Abstract
The development of antimicrobial resistance (AMR), along with the relative reduction in the production of new antimicrobials, significantly limits the therapeutic options in infectious diseases. Thus, novel treatments, especially in the current era, where AMR is increasing, are urgently needed. There are several ongoing studies on non-classical therapies for infectious diseases, such as bacteriophages, antimicrobial peptides, and nanotechnology, among others. Nanomaterials involve materials on the nanoscale that could be used in the diagnosis, treatment, and prevention of infectious diseases. This review provides an overview of the applications of nanotechnology in the diagnosis and treatment of infectious diseases from a clinician’s perspective, with a focus on pathogens with AMR. Applications of nanomaterials in diagnosis, by taking advantage of their electrochemical, optic, magnetic, and fluorescent properties, are described. Moreover, the potential of metallic or organic nanoparticles (NPs) in the treatment of infections is also addressed. Finally, the potential use of NPs in the development of safe and efficient vaccines is also reviewed. Further studies are needed to prove the safety and efficacy of NPs that would facilitate their approval by regulatory authorities for clinical use.
1. Introduction
Infectious diseases are an important cause of mortality worldwide [1]. During the last decades, there has been a reduction in morbidity and mortality of these communicable diseases in higher-income countries; however, infectious diseases are still a cause for significant morbidity, even in these countries [2,3,4]. Importantly, antimicrobial resistance (AMR) is a global problem that poses a significant threat to millions of people [5,6]. For example, 4.95 million deaths were estimated to be associated with bacterial AMR in 2019, and it is predicted that this number will greatly increase [5]. Unfortunately, the pharmaceutical pipeline has not met the growing need for antimicrobials to combat AMR [7]. Thus, academia and the pharmaceutical industry have also tried to identify methods that involve treatments other than antimicrobial use to fight resistant pathogens that very few, if any, antimicrobials are potent to kill. To that end, non-antibiotic treatments may include simple nutrients or amino-acids, such as D-mannose, antimicrobial peptides, bacteriophages, or even more technically elegant methods, such as nanobiotics [8,9,10,11,12].
Nanoscience involves the use of materials on the scale of nanometers in an attempt to develop technology (nanotechnology) that could have several applications in electronics or medicine [13]. More specifically, several applications of nanotechnology in medicine have been developed in the last decades, such as the development of liposomes [14,15], polymer-drug conjugates [16], DNA-drug complexes [17], polymer nanocapsules [18,19], antibody-drug conjugates [20], albumin-drug conjugates [21], polymer-protein conjugates [22], antimicrobial silver nanoparticles [23], and gold nanoparticles for rheumatologic disorders [24,25]. Nanotechnology has numerous applications in medicine, such as in drug delivery, where several nanostructures can be used for the direct delivery of a specific drug to a specific biological target with controlled drug release and reduced toxicity, and in in vivo imaging with nanoparticles used as imaging contrast agents, such as in computed tomography and magnetic resonance imaging [26]. Other applications of nanotechnology in medicine include in vitro diagnosis, tissue regeneration and engineering, as well as its use with implantable or wearable devices for diagnosis or treatment [26].
This study aimed to provide an overview of the current medical applications of nanotechnology in infectious diseases and, more specifically, against pathogens with significant AMR. In particular, applications of nanotechnology in the diagnosis and treatment of infectious diseases are discussed, along with specific representative examples of the different technologies that have been developed so far.
2. Search Methodology
This narrative review aims to present the basic principles of the applications of nanotechnology in the diagnosis and treatment of infectious diseases with a focus on pathogens with AMR, and it also aims to provide representative examples of the different technologies that have been developed so far. Importantly, this manuscript was written from a clinician’s perspective; thus, its main focus is to inform the reader of the spectrum of the applications of nanotechnology in infectious diseases by providing the necessary emphasis on technical information and, more importantly, underlining the current and future potential of nanotechnology.
For this review, a search of the PubMed/Medline database up until 18 January 2024 for eligible articles was conducted, using the search terms ‘nanotechnology AND infectious diseases’. Relevant reviews and original studies providing information on the topic were retrieved, evaluated, and added to the evidence synthesis in a liberal, non-systematic way. Two investigators (PI and SB) performed the process of screening, extracting, and synthesizing the evidence. The references of the included articles were searched for the identification of other relevant articles.
3. Principles of Nanotechnology
The basic functional unit of a material used in nanotechnology is a nanoparticle (NP). NPs are composed of metal, metal oxides, carbon, or organic matter and have higher surface charge, stability, strength, reactivity, sensitivity, surface area, and absorption compared to other older materials [27]. Their size is in the nanoscale, most commonly between 1 and 100 nm [28]. They have unique magnetic, electric, optical, or other properties in the nanoscale that allow them to function, and they have been recently placed at the center of many research studies because of their great potential to produce materials, with uses in several fields, such as medicine and many others, like industry [13,28,29].
Even though this field of research may sound innovative and quite recent, the principles of nanotechnology have been used for a long time, as in the case of the reinforcement of ceramic matrices with natural asbestos nanofibers approximately 4500 years ago or in the case of lead sulfide (PbS) NPs with a size of approximately 5 nm that were chemically synthesized in the same period for use in hair dye [28]. Ever since, principles of nanotechnology have been used through metallic NPs, Cu NPs, Ag NPs, Au NPs, or SiO2 NPs for decorative purposes, antibacterial use, and rubber reinforcement, among other uses [28].
Some common types of NPs include carbon nanotubes, dendrimers, liposomes, metallic NPs, micelles, and quantum dots. Carbon nanotubes are cylindrical molecules consisting of folded sheets of carbon atoms that may be single-walled, multi-walled, or that may be formed by several nanotubes that are concentrically interlinked [13,30]. They can cross cell membranes in both an endocytosis-independent and an endocytosis-dependent manner [31,32]. They can serve as efficient carriers for drugs due to their high external surface [13]. Moreover, they have optical, electronic, and mechanical properties that allow them to be used as biological sensors and imaging contrast agents [32,33,34]. They can be used in photoacoustic imaging, drug delivery, phototherapy, gene delivery, photoluminescence imaging, thermal therapy, gas storage, diagnostics, and elsewhere [35]. Some of their disadvantages are their relatively low solubility in water, the variable pharmacokinetics that depend on their physicochemical characteristics, and the potential for toxicity, more likely to the lungs [36]. Dendrimers are highly ordered macromolecules that have branching repeating functional units and usually have symmetry and a spherical three-dimensional morphology [13,37,38,39]. These molecules can have cationic, neutral, or anionic terminals, and they can encapsulate therapeutic molecules in their interior or at their surface and, thus, facilitate their delivery due to their bioavailability. They can be conjugated to peptides or saccharides to enhance their antimicrobial activity [40]. More specifically, they can be used as conjugates to drug molecules, fluorescent trackers, antibodies, enzymes, and targeting ligands that can be released either actively or passively to their target; they could also be used as drug carriers through the encapsulation of the drug or as gene carriers through complexation with the target gene [41]. Some of their limitations include the difficulty in their production as well as their toxicity, which depends on their net charge as well as on their size, with dendrimers of different sizes being also eliminated from different routes [41]. For example, smaller dendrimers have renal elimination, while larger ones rely on hepatic elimination [41]. Liposomes are spherical particles with a size as low as 30 nm that are composed of lipid bilayers. They are typically used to enhance the delivery of drugs by incorporating them in their hydrophilic center, if the drugs are hydrophilic, or into their hydrophobic membrane, if the drugs are hydrophobic [13]. Their characteristics can also be modified with peptides, antibodies, or polymers, allowing for the formation of macromolecular drugs [38]. Several liposomes are currently in clinical use, mostly in oncology but also in the treatment and prevention of infectious diseases [42]. Some of their limitations include the relatively high cost of production, their short half-life, their low solubility, and the possibility of leakage of the encapsulated drug [43]. Metallic NPs mainly refer to gold and iron NPs. Iron oxide NPs are composed of a magnetic core 4–5 nm in size and hydrophilic polymers such as dextran [30,32,34]. Gold NPs consist of a gold atom core in the center and negative reactive groups in the periphery that can be modified with the addition of surface moieties, such as ligands, to provide active molecular targeting [13,30,32,34]. They have the potential to carry proteins, nucleic acids, drugs, cell-penetrating agents, imaging agents, or specific targeting moieties, such as monoclonal antibodies [44]. Their biosynthesis can be mediated by microorganisms and plants, which leads to a lower production cost [45]. Several clinical studies are currently ongoing and are evaluating the activity and safety of metallic NPs in the treatment of cancer and infectious diseases [44]. Some of their limitations may include issues regarding biocompatibility and their relatively weak optical signal [46,47]. Micelles are surfactants of lipids with amphiphilic properties due to their polarized heads. They have the ability to assemble spherical vesicles in a spontaneous manner in aqueous conditions, having a hydrophobic inner core and a hydrophilic outer layer due to the hydrophilic head of their amphiphilic molecules [48]. They have several applications, mainly due to their ability to solubilize hydrophobic drugs, thus increasing their bioavailability [13]. For this reason, they are used as imaging agents, therapeutics, and drug delivery agents [13,49]. More specifically, there are several clinical trials with micelles being evaluated in the treatment of various cancers, such as breast, lung, bladder, and pancreatic cancer [50]. One of their limitations includes their relatively limited loading capacity [51,52,53]. Quantum dots are nanocrystals with fluorescent semiconductor properties with the potential for use in cellular imaging and drug delivery [30,54,55]. They have a shell structure consisting of elements from groups II-VI or III-V of the periodic table and have optical properties and sizes that allow them to be used in medical imaging [13,55]. Several clinical trials with quantum dots in the detection and diagnosis of cancer, acute myocardial infarction, and type one diabetes mellitus are ongoing [56]. Moreover, their potential in the detection of cells expressing specific target receptors is also being evaluated [56]. Some of their limitations include pharmaceutical issues regarding their relative instability that could lead to aggregation or chemical redox alterations [56]. Furthermore, the requirements for their production could make their mass production difficult. Moreover, the possibility for adverse reactions and their non-specificity for interaction with all host cells are factors that may limit their use. Table 1 shows a comparison of the characteristics, advantages, and disadvantages of the different NPs.

Table 1.
Comparison of different nanoparticle categories.
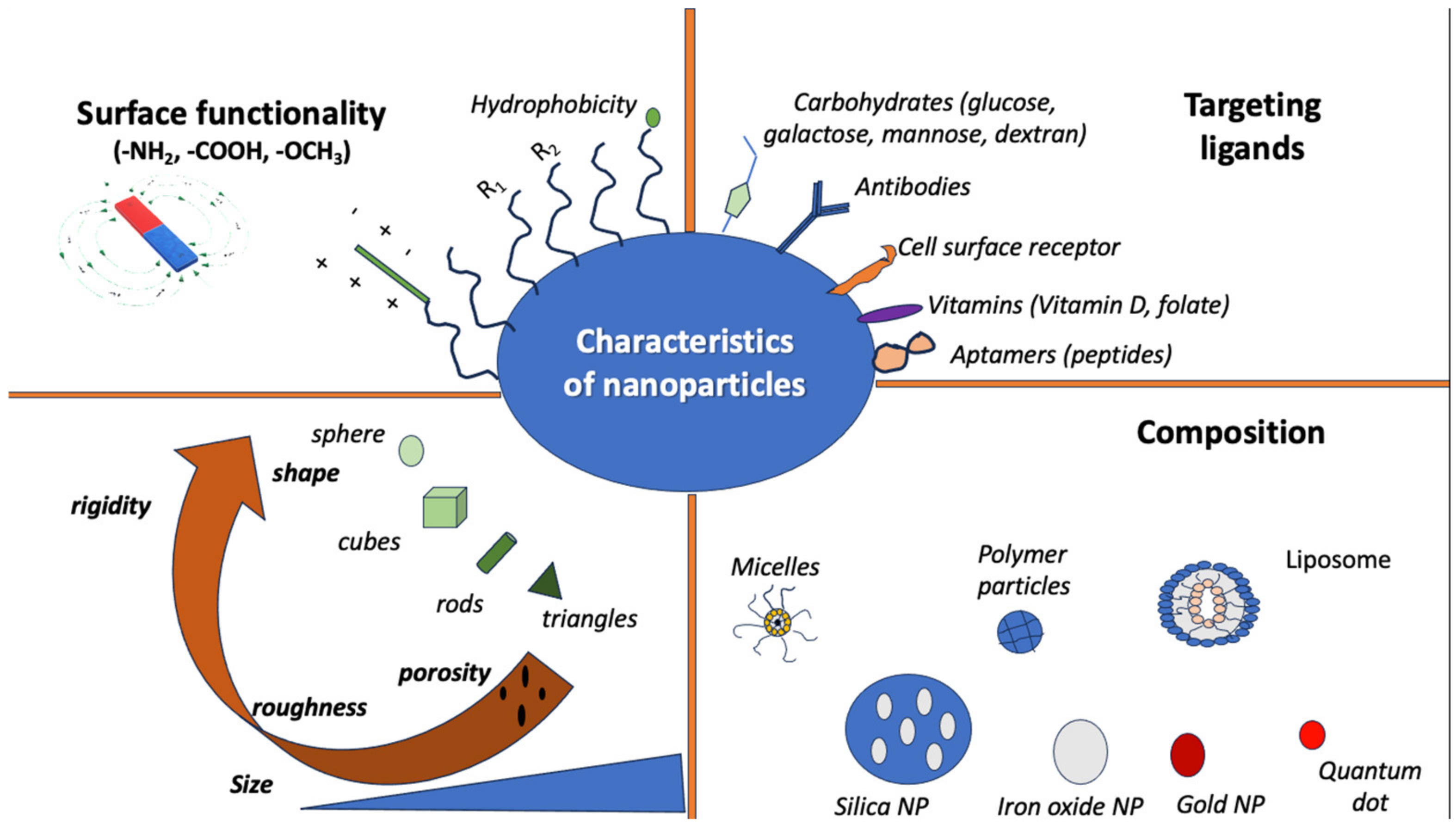
Additionally, the use and properties of NPs depend on factors like shape and size. More specifically, the function of NPs often depends on their surface area, which provides a relatively high area-to-volume index, given their very small size [28]. Some of their possible properties include the following: mechanical, thermal, optical, electronic, and magnetic properties. Mechanical properties include hardness, friction, interfacial adhesion, and elastic modulus, among others, and they are primarily used in surface engineering, nanofabrication, painting materials, and nanomanufacturing [28,64,65]. The optical and electronic properties of NPs are interconnected, and they are mostly used in light-emitting diodes (LEDs), sensors, biophotonics, batteries, and semiconductors [28,66,67]. The magnetic properties of NPs seem apparent in NPs of 10–20 nm in size that have uneven electron distribution [28]. NPs with magnetic properties are used in catalysts, data storage, water and soil purification, magnetic resonance imaging (MRI), and elsewhere [65,68,69]. Figure 1 shows the characteristics of different nanoparticles.
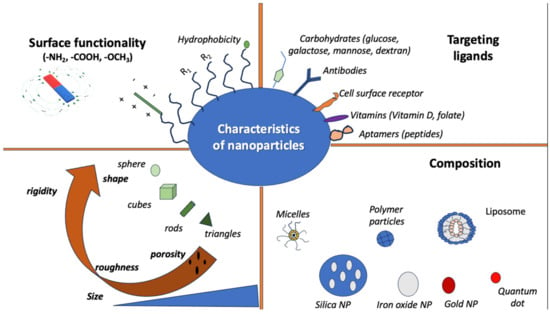
Figure 1.
Characteristics of nanoparticles in terms of shape, composition, targeting ligands, and surface function.
A new field, nanomedicine, has been developed, encompassing all of these nanotechnology applications in medicine. Several applications have emerged, such as cancer, cardiovascular disease, diabetes, neurodegenerative diseases, and infectious diseases. In infectious diseases, nanomedicine has applications in diagnosis as well as in therapy [13,38,70,71,72,73]. More specifically, nanomedicine in the field of infectious diseases may aid in the diagnosis and treatment of bacterial, viral, parasitic, or mycobacterial disease [74,75,76,77,78,79]. The following sections of this review specifically address the applications of nanotechnology in diagnosing and treating infectious diseases.
4. Nanotechnology in the Diagnosis of Infectious Diseases
4.1. Basic Principles in the Diagnosis of Infectious Diseases
Due to the large area-to-volume ratio and the reactivity of the molecules at the nanomaterials’ surface, these nanomaterials have great potential in diagnosing infectious diseases. The use of nanomaterials as diagnostic tools has been attributed to their ability to bind infectious agents and occupy mechanical, optical, electronic, and magnetic signals [80]. They also provide the ability to perform point-of-care diagnostic tests, identify resistance determinants, and provide multiplexing capacity, thus being of utmost importance in diagnosis [81,82,83]. Until now, many nanobiosensing assays have been used in the diagnosis of infectious diseases in humans, but their efficacy and safety remain to be elucidated [80,84,85]. Different principles occupied by nanosensors include colorimetric, electrochemical, fluorescent, surface-enhanced Raman spectroscopy (SERS), and others [86].
4.2. Nanosensors Occupying Colorimetric Properties
Colorimetric biosensors create visible signals to the naked eye and have long been used in diagnosing infectious diseases [86]. One of their primary disadvantages is their relatively low sensitivity, which may limit their broad use. Nanomaterials with optical properties may provide a higher sensitivity in such assays in diagnosing infectious diseases [86]. Nanotechnology offers many different substrates for use as nanosensors. For example, gold NPs (AuNPs) are commonly used in that direction due to their unique surface plasmon resonance (SPR) phenomenon that gives them strong absorption in visible light [86]. Thus, many sensitive colorimetric assays have been developed occupying AuNPs, such as assays for the detection of pathogen nucleic acid in paper-based biosensors combined with loop-mediated amplification (LAMP) [86,87]. In this case, the biosensor functions in nucleic acid extraction, amplification, and detection. After the amplification, amplicons labeled with biotin bind to AuNPs that are detection probes. Then, due to the biotin–streptavidin interaction, this complex is immobilized at the test zone and emits a visible signal that is read as a positive test [86]. Other techniques also take advantage of the optical abilities of AuNPs, such as in the case of enzyme-linked immunosorbent assays (ELISA) that are used for the detection of biomarkers with increased sensitivity [88,89,90]. Other nanomaterials, such as AgNPs, are occupied in colorimetric applications of nanotechnology for diagnosing infectious diseases [91,92,93]. Another application of NPs has to do with their potential to be used as enzymes. These NPs, called nanozymes, have similar catalytic activity but may have increased stability compared to enzymes [86,94]. One such example involves the use of copper-based metal-organic framework (Cu-MOF) NPs with enzymatic activity similar to peroxidase that is used for the detection of Staphylococcus aureus [95].
4.3. Nanosensors Occupying Electrochemical Properties
The electrochemical properties of some NPs are important for the detection of pathogens [86,96]. The principle of the method relies on the production of measurable electrochemical signals upon pathogen detection, such as impedance, current, or potential. Hence, nanomaterials based on carbon, such as graphene or carbon nanotubes, are used for the production of biosensors due to their high conductivity [86]. For example, the detection of uropathogenic Escherichia coli is possible using a multiwall carbon nanotube-chitosan composite-based electrochemical immunosensor, while this technique has also been used for the detection of genetic material, like in the case of human papillomavirus DNA [97,98]. An example of a biosensor occupying the measurement of potential is the potentiometric graphene-based aptasensor that was developed for detecting S. aureus in a concentration of even a single cfu/mL level in just one or two minutes [99].
4.4. Nanosensors Occupying Fluorescent Properties
Fluorescent biosensors are of great value in pathogen detection assays due to their high sensitivity, even though these assays may be limited because of the relatively low stability of fluorophores and their tendency to bleach [86]. Nanosensors with fluorescent properties have been evaluated for diagnosing infectious diseases, with various examples available. Hence, quantum dots may be valuable tools in that direction due to their narrow emission peak, their high photostability, and tunable emission wavelength [100,101]. Likewise, CdSe@ZnS quantum dots are used in a lateral flow assay for pathogen detection [101]. More specifically, the conjugates of antibodies with quantum dots are immobilized at the test and the control lines. Graphene oxide is added; thus the quantum dots are quenched if pathogens are absent. However, in the presence of pathogens, the distance between quantum dots and the graphene oxide is increased, allowing fluorescence detection from the quantum dots [101]. Other examples of NPs used as biosensors due to their fluorescent properties include silver nanoclusters, along with graphene oxide for the detection of pathogen DNA, carbon dots that, similar to quantum dots, have the photoluminescent emission properties that can be used for bioimaging and bioanalysis, such as, for example, in the case of Gram-positive bacterial detection [102,103,104].
4.5. Nanosensors Occupying SERS Properties
SERS is a sensitive method that uses optical detection [86,105]. Its principle relies on the enhancement of Raman scattering by nanomolecules, such as plasmonic-magnetic nanotubes or by molecules adsorbed on rough metal surfaces [106]. This method allows for the enhancement of scattering intensity up to 1014 times, thus making this a very sensitive method for diagnosis even at a single-molecule level [86,107,108]. This method has been used for viral detection. An SERS-based lateral flow assay has been used for the detection of HIV-1 genetic material or the detection of the DNA of Kaposi sarcoma-associated herpesvirus [109,110].
4.6. Nanosensors in Point-of-Care Testing
Point-of-care testing has the advantage of providing diagnostic abilities to people who do not have either the expertise or the high technical capacities nearby, thus allowing the use of sophisticated technology in the field where the diagnostic abilities of these tests are mostly needed [86]. Such examples of point-of-care testing include the use of magnetic NPs. For example, magnetic NP-DNA complexes and conjugates of DNA-invertase are used for the detection of hepatitis B virus (HBV) DNA [111]. More specifically, the DNA fragments of HBV are captured by the magnetic NPs labeled with a DNA fragment partially complementary to the target DNA. Then, the DNA hybridizes with the DNA-invertase conjugate. After separation of the sandwich complex and incubation with sucrose, glucose is produced, and this can be measured with a glucose meter for the quantification of the DNA fragment [111]. Other point-of-care tests have been developed for microorganism detection, such as for severe acute respiratory syndrome coronavirus 2019 (SARS-CoV-2) [112]. Nanotechnology can also occupy newer scientific and technological methods, such as CRISP-Cas technology or even smartphone-based technology [113,114,115]. Table 2 shows some examples of nanosensors occupying different principles that have been evaluated in diagnosing infectious diseases.

Table 2.
Examples of nanosensors that have been evaluated in the diagnosis of infectious diseases.
4.7. Future Perspectives in Nanotechnology and the Diagnosis of Infectious Diseases
The current era provides huge potential for the advancement of diagnostics in infectious diseases. The implementation of the abovementioned principles of nanotechnology in the diagnosis of infectious diseases provides a great opportunity for diagnosing infections with high sensitivity and specificity, even with point-of-care tests. Optimization of the diagnostic techniques, overcoming technical barriers to increase sensitivity and specificity, large-scale production of the tests, and reduction of cost should be the next steps toward the increased implementation of this technology in the diagnosis of infectious diseases. Mass production and reduction of cost are of particular significance since many of the tests that are either available or under development could be of great use in countries with limited resources but with a high burden of disease, such as in the case of HIV infection and AIDS in Africa [122].
Moreover, the introduction of artificial intelligence in medicine could also aid in the diagnosis of infectious diseases. Combining nanotechnology applications in diagnosis with artificial intelligence could also lead to more potent diagnostic tools through increasing automation in diagnosis, reducing human error, increasing speed in providing diagnostic results, and increasing diagnostic accuracy [122,123,124].
5. Nanotechnology in the Treatment of Infectious Diseases
5.1. The Need for Non-Antibiotic Interventions in the Treatment of Infectious Diseases
Even though antimicrobials have changed the face of human history by allowing the effective treatment of many infectious diseases and also enabling surgeons to conduct major surgical managements with significant reduction of surgical site infections, antimicrobial resistance developed rapidly after the initiation of antimicrobial use, thus making some of the previously mentioned treatments unavailable [125]. The development of extensively-drug-resistant and pan-drug-resistant pathogens has posed severe therapeutic limitations in the everyday clinical practice of infectious diseases [126,127]. Given the imbalance in the relatively slow production of new antimicrobials, from the pharmaceutical pipeline and the rapid development of AMR, new non-classical therapeutic options could be of great use in the fight against pathogens with significant AMR [128]. Several therapeutic strategies have been employed in this direction involving natural and chemical products, probiotics and prebiotics, antimicrobial peptides, bacteriophages, predatory bacteria, immunotherapy, vaccines, and other methods [10,11,129,130,131,132,133]. Nanotechnology may offer valuable tools in this fight against pathogens harboring significant AMR.
5.2. Basic Principles of Nanotechnology in the Treatment of Infectious Diseases
Due to their small size and specific electrical, magnetic, and binding properties, the NPs could be used in the fight against infectious diseases, even against resistant pathogens. Their properties and size could allow them to easily cross bacterial membranes and target specific biosynthetic and enzymatic pathways [134,135]. This is in contrast to classic antimicrobials that may not enter in adequate concentrations inside the target cells due to the rarity of pores and the transport mechanisms needed to enter the cells, as well as due to mechanisms employed by target cells aimed towards antimicrobial drug efflux outside the cell or its enzymatic inhibition [134,136]. NPs may act through various mechanisms against pathogens. More specifically, they may have inherently antibacterial activity or act as delivery vehicles for antibiotics that may be carried on or inside them. In these cases, they are called nanobiotics or nanoantibiotics [134]. Inorganic NPs may have inherent antibiotic activity and present many different mechanisms of antibacterial activity. These are called nanobacteriocides, while NPs acting as NP-based delivery systems transferring old antibiotics are called nanocarriers [134].
5.3. Nanotechnology and Enhanced Drug Delivery in the Treatment of Infectious Diseases
Nanocarriers may be used to treat infectious diseases due to their ability to transfer antibiotics to the target, thus altering the pharmacological properties of antibiotics by increasing their absorption or increasing their delivery to the tissues and the target microbial cells [134,137,138,139]. One typical example is encochleated amphotericin B, a novel formulation of a well-known antifungal drug [138,139]. Encochleated amphotericin B is a novel nanoparticle-based formulation that encapsulates and delivers amphotericin B in the cells. It does so by protecting amphotericin B in the interior of an anhydrous crystal of calcium and phospholipids [139]. This formulation completely alters the pharmacokinetics of the drug, allowing, for the first time now, the oral administration of this drug that had for decades been administered only intravenously [139]. However, this drug has not yet received FDA approval for clinical use.
Liposomes are the most commonly used NPs for enhancing drug delivery for topical and systemic use. They are appropriate for both hydrophobic and hydrophilic molecules [134]. Liposomes have multiple advantages that make them ideal candidates for drug delivery. They are non-toxic and biodegradable since they comprise material resembling human cell membranes. Moreover, they can be modified in multiple ways, including size, lipid composition, and net surface charge, allowing modification of their half-life, their pharmacokinetics, and the way they carry the drug to be delivered [134]. Finally, they have the ability to fuse with membranes, either those of the human cells or the ones of the pathogens, and this allows for the delivery of the antibiotic inside the pathogen or even inside the human cells in the case of infection by an intracellular pathogen [140,141]. An example of liposomes used as carrier molecules in the therapeutics of infection includes the delivery of antiretrovirals, such as zidovudine or indinavir in the case of infection by HIV that leads to more efficient delivery of the drug to the lymphoid tissues compared to administration without liposomes [142,143,144].
Dendrimers can also be used as carriers of drugs in the treatment of infectious diseases. Their hyper-branched structure with the central core and the three-dimensional branches can increase the solubility of hydrophobic drugs, thus increasing their efficacy [145,146]. For example, the delivery of efavirenz with dendrimers has been studied, leading to the enhanced delivery of the drug to cells of the immune system [147].
5.4. Nanoparticles as Antimicrobial Drugs
Metallic NPs can have toxic effects on microorganisms since they may induce the production of reactive oxygen species under specific circumstances, such as under ultraviolet light [148]. Metallic NPs with Au, Ag, Ti, Cu, Zn, Fe, Ti, or metal oxides can have significant antimicrobial activity against bacteria, viruses, or fungi [145,149,150,151,152,153,154,155].
For example, AgNPs act as antimicrobials based on four mechanisms: binding to the cell of the microorganisms, destabilization of its cell membrane and induction of changes in its permeability, generation of reactive oxygen species and free radicals, leading to cell toxicity of the microorganisms, and induction of alterations in signal transduction within the pathogen [156,157]. As a first step towards their antimicrobial activity, AgNPs bind to the pathogen’s membrane, mainly driven by their net charge. More specifically, the AgNPs with a more positive net charge have an increased antimicrobial effect due to their higher ability to bind to the pathogen’s membrane, even though a high concentration of AgNPs can also lead to a potent antimicrobial effect through the saturation of the microbial surface [156,158]. After binding to the microorganism’s cell membrane, small AgNPs can penetrate the membrane, while larger AgNPs may remain on the microorganism’s membrane. In both cases, the NPs release Ag+ ions, destabilizing the microorganism’s membrane and causing leakage of the contents of the microbial cell. This membrane destabilization may allow even large AgNPs to enter the pathogen, thus allowing them to act intracellularly as well [159]. After entering the target microorganism, AgNPs, and the Ag+ ions that are released, may interact with various structures of the microorganism, such as proteins, DNA, and lipids, blocking several critical biological functions. They can produce free radicals, reactive oxygen species, and oxidative stress that interact with carboxyl, thiol, and phosphate groups of proteins, thus affecting their activity and blocking microbial growth [156,160]. Finally, AgNPs were shown to have better penetration in bacterial biofilms in a pH-dependent manner. This leads to the persistence of AgNPs in the biofilms for a long time, the disruption of bacterial biofilm formation, and enhanced antibacterial activity [161].
The antimicrobial activity of AgNPs against wild strains of Salmonella was examined in a study by Losasso et al. [159]. AgNPs of spherical and sporadically regular polygonal shape with a median diameter of 6 nm and 18 nm appeared at the electron microscope. The addition of AgNPs effectively reduced counts in a dose-dependent manner, with doses of 200 mg/L being the most effective [159]. In the same study, the authors also described the mechanism of resistance of Salmonella to AgNPs. Expression of the SilB gene, which has been implicated in bacterial resistance to silver and copper, was confirmed and was further identified to be positioned on the plasmidic portion of the bacterial genetic material [159].
Similarly, CuONPs have potent antimicrobial effects by first binding on the microbial cell wall through electrostatic interactions and then by the delivery of Cu2+ ions that lead to an intracellular increase of reactive oxygen species as well as to increased permeability of the microbial wall membrane, leading to the leakage of intracellular material [162]. To this end, the antibacterial activity of CuONPs has been shown against many bacteria, including V. cholera, P. aeruginosa, E. coli, S. aureus, and E. faecalis [156,163,164,165].
More specifically, in a relatively old study, Sabbatini et al. established polymer-based NPs loaded with CuNPs [155]. Copper was embedded in three different polymers, namely, poly-(vinyl chloride) (PVC), polyvinylmethyl ketone (PVMK), and polyvinylidenefluoride (PVDF), leading to the formation of CuNPs of 80–530 nm in size. The in vitro kinetics of copper release were examined, and the CuNPs were shown to have potent antimicrobial activity against Saccharomyces cerevisiae, Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes [155].
In a more recent study, Lv et al. used Shewanella loihica PV-4 as a means for the production of CuNPs that were purified, characterized with electron microscopy, and found to have a size of 6–20 nm [165]. In vitro application of 100 µg/mL of the CuNPs could inhibit 86% of E. coli. The mechanism of action of CuNPs was studied, and the bacterial cells had become amorphous and wizened after a 12 h incubation with the CuNPs. The bacterial membrane had accumulated CuNPs as well as lack of its continuity [165]. Deposits of CuNPs were also seen inside the bacterial cells and could be associated with bacterial damage [165].
AuNPs have antibacterial activity against Gram-negative and Gram-positive bacteria, such as P. aeruginosa, E. coli, K. pneumoniae, S. aureus, and E. faecalis [156]. Even though AuNPs also bind to the bacterial surface based on electrostatic interactions, depending on their net charge, they are thought to have minimal inherent antibacterial activity; thus, their mechanism of action mostly relies on the induction of changes in the bacterial membrane potential. Moreover, they may also reduce the adenosine triphosphatase (ATPase) activity, leading to a reduction in the bacterial ATP, and inhibit protein translation by inhibiting tRNA binding on the ribosomes [156,166]. Gold ion release and reactivity leading to the production of reactive oxygen species are considered to contribute to a much lower extent in the activity of AuNPs [156].
In a study by Nagalingam et al., AuNPs were synthesized using leaf extracts of Alternanthera bettzickiana. Using scanning electron microscopy, the AuNPs were identified as spherical, and they had a size of 80–120 nm. The zeta potential was found to be −41.4. The AuNPs had adequate antimicrobial activity against S. typhi, P. aeruginosa, E. aerogenes, S. aureus, B. subtilis, and M. luteus [167].
In another study by Arockiya Aarthi Rajathi et al., biosynthesis of AuNPs by the brown alga Stoechospermum marginatum was performed [168]. Scanning electron microscopy revealed that polydispersed NPs were formed and had a size that was within the range of 40–85 nm. Transmission electron microscopy revealed that the AuNPs had variable shapes and were mostly spherical, even though triangular and hexagonal NPs were also seen. The AuNPs had adequate antibacterial activity when tested against P. aeruginosa, K. oxytoca, E. faecalis, K. pneumoniae, V. cholerae, E. coli, S. typhi, S. paratyphii, V. parahaemolyticus, and P. vulgaris [168].
ZnONPs exhibit antimicrobial activity that spans Gram-positive and Gram-negative bacteria, such as S. aureus, E. faecium, E. coli, P. aeruginosa, and K.pneumoniae [169]. The antibacterial activity of ZnONPs is attributed to the blockage of potassium ion channels on the microbial membrane that is performed by Zn2+ ions produced after their release in aqueous medium [170]. This leads to the destabilization of the membrane and microbial cell death. Moreover, the production of reactive oxygen species, binding to DNA and proteins, the blockage of DNA amplification, and the alteration of the expression of several genes have also been proposed as mechanisms of action of ZnONPs [170,171,172,173,174,175,176].
In a study by Vijayakumar et al., ZnONPs were synthesized from the methanolic leaf extract of Glycosmis pentaphylla [169]. Scanning electron microscopy revealed that most NPs were spherical in shape and had a diameter of 32–40 nm. The antimicrobial activity of these NPs against several Gram-positive and Gram-negative pathogens and fungi was studied at different concentrations. The maximum zone of inhibition was noted for the concentration of 100 μg/mL for all pathogens that were tested, namely, Shigella dysenteriae, Bacillus cereus, Salmonella paratyphi, Candida albicans, A. niger, Staphylococcus aureus, Salmonella paratyphi, and Bacillus cereus [169].
In another study, Reza Rajabi et al. used microwave-assisted extraction to allow the synthesis of ZnONPs from the aqueous extract of the Suaeda aegyptiaca plant [177]. Electron microscopy studies identified the NPs to be spherical with a size of 60 nm. The antimicrobial activity of ZnONPs was examined against P. aeruginosa, E. coli, S. aureus, and B. subtilis. For all these microorganisms, inhibitory and bactericidal activity was demonstrated, with minimum inhibitory concentrations of less than 0.5 mg/mL for P. aeruginosa, S. aureus, and B. subtilis and 1.56 mg/mL for E. coli [177].
Quantum dots are metallic and contain semiconductor materials as a core, like Zn or Cd. They have antimicrobial activity that is mainly attributed to the induction of membrane damage, the production of reactive oxygen species, damage to the microbial genetic material, and the inhibition of energy production [12,178]. In a relatively recent study, Courtney et al. synthesized 2.4 eV CdTe photoexcited quantum dots from NaHSe and NaHTe with CdCl2 and extensively described their properties [179]. Their size was in the range of 3 nm, and their antimicrobial activity against multidrug-resistant pathogens was evaluated. Quantum dots were able to kill many multidrug-resistant clinical isolates of bacteria, including methicillin-resistant Staphylococcus aureus, Salmonella typhimurium, carbapenem-resistant Escherichia coli, and extended-spectrumβ-lactamase (ESBL)-producing Klebsiella pneumoniae. Their bactericidal activity is independent of the material and appears to be controlled by the redox potentials of the charge carriers that are photogenerated and that selectively alter the cellular redox state [179].
In another study, Hao et al. developed a one-pot method for the preparation of positively charged carbon quantum dots that had a size of about 2.5 nm and showed potent antibacterial activity against many Gram-positive, Gram-negative, and drug-resistant bacteria. In a detailed investigation of the mechanism of their antimicrobial activity, the small-sized quantum dots strongly adhered to the bacterial cell membrane. Additionally, the entry of the quantum dots in the bacterial cells led to conformational changes in the DNA due to quantum dot adsorption, while reactive oxygen species were also produced intracellularly. Antimicrobial resistance against quantum dots was not detected. Moreover, quantum dots had significant antibacterial activity in a mixed infected wound rat animal model with S. aureus and E. coli, while the in vivo toxicity was minimal [180].
Organic NPs, such as polymeric micelles, liposomes, and other NPs, may carry either hydrophilic or hydrophobic antimicrobial agents [181]. Lipid NPs and liposomes are spherical vesicles consisting of phospholipid bilayers. These can fuse with the membranes of the target microorganisms, allowing for the delivery of the antimicrobial substance carried by the NPs in the pathogen [182]. Polymeric NPs, such as nanospheres, may also act as carriers of antimicrobial drugs [12]. For example, cellulose fibers reformed with AgNPs can act as potent antimicrobials against S. aureus and E. coli [183]. In another example, graphene quantum dots and silica nano-fabrications can lead to increased reactive oxygen species production after exposure to light by converting the energy of light to thermal energy, leading to potent bacterial killing [12]. Polymeric micelles include polystyrene, polylactic acid, poly(butyl methacrylate), poly(ethylene oxide), and poly(propylene oxide). These NPs generally have a hydrophobic inner core and a hydrophilic outer core. This allows them to be loaded with hydrophobic or hydrophilic antimicrobial drugs and to deliver them to the target microorganisms [184].
One example of an organic NP is encochleated amphotericin B. More specifically, due to several issues regarding the bioavailability of amphotericin B, encochleated amphotericin B has emerged as a potential solution towards the oral administration of this drug that had been traditionally used intravenously [138,185]. More specifically, amphotericin B is embedded within phosphatidylserine bilayers, thus creating a cochleate structure due to the action of calcium ions. This structure protects the drug from being digested in the gastrointestinal tract and allows for its absorption. Then, the cochleate structure is phagocytosed by human macrophages, and the bilayers pull open due to the difference in the calcium concentration, thus allowing for the intracellular delivery of the drug [138]. An in vivo study in a mouse model of cryptococcal meningoencephalitis showed that the oral encochleated amphotericin B with flucytosine had equal activity to parenteral amphotericin B with flucytosine and was superior to oral fluconazole without excess toxicity [185]. A phase-one study in HIV-positive survivors of cryptococcosis assessed the tolerability of the drug and proved that the oral encochleated amphotericin B was well tolerated when given in up to six divided daily doses without the occurrence of the toxicities that are commonly seen with the intravenous forms of amphotericin B [138]. Recently, a randomized clinical trial of encochleated amphotericin B (oral Lipid Nanocrystal Amphotericin B—MAT2203, Matinas Biopharma) showed promising results for the treatment of patients with cryptococcal meningitis with similar antifungal activity, survival, and less toxicity than the intravenous forms of amphotericin B [186,187].
Liposomal amphotericin B is one of the most widespread intravenous forms of amphotericin B and also consists of lipid-based NPs. With a size of about 80 nm, these NPs consist of liposomes carrying amphotericin B, thus reducing the likelihood of adverse events compared to other formulations [188]. The liposomes preferentially attach to the cell wall of the fungus and release the active amphotericin B molecule that is then transferred to the fungal cell membrane, where it can exert its activity, forming pores and leading to ion leakage and cell death [188]. Its efficacy and safety have been demonstrated in several studies of cryptococcal meningitis, candidiasis, visceral leishmaniasis, and invasive aspergillosis [188,189,190,191].
Nanozymes are NPs that have inherent enzymatic activity. Nanozymes were first reported in 2007, when ferromagnetic–oxide NPs were found to have inherent peroxidase-like activity and to be able to catalyze the peroxidase substrates 3,3,5,5-tetramethylbenzidine (TMB), di-azo-aminobenzene (DAB), and o-phenylenediamine (OPD) [94,192]. Nanozymes have lower cost and better stability compared to natural or other artificial enzymes, while their catalytic activity may be adjustable [193,194]. The most common enzymatic activity by nanozymes that could be used in the fight against infectious diseases is that of peroxidase, even though nanozymes with oxidase, haloperoxidase, or other enzymatic activities have also been implemented [192].
Qiu et al., in a recent study, developed a novel artificial enzyme possessing effective antibacterial activity and the potential for promoting wound healing [195]. They synthesized a hydrogel-based artificial enzyme consisting of copper and amino acids with an intrinsic peroxidase-like catalytic activity with a size of 50–70 nm that could effectively kill microorganisms in wounds and facilitate wound healing by increasing collagen deposition and angiogenesis [195]. Using in vitro studies, they proved that this nanozyme was effective against drug-resistant S. aureus and drug-resistant E. coli. Furthermore, they used local application of the nanozyme in a mouse model of an infectious wound and proved its efficacy towards wound healing [195].
In another example, Fang et al. synthesized a peroxidase mimic from nanodiamonds with a size of 2–10 nm to treat periodontal infections [196]. In vitro experiments revealed that the oxygenated nanodiamonds had potent antibacterial activity against Fusobacterium nucleatum, Porphyromonas gingivalis, and S. sanguis. In vivo experiments in a murine periodontal infection model showed that oral cavity flushing once a day with the combination of nanozyme and hydrogen peroxide could lead to accelerated wound healing around sites of periodontal infection in time [196]. Table 3 shows representative examples of the different NPs that have been used in the treatment of infectious diseases.

Table 3.
Examples of nanoparticles that have been used in the treatment of infectious diseases.
5.5. Nanoparticles and Biofilms
Biofilms are microbial communities formed on surfaces that are surrounded by a polymer matrix consisting of extracellular polymeric substances, such as extracellular DNA, proteins, and exopolysaccharides [197,198]. The bacteria in the biofilms may exist in two different forms, with bacteria alternating from one to the other during the formation and maturation of the biofilm [199]. In the planktonic form, the bacteria are metabolically active and are not attached to the surface of the biofilm, while the sessile bacteria are metabolically relatively inactive and are the ones that are attached to the surface of the biofilms and constitute their main mass [200]. This transition is associated with a great change in their gene expression profile, the reduction of their metabolic activity, and an associated significant increase in their antimicrobial resistance to common antimicrobials [201,202].
Bacterial biofilms could be a target for NPs since some of them appear to have potent activity against them. More specifically, SeNPs could retard the formation of biofilm by P. aeruginosa [203]. Moreover, in vitro tests with antimicrobials and biofilm tube ring formation studies show that ZnO NPs have significant activity against bacteria with significant AMR, such as Staphylococcus and Klebsiella, and they can also inhibit the formation of bacterial biofilm [204]. Additionally, large aggregated AgNPs can penetrate infected areas better, and they may also resemble longer retention in bacterial biofilms, thus leading to an inhibition of biofilm formation and even the effective elimination of the bacterial populations [12]. The aggregated AgNPs may also have a longer retention duration in the tissues, while exocytosis from the cells may be slower compared to that of small non-aggregated particles, allowing for a longer antimicrobial effect in the biofilms [12].
5.6. Combination of Nanoparticles with Antibiotics
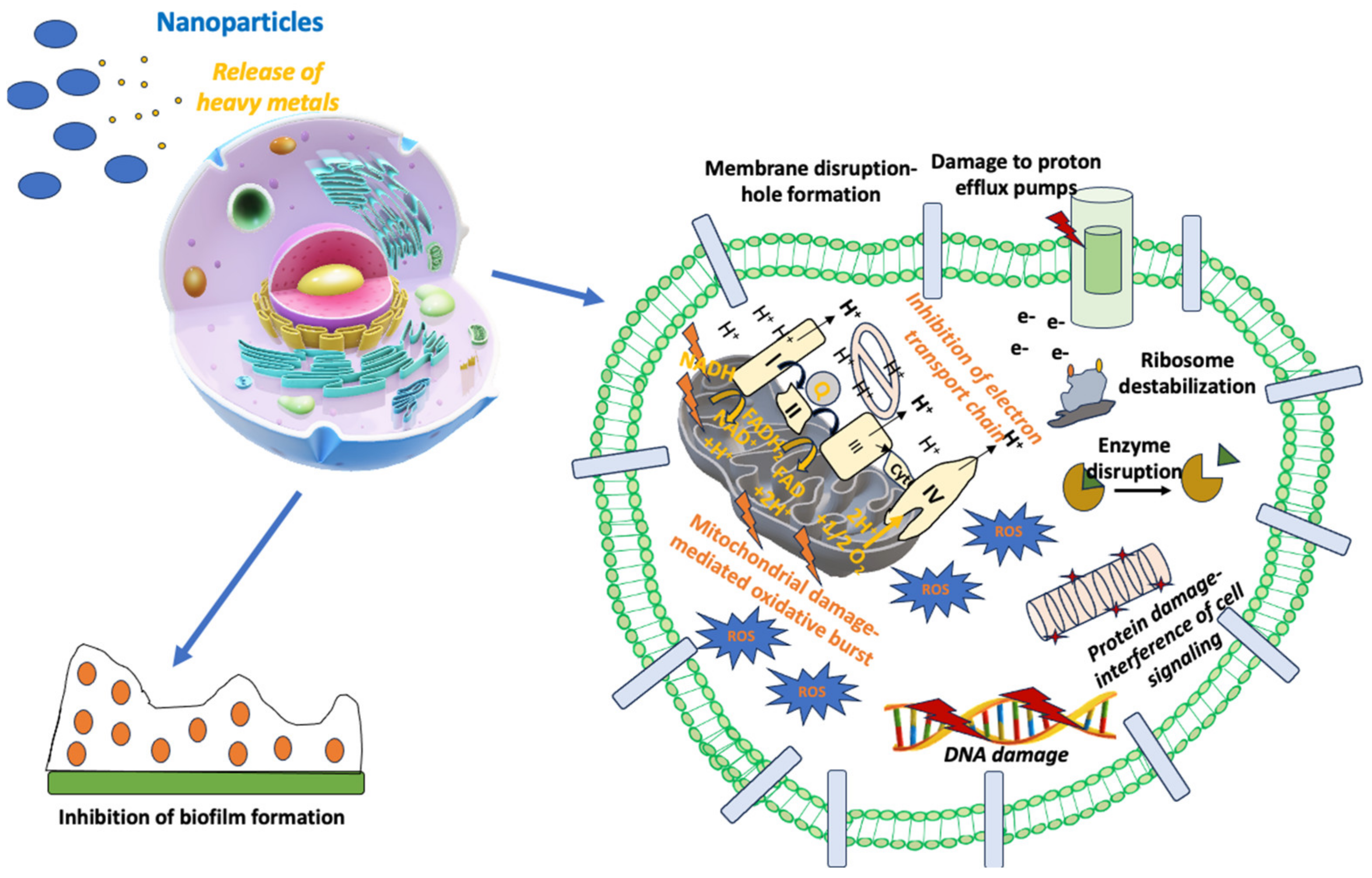
Combining NPs with already approved antibiotics may be a very promising option for the therapy of infectious diseases, especially for pathogens harboring significant AMR [205]. Several research groups have published reports studying different NPs in combination with antibiotics against many different bacteria since this effort can overcome AMR and reduce the toxic adverse effects of systemically administered antibiotics [206]. In this direction, Ag, Au, and ZnONPs conjugated with beta-lactams have shown strong bactericidal activity against E. coli, S. aureus, A. baumannii, E. faecium, and P. aeruginosa by their incorporation into the cell membrane and their interference with signal transduction pathways [206]. As a result, NPs seem to be effective in the fight against resistant Gram-positive and Gram-negative bacterial strains [206]. The different NPs based on Ag, Au, or Zn seem to potentiate the bactericidal effect of several antibiotics, like vancomycin, ceftazidime, clindamycin, ciprofloxacin, polymyxin B, and ampicillin, against bacterial strains with significant AMR [206]. Another mechanism explaining the synergistic effect of NPs with antibiotics is the enhancement of bacterial vulnerability to antibiotics. For example, NPs can allow antibiotics to penetrate into the bacterial cell membrane, thus facilitating its killing through the induction of microbial cell damage [207]. Hence, AgNPs have been combined with antibiotics like vancomycin, erythromycin, streptomycin, and chloramphenicol and have shown strong antimicrobial activity, achieving significant growth inhibition of Gram-positive and Gram-negative bacteria [208]. The bactericidal mechanism of AgNPs with the antibiotics mentioned above was dependent on the type of antibiotic used [208]. The most significant escalation of antibacterial action was shown by the streptomycin-conjugated AgNPs against E. coli [208]. Figure 2 summarizes the mechanisms of action of NPs in the treatment of infectious diseases.
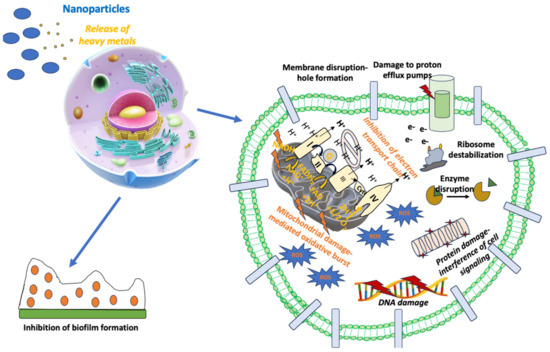
Figure 2.
Mechanism of action of nanoparticles in the treatment of infectious diseases.
5.7. Choosing the Right NP
Choosing the right antimicrobial substance for the treatment of a particular infectious disease, irrespective of whether it is a classic antibiotic, a bacteriophage, an antimicrobial peptide, an NP, or another substance, may be challenging. Several differences exist among the different NPs that were previously described herein. However, the pharmacological properties of the final drug may not be that easy to predict, and the possible adverse events may also differ from those that could be seen in a clinical trial in humans or in real-world data after the approval of the drug. Several such examples exist in cancer, as reviewed in a recent study by Mukherjee et al. [209]. Indeed, several issues regarding the translation of nanomedicines from bench to bedside exist and include issues regarding the production of the NP, its physicochemical characteristics, and issues regarding its pharmacokinetics, pharmacodynamics, and issues regarding long-term safety [210]. Thus, even though quantum dots may be more appropriate in the diagnosis of infectious diseases rather than in treatment, while the predominant NPs used for the treatment of infectious diseases that have been accepted for clinical use until now are lipid-based, the right choice of NP for the treatment of an infectious disease may require preclinical studies in animals and clinical studies in humans. For example, liposomal amphotericin B and encochleated amphotericin B are both lipid-based and seem to be effective in the treatment of fungal diseases [185,186,188,189,190,191]. However, encochleated amphotericin B seems to be stable for oral use and has adequate pharmacokinetics and pharmacodynamics, which is an obvious advantage compared to the other lipid-based forms of amphotericin B. Indeed, for two NPs with similar bioavailability, the one that can be administered orally would be preferable, since this would be associated with a reduced need for healthcare utilization, as well as fewer complications, such as those associated with maintaining an intravenous route of administration [211,212].
In the future, more NPs may be available for the treatment of infectious diseases and from many different NP categories. At that time, more parameters should be considered when choosing the appropriate NP for treatment. Factors such as bioavailability, cost, adverse events, required duration of treatment, and route of administration should be considered when deciding which NPs to use.
6. Nanotechnology in the Prevention of Infectious Diseases
6.1. Application of Nanotechnology in Vaccine Technology
Vaccines have changed the course of human history by significantly reducing the likelihood of specific transmissible diseases and even by eliminating some of them [213,214]. Even though much has been achieved with the vaccine technology that was previously available, further studies are being performed toward the optimization of vaccines by also implementing the latest advances in science [215,216].
