Implementation of Whole Genome Sequencing of Tuberculosis Isolates in a Referral Center in Rome: Six Years’ Experience in Characterizing Drug-Resistant TB and Disease Transmission
Abstract
1. Introduction
2. Results
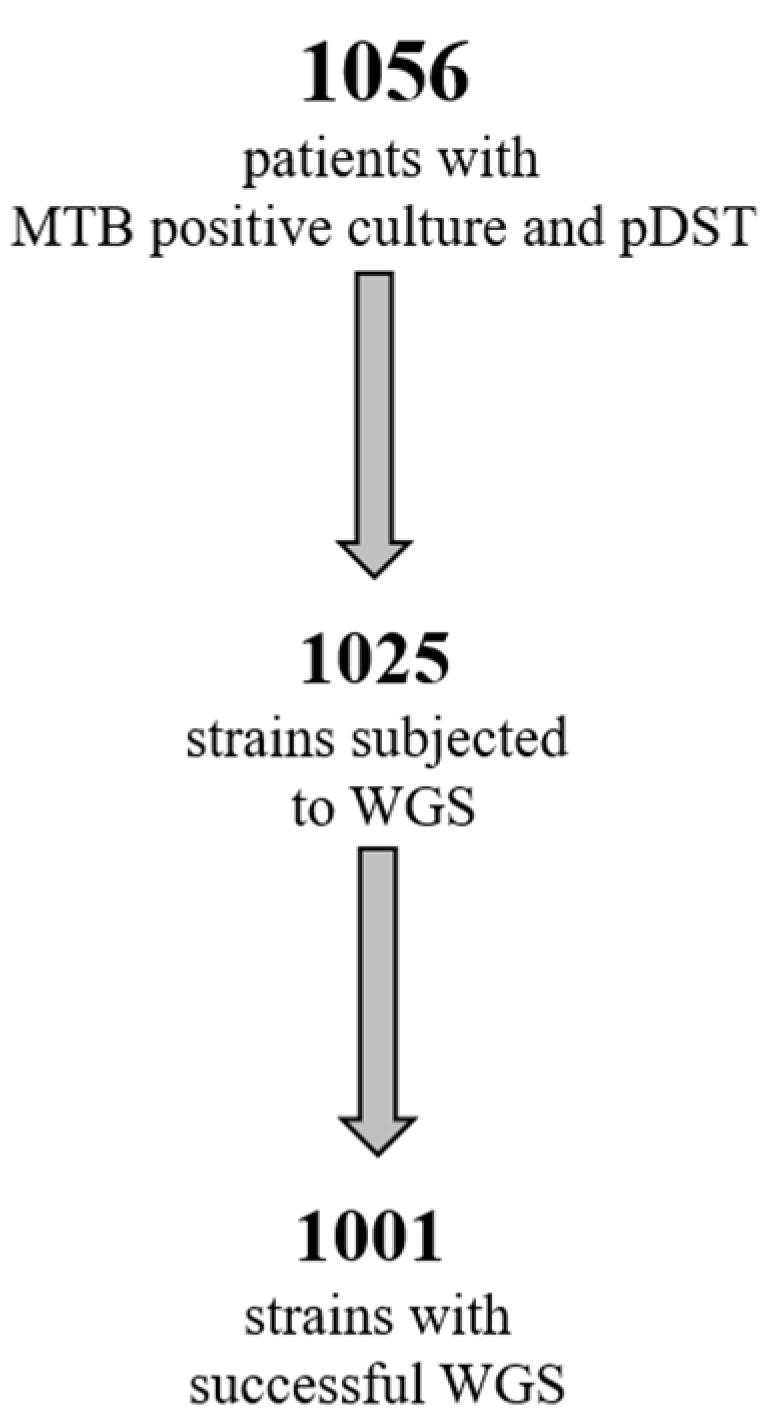
2.1. Study Population
2.2. Prediction of TB Drug Resistance Based on WGS
2.3. Characteristics of the MDR Population
2.4. Frequency of Mutations Predictive of RIF and INH Resistance in MDR Strains
2.5. Comparison of WGS and pDST
2.6. Cluster Analysis
3. Discussion
4. Materials and Methods
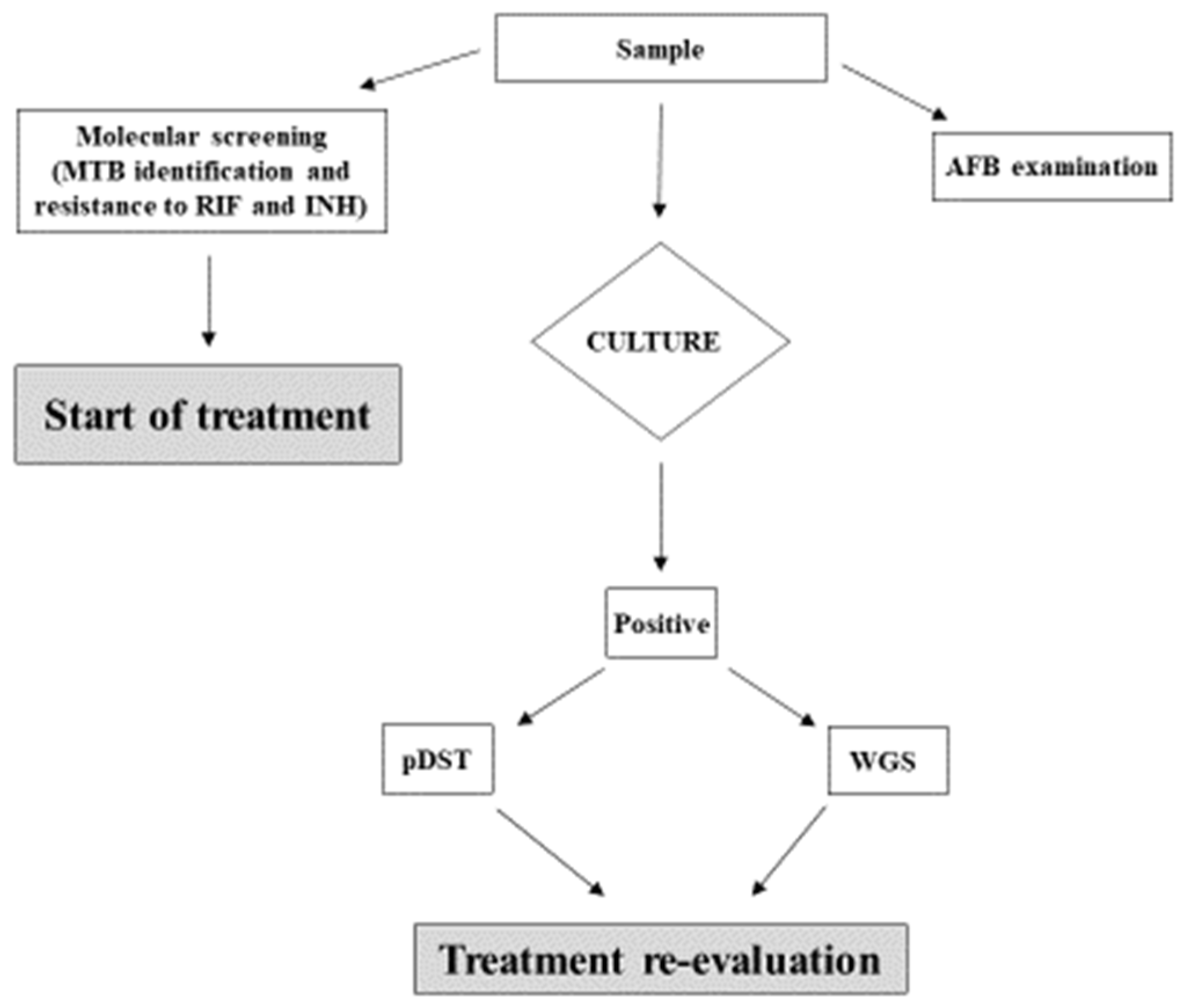
4.1. Study Population and Setting
4.2. Diagnosis and Drug Susceptibility Testing
4.3. Whole Genomic Sequencing
4.4. Sequence Analysis for Prediction of Drug Resistance and Cluster Identification
4.5. Ethics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (accessed on 15 January 2024).
- Tuberculosis Surveillance and Monitoring in Europe-2023–2021 Data. Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2023-2021-data (accessed on 15 January 2024).
- Tuberculosis—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-annual-epidemiological-report-2021 (accessed on 15 January 2024).
- REPORT SERESMI 2019–2020. Available online: https://www.inmi.it/wp-content/uploads/2022/05/Tubercolosi-Regione-Lazio.-Aggiornamento-2020.pdf (accessed on 15 January 2024).
- Domínguez, J.; Boeree, M.J.; Cambau, E.; Chesov, D.; Conradie, F.; Cox, V.; Dheda, K.; Dudnyk, A.; Farhat, M.R.; Gagneux, S.; et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: A 2023 TBnet/RESIST-TB consensus statement. Lancet Infect Dis. 2023, 23, e122–e137. [Google Scholar] [CrossRef] [PubMed]
- Meeting Report of WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis. Available online: https://www.who.int/publications/i/item/9789240018662 (accessed on 15 January 2024).
- WHO. Consolidated Guidelines on Tuberculosis. Module 4: Treatment—Drug-Resistant Tuberculosis Treatment, 2022 Update; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240063129 (accessed on 15 January 2024).
- Pankhurst, L.J.; Del Ojo Elias, C.; Votintseva, A.A.; Walker, M.T.; Cole, C.; Davies, J.; Fermont, J.M.; Gascoyne-Binzi, D.M.; Kohl, T.A.; Kong, C.; et al. Rapid, comprehensive, and affordable mycobacterial diagnosis with whole-genome sequencing: A prospective study. Lancet Respir. Med. 2016, 4, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Genestet, C.; Hodille, E.; Berland, J.-L.; Ginevra, C.; Bryant, J.E.; Ader, F.; Lina, G.; Dumitrescu, O. Whole-genome sequencing in drug susceptibility testing of Mycobacterium tuberculosis in routine practice in Lyon, France. Int. J. Antimicrob. Agents 2020, 55, 105912. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, K.; Reichmuth, M.L.; Ballif, M.; Loiseau, C.; Borrell, S.; Reinhard, M.; Skrivankova, V.; Hömke, R.; Sander, P.; Avihingsanon, A.; et al. Mortality from drug-resistant tuberculosis in high-burden countries comparing routine drug susceptibility testing with whole-genome sequencing: A multicentre cohort study. Lancet Microbe 2021, 2, e320–e330. [Google Scholar] [CrossRef] [PubMed]
- Holicka, Y.; Tagliani, E.; Cirillo, D.M.; Nikolayevskyy, V. Utility of the Whole Genome Sequencing based methodologies in routine European tuberculosis reference laboratory network setting. Tuberculosis 2022, 134, 102185. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lalvani, A.; Satta, G.; Kon, O.M. Evaluating the clinical impact of routine whole genome sequencing in tuberculosis treatment decisions and the issue of isoniazid mono-resistance. BMC Infect. Dis. 2022, 22, 349. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, F.; Gualano, G.; Timelli, L.; Vittozzi, P.; Di Bari, V.; Libertone, R.; Cerva, C.; Pinnarelli, L.; Nisii, C.; Ianniello, S.; et al. Increase in Tuberculosis Diagnostic Delay during First Wave of the COVID-19 Pandemic: Data from an Italian Infectious Disease Referral Hospital. Antibiotics 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Catalogue of Mutations in Mycobacterium tuberculosis Complex and Their Association with Drug Resistance WHO 2023. Available online: https://www.who.int/publications/i/item/9789240082410 (accessed on 15 January 2024).
- Rigouts, L.; Gumusboga, M.; de Rijk, W.B.; Nduwamahoro, E.; Uwizeye, C.; de Jong, B.; Van Deun, A. Rifampin Resistance Missed in Automated Liquid Culture System for Mycobacterium tuberculosis Isolates with Specific rpoB Mutations. J. Clin. Microbiol. 2013, 51, 2641–2645. [Google Scholar] [CrossRef] [PubMed]
- Ocheretina, O.; Escuyer, V.E.; Mabou, M.-M.; Royal-Mardi, G.; Collins, S.; Vilbrun, S.C.; Pape, J.W.; Fitzgerald, D.W. Correlation between Genotypic and Phenotypic Testing for Resistance to Rifampin in Mycobacterium tuberculosis Clinical Isolates in Haiti: Investigation of Cases with Discrepant Susceptibility Results. PLoS ONE 2014, 9, e90569. [Google Scholar] [CrossRef] [PubMed]
- Miotto, P.; Cabibbe, A.M.; Borroni, E.; Degano, M.; Cirillo, D.M. Role of Disputed Mutations in the rpoB Gene in Interpretation of Automated Liquid MGIT Culture Results for Rifampin Susceptibility Testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 2018, 56, e01599-17. [Google Scholar] [CrossRef] [PubMed]
- Mustazzolu, A.; Iacobino, A.; Giannoni, F.; Piersimoni, C.; Fattorini, L. Italian Multicentre Study on Resistance to Antituberculosis Drugs (SMIRA) Group Improved Bactec MGIT 960 Pyrazinamide Test Decreases Detection of False Mycobacterium tuberculosis Pyrazinamide Resistance. J. Clin. Microbiol. 2017, 55, 3552–3553. [Google Scholar] [CrossRef] [PubMed]
- CRyPTIC Consortium and the 100,000 Genomes Project; Allix-Béguec, C.; Arandjelovic, I.; Bi, L.; Beckert, P.; Bonnet, M.; Bradley, P.; Cabibbe, A.M.; Cancino-Muñoz, I.; Caulfield, M.J.; et al. Prediction of Susceptibility to First-Line Tuberculosis Drugs by DNA Sequencing. N. Engl. J. Med. 2018, 379, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- PDTA SULLA GESTIONE DEL PAZIENTE CON INFEZIONE/MALATTIA TUBERCOLARE. Available online: https://www.inmi.it/wp-content/uploads/2023/02/PDTA-Tubercolosi-Rev.-9_2023_DS_PW.pdf (accessed on 15 January 2024).
- WHO-FIND. The Use of Next-Generation Sequencing Technologies for the Detection of Mutations Associated with Drug Resistance in Mycobacterium tuberculosis Complex: Technical Guide. Available online: https://apps.who.int/iris/handle/10665/274443 (accessed on 15 January 2024).
- Schön, T.; Miotto, P.; Köser, C.; Viveiros, M.; Böttger, E.; Cambau, E. Mycobacterium tuberculosis drug-resistance testing: Challenges, recent developments and perspectives. Clin. Microbiol. Infect. 2016, 23, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Finci, I.; Albertini, A.; Merker, M.; Andres, S.; Bablishvili, N.; Barilar, I.; Cáceres, T.; Crudu, V.; Gotuzzo, E.; Hapeela, N.; et al. Investigating resistance in clinical Mycobacterium tuberculosis complex isolates with genomic and phenotypic antimicrobial susceptibility testing: A multicentre observational study. Lancet Microbe 2022, 3, e672–e682. [Google Scholar] [CrossRef] [PubMed]
- Tagliani, E.; Anthony, R.; Kohl, T.A.; de Neeling, A.; Nikolayevskyy, V.; Ködmön, C.; Maurer, F.P.; Niemann, S.; van Soolingen, D.; van der Werf, M.J.; et al. Use of a Whole Genome Sequencing-based approach for Mycobacterium tuberculosis surveillance in Europe in 2017–2019: An ECDC pilot study. Eur. Respir. J. 2020, 57, 2002272. [Google Scholar] [CrossRef] [PubMed]
- Merker, M.; Rasigade, J.-P.; Barbier, M.; Cox, H.; Feuerriegel, S.; Kohl, T.A.; Shitikov, E.; Klaos, K.; Gaudin, C.; Antoine, R.; et al. Transcontinental spread and evolution of Mycobacterium tuberculosis W148 European/Russian clade toward extensively drug resistant tuberculosis. Nat. Commun. 2022, 13, 5105. [Google Scholar] [CrossRef] [PubMed]
- WHO. Consolidated Guidelines on Drug-Resistant Tuberculosis. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf (accessed on 15 January 2024).
- Kadura, S.; King, N.; Nakhoul, M.; Zhu, H.; Theron, G.; Köser, C.U.; Farhat, M. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J. Antimicrob. Chemother. 2020, 75, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Aono, A.; Borroni, E.; Cirillo, D.M.; Desmaretz, C.; Hasan, R.; Mitarai, S.; Shakoor, S.; Torrea, G.; Kaniga, K.; et al. A Multimethod, Multicountry Evaluation of Breakpoints for Bedaquiline Resistance Determination. Antimicrob. Agents Chemother. 2020, 64, e00479-20. [Google Scholar] [CrossRef] [PubMed]
- WHO. Announces Updated Critical Concentrations for Susceptibility Testing to Rifampicin. 5 February 2021. Available online: https://www.who.int/news/item/05-02-2021-who-announces-updated-critical-concentrations-for-susceptibility-testing-to-rifampicin (accessed on 15 January 2024).
- Feuerriegel, S.; Schleusener, V.; Beckert, P.; Kohl, T.A.; Miotto, P.; Cirillo, D.M.; Cabibbe, A.M.; Niemann, S.; Fellenberg, K. PhyResSE: A Web Tool Delineating Mycobacterium tuberculosis Antibiotic Resistance and Lineage from Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015, 53, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
| Sensitive N. (%) | Resistant N. (%) | Single Resistance, Not MDR N. Cases (%) | RR/MDR N. Cases (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| INH | EMB | S | PZA | FQN | I.D. | RR | MDR | Pre-XDR | XDR | ||
| 852 (85) | 149 (15) | 38 (3.8) | 4 (0.4) | 8 (0.8) | 6 (0.6) | 22 (2.2) | 20 (2.0) | 5 (0.5) | 34 (3.4) | 10 (1.0) | 1 (0.1) |
| Total N. 1001 (100) | Total N. 99 (10) | Total N. 50 (5) | |||||||||
| RIF-R N. (%) | Leu452Pro | Ser450Leu | Asp435Val | Gln432Pro | Leu430Pro | His445Tyr | Asp435Tyr | Total | |
|---|---|---|---|---|---|---|---|---|---|
| N. (%) | |||||||||
| INH-R N. (%) | Ser315Thr | 1 (2.22) | 21 (46.7) | 5 (11.1) | 2 (4.42) | 1 (2.22) | 1 (2.22) | 3 (6.7) | 34 (75.56) |
| inhA-prom −15 | 1 (2.22) | 1 (2.22) | |||||||
| Ser315Thr + inhA prom | 8 (17.8) | 2 (4.44) | 10 (22.2) | ||||||
| Total N. (%) | 1 (2.22) | 30 (66.7) | 5 (11.1) | 2 (4.42) | 1 (2.22) | 3 (6.7) | 3 (6.7) | 45 (100) | |
| Level of Resistance | |||||
|---|---|---|---|---|---|
| rpoB Mutation N. (%) | RIF-R | MDR | Pre-XDR | XDR | Total N. (%) |
| Leu452Pro | 1 (2) | 1 (2) | |||
| Ser450Leu | 2 (4) | 24 (48) | 5 (10) | 1 (2) | 32 (64) |
| Asp435Val | 2 (4) | 3 (6) | 5 (10) | ||
| Gln432Pro | 2 (4) | 2 (4) | |||
| Leu430Pro | 1 (2) | 1 (2) | 2 (4) | ||
| His445Tyr | 1 (2) | 3 (6) | 4 (8) | ||
| Asp435Tyr | 3 (6) | 3 (6) | |||
| His445Asp | 1 (2) | 1 (2) | |||
| Total N. (%) | 5 (10) | 35 (70) | 9 (18) | 1 (2) | 50 (100) |
| pDST | ||||
| WGS | S | R | Total | |
| S | 951 | 0 | 951 | |
| R | 6 | 44 | 50 | |
| Total | 957 | 44 | 1001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannas, A.; Butera, O.; Mazzarelli, A.; Messina, F.; Vulcano, A.; Parracino, M.P.; Gualano, G.; Palmieri, F.; Di Caro, A.; Nisii, C.; et al. Implementation of Whole Genome Sequencing of Tuberculosis Isolates in a Referral Center in Rome: Six Years’ Experience in Characterizing Drug-Resistant TB and Disease Transmission. Antibiotics 2024, 13, 134. https://doi.org/10.3390/antibiotics13020134
Cannas A, Butera O, Mazzarelli A, Messina F, Vulcano A, Parracino MP, Gualano G, Palmieri F, Di Caro A, Nisii C, et al. Implementation of Whole Genome Sequencing of Tuberculosis Isolates in a Referral Center in Rome: Six Years’ Experience in Characterizing Drug-Resistant TB and Disease Transmission. Antibiotics. 2024; 13(2):134. https://doi.org/10.3390/antibiotics13020134
Chicago/Turabian StyleCannas, Angela, Ornella Butera, Antonio Mazzarelli, Francesco Messina, Antonella Vulcano, Mario Pasquale Parracino, Gina Gualano, Fabrizio Palmieri, Antonino Di Caro, Carla Nisii, and et al. 2024. "Implementation of Whole Genome Sequencing of Tuberculosis Isolates in a Referral Center in Rome: Six Years’ Experience in Characterizing Drug-Resistant TB and Disease Transmission" Antibiotics 13, no. 2: 134. https://doi.org/10.3390/antibiotics13020134
APA StyleCannas, A., Butera, O., Mazzarelli, A., Messina, F., Vulcano, A., Parracino, M. P., Gualano, G., Palmieri, F., Di Caro, A., Nisii, C., Fontana, C., & Girardi, E. (2024). Implementation of Whole Genome Sequencing of Tuberculosis Isolates in a Referral Center in Rome: Six Years’ Experience in Characterizing Drug-Resistant TB and Disease Transmission. Antibiotics, 13(2), 134. https://doi.org/10.3390/antibiotics13020134









