5 µg/mL. On the other hand, isolates with unknown status still form a bimodal distribution and, hence might consist of at least two subpopulations of phenotypes tolerating different concentrations of maduranic acid. The same observation is true for closthioamide, except for a few isolates with known resistance being susceptible at ≤0.125 µg/mL, and for celastramycin A, only less pronounced. A plausible explanation for this trend towards higher MIC values for the compounds tested against isolates with known resistance could be cross-resistance (i.e., possibly due to efflux), mediating higher tolerance towards the substances tested. This was previously reported for MDR isolates of S. aureus, which were able to tolerate higher doses of seaweed extract with antimicrobial activity than isolates with fewer resistance determinants [67]. Isolates with unknown resistance status, thus, should consist of (i) so-called wild-type (WT) isolates carrying no resistance determinants and hence tolerating only lower concentrations of test substances, and (ii) non-WT isolates that have acquired resistance determinants, hence tolerating higher concentrations [68]. A deeper analysis of the underlying mechanisms mediating resistance against these natural products could be helpful in identifying AMR determinants relevant for cross-resistance. Further investigation is required to determine the resistance profile of those isolates, which would allow us to clarify whether or not this explanation holds true.
4. Materials and Methods
4.1. Bacterial Isolates
A total of 41 bacterial reference (type and/or control) strains and field isolates from eight different families representing six phyla (Table 2) were pre-screened to determine bacterial susceptibility towards eight different natural products (Table 3). The respective isolates and their AMR profiles are summarized in Supplemental Table S1. Two strains (E. coli ATCC® 25922 and S. aureus ATCC® 29213), which were being used as controls, were single representatives of their species. Therefore, they were excluded from evaluating the activity testing of the included natural products.

Table 2.
Bacterial isolates used for pre-screening of the natural products.

Table 3.
Summary of natural products and their known characteristics.
Based on the results of the pre-screening, a second round of testing was performed in which an additional 51 Mannheimia haemolytica and 59 Pasteurella multocida field isolates were tested. These species included isolates with known resistance phenotypes against commonly used chemotherapeutic substances (n = 23 and n = 12, respectively), as well as isolates without information on their resistance profile (“unknown status”) (Supplemental Table S2). The isolates originated from farm animals (cattle and pigs) with no documented antibiotic treatment at least four weeks before sampling, and for some with a known clinical history. After revitalization from the cryo-conservation culture, all isolates, except the mycoplasma and chlamydia isolates, were at least twice freshly re-streaked on the respective agar prior to MIC testing.
4.2. Natural Products
The natural products to be tested were produced by Leibniz-HKI following published protocols (Table 3) and available as pure solid powders. Identity and purity were analyzed by LC–HRMS and HPLC, respectively, prior to dispatch. Stock solutions (10 mg/mL) of the substances were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Germany). The respective working solutions (320 µg/mL) of cervimycin C, cervimycin D, cervimycin K1, closthioamide, griseochelin, micacocidin, and maduranic acid were prepared by diluting the respective stock solution 1:31.25 [v/v] in sterile, deionized H2O (diH2O). For celastramycin A, a first 1:10 [v/v] dilution step in diH2O/DMSO (1:1) was necessary, before it was further diluted 1:3.125 [v/v] in diH2O. Pre-testing of DMSO in the maximum working concentration used showed no inhibitory effects on the isolates themselves. Prior to their use, all working solutions were 1:10 [v/v] diluted in the respective test medium. Due to limited amounts of the natural products and the search for highly potent active ingredients, the natural products were tested for their antimicrobial activity up to a concentration of 16 µg/mL.
4.3. Determination of Minimal Inhibitory Concentrations (MICs) of the Natural Products
For most bacterial species to be tested, the MIC values were determined using broth microdilution methodology (Table 2). To this end, CLSI protocols (broth microdilution dilution and agar dilution assays) were followed as closely as possible [80] with only minor modifications as specified below. Each test protocol included sterility (medium without bacteria and natural products) and growth controls (medium with bacteria but without natural products).
The MIC values for the quality control strains Escherichia (E.) coli strain ATCC 25922 and Staphylococcus (S.) aureus ATCC 29213 were determined following the CLSI protocol Vet01-A4 [81]. In brief, the control strains were grown on 5% sheep blood agar plates (35 °C, 16–18 h) and 3–5 single colonies were suspended in 5 mL sterile 0.9% NaCl solution, corresponding to a 0.5 McFarland standard; thereof, 50 µL were diluted in 11 mL Müller-Hinton broth (MHB; Oxoid, Germany) and served as inoculum containing approx. 5 × 105 cfu/mL. The cfu of the inoculum was controlled by diluting each inoculum 1:1000 in sterile 0.9% NaCl solution and plating 100 µL of the diluted inoculum on a sheep blood agar plate. After incubation (37 °C, 18–24 h), colonies were counted and the cfu in the inoculum was calculated. The MIC testing was performed in 96-U-well PS-microtiter plates (Greiner Bio-One, Germany) with 100 µL of the natural products freshly diluted in MHB and 100 µL of the inoculum. After incubation (35 °C, 16–20 h), the wells were checked visually for bacterial growth and the MIC determined as the lowest concentration of the natural product that completely inhibited bacterial growth.
MIC testing of Pasteurellaceae was carried out as described above with the following modifications: the isolates were freshly grown on 5% sheep blood agar plates (37 °C, 20–24 h) and 5 single colonies were suspended in 5 mL sterile 0.9% NaCl solution. Thereof, 400 µL were diluted in 11 mL Cation-Adjusted MHB (CAMHB; Oxoid, Germany) and used as inoculum with approx. 5 × 105 cfu/mL. The cfu counts of the inoculum were controlled as described above. MIC testing was performed as described above, but with CAMHB instead of MHB and incubation of the microtiter plates at 37 °C for 20–24 h.
Mycobacterium avium subsp. avium (MAA) and subsp. hominissuis (MAH) were tested following the CLSI protocol M24-A02 [82]. The isolates were grown on Löwenstein–Jensen agar slants (with glycerol, without pyruvate, Artelt-Enclit, Borna, Germany). Thereof, colony material was collected and resuspended in 5 mL sterile 0.9% NaCl solution. To resolve agglomerated bacteria, 3–5 glass beads were added followed by vortexing the suspension for 10–20 s. Then, 100 µL were transferred to 11 mL CAMHB with 5% oleic acid–albumin–dextrose–catalase solution (OADC, Sigma-Aldrich, Germany). Inoculation of the microtiter plates and cfu control of the inoculum on Middlebrook 7H11 agar plates with OADC (Sigma-Aldrich, Germany) were performed as described above. The incubation period of the microtiter and agar plates was extended to 7–10 d (35 °C).
The MIC testing of Brachyspira (B.) hyodysenteriae isolates was adapted to the in-house protocol published by Herbst et al., 2014 [27]. B. hyodysenteriae isolates were grown on tryptic soy agar plates with 5% sheep blood (TSB) under anaerobic conditions (37 °C, 3–5 d). To prepare the inoculum, 5 loops with bacteria were resuspended in 14 mL brain heart infusion broth supplemented with 20% fetal calf serum (BHIF; Becton Dickinson, Heidelberg, Germany; Biochrom, Berlin, Germany) to reach 105 cfu/mL. The inoculum was controlled by plating log10 dilutions on TSB and checking for growth of B. hyodysenteriae indicated by strong hemolysis. The natural products were diluted in BHIF and 100 µL per dilution step were dispensed into 96-U-well PS-microtiter plates. After adding 100 µL of the inoculum, the microtiter plates were incubated (anaerobic, 37 °C, 5 d, shaking [130 rpm]). Using a dark light table with lateral light fall, the MIC was determined as the macroscopically visible lowest concentration that completely inhibited bacterial growth.
For titration and MIC analyses, Mycoplasma (M.) bovis isolates were grown in commercially available ML10 broth (Mycoplasma Experience, Redhill, UK) without antimicrobial agents and supplemented with sodium pyruvate (0.5%) and phenol red (0.005%) as described by Hannan, 2000 [69]. Test culture batches were prepared for each isolate through 48 h growth in ML10, which were then aliquoted and stored frozen at −80 °C. The number of color-changing units (CCUs) was determined via broth microdilution [83]. The natural products were diluted in ML10 broth and 100 µL applied to each well on microtiter plates. The inoculum of 104 CCU/mL was prepared from titrated M. bovis culture batches and freshly cultured for 2 h at 37 °C before 100 µL were applied to each well to give a final concentration of 5 × 105 CCU/mL. Individual isolates were tested at least twice. Plates were sealed with a sterile plastic cover, incubated at 37 °C, and analyzed after 40–48 h, as soon as a color shift became evident in the wells lacking any natural products that served as growth controls. MIC values were determined as the lowest concentration of a natural product that completely suppressed M. bovis growth. When individual MIC values for an isolate differed by a maximum factor of two, the higher concentration was recorded, otherwise, testing was repeated.
Two Chlamydia (C.) suis isolates with known tetracycline resistance (see Supplemental Table S1) were used to determine the inhibitory effect of the natural products on intracellular bacteria. The natural products were pre-diluted in Ultra-MDCK medium (Lonza, Basel, Switzerland) in a final volume of 100 µL per well and applied to microtiter plates. For the inoculum, 100 µL of a buffalo green monkey (BGM) cell suspension containing 4 × 104 cells in Ultra-MDCK and chlamydiae adjusted to a multiplicity of infection (MOI) of 0.05 were added. Plates were centrifuged at 2000× g and 37 °C for 1 h and incubated at 37 °C, 5% CO2 for 30–32 h. Then, the medium was removed and the cells were fixed with 200 µL methanol per well overnight and stained using the IMAGEN Chlamydia kit (Oxoid, Wesel, Germany) according to the manufacturer’s instructions. Fluorescent inclusion forming units (IFUs) were counted under a fluorescence microscope and the MIC was defined as the lowest concentration of the natural product preventing the detection of more than 90% of the chlamydial inclusions compared with the drug-free control. All tests were run in duplicate.
The MIC profiles for Clostridioides (C.) difficile were determined following the Wadsworth method in document M11-A8 [84] as an agar dilution assay. Briefly, enriched Brucella agar (Carl Roth, Germany) plates, additionally containing vitamin K [1 µg/mL], haemin [5 µg/mL], and defibrinated sheep blood [5%] (Oxoid, Germany), were prepared and supplemented with the natural products in a log2 dilution series ranging from 16 µg/mL to 0.0675 µg/mL. Assays were performed in Petri dishes or 6-well cell culture plates. The C. difficile isolates were cultivated on enriched Brucella agar and incubated under anaerobic conditions (37 °C, 48 h). To prepare the suspension used to inoculate the plates, 3 to 7 single C. difficile colonies were suspended in 5 mL Brucella broth (Carl Roth, Karlsruhe, Germany) and 1.5 µL of the inoculum (corresponding to 5 × 105 cfu/spot) spotted on enriched and supplemented Brucella agar plates, as well as on enriched Brucella agar without supplemented natural products. After the drops had dried, the plates were inverted and incubated (anaerobic conditions, 37 °C, 42 to 48 h). The MIC was defined as the dilution step at which a marked reduction in colony growth is macroscopically visible compared to the control plate without supplemented natural products. The cfu of the inoculum was controlled each test day by plating 100 µL of a 1:1000 dilution of the inoculum in sterile 0.9% NaCl solution on an enriched Brucella agar plate and colony counting after incubation (anaerobic conditions, 37 °C, 42 to 48 h).
5. Conclusions
The study carried out here shows that natural products still yield interesting and worthwhile candidates for the development of antibiotically active therapeutics. This study also shows that it is important that the screening of natural products covers a spectrum of target pathogens/species as broad and heterogeneous as possible (e.g., Gram-positive, Gram-negative, cell-wall-free, spore-forming, and acid-fast species and/or intracellular growth), including field and clinical isolates, in order to be able to fully evaluate the true potential of the compounds.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics13020135/s1, Table S1: Results of pre-screening the natural products against different bacterial species; Table S2: Results of testing additional Mannheimia haemolytica and Pasteurella multocida isolates. References [85,86,87,88] are cited in the supplemenatry materials.
Author Contributions
Conceptualization, F.K. and C.M.; methodology, A.T.F., M.H., W.H., C.S. and S.S.; investigation, J.P., M.H., W.H. and C.S.; formal analysis, S.A.B. and D.P.; visualization, D.P. and S.A.B.; resources, F.K.; data curation, J.P. and S.A.B.; writing—original draft preparation, D.P., C.B. and S.A.B.; writing—review and editing, C.M., S.S. and A.T.F.; project administration, C.M.; funding acquisition, F.K., C.M. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by consortium InfectControl and the German Ministry for Education and Research, grant numbers 03ZZ0803A for F.K.; 03ZZ0803B for J.P., and 03ZZ0835B for D.P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are included in the Supplemental Material and openly available in Zenodo at https://zenodo.org/records/10214242 (created on 4 December 2023).
Acknowledgments
For the provision of isolates, the authors are particularly grateful to Roger D. Ayling (VLA Weybridge, United Kingdom), Nicole Borel (Vetsuisse Uni Zürich, Switzerland), as well as Heike Köhler and Christian Seyboldt (both FLI, Jena). Excellent technical support was provided by Susan Bahrmann and Christopher Sust (both FLI, Jena). We thank Kirstin Scherlach for the provision of cervimycin C and D and Markus Nett (both Leibniz-HKI, Jena) for micacocidin samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Cui, C.Y.; Li, X.J.; Chen, C.; Wu, X.T.; He, Q.; Jia, Q.L.; Zhang, X.J.; Lin, Z.Y.; Li, C.; Fang, L.X.; et al. Comprehensive analysis of plasmid-mediated tet(X4)-positive Escherichia coli isolates from clinical settings revealed a high correlation with animals and environments-derived strains. Sci. Total Environ. 2022, 806, 150687. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, R.; Liu, D.J.; Walsh, T.R.; Zhang, R.; Lv, Y.; Ke, Y.B.; Ji, Q.J.; Wei, R.C.; Liu, Z.H.; et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.R.; Xiao, X.; Wang, Z.Q.; Li, R.C. Global distribution and genomic characteristics of tet(X)-positive Escherichia coli among humans, animals, and the environment. Sci. Total Environ. 2023, 887, 164148. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Allel, K.; Day, L.; Hamilton, A.; Lin, L.S.; Furuya-Kanamori, L.; Moore, C.E.; Van Boeckel, T.; Laxminarayan, R.; Yakob, L. Global antimicrobial-resistance drivers: An ecological country-level study at the human-animal interface. Lancet Planet. Health 2023, 7, E291–E303. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kalemaki, D. Antimicrobial overuse and misuse in the community in Greece and link to antimicrobial resistance using methicillin-resistant S. aureus as an example. J. Infect. Public Health 2019, 12, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef] [PubMed]
- EU Health. EU Action on Antimicrobial Resistance. Available online: https://health.ec.europa.eu/antimicrobial-resistance/eu-action-antimicrobial-resistance_en (accessed on 7 October 2022).
- Pollock, J.; Low, A.S.; McHugh, R.E.; Muwonge, A.; Stevens, M.P.; Corbishley, A.; Gally, D.L. Alternatives to antibiotics in a One Health context and the role genomics can play in reducing antimicrobial use. Clin. Microbiol. Infect. 2020, 26, 1617–1621. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Bernal, F.A.; Hammann, P.; Kloss, F. Natural products in antibiotic development: Is the success story over? Curr. Opin. Biotechnol. 2022, 78, 102783. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. 2020. Available online: https://www.who.int/publications/i/item/9789240021303 (accessed on 7 October 2022).
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 2022, 66, e0199121. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.; Chan, A.N.; Wright, G.D. The Antibiotic Resistome: A Guide for the Discovery of Natural Products as Antimicrobial Agents. Chem. Rev. 2021, 121, 3464–3494. [Google Scholar] [CrossRef]
- Privalsky, T.M.; Soohoo, A.M.; Wang, J.; Walsh, C.T.; Wright, G.D.; Gordon, E.M.; Gray, N.S.; Khosla, C. Prospects for Antibacterial Discovery and Development. J. Am. Chem. Soc. 2021, 143, 21127–21142. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.; Gollmick, F.A.; Groth, I.; Roth, M.; Menzel, K.D.; Mollmann, U.; Grafe, U.; Hertweck, C. Cervimycin A-D: A polyketide glycoside complex from a cave bacterium can defeat vancomycin resistance. Chemistry 2005, 11, 5523–5530. [Google Scholar] [CrossRef]
- Herold, K.; Xu, Z.; Gollmick, F.A.; Grafe, U.; Hertweck, C. Biosynthesis of cervimycin C, an aromatic polyketide antibiotic bearing an unusual dimethylmalonyl moiety. Org. Biomol. Chem. 2004, 2, 2411–2414. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare; Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; Herskin, M.; et al. Scientific opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: Cattle. EFSA J. 2021, 19, e06955. [Google Scholar] [CrossRef]
- Hampson, D.J.; Burrough, E.R. Swine Dysentery and Brachyspiral Colitis. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2019; pp. 951–970. [Google Scholar]
- Hampson, D.J.; Lugsomya, K.; La, T.; Phillips, N.D.; Trott, D.J.; Abraham, S. Antimicrobial resistance in Brachyspira-An increasing problem for disease control. Vet. Microbiol. 2019, 229, 59–71. [Google Scholar] [CrossRef]
- Herbst, W.; Schlez, K.; Heuser, J.; Baljer, G. Antimicrobial susceptibility of Brachyspira hyodysenteriae determined by a broth microdilution method. Vet. Rec. 2014, 174, 382. [Google Scholar] [CrossRef]
- La, T.; Phillips, N.D.; Dunlop, H.; Lugsomya, K.; Coiacetto, F.; Hampson, D.J. Testing the efficacy of kitasamycin for use in the control and treatment of swine dysentery in experimentally infected pigs. Aust. Vet. J. 2019, 97, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.G.; Andersen, A.A. Conjunctivitis caused by a swine Chlamydia trachomatis-like organism in gnotobiotic pigs. J. Vet. Diagn. Investig. 1999, 11, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, P.; Kirschvink, N.; Theegarten, D.; Berndt, A. An experimentally induced Chlamydia suis infection in pigs results in severe lung function disorders and pulmonary inflammation. Vet. Res. 2008, 39, 35. [Google Scholar] [CrossRef] [PubMed]
- Guscetti, F.; Schiller, I.; Sydler, T.; Heinen, E.; Pospischil, A. Experimental enteric infection of gnotobiotic piglets with Chlamydia suis strain S45. Vet. Microbiol. 2009, 135, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Bommana, S.; Polkinghorne, A. Mini Review: Antimicrobial Control of Chlamydial Infections in Animals: Current Practices and Issues. Front. Microbiol. 2019, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, P.; Liebler-Tenorio, E.; Sattler, S.; Sachse, K. Recurrence of Chlamydia suis infection in pigs after short-term antimicrobial treatment. Vet. J. 2011, 187, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Clostridium (Clostridioides) difficile in animals. J. Vet. Diagn. Investig. 2020, 32, 213–221. [Google Scholar] [CrossRef]
- Dureja, C.; Olaitan, A.O.; Hurdle, J.G. Mechanisms and impact of antimicrobial resistance in Clostridioides difficile. Curr. Opin. Microbiol. 2022, 66, 63–72. [Google Scholar] [CrossRef]
- Steele, J.; Feng, H.; Parry, N.; Tzipori, S. Piglet models of acute or chronic Clostridium difficile illness. J. Infect. Dis. 2010, 201, 428–434. [Google Scholar] [CrossRef]
- Michael, G.B.; Bossé, J.T.; Schwarz, S. Antimicrobial Resistance in Pasteurellaceae of Veterinary Origin. Microbiol. Spectr. 2018, 6, ARBA-0022-2017. [Google Scholar] [CrossRef]
- Schroedl, W.; Fuerll, B.; Reinhold, P.; Krueger, M.; Schuett, C. A novel acute phase marker in cattle: Lipopolysaccharide binding protein (LBP). J. Endotoxin Res. 2001, 7, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, M.E. Paratuberculosis in Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 209–222. [Google Scholar] [CrossRef]
- Saxena, S.; Spaink, H.P.; Forn-Cuni, G. Drug Resistance in Nontuberculous Mycobacteria: Mechanisms and Models. Biology 2021, 10, 96. [Google Scholar] [CrossRef]
- Köhler, H.; Soschinka, A.; Meyer, M.; Kather, A.; Reinhold, P.; Liebler-Tenorio, E. Characterization of a caprine model for the subclinical initial phase of Mycobacterium avium subsp. paratuberculosis infection. BMC Vet. Res. 2015, 11, 74. [Google Scholar] [CrossRef]
- Dudek, K.; Nicholas, R.A.J.; Szacawa, E.; Bednarek, D. Mycoplasma bovis Infections-Occurrence, Diagnosis and Control. Pathogens 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Bednarek, D.; Ayling, R.D.; Kycko, A.; Reichert, M. Preliminary study on the effects of enrofloxacin, flunixin meglumine and pegbovigrastim on Mycoplasma bovis pneumonia. BMC Vet. Res. 2019, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Byrne, W.; Markey, B.; McCormack, R.; Egan, J.; Ball, H.; Sachse, K. Persistence of Mycoplasma bovis infection in the mammary glands of lactating cows inoculated experimentally. Vet. Rec. 2005, 156, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, P.; Rabeling, B.; Günther, H.; Schimmel, D. Comparative evaluation of ultrasonography and lung function testing with the clinical signs and pathology of calves inoculated experimentally with Pasteurella multocida. Vet. Rec. 2002, 150, 109–114. [Google Scholar] [CrossRef]
- Coetzee, J.F.; Magstadt, D.R.; Sidhuid, P.K.; Follett, L.; Schuler, A.M.; Krull, A.C.; Cooper, V.L.; Engelken, T.J.; Kleinhenz, M.D.; O’Connor, A.M. Association between antimicrobial drug class for treatment and retreatment of bovine respiratory disease (BRD) and frequency of resistant BRD pathogen isolation from veterinary diagnostic laboratory samples. PLoS ONE 2019, 14, e0219104. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.S.; Pors, S.E.; Jensen, H.E.; Bille-Hansen, V.; Bisgaard, M.; Flachs, E.M.; Nielsen, O.L. An Investigation of the Pathology and Pathogens Associated with Porcine Respiratory Disease Complex in Denmark. J. Comp. Pathol. 2010, 143, 120–131. [Google Scholar] [CrossRef]
- Truswell, A.; Laird, T.J.; Jones, S.; O’Dea, M.; Blinco, J.; Abraham, R.; Morison, D.; Jordan, D.; Hampson, D.J.; Pang, S.; et al. Antimicrobial Resistance of and Genomic Insights into Pasteurella multocida Strains Isolated from Australian Pigs. Microbiol. Spectr. 2023, 11, e03784-22. [Google Scholar] [CrossRef] [PubMed]
- Brockmeier, S.L.; Halbur, P.G.; Thacker, E.L. Porcine Respiratory Disease Complex. In Polymicrobial Diseases, 1st ed.; Brogden, K.A., Guthmiller, J.M., Eds.; ASM Press: Washington, DC, USA; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 231–258. [Google Scholar]
- Uchil, R.R.; Kohli, G.S.; Katekhaye, V.M.; Swami, O.C. Strategies to combat antimicrobial resistance. J. Clin. Diagn. Res. 2014, 8, ME01–ME04. [Google Scholar] [CrossRef]
- Chiriac, A.I.; Kloss, F.; Kramer, J.; Vuong, C.; Hertweck, C.; Sahl, H.G. Mode of action of closthioamide: The first member of the polythioamide class of bacterial DNA gyrase inhibitors. J. Antimicrob. Chemother. 2015, 70, 2576–2588. [Google Scholar] [CrossRef]
- Lachowicz-Wolak, A.; Klimowicz-Bodys, M.D.; Ploneczka-Janeczko, K.; Bykowy, M.; Siedlecka, M.; Cinciala, J.; Rypula, K. The Prevalence, Coexistence, and Correlations between Seven Pathogens Detected by a PCR Method from South-Western Poland Dairy Cattle Suffering from Bovine Respiratory Disease. Microorganisms 2022, 10, 1487. [Google Scholar] [CrossRef]
- Cid, D.; Pinto, C.; Dominguez, L.; Vela, A.I.; Fernandez-Garayzabal, J.F. Strength of association between isolation of Pasteurella multocida and consolidation lesions in ovine pneumonic pasteurellosis. Vet. Microbiol. 2020, 248, 108823. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef]
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef]
- Dubrovsky, S.A.; Van Eenennaam, A.L.; Karle, B.M.; Rossitto, P.V.; Lehenbauer, T.W.; Aly, S.S. Bovine respiratory disease (BRD) cause-specific and overall mortality in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7320–7328. [Google Scholar] [CrossRef]
- Teixeira, A.G.V.; McArt, J.A.A.; Bicalho, R.C. Thoracic ultrasound assessment of lung consolidation at weaning in Holstein dairy heifers: Reproductive performance and survival. J. Dairy Sci. 2017, 100, 2985–2991. [Google Scholar] [CrossRef]
- Bach, A. Associations between several aspects of heifer development and dairy cow survivability to second lactation. J. Dairy Sci. 2011, 94, 1052–1057. [Google Scholar] [CrossRef]
- Dunn, T.R.; Ollivett, T.L.; Renaud, D.L.; Leslie, K.E.; LeBlanc, S.J.; Duffield, T.F.; Kelton, D.F. The effect of lung consolidation, as determined by ultrasonography, on first-lactation milk production in Holstein dairy calves. J. Dairy Sci. 2018, 101, 5404–5410. [Google Scholar] [CrossRef] [PubMed]
- Melchner, A.; van de Berg, S.; Scuda, N.; Feuerstein, A.; Hanczaruk, M.; Schumacher, M.; Straubinger, R.K.; Marosevic, D.; Riehm, J.M. Antimicrobial Resistance in Isolates from Cattle with Bovine Respiratory Disease in Bavaria, Germany. Antibiotics 2021, 10, 1538. [Google Scholar] [CrossRef]
- Schink, A.K.; Hanke, D.; Semmler, T.; Brombach, J.; Bethe, A.; Lübke-Becker, A.; Teske, K.; Müller, K.E.; Schwarz, S. Novel multiresistance-mediating integrative and conjugative elements carrying unusual antimicrobial resistance genes in Mannheimia haemolytica and Pasteurella multocida. J. Antimicrob. Chemother. 2022, 77, 2033–2035. [Google Scholar] [CrossRef]
- Heinisch, L.; Roemer, E.; Jutten, P.; Haas, W.; Werner, W.; Mollmann, U. Semisynthetic derivatives of madurahydroxylactone and their antibacterial activities. J. Antibiot. 1999, 52, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, R.; Satoh, K.; Kikuchi, N.; Kikuchi, H.; Saigusa, D.; Al-Mamun, M.E.; Siddique, M.A.H.; Omura, J.; Satoh, T.; Sunamura, S.; et al. Identification of Celastramycin as a Novel Therapeutic Agent for Pulmonary Arterial Hypertension. Circ. Res. 2019, 125, 309–327. [Google Scholar] [CrossRef]
- Altenburg, J.; de Graaff, C.S.; van der Werf, T.S.; Boersma, W.G. Immunomodulatory effects of macrolide antibiotics-part 1: Biological mechanisms. Respiration 2011, 81, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Diedrich, B.; Muenster, S.; Hentschel, V.; Weisheit, C.; Rommelsheim, K.; Hoeft, A.; Meyer, R.; Boehm, O.; Knuefermann, P.; et al. Antibiotics regulate the immune response in both presence and absence of lipopolysaccharide through modulation of Toll-like receptors, cytokine production and phagocytosis in vitro. Int. Immunopharmacol. 2014, 18, 27–34. [Google Scholar] [CrossRef]
- Yang, J.H.; Bhargava, P.; McCloskey, D.; Mao, N.; Palsson, B.O.; Collins, J.J. Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function. Cell Host Microbe 2017, 22, 757–765.e3. [Google Scholar] [CrossRef]
- Colclough, A.; Corander, J.; Sheppard, S.K.; Bayliss, S.C.; Vos, M. Patterns of cross-resistance and collateral sensitivity between clinical antibiotics and natural antimicrobials. Evol. Appl. 2019, 12, 878–887. [Google Scholar] [CrossRef]
- Michael, A.; Kelman, T.; Pitesky, M. Overview of Quantitative Methodologies to Understand Antimicrobial Resistance via Minimum Inhibitory Concentration. Animals 2020, 10, 1405. [Google Scholar] [CrossRef]
- Hannan, P.C. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet. Res. 2000, 31, 373–395. [Google Scholar] [CrossRef]
- Kikuchi, H.; Sekiya, M.; Katou, Y.; Ueda, K.; Kabeya, T.; Kurata, S.; Oshima, Y. Revised structure and synthesis of celastramycin a, a potent innate immune suppressor. Org. Lett. 2009, 11, 1693–1695. [Google Scholar] [CrossRef]
- Pullen, C.; Schmitz, P.; Meurer, K.; Bamberg, D.D.; Lohmann, S.; De Castro Franca, S.; Groth, I.; Schlegel, B.; Mollmann, U.; Gollmick, F.; et al. New and bioactive compounds from Streptomyces strains residing in the wood of Celastraceae. Planta 2002, 216, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Lincke, T.; Behnken, S.; Ishida, K.; Roth, M.; Hertweck, C. Closthioamide: An unprecedented polythioamide antibiotic from the strictly anaerobic bacterium Clostridium cellulolyticum. Angew. Chem. Int. Ed. 2010, 49, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Steffens, U.; Gajdiss, M.; Boschert, A.L.; Droge, J.K.; Szekat, C.; Sass, P.; Malik, I.T.; Bornikoel, J.; Reinke, L.; et al. Cervimycin-Resistant Staphylococcus aureus Strains Display Vancomycin-Intermediate Resistant Phenotypes. Microbiol. Spectr. 2022, 10, e0256722. [Google Scholar] [CrossRef] [PubMed]
- Gräfe, U.; Schade, W.; Roth, M.; Radics, L.; Incze, M.; Ujszaszy, K. Griseochelin, a novel carboxylic acid antibiotic from Streptomyces griseus. J. Antibiot. 1984, 37, 836–846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walther, E.; Boldt, S.; Kage, H.; Lauterbach, T.; Martin, K.; Roth, M.; Hertweck, C.; Sauerbrei, A.; Schmidtke, M.; Nett, M. Zincophorin-biosynthesis in Streptomyces griseus and antibiotic properties. GMS Infect. Dis. 2016, 4, Doc08. [Google Scholar] [CrossRef]
- Ino, A.; Hasegawa, Y.; Murabayashi, A. Total synthesis of the antimycoplasma antibiotic micacocidin. Tetrahedron Lett. 1998, 39, 3509–3512. [Google Scholar] [CrossRef]
- Kage, H.; Kreutzer, M.F.; Wackler, B.; Hoffmeister, D.; Nett, M. An iterative type I polyketide synthase initiates the biosynthesis of the antimycoplasma agent micacocidin. Chem. Biol. 2013, 20, 764–771. [Google Scholar] [CrossRef]
- Fleck, W.F.; Strauss, D.G.; Meyer, J.; Porstendorfer, G. Fermentation, isolation, and biological activity of maduramycin: A new antibiotic from Actinomadura rubra. Z. Allg. Mikrobiol. 1978, 18, 389–398. [Google Scholar] [CrossRef]
- Kim, B.M.; Choi, H.Y.; Kim, G.W.; Zheng, C.J.; Kim, Y.H.; Kim, W.G. Madurahydroxylactone, an Inhibitor of Staphylococcus aureus FtsZ from Nonomuraea sp. AN100570. J. Microbiol. Biotechnol. 2017, 27, 1994–1998. [Google Scholar] [CrossRef]
- Feßler, A.T.; Wang, Y.; Burbick, C.R.; Diaz-Campos, D.; Fajt, V.R.; Lawhon, S.D.; Li, X.-Z.; Lubbers, B.V.; Maddock, K.; Miller, R.A.; et al. Antimicrobial susceptibility testing in veterinary medicine: Performance, interpretation of results, best practices and pitfalls. One Health Adv. 2023, 1, 26. [Google Scholar] [CrossRef]
- CLSI Document Vet01-A4; Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard-Fourth Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013; Volume 33.
- CLSI Document M24-A2; Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard-Second Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011.
- Rodwell, A.W.; Whitcomb, R.F. Methods for direct and indirect measurement of mycoplasma growth. In Methods in Mycoplasmology; Razin, S., Tully, J.G., Eds.; Academic Press: NewYork, NY, USA, 1983; pp. 185–196. [Google Scholar]
- CLSI Document M11-A8; Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard-Eighth Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32.
- Kehrenberg, C.; Catry, B.; Haesebrouck, F.; de Kruif, A.; Schwarz, S. tet(L)-mediated tetracycline resistance in bovine Mannheimia and Pasteurella isolates. J. Antimicrob. Chemother. 2005, 56, 403–406. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Salmon, S.A.; Watts, J.L.; Schwarz, S. Tetracycline resistance genes in isolates of Pasteurella multocida, Mannheimia haemolytica, Mannheimia glucosida and Mannheimia varigena from bovine and swine respiratory disease: Intergeneric spread of the tet(H) plasmid pMHT1. J. Antimicrob. Chemother. 2001, 48, 631–640. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Schwarz, S. Identification of a truncated, but functionally active tet(H) tetracycline resistance gene in Pasteurella aerogenes and Pasteurella multocida. FEMS Microbiol. Lett. 2000, 188, 191–195. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Werckenthin, C.; Schwarz, S. Tn5706, a transposon-like element from Pasteurella multocida mediating tetracycline resistance. Antimicrob. Agents. Chemother. 1998, 42, 2116–2118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).