NPs are being currently evaluated as a means to increase vaccine efficacy, increase antigen uptake, and produce a stronger immunologic response from the host against specific antigens [217,218]. NPs may have several advantages compared to other novel methods for vaccine development, such as nucleic acid vaccines, viral vector vaccines, and protein and synthetic peptide vaccines, such as the high stability, the low rate of serious adverse events, and the lack of requirement for an adjuvant [216]. They can also be designed to enhance specific cells of the immune system or intracellular compartments [218]. As in the case of their use as antimicrobial agents, their properties, such as their surface charge, hydrophobicity, and size, mainly define their function and, eventually, the efficacy of the immunological response produced by the antigen delivered by the NPs [216]. For example, the mechanism of the cellular uptake of the NPs and the antigen depends on the size of the NPs, which defines if phagocytosis, endocytosis, or micropinocytosis will be performed, thus affecting the immunogenicity [219,220].
The interaction between the NP and the antigen may involve three different mechanisms, namely antigen adsorption on the surface of the NP, covalent binding of the antigen to the NP (conjugation), and finally, antigen encapsulation in the NP. The type of interaction used depends on the nature of the antigen and the NP [216]. The most commonly used types of NPs in vaccine technology involve liposomes, polymeric NPs, emulsions, virus-like particles (VLPs), and immunostimulatory complexes (ISCOMs) [221].
VLPs are multi-protein nanostructures that are self-assembled by using viral structural proteins and closely resemble the formation of naturally occurring viruses. They can be formed in several different protein expression systems, such as in bacteria or mammalian cells. Since they do not have genetic material, they have no infective potential and are, thus, safe for administration in humans [216]. Their small size allows for efficient drainage in the lymph nodes [222,223], while, their stable structure allows for adequate uptake and antigen presentation by the antigen-presenting cells that lead to the activation of adaptive immunity [224].
Liposomes can carry antigens in their hydrophobic lipid bilayer or their hydrophilic core. Furthermore, some antigens can be attached to their outer layer by chemical cross-linking or by adsorption [225,226]. Additionally, liposomes can achieve more potent immunostimulatory properties by adding molecules like the monophosphoryl lipid A or lectin-binding mannose. This may increase delivery to antigen-presenting cells, like dendritic cells [227]. Finally, designing smaller or larger liposomes as methods of antigen delivery may affect the arm of the immunity that is mostly activated. For example, immunization with liposomes larger than 400 nm favored a Th1 immune response, while the use of smaller liposomes favored a Th2 response [228].
ISCOMs are cage particles with a size of about 40 nm that also self-assemble and can be used as delivery NPs for vaccines [216]. They are formed by the combination of a protein antigen, cholesterol, or phospholipids through the formation of hydrophobic interactions. They can entrap hydrophobic antigens and have been shown to effectively produce humoral and cellular immunity [229,230].
6.2. Limitations of Nanotechnology Applications in Vaccine Technology
NPs could increase the delivery of antigens and increase immune stimulation when applied in vaccine technology, as mentioned above. However, NPs can enter human organs and cells since they may easily cross membrane bilayers [231]. The clearance of NPs would depend on their size, but in general, it involves their phagocytosis from mononuclear phagocytes, kidney, and biliary excretion. Metallic NPs may negatively affect innate immunity through cytotoxicity and the interference of cytokine production, leading to the production of free radicals and reactive oxygen species, thus leading to cell death and, potentially, oncogenesis [231,232]. Due to their small size, their high surface-to-mass ratio, and the physicochemical properties of the NPs, they can increase phagocytosis of the antigen, thus increasing the immune response of the host against the antigen; however, they can generate adverse effects, such as necrosis or apoptosis, in other tissues. For example, intranasal immunization with NPs could be associated with the possibility of inducing lung injury through the induction of oxidative stress and the production of inflammatory cytokines and cytotoxic cellular responses [233]. Additionally, NPs can be aggregated, thus blocking the flow in the blood vessels of the host [233]. Finally, the possibility of germline alterations or carcinogenesis has been previously noted in animal models, in which VLPs could disperse widely in the body, thus ending up in the testes and ovaries of the host [234].
7. Challenges in the Use of Nanotechnology in the Treatment of Infectious Diseases
Applications of nanotechnology may have many advantages in the treatment of infectious diseases in the future; however, several shortcomings need to be addressed before these are widely implemented [235]. For example, further studies of the potential effects of NPs on human cells and tissues, issues regarding pharmacokinetics and pharmacodynamics, optimal dosing, potential short-term and long-term adverse events, and the optimal route of administration should be performed to allow for the safe and efficient use of NPs [154,236]. For example, the possibility for human toxicity by NP accumulation in the spleen, bone marrow, and lung exists after intravenous administration [237]. Furthermore, inhaled NPs may lead to accumulation and toxicity in the lung, liver, heart, spleen, liver, and brain [238]. This toxicity may be associated with the induction of oxidative stress and metabolic modifications, such as ketogenesis and beta-oxidation of lipids [238,239,240]. Thus, nanotechnology has led to very few FDA-approved medications until now. Finally, the cost-effectiveness of these therapies needs to be evaluated [235,241].
8. Conclusions
The increasing prevalence of AMR among Gram-positive and Gram-negative bacteria is associated with high morbidity and mortality. This, in combination with the relative reduction of the production of novel antimicrobials by the pharmaceutical industry, underlines the need for the identification of novel ways to fight infectious diseases with other means, beyond antimicrobials. To this end, nanotechnology emerges as a valuable tool both in the diagnosis and the treatment of infectious diseases. The ability of NPs to directly kill bacteria without being affected by AMR may allow for efficient treatment in cases with very few therapeutic options. However, the short-term and long-term safety, as well as the efficacy of NPs in the treatment of infectious diseases, should be confirmed in well-designed, randomized, and controlled trials in the future.
Author Contributions
Conceptualization, G.S. and P.I.; software, P.I. and S.B.; writing—original draft preparation, P.I. and S.B.; writing—review and editing, G.S.; visualization, S.B.; supervision, G.S.; project administration, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Michaud, C.M. Global Burden of Infectious Diseases. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 444–454. ISBN 978-0-12-373944-5. [Google Scholar]
- Armstrong, G.L.; Conn, L.A.; Pinner, R.W. Trends in Infectious Disease Mortality in the United States during the 20th Century. JAMA 1999, 281, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Lewis, M.; Powles, J. The Australian Mortality Decline: Cause-Specific Mortality 1907–1990. Aust. N. Z. J. Public Health 1998, 22, 37–44. [Google Scholar] [CrossRef] [PubMed]
- GBD; 2019 Child and Adolescent Communicable Disease Collaborators. The Unfinished Agenda of Communicable Diseases among Children and Adolescents before the COVID-19 Pandemic, 1990–2019: A Systematic Analysis of the Global Burden of Disease Study 2019. Lancet Lond. Engl. 2023, 402, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet Lond. Engl. 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, W.Y.; Bisht, A.; Gopinath, A.; Cheema, A.H.; Chaludiya, K.; Khalid, M.; Nwosu, M.; Konka, S.; Khan, S. A Systematic Review of Antibiotic Resistance Trends and Treatment Options for Hospital-Acquired Multidrug-Resistant Infections. Cureus 2022, 14, e29956. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 2022, 66, e0199121. [Google Scholar] [CrossRef] [PubMed]
- Ala-Jaakkola, R.; Laitila, A.; Ouwehand, A.C.; Lehtoranta, L. Role of D-Mannose in Urinary Tract Infections—A Narrative Review. Nutr. J. 2022, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Lenger, S.M.; Bradley, M.S.; Thomas, D.A.; Bertolet, M.H.; Lowder, J.L.; Sutcliffe, S. D-Mannