Sequential versus Standard Triple Therapy for First-Line Helicobacter pylori Eradication: An Update
Abstract
1. Introduction
2. Results
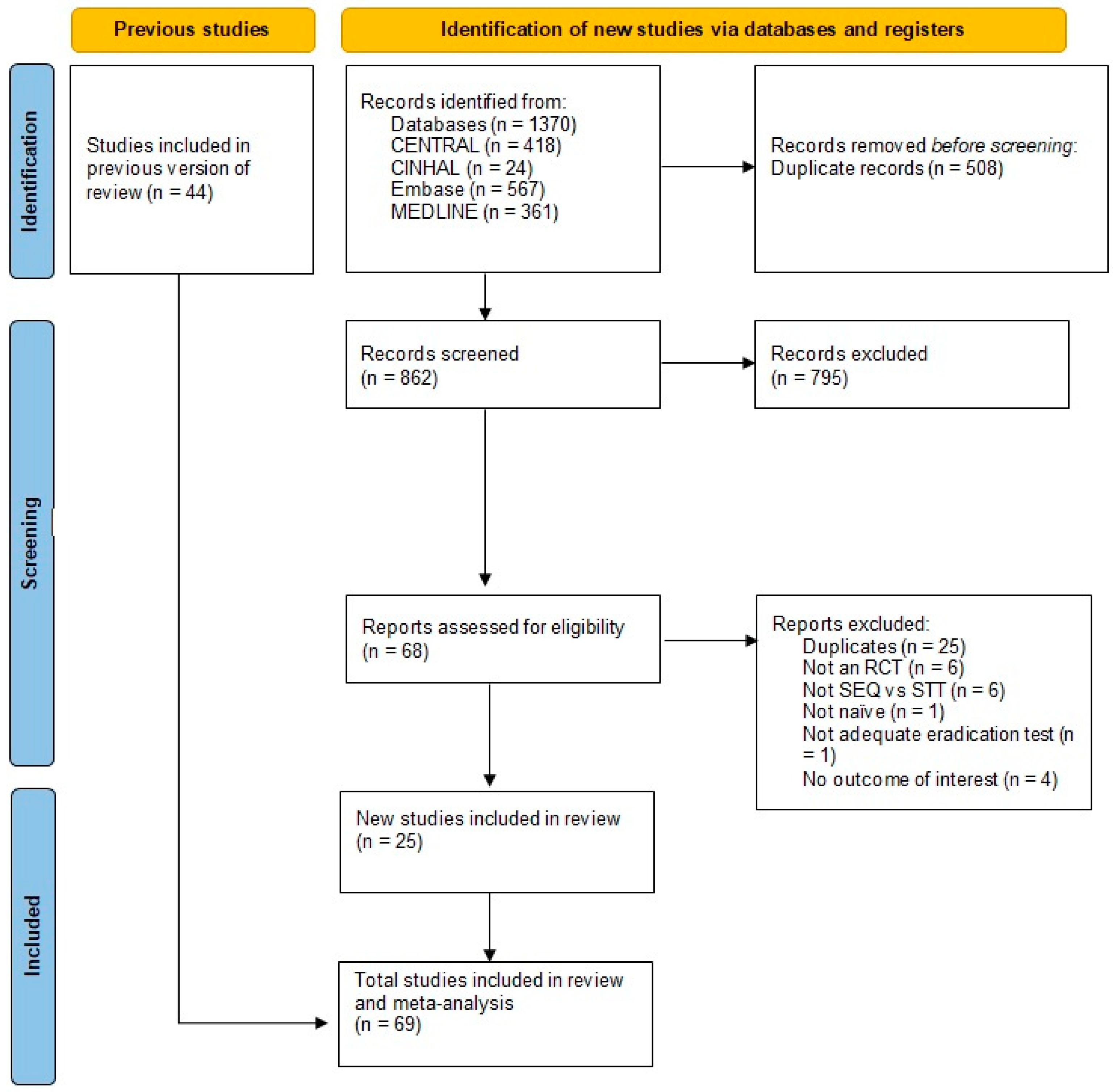
2.1. Overview of the Search
2.1.1. Included Studies
2.1.2. Excluded Studies
2.2. Quality of the Included Studies
2.3. Effects of Interventions
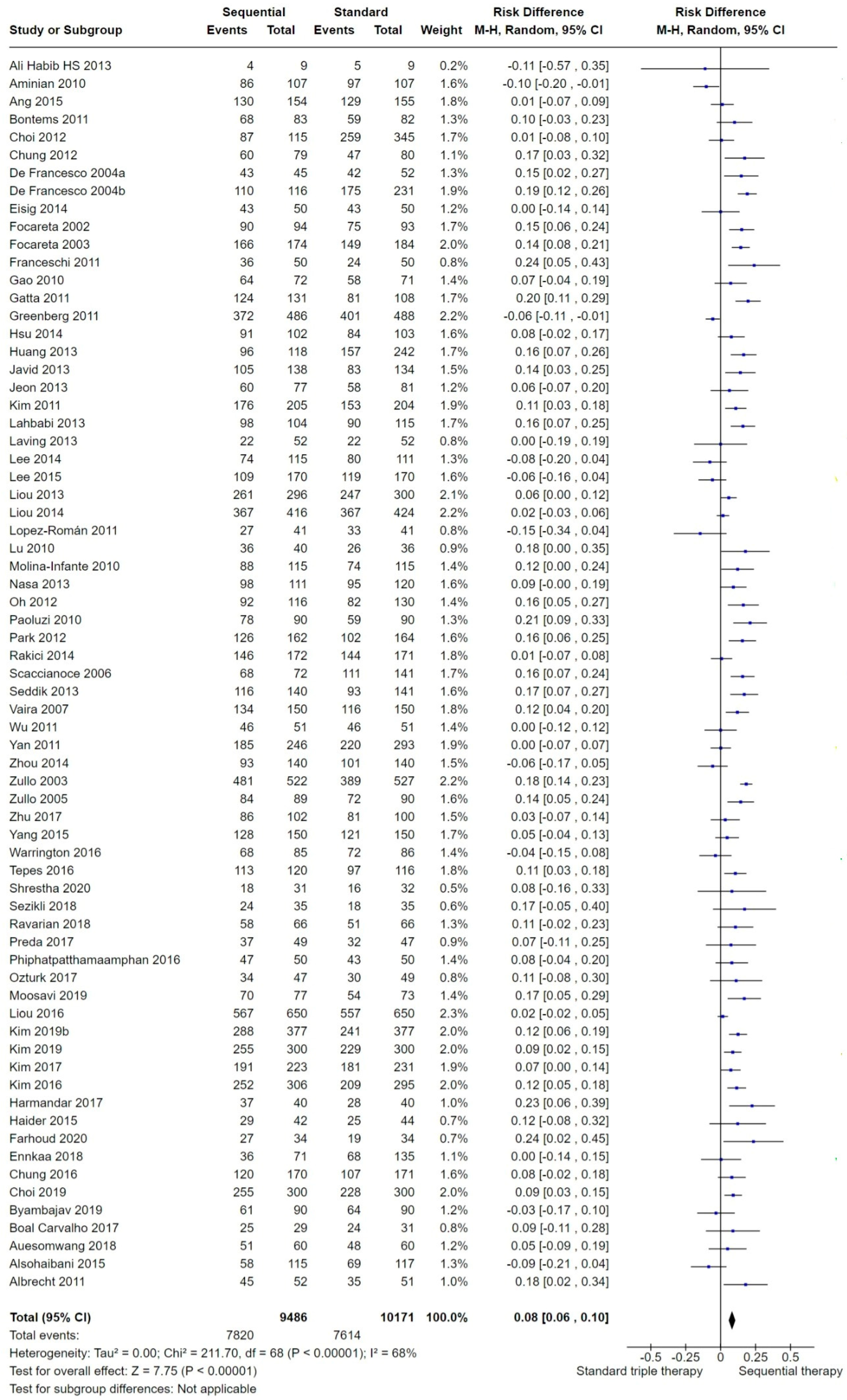
2.3.1. Overall H. pylori Eradication
2.3.2. Geographic Region
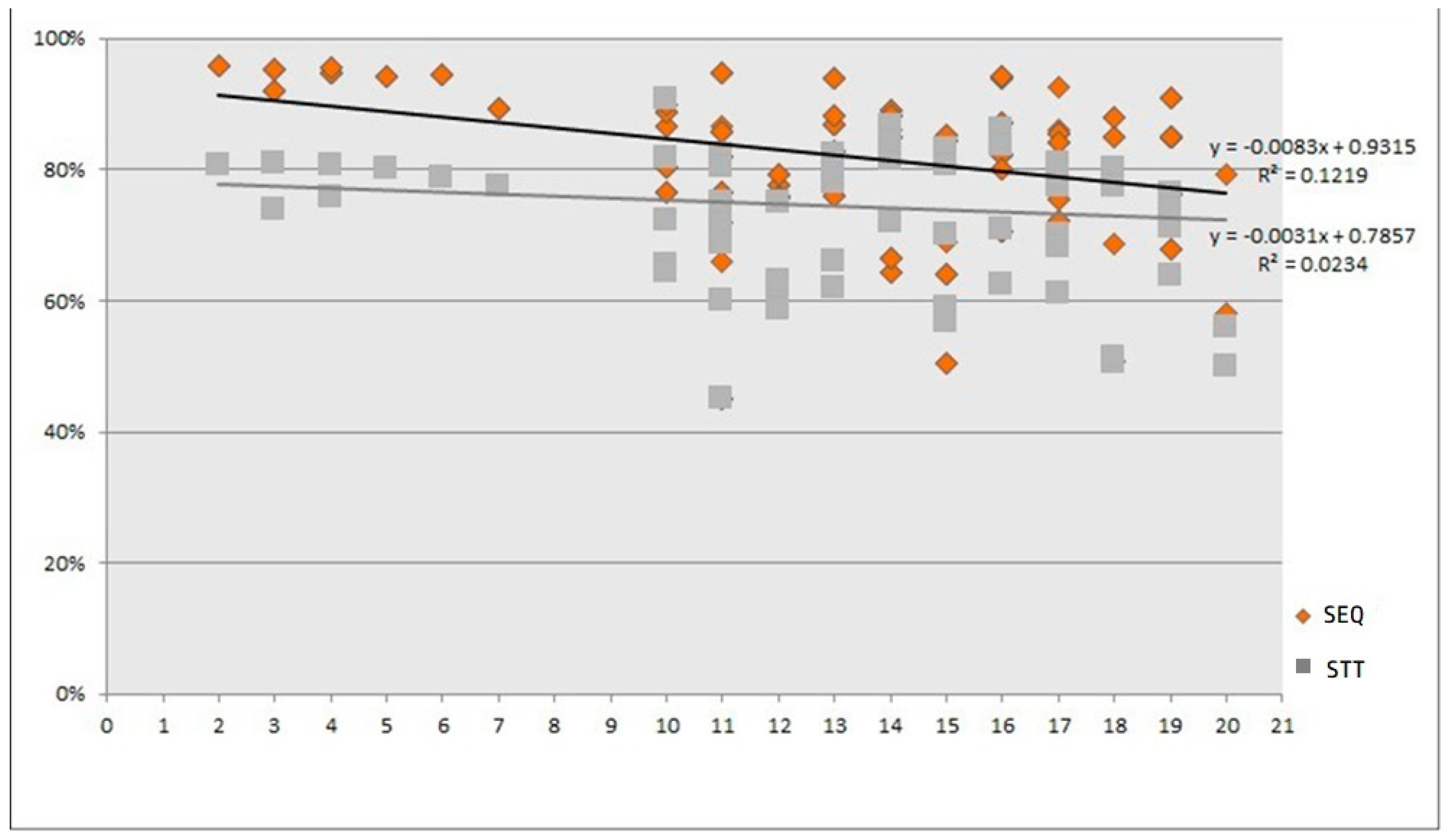
2.3.3. Publication Date
2.3.4. Age of the Population
2.3.5. Medical Condition: Non-Ulcer Disease (NUD) versus Peptic Ulcer Disease (PUD)
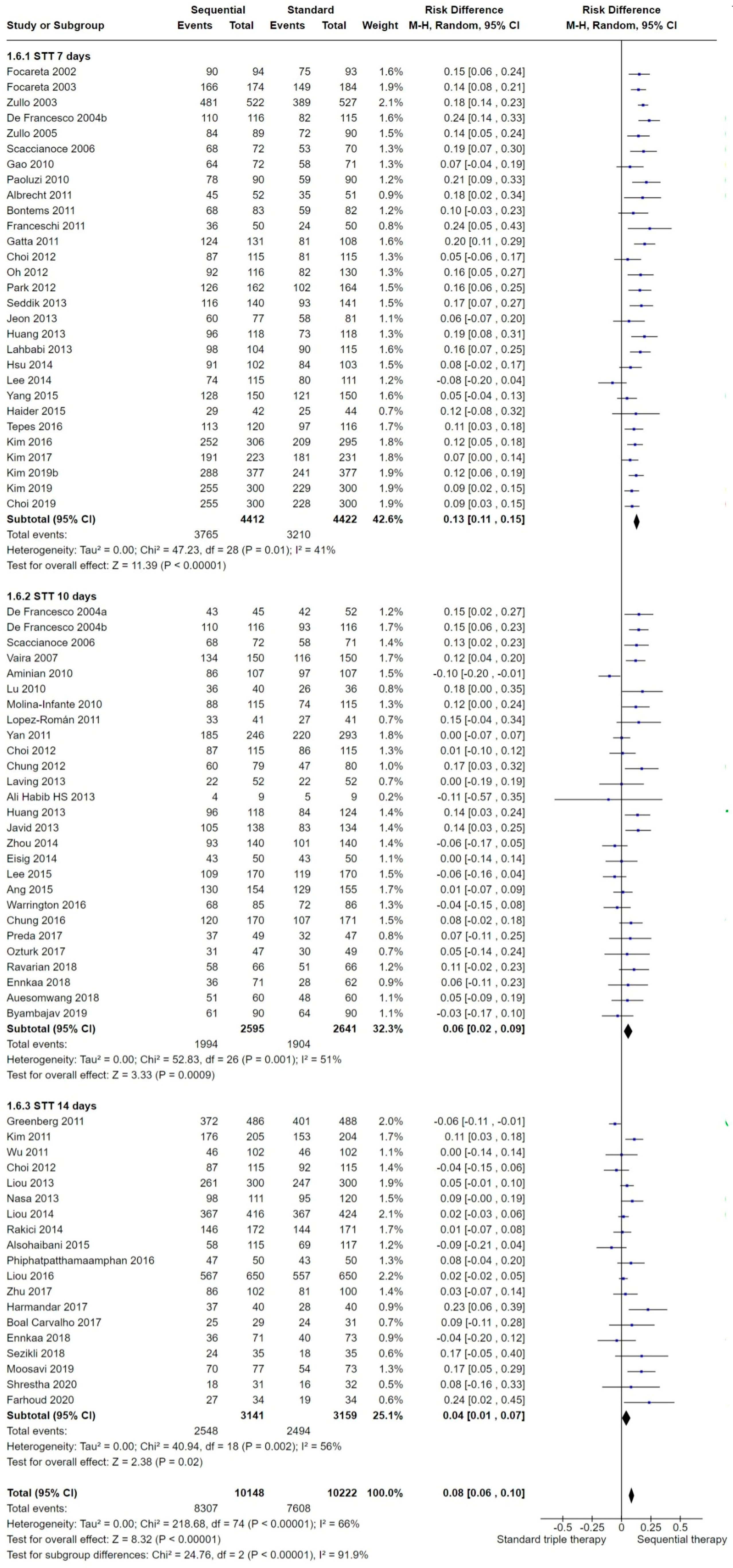
2.3.6. Length of the Standard Triple Therapy (STT)
2.3.7. Type of Nitroimidazole
2.3.8. Acid Inhibition with Proton Pump Inhibitors (PPIs)
2.3.9. Bacterial Antibiotic Resistance
2.4. Safety Profile
2.5. Compliance
2.6. Sensitivity Analysis
2.6.1. Risk of Bias
2.6.2. Year of Publication
2.6.3. Length of STT
3. Discussion
3.1. Summary of Main Results
3.2. Overall Efficacy of SEQ versus STT
3.3. Subgroup Analyses: Variables Influencing Efficacy of Both Treatments
3.3.1. Geographic Region
3.3.2. Publication Date
3.3.3. Effect Modifiers over Time
3.3.4. Age of the Population
3.3.5. Medical Condition
3.3.6. STT Length
3.3.7. Acid Inhibition with PPIs
3.3.8. Bacterial Antibiotic Resistance
3.4. Safety
3.5. Overall Completeness and Applicability of Evidence
3.6. Quality of the Evidence
3.7. Intention-to-Treat Reporting
3.8. Reporting of Baseline Characteristics by Treatment Arm versus Not Reporting Findings by Treatment Arm
3.9. Masking of Personnel and Participants
3.10. Sample Size
3.11. Recommendations, Other Treatments for H. pylori Eradication and Further Research
3.12. Potential Biases in the Review Process
3.13. Agreements and Disagreements with Other Studies or Reviews
4. Methods
4.1. Selection Criteria
4.2. Types of Interventions
4.3. Types of Outcome Measures
4.4. Search Methods for Identification of Studies
4.4.1. Electronic Searches
4.4.2. Other Sources
4.5. Data Collection and Analysis
Data Extraction and Management
- The first author’s name; year of publication; country;
- The format of publication (abstract versus journal article); age of the population (adult versus children);
- Medical condition (PUD or NUD or other);
- Number of participants in each treatment group;
- Name, dose and timing of antibiotic administration; length of STT;
- Eradication proportion per treatment regimen (ITT and PP); if only the PP sample was reported, we calculated the ITT sample on the basis of the randomization and dropout information;
- Definition of compliance and the level of compliance in the ITT sample;
- Details of the method of assessment of H. pylori infection both before and after treatment;
- Whether the antibiotic sensitivity and resistance were tested before and after eradication; if so, the primary and secondary antibiotic resistance;
- Incidence, type and severity of AEs;
- Study quality: generation of the treatment allocation, concealment of the treatment allocation at randomization, implementation of masking, completeness of follow-up and use of ITT analysis.
4.6. Quality of the Evidence
4.7. Completeness of Follow-Up and Use of Intention-to-Treat (ITT) Analysis
4.8. Assessment of Heterogeneity
4.9. Assessment of Reporting Biases
4.10. Data Synthesis
- Geographic region;
- Publication date;
- Age (children versus adults);
- Length of STT (7 versus 10 versus 14 days);
- Type of nitroimidazole (metronidazole versus tinidazole); resistance of each antibiotic;
- Dosing for PPI (SEQ treatment versus STT), where the PPI dosage was categorized in three categories as follows: (1) low-dose PPI ranging between 4.5 and 27 mg of omeprazole equivalents, two times per day (i.e., 20 mg of omeprazole equivalents, two times per day); (2) standard-dose PPI ranging between 32 and 40 mg of omeprazole equivalents, two times per day (i.e., 40 mg of omeprazole equivalents, two times per day) and (3) high-dose PPI ranging between 54 and 128 mg of omeprazole equivalents, two times per day (i.e., 80 mg of omeprazole equivalents, two times per day). These dosage categories were calculated based on the definitions of PPI dosage standardization reported by Graham et al. [144] and Kirchheiner et al [145].
- Type of disease at enrolment (PUD versus NUD).
5. Conclusions
5.1. Implications for Practice
5.2. Implications for Research
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Martel, C.; Parsonnet, J. Helicobacter pylori infection and gender: A meta-analysis of population-based prevalence surveys. Dig. Dis. Sci. 2006, 51, 2292–2301. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef]
- Malfertheiner, P. Current European concepts in the management of Helicobacter pylori infection; The Maastricht consensus report. Gut 1997, 41, 8–13. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Bazzoli, F.; El-Omar, E.; Graham, D. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut 2007, 56, 772–781. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Hungin, A.P.; Jones, R.; Axon, A. Current concepts in the management of Helicobacter pylori infection—the Maastricht 2-2000 Consensus Report. Aliment. Pharmacol. Ther. 2002, 16, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.; Bazzoli, F. Management of Helicobacter pylori infection–the Maastricht IV/ Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Pajares, R.; Pajares, J.M. Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter 2007, 12 (Suppl. S2), 50–58. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Zullo, A.; Hassan, C.; Faleo, D.; Ierardi, E.; Panella, C. Two new treatment regimens for Helicobacter pylori eradication: A randomised study. Dig. Liver Dis. 2001, 33, 676–679. [Google Scholar] [CrossRef]
- Zullo, A.; Rinaldi, V.; Winn, S.; Meddi, P.; Lionetti, R.; Hassan, C. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2000, 14, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Yang, J.C.; Huang, S.H. Pre-treatment urea breath test results predict the efficacy of Helicobacter pylori eradication therapy in patients with active duodenal ulcers. World J. Gastroenterol. 2004, 10, 991–994. [Google Scholar] [CrossRef]
- Perri, F.; Clemente, R.; Festa, V.; Quitadamo, M.; Conoscitore, P.; Niro, G. Relationship between the results of pre-treatment urea breath test and efficacy of eradication of Helicobacter pylori infection. Ital. J. Gastroenterol. 1998, 30, 146–150. [Google Scholar]
- Moshkowitz, M.; Konikoff, F.M.; Peled, Y.; Santo, M.; Hallak, A.; Bujanover, Y. High Helicobacter pylori numbers are associated with low eradication rate after triple therapy. Gut 1995, 36, 845–847. [Google Scholar] [CrossRef]
- Zullo, A.; De Francesco, V.; Hassan, C.; Morini, S.; Vaira, D. The sequential therapy regiment for Helicobacter pylori eradication: A pooled data analysis. Gut 2007, 56, 1353–1357. [Google Scholar] [CrossRef]
- Murakami, K.; Fujioka, T.; Okimoto, T.; Sato, R.; Kodama, M.; Nasu, M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int. J. Antimicrob. Agents 2002, 19, 67–70. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lu, H.; Yamaoka, Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007, 12, 275–278. [Google Scholar] [CrossRef]
- Graham, D.Y.; Yamaoka, Y. Ethical considerations of comparing sequential and traditional anti Helicobacter pylori therapy. Ann. Intern. Med. 2007, 147, 434–435. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21,533 patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Vaira, D.; Tepes, B.; Kupcinskas, L.; Bordin, D.; Pérez-Aisa, Á.; Gasbarrini, A.; Castro-Fernández, M.; Bujanda, L.; Garre, A.; et al. Room for Improvement in the Treatment of Helicobacter pylori Infection: Lessons from the European Registry on H. pylori Management (Hp-EuReg). J. Clin. Gastroenterol. 2002, 56, e98–e108. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin. Exp. Gastroenterol. 2012, 5, 23–24. [Google Scholar] [CrossRef]
- Mégraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.D.; Hoebeke, M.; Benejat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y.; et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef]
- Bujanda, L.; Nyssen, O.P.; Vaira, D.; Saracino, I.M.; Fiorini, G.; Lerang, F.; Georgopoulos, S.; Tepes, B.; Heluwaert, F.; Gasbarrini, A.; et al. Antibiotic Resistance Prevalence and Trends in Patients Infected with Helicobacter pylori in the Period 2013–2020: Results of the European Registry on H. pylori Management (Hp-EuReg). Antibiotics 2021, 10, 1058. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lu, H.; Yamaoka, Y. Therapy for Helicobacter pylori infection can be improved: Sequential therapy and beyond. Drugs 2008, 68, 725–736. [Google Scholar] [CrossRef]
- Moayyedi, P. Sequential regimes for Helicobacter pylori eradication. Lancet 2007, 370, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chun, H.J.; Kim, E.S.; Park, S.C.; Jung, E.S.; Lee, S.D. The 10-day sequential therapy for Helicobacter pylori eradication in Korea: Less effective than expected. Gastroenterology 2009, 136, M1053. [Google Scholar] [CrossRef]
- Vaira, D.; Zullo, A.; Hassan, C.; Fiorini, G.; Vakil, N. Sequential Therapy for Helicobacter Pylori Eradication: The Time is Now! Ther. Adv. Gastroenterol. 2009, 2, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Jafri, N.S.; Hornung, C.A.; Howden, C.W. Meta-analysis: Sequential Therapy Appears Superior to Standard Therapy for Helicobacter pylori Infection in Patients Naive to Treatment. Ann. Intern. Med. 2008, 148, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.L.; Ran, Z.H.; Shen, J.; Xiao, S.D. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: A meta-analysis. J. Clin. Pharm. Ther. 2009, 34, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Vakil, N.; Leandro, G.; Di Mario, F.; Vaira, D. Sequential therapy or triple therapy for Helicobacter pylori infection: Systematic review and meta-analysis of randomized controlled trials in adults and children. Am. J. Gastroenterol. 2009, 104, 3069–3079. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X.; O´Connor, A.; Mégraud, F.; O´Morain, C.A. Sequential therapy for Helicobacter pylori eradication. A critical review. J. Clin. Gastroenterol. 2010, 44, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; McNicholl, A.G.; Megraud, F.; Savarino, V.; Oderda, G.; Fallone, C.A.; Fischbach, L.; Bazzoli, F.; Gisbert, J.P. Sequential versus standard triple first-line therapy for Helicobacter pylori eradication. Cochrane Database Syst. Rev. 2016, 2016, CD009034. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Zullo, A.; Hassan, C.; Della Valle, N.; Pietrini, L.; Minenna, M.F.; Winn, S.; Monno, R.; Stoppino, V.; Morini, S.; et al. The prolongation of triple therapy for Helicobacter pylori does not allow reaching therapeutic outcome of sequential scheme: A prospective, randomised study. Dig. Liver Dis. 2004, 36, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Perez-Gallardo, B.; Fernandez-Bermejo, M.; Hernandez-Alonso, M.; Vinagre, G.; Dueñas, C.; Mateos-Rodriguez, J.M.; Gonzalez-Garcia, G.; Abadia, E.G.; Gisbert, J.P. Clinical trial: Clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2010, 31, 1077–1084. [Google Scholar] [CrossRef]
- Focareta, R.; Forte, G.; Ciarlegio, A. Helicobacter pylori eradication: One week triple therapy versus 10-day sequential regimen introduction. Dig. Liver Dis. 2002, 34, A17. [Google Scholar] [CrossRef]
- Focareta, R.; Forte, G.; Forte, F.; Ciarleglio, A.; Grimaldi, E.; Ievoli, F. Could the 10-days sequential therapy be considered a first choice treatment for the eradication of Helicobacter pylori infection? Dig. Liver Dis. 2003, 33, C091. [Google Scholar]
- Zullo, A.; Hassan, C.; Lorenzetti, R.; Winn, S.; Morini, S. A clinical practice viewpoint: To culture or not to culture Helicobacter pylori? Dig. Liver Dis. 2003, 35, 357–361. [Google Scholar] [CrossRef]
- Zullo, A.; Gatta, L.; De Francesco, V.; Hassan, C.; Ricci, C.; Bernabucci, V.; Cavina, M.; Ierardi, E.; Morini, S.; Vaira, D. High rate of Helicobacter pylori eradication with sequential therapy in elderly patients with peptic ulcer: A prospective controlled study. Aliment. Pharmacol. Ther. 2005, 21, 1419–1424. [Google Scholar] [CrossRef]
- De Francesco, V.; Zullo, A.; Margiotta, M.; Marangi, S.; Burattini, O.; Berloco, P.; Russo, F.; Barone, M.; Di Leo, A.; Minenna, M.F.; et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment. Pharmacol. Ther. 2004, 19, 407–414. [Google Scholar] [CrossRef]
- Scaccianoce, G.; Hassan, C.; Panarese, A.; Piglionica, D.; Morini, S.; Zullo, A. Helicobacter pylori eradication with either 7-day or 10-day triple therapies, and with a 10-day sequential regimen. Can. J. Gastroenterol. 2006, 20, 113–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaira, D.; Zullo, A.; Vakil, N.; Gatta, L.; Ricci, C.; Perna, F.; Hassan, C.; Bernabucci, V.; Tampieri, A.; Morini, S. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: A randomized trial. Ann. Intern. Med. 2007, 146, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Paoluzi, O.A.; Visconti, E.; Andrei, F.; Tosti, C.; Lionetti, R.; Grasso, E.; Ranaldi, R.; Stroppa, I.; Pallone, F. Ten and eight-day sequential therapy in comparison to standard triple therapy for eradicating Helicobacter pylori infection: A randomized controlled study on efficacy and tolerability. J. Clin. Gastroenterol. 2010, 44, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Ojetti, V.; Gabrielli, M.; Petruzziello, C.; Tortora, A.; Gasbarrini, G.; Lopetuso, L.R.; Scaldaferri, F.; Gasbarrini, A. High dose amoxicillin-based first line regimen is equivalent to sequential therapy in the eradication of H. pylori infection. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 297–300. [Google Scholar]
- Kim, Y.S.; Kim, S.J.; Yoon, J.H.; Suk, K.T.; Kim, J.B.; Kim, D.J.; Kim, D.Y.; Min, H.J.; Park, S.H.; Shin, W.G.; et al. Randomised clinical trial: The efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment. Pharmacol. Ther. 2011, 34, 1098–1105. [Google Scholar] [CrossRef]
- Choi, H.S.; Chun, H.J.; Park, S.H.; Keum, B.; Seo, Y.S.; Kim, Y.S.; Jeen, Y.T.; Um, S.H.; Lee, H.S.; Kim, C.D.; et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J. Gastroenterol. 2012, 18, 2377–2382. [Google Scholar] [CrossRef]
- Chung, J.W.; Jung, Y.K.; Kim, Y.J.; Kwon, K.A.; Kim, J.H.; Lee, J.J.; Lee, S.M.; Hahm, K.B.; Lee, S.M.; Jeong, J.Y.; et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: A prospective, open-label, randomized trial. J. Gastroenterol. Hepatol. 2012, 27, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Jung, M.K.; Jung, J.T.; Kwon, J.G.; Kim, E.Y.; Seo, H.E.; Lee, J.H.; Yang, C.H.; Kim, E.S.; Cho, K.B.; et al. Randomised clinical trial: A comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment. Pharmacol. Ther. 2012, 35, 56–65. [Google Scholar] [CrossRef]
- Jeon, W.K.; Park, D.; Song, C. Effectiveness of 10 day-sequential treatment for Helicobacter pylori eradication in Korea. Gastroenterology 2013, 144, S567–S568. [Google Scholar] [CrossRef]
- Oh, H.S.; Lee, D.H.; Seo, J.Y.; Cho, Y.R.; Kim, N.; Jeoung, S.H.; Kim, J.W.; Hwang, J.H.; Park, Y.S.; Lee, S.H.; et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: A prospective, randomized study. J. Gastroenterol. Hepatol. 2012, 27, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, N.; Kim, J.M.; Nam, R.H.; Kim, J.Y.; Lee, J.Y.; Lee, D.H.; Jung, H.C. A comparison between 15-day sequential, 10-day sequential and proton pump inhibitor-based triple therapy for Helicobacter pylori infection in Korea. Scand. J. Gastroenterol. 2014, 49, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.I.; Lee, J.S.; Jun, E.J.; Oh, J.H.; Cheung, D.Y. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J. Gastroenterol. 2015, 21, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Han, J.P.; Kim, K.O.; Kim, S.Y.; Hong, S.J.; Kim, T.H.; Kim, C.W.; Kim, J.S.; Kim, B.W.; Bang, B.W.; et al. Ten-day empirical sequential or concomitant therapy is more effective than triple therapy for Helicobacter pylori eradication: A multicenter, prospective study. Dig. Liver Dis. 2016, 48, 888–892. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.W.; Hong, S.J.; Kim, J.I.; Shim, K.N.; Kim, J.H.; Baik, G.H.; Kim, S.W.; Song, H.J.; Kim, J.H. Sequential Therapy versus Triple Therapy for the First Line Treatment of Helicobacter pylori in Korea: A Nationwide Randomized Trial. Gut Liver 2016, 10, 556–561. [Google Scholar] [CrossRef]
- Kim, J.; Cheung, D.Y. Su1282—Eradication Rates of Triple, Triple with Probiotics, Sequential, Concomitant, and Tailored Therapy for Helicobacter Pylori. Gastroenterology 2019, 156, S-529. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, H.; Lee, Y.C.; Jeon, S.W.; Kim, G.H.; Kim, H.S.; Sung, J.K.; Lee, D.H.; Kim, H.U.; Park, M.I.; et al. Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea. Gut Liver 2019, 13, 531–540. [Google Scholar] [CrossRef]
- Gao, X.Z.; Qiao, X.L.; Song, W.C.; Wang, X.F.; Liu, F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World J. Gastroenterol. 2010, 16, 4357–4362. [Google Scholar] [CrossRef]
- Lu, J.H.; Xu, M.Y.; Sheng, Y.; Yang, W.X. Comparison of the efficacy of 10-day sequential therapy and conventional triple therapy for Helicobacter pylori eradication in children. Zhongguo Dang Dai Er Ke Za Zhi 2010, 12, 988–990. [Google Scholar]
- Wu, G.-L.; Lan, Y.; Zhang, X.-J. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication. World J. Gastroenterol. 2011, 19, 3100. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, L.; Song, Z.; Xue, Y.; Wang, Y.; Bai, P.; Hou, X.; Xu, S.; Chen, M.; Xiong, L.; et al. Sequential therapy for helicobacter pylori eradication in adults compared with triple therapy in China: A multiple- centre, prospective, randomized, controlled trial. Helicobacter 2011, 16, 77–143. [Google Scholar]
- Huang, J.; Zhou, L.; Geng, L.; Yang, M.; Xu, X.W.; Ding, Z.L.; Mao, M.; Wang, Z.L.; Li, Z.L.; Li, D.Y.; et al. Randomised controlled trial: Sequential vs. standard triple therapy for Helicobacter pylori infection in Chinese children-a multicentre, open-labelled study. Aliment. Pharmacol. Ther. 2013, 38, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Chen, C.C.; Chen, M.J.; Chen, C.C.; Chang, C.Y.; Fang, Y.J.; Lee, J.Y.; Hsu, S.J.; Luo, J.C.; Chang, W.H.; et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: A multicentre, open-label, randomised trial. Lancet 2013, 381, 205–213. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Chen, M.; Hou, X.; Li, Z.; Song, Z.; He, L.; Lin, S. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: A randomized multicenter trial. Am. J. Gastroenterol. 2014, 109, 535–541. [Google Scholar] [CrossRef]
- Zhu, X.L.; Liu, Z.; Wu, Z.Q.; Li, D.; Jiang, A.P.; Yu, G.X. Clinical effects of different therapeutic regimens for Helicobacter pylori infection in children. Zhongguo Dang Dai Er Ke Za Zhi 2017, 19, 672–676. [Google Scholar]
- Liou, J.-M.; Chen, C.-C.; Chang, C.-Y.; Wu, J.-Y.; Fang, Y.-J.; Luo, J.-C.; Lee, Y.-C.; Lin, J.-T.; Wu, M.-S. Sequential therapy for 10 days versus triple therapy for 14 days in the first-line treatment of Helicobacter pylori infection—A multicenter, open-label, randomized trial. Clin. Gastroenterol. Hepatol. 2014, 12, 161–162. [Google Scholar] [CrossRef]
- Javid, G.; Zargar, S.A.; Bhat, K.; Khan, B.A.; Yatoo, G.N.; Gulzar, G.M.; Shah, A.H.; Sodhi, J.S.; Khan, M.A.; Shoukat, A.; et al. Efficacy and safety of sequential therapy versus standard triple therapy in Helicobacter pylori eradication in Kashmir India: A randomized comparative trial. Indian J. Gastroenterol. 2013, 32, 190–194. [Google Scholar] [CrossRef]
- Nasa, M.; Choksey, A.; Phadke, A.; Sawant, P. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: A randomized study. Indian J. Gastroenterol. 2013, 32, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Lahbabi, M.; Alaoui, S.; El Rhazi, K.; El Abkari, M.; Nejjari, C.; Amarti, A.; Bennani, B.; Mahmoud, M.; Ibrahimi, A.; Benajah, D.A. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: Result of the HPFEZ randomised study. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 416–421. [Google Scholar] [CrossRef]
- Seddik, H.; Ahid, S.; El Adioui, T.; El Hamdi, F.Z.; Hassar, M.; Abouqal, R.; Cherrah, Y.; Benkirane, A. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: A prospective randomized study. Eur. J. Clin. Pharmacol. 2013, 69, 1709–1715. [Google Scholar] [CrossRef]
- Aminian, K.; Farsad, F.; Ghanbari, A.; Fakhreih, S.; Hasheminasab, S.M. A randomized trial comparing four Helicobacter pylori eradication regimens: Standard triple therapy, ciprofloxacin based triple therapy, quadruple and sequential therapy. Trop. Gastroenterol. 2010, 31, 303–307. [Google Scholar]
- Ravarian, B.; Esmaeilzadeh, A.; Saeedi, S.; Moghbeli, M.; Ganji, A. Comparison of Ten-Day Sequential and Standard Triple Therapy for Helicobacter pylori Eradication. Acta Medica Iran. 2019, 57, 224–229. [Google Scholar] [CrossRef]
- Moosavi, S.; Mahboobi, H.; Dadvand, H. Sequential Therapy versus Standard Triple Therapy for Helicobacter Pylori Eradication in Iran: A double-blind, randomized, placebo-controlled trial. Electron. Physician 2019, 11, 7378–7385. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Roman, O.; Warrington, E.; Cruz-Correa, M.R.; Toro, D.H. 10-day and 14-day sequential therapy vs. standard triple therapy for Helicobacter pylori infection in a Puerto Rican treatment-naive population: An interim analysis. Gastroenterology 2011, 140, S-149. [Google Scholar] [CrossRef]
- Warrington, E.; López-Román, O.; Tirado Montijo, R.; Urbina, R.; Cruz-Correa, M.; Toro, D.H. Neither 10- nor 14-Day Sequential Treatment is better than Standard Triple Therapy for Helicobacter Pylori Eradication. Puerto Rico Health Sci. J. 2016, 35, 203–208. [Google Scholar]
- Rakici, H.; Akdoğan, R.A.; Bedir, R.; Copur, A.; Yilmaz, A. Comparison of standard triple therapy, sequential therapy and moxifloxacin-based triple therapy for Helicobacter pylori infection: Patients’ compliance and bacterial eradication rates. J. Dig. Dis. 2014, 15, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Harmandar, F.; İlikhan, S.U.; Üstüündağn, Y.; Harmandar, O. The efficacy of sequential therapy in eradication of Helicobacter pylori in Turkey. Niger. J. Clin. Pract. 2017, 20, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, O.; Doganay, L.; Colak, Y.; Yilmaz Enc, F.; Ulasoglu, C.; Ozdil, K.; Tuncer, I. Therapeutic success with bismuth-containing sequential and quadruple regimens in Helicobacter pylori eradication. Arab J. Gastroenterol. 2017, 18, 62–67. [Google Scholar] [CrossRef]
- Sezikli, M.; Sirin, G.; Cetinkaya, Z.A.; Tanoglu, A.; Guzelbulut, F.; Bunul, F.; Dindar, G. Comparison of the efficacy of six different Helicobacter pylori eradication regimens: Greater than or equal to another. Biomed. Res. 2018, 29, 1143–1148. [Google Scholar] [CrossRef]
- Ennkaa, A.; Shaath, N.; Salam, A.; Mohammad, R.M. Comparison of 10 and 14 days of triple therapy versus 10 days of sequential therapy for Helicobacter pylori eradication: A prospective randomized study. Turk. J. Gastroenterol. 2018, 29, 549–554. [Google Scholar] [CrossRef]
- Phiphatpatthamaamphan, K.; Vilaichone, R.K.; Siramolpiwat, S.; Tangaroonsanti, A.; Chonprasertsuk, S.; Bhanthumkomol, P.; Pornthisarn, B.; Mahachai, V. Effect of IL-1 Polymorphisms, CYP2C19 Genotype and Antibiotic Resistance on Helicobacter pylori Eradication Comparing Between 10-day Sequential Therapy and 14-day Standard Triple Therapy with Four-Times-Daily-Dosing of Amoxicillin in Thailand: A Prospective Randomized Study. Asian Pac. J. Cancer Prev. 2016, 17, 1903–1907. [Google Scholar] [CrossRef]
- Auesomwang, C.; Maneerattanaporn, M.; Chey, W.D.; Kiratisin, P.; Leelakusolwong, S.; Tanwandee, T. Ten-day high-dose proton pump inhibitor triple therapy versus sequential therapy for Helicobacter pylori eradication. J. Gastroenterol. Hepatol. 2018, 33, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Chen, C.C.; Chang, C.Y.; Chen, M.J.; Chen, C.C.; Fang, Y.J.; Lee, J.Y.; Yang, T.H.; Luo, J.C.; Wu, J.Y.; et al. Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: A randomised trial. Gut 2016, 65, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Lin, C.J.; Wang, H.L.; Chen, J.D.; Kao, J.Y.; Shun, C.T.; Lu, C.W.; Lin, B.R.; Shieh, M.J.; Chang, M.C.; et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. 2015, 13, 895–905.e5. [Google Scholar] [CrossRef] [PubMed]
- Alsohaibani, F.; Al Ashgar, H.; Al Kahtani, K.; Kagevi, I.; Peedikayil, M.; Alfadda, A.; Khan, M. Prospective trial in Saudi Arabia comparing the 14-day standard triple therapy with the 10-day sequential therapy for treatment of Helicobacter pylori infection. Saudi J. Gastroenterol. 2015, 21, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ali Habib, H.S.; Murad, H.A.; Amir, E.M.; Halawa, T.F. Effect of sequential versus standard Helicobacter pylori eradication therapy on the associated iron deficiency anemia in children. Indian J. Pharmacol. 2013, 45, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.R.; Anderson, G.L.; Morgan, D.R.; Torres, J.; Chey, W.D.; Bravo, L.E.; Dominguez, R.L.; Ferreccio, C.; Herrero, R.; Lazcano-Ponce, E.C.; et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: A randomised trial. Lancet 2011, 378, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.; Kotowska, M.; Szajewska, H. Sequential therapy compared with standard triple therapy for Helicobacter pylori eradication in children: A double-blind, randomized, controlled trial. J. Pediatr. 2011, 159, 45–49. [Google Scholar] [CrossRef]
- Bontems, P.; Kalach, N.; Oderda, G.; Salame, A.; Muyshont, L.; Miendje, D.Y.; Raymond, J.; Cadranel, S.; Scaillon, M. Sequential therapy versus tailored triple therapies for Helicobacter pylori infection in children. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 646–650. [Google Scholar] [CrossRef]
- Tepes, B.; Vujasinovic, M.; Seruga, M.; Stefanovic, M.; Jeverica, S. Sequential and Quadruple Therapies for Helicobacter Pylori Eradication Compared with Triple Therapy in Slovenia: A Multicenter, Prospective, Randomized, Controlled Trial. Helicobacter 2012, 17, 73. [Google Scholar]
- Laving, A.; Kamenwa, R.; Sayed, S.; Kimang’a, A.N.; Revathi, G. Effectiveness of sequential v. standard triple therapy for treatment of Helicobacter pylori infection in children in Nairobi, Kenya. S. Afr. Med. J. 2013, 103, 921–924. [Google Scholar] [CrossRef][Green Version]
- Eisig, J.N.; Navarro-Rodriguez, T.; Teixeira, A.C.; Silva, F.M.; Mattar, R.; Chinzon, D. Standard triple therapy for Helicobacter pylori is still the best first line treatment in Brazil, compared with sequential therapy: A randomized, prospective, double-blind, placebo-controlledStudy. Gastroenterology 2014, 146, S-390. [Google Scholar] [CrossRef]
- Hsu, P.I.; Wu, D.C.; Chen, W.C.; Tseng, H.H.; Yu, H.C.; Wang, H.M.; Kao, S.S.; Lai, K.H.; Chen, A.; Tsay, F.W. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob. Agents Chemother. 2014, 58, 5936–5942. [Google Scholar] [CrossRef]
- Ang, T.L.; Fock, K.M.; Song, M.; Ang, D.; Kwek, A.B.; Ong, J.; Tan, J.; Teo, E.K.; Dhamodaran, S. Ten-day triple therapy versus sequential therapy versus concomitant therapy as first-line treatment for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2015, 30, 1134–1139. [Google Scholar] [CrossRef]
- Byambajav, T.O.; Bira, N.; Choijamts, G.; Davaadorj, D.; Gantuya, B.; Sarantuya, T.; Sarantuya, G.; Enkhtsetseg, A.; Erdenetsogt, D.; Battulga, A.; et al. Initial Trials with Susceptibility-Based and Empiric Anti-H. pylori Therapies in Mongolia. Front. Pharmacol. 2019, 10, 394. [Google Scholar] [CrossRef]
- Farhoud, N.S.; Ibrahim, O.M.; Ezzat, S.E. Efficacy and Cost-effectiveness Comparison of 10-Day, 14-Day Sequential Versus 14-Day Triple Therapies for Treating Helicobacter pylori Infection in Egyptian Patients. J. Clin. Gastroenterol. 2020, 54, 806–812. [Google Scholar] [CrossRef]
- Preda, C.M.; Proca, D.; Sandra, I.; Fulger, L.E.; Horeanga, B.C.; Manuc, M.; Manuc, T.; Dutei, C.A.; Barbu, M.; Tugui, L.; et al. A Comparative Study of Efficacy and Safety of Two Eradication Regimens for Helicobacter pylori Infection. Maedica 2017, 12, 157–163. [Google Scholar]
- Boal Carvalho, P.; Magalhães, J.; Dias de Castro, F.; Rosa, B.; Cotter, J. Randomized Controlled Trial for Helicobacter pylori Eradication in a Naive Portuguese Population: Is Sequential Treatment Superior to Triple Therapy in Real World Clinical Setting? Acta Med. Port. 2017, 30, 185–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haider, R.B.; Brennan, D.E.; Omorogbe, J.; Holleran, G.; Hall, B.; O’Morain, C.; Breslin, N.; O’Connor, H.J.; Smith, S.M.; McNamara, D. A randomized-controlled study to compare the efficacy of sequential therapy with standard triple therapy for Helicobacter pylori eradication in an Irish population. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Poudel, R.; Shakya, S.; Gurung, R.B.; Makaju, R.; Koju, P. Investigating the Efficacy of Triple Drug Therapy and Sequential Drug Therapy in the Eradication of Helicobacter Pylori with Respect to Antigen Stool test: A Pilot Study. Kathmandu Univ. Med. J. 2020, 18, 74–83. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, J.I.; Cheung, D.Y. Comparison of the eradication rates of five treatment regimens for helicobacter pylori infection. AVES Ibrahim Kara 2019, 30, S243. [Google Scholar]
- Eisig, J.N.; Navarro-Rodriguez, T.; Teixeira, A.C.; Silva, F.M.; Mattar, R.; Chinzon, D.; Haro, C.; Diniz, M.A.; Moraes-Filho, J.P.; Fass, R.; et al. Standard Triple Therapy versus Sequential Therapy in Helicobacter pylori Eradication: A Double-Blind, Randomized, and Controlled Trial. Gastroenterol. Res. Pract. 2015, 2015, 818043. [Google Scholar] [CrossRef]
- Franceschi, F.; Campanale, M.; Finizio, R.; Barbaro, F.; Tortora, A.; Gigante, G. High dose amoxicillin-based first line regimen is equivalent to sequential therapy in the eradication of H. pylori infection. Gastroenterology 2011, 140, S-149. [Google Scholar] [CrossRef]
- Kim, J.I.J.G. Comparison of AOC and AOM Triple, Sequential, and Concomitant Therapy as the First Line Eradication Therapy for Helicobacter Pylori. Gastroenterology 2017, 152, S249. [Google Scholar] [CrossRef]
- Kim, E.H.; Park, C.H. Vonoprazan-Based Helicobacter pylori Eradication Therapy: Time to Get Kompetitive? Dig. Dis. Sci. 2017, 62, 2955–2957. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; McNicholl, A.G.; Gisbert, J.P. Meta-analysis of three-in-one single capsule bismuth-containing quadruple therapy for the eradication of Helicobacter pylori. Helicobacter 2019, 24, e12570. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; Perez-Aisa, A.; Castro-Fernandez, M.; Pellicano, R.; Huguet, J.M.; Rodrigo, L.; Ortuñ, J.; Gomez-Rodriguez, B.J.; Pinto, R.M.; Areia, M.; et al. European Registry on Helicobacter pylori management: Single-capsule bismuth quadruple therapy is effective in real-world clinical practice. United Eur. Gastroenterol. J. 2021, 9, 38–46. [Google Scholar] [CrossRef]
- Delchier, J.C.; Bastuji-Garin, S.; Raymond, J.; Megraud, F.; Amiot, A.; Cambau, E.; Burucoa, C. Efficacy of a tailored PCR-guided triple therapy in the treatment of Helicobacter pylori infection. Med. Mal. Infect. 2020, 50, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Shiotani, A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 321–331. [Google Scholar] [CrossRef]
- Vilaichone, R.K.; Mahachai, V.; Graham, D.Y. Helicobacter pylori diagnosis and management. Gastroenterol. Clin. N. Am. 2006, 35, 229–247. [Google Scholar] [CrossRef]
- Graham, D.Y.; Javed, S.U.; Keihanian, S.; Abudayyeh, S.; Opekun, A.R. Dual proton pump inhibitor plus amoxicillin as an empiric anti-H. pylori therapy: Studies from the United States. J. Gastroenterol. 2010, 45, 816–820. [Google Scholar] [CrossRef]
- He, J.D.; Liu, L.; Zhu, Y.J. Ten-day sequential therapy for Helicobacter pylori eradication in children: A systematic review of randomized controlled trials. Zhonghua Yi Xue Za Zhi 2013, 93, 3500–3505. [Google Scholar] [PubMed]
- Horvath, A.; Dziechciarz, P.; Szajewska, H. Meta-analysis: Sequential therapy for Helicobacter pylori eradication in children. Aliment. Pharmacol. Ther. 2012, 36, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Calvet, X.; Garcia, N.; Lopez, T.; Gisbert, J.P.; Gene, E.; Roque, M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2000, 14, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Moayyedi, P. How can the current strategies for Helicobacter pylori eradication therapy be improved? Can. J. Gastroenterol. Hepatol. 2003, 17 (Suppl. B), 36B–40B. [Google Scholar]
- Fuccio, L.; Minardi, M.E.; Zagari, R.M.; Grilli, D.; Magrini, N.; Bazzoli, F. Meta-analysis: Duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann. Intern. Med. 2007, 147, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Calvet, X.; Bermejo, F.; Boixeda, D.; Bory, F.; Bujanda, L. III Spanish Consensus Conference on Helicobacter pylori infection. Gastroenterol. Hepatol. 2013, 36, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F.; Lehours, P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 2007, 20, 280–322. [Google Scholar] [CrossRef]
- Mégraud, F. Helicobacter pylori and antibiotic resistance. Gut 2007, 56, 1502. [Google Scholar] [CrossRef]
- Mégraud, F. H pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut 2004, 53, 1374–1384. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Perez-Aisa, A.; Tepes, B.; Castro-Fernandez, M.; Kupcinskas, J.; Jonaitis, L.; Bujanda, L.; Lucendo, A.; Jurecic, N.B.; Perez-Lasala, J.; et al. Adverse Event Profile During the Treatment of Helicobacter pylori: A Real-World Experience of 22,000 Patients From the European Registry on H. pylori Management (Hp-EuReg). Am. J. Gastroenterol. 2021, 116, 1220–1229. [Google Scholar] [CrossRef]
- Gupta, S.K. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011, 2, 109–112. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, D.H.; Choi, C.; Lee, S.T.; Kim, N.; Jeong, S.H. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: A randomized pilot study. Helicobacter 2013, 18, 180–186. [Google Scholar] [CrossRef]
- McNicholl, A.G.; Linares, P.M.; Nyssen, O.P.; Calvet, X.; Gisbert, J.P. Meta-analysis: Esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2012, 36, 414–425. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; Nyssen, O.P.; Gisbert, J.P. Sequential and concomitant treatments in H. pylori eradication: A network meta-analysis. United Eur. Gastroenterol. J. 2014, 2, A64. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F. Efficacy and safety comparison of Helicobacter pylori eradication between vonoprazan dual therapy versus triple therapy: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2022, 15, 17562848221125308. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Fischbach, L.A. Letter: The ethics of using inferior regimens in H. pylori randomised trials. Aliment. Pharmacol. Ther. 2012, 35, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, L.H.; He, X.X. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication in Chinese patients: A meta-analysis. World J. Gastroenterol. 2009, 17, 3365–3369. (In Chinese) [Google Scholar] [CrossRef]
- Janssen, M.J.; Van Oijen, A.H.; Verbeek, A.L.; Jansen, J.B.; De Boer, W.A. A systematic comparison of triple therapies for treatment of Helicobacter pylori infection with proton pump inhibitor/ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment. Pharmacol. Ther. 2001, 15, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Laheij, R.J.; Rossum, L.G.; Jansen, J.B.; Straatman, H.; Verbeek, A.L. Evaluation of treatment regimens to cure Helicobacter pylori infection—A meta-analysis. Aliment. Pharmacol. Ther. 1999, 13, 857–864. [Google Scholar] [CrossRef]
- Wong, B.C.Y.; Chang, F.Y.; Abid, S.; Abbas, Z.; Lin, B.R.; Van, R.C. Triple therapy with clarithromycin, omeprazole, and amoxicillin for eradication of Helicobacter pylori in duodenal ulcer patients in Asia and Africa. Aliment. Pharmacol. Ther. 2000, 14, 1529–1535. [Google Scholar] [CrossRef]
- Laine, L.; Fennerty, M.B.; Osato, M.; Sugg, M.S.J.; Suchower, L.; Probst, P. Esomeprazole-based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: Results of three US multicenter, double-blind trials. Am. J. Gastroenterol. 2000, 95, 3393–3398. [Google Scholar] [CrossRef]
- Hunt, R.; Fallone, C.; Veldhuyzan van Zanten, S.; Sherman, P.; Smaill, F.; Flook, N. Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori—An evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can. J. Gastroenterol. Hepatol. 2004, 18, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.2; Updated February 2021; John Wiley & Sons: Chichester, UK, 2021. [Google Scholar]
- Kjaergard, L.L.; Villumsen, J.; Gluud, C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann. Intern. Med. 2001, 135, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Hollis, S.; Campbell, F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999, 319, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Moyer, A.; Finney, J.W. Rating methodological quality: Toward improved assessment and investigation. Account. Res. 2005, 12, 299–313. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Altman, D.; Deeks, J. Meta-analysis, Simpson’s paradox, and the number needed to treat. BMC Med. Res. Methodol. 2002, 2, 3. [Google Scholar] [CrossRef]
- Cates, C.J. Simpson’s paradox and calculation of number needed to treat from meta-analysis. BMC Med. Res. Methodol. 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Gavaghan, D.J.; Edwards, J.E.; Wiffen, P.; McQuay, H.J. Pooling data for number needed to treat: No problems for apples. BMC Med. Res. Methodol. 2002, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Lu, H.; Dore, M.P. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019, 24, e12554. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, J.; Glatt, S.; Fuhr, U.; Klotz, U.; Meineke, I.; Seufferlein, T.; Brockmöller, J. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur. J. Clin. Pharmacol. 2009, 65, 19–31. [Google Scholar] [CrossRef]





| Risk of Bias Item | RD (95% CI) in Sensitivity Analysis | Impact on the Overall Eradication |
|---|---|---|
| Randomization (n = 2 excluded studies) | 0.08 (0.06 to 0.11) | Differences between therapies are still significant |
| Allocation concealment (n = 43 excluded studies) |
| Differences between therapies are still significant |
| Blinding (n = 60 excluded studies) |
| Differences between therapies are still significant |
| Incomplete outcome data (n = 22 excluded studies) | 0.10 (0.07 to 0.12) ** | Differences between therapies are still significant |
| Publication format (n = 13 excluded studies) | 0.08 (0.06 to 0.11) | Differences between therapies are still significant |
| Subgroups by Year of Publication (after 2010) | RD (95% CI) in Sensitivity Analyses | Impact on the Overall Eradication |
|---|---|---|
| Eradication proportion | 0.07 (0.05 to 0.09) | Tendency toward lower/no differences between therapies |
| Geographic region (Europe) | 0.14 (0.10 to 0.18) (Europe) 0.07 (0.05 to 0.10) (Total) | Tendency toward lower/no differences between therapies |
| Age of the population | 0.07 (0.04 to 0.09) (Adults) 0.07 (0.05 to 0.09) (Total) | Tendency toward lower/no differences between therapies |
| Baseline medical condition—PUD participants | 0.02 (−0.07 to 0.12) | Tendency toward lower/no differences between therapies |
| Baseline medical condition—NUD participants | 0.03 (−0.04 to 0.09) | Tendency toward lower/no differences between therapies |
| Length of STT regimen—7 days | 0.11 (0.09 to 0.14) | Tendency toward lower/no differences between therapies |
| Length of STT regimen—10 days | 0.04 (0.00 to 0.07) | Tendency toward lower/no differences between therapies |
| Metronidazole type (tinidazole) | 0.09 (0.05 to 0.14) | Tendency toward lower/no differences between therapies |
| PPI acid inhibition (standard dose) | 0.07 (0.04 to 0.09) | Tendency toward lower/no differences between therapies |
| Bacterial antibiotic resistance (clarithromycin) | 0.23 (0.07 to 0.39) | Tendency toward lower/no differences between therapies |
| Bacterial antibiotic resistance (nitroimidazole) | −0.02 (−0.07 to 0.04) | Tendency toward lower/no differences between therapies |
| Bacterial antibiotic resistance (dual) | 0.10 (−0.00 to 0.20) | Tendency toward lower/no differences between therapies |
| Subgroups by STT Length of 10 Days | RD (95% CI) in Sensitivity Analyses | Impact on the Overall Eradication |
|---|---|---|
| Baseline medical condition—PUD participants | 0.02 (−0.10 to 0.13) | Tendency toward lower/no differences between therapies |
| Baseline medical condition—NUD participants | 0.08 (−0.02 to 0.19) | Tendency toward higher differences between therapies |
| Clarithromycin resistance | 0.56 (0.36 to 0.75) | Tendency toward higher differences between therapies |
| Nitroimidazole resistance | 0.01 (−0.08 to 0.11) | Tendency toward lower/no differences between therapies |
| Dual resistance | −0.12 (−0.32 to 0.08) | Tendency shift toward higher efficacy with STT |
| PPI dose—standard acid inhibition | 0.06 (0.01 to 0.10) | Tendency toward lower/no differences between therapies |
| PPI dose—high acid inhibition | −0.01 (−0.14 to 0.11) | Tendency shift toward lower/no differences between therapies |
| Geographic region—Latin America | −0.04 (−0.12 to 0.04) | Tendency toward lower/no differences between therapies |
| Geographic region—Africa | 0.00 (−0.19 to 0.19) | Tendency toward lower/no differences between therapies |
| Geographic region—Asia | 0.03 (−0.02 to 0.08) | Tendency toward lower/no differences between therapies |
| Nitroimidazole type—metronidazole | 0.05 (−0.00 to 0.09) | Tendency toward lower/no differences between therapies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyssen, O.P.; Martínez, B.; Mégraud, F.; Savarino, V.; Fallone, C.A.; Bazzoli, F.; Gisbert, J.P. Sequential versus Standard Triple Therapy for First-Line Helicobacter pylori Eradication: An Update. Antibiotics 2024, 13, 136. https://doi.org/10.3390/antibiotics13020136
Nyssen OP, Martínez B, Mégraud F, Savarino V, Fallone CA, Bazzoli F, Gisbert JP. Sequential versus Standard Triple Therapy for First-Line Helicobacter pylori Eradication: An Update. Antibiotics. 2024; 13(2):136. https://doi.org/10.3390/antibiotics13020136
Chicago/Turabian StyleNyssen, Olga P., Belén Martínez, Francis Mégraud, Vincenzo Savarino, Carlo A. Fallone, Franco Bazzoli, and Javier P. Gisbert. 2024. "Sequential versus Standard Triple Therapy for First-Line Helicobacter pylori Eradication: An Update" Antibiotics 13, no. 2: 136. https://doi.org/10.3390/antibiotics13020136
APA StyleNyssen, O. P., Martínez, B., Mégraud, F., Savarino, V., Fallone, C. A., Bazzoli, F., & Gisbert, J. P. (2024). Sequential versus Standard Triple Therapy for First-Line Helicobacter pylori Eradication: An Update. Antibiotics, 13(2), 136. https://doi.org/10.3390/antibiotics13020136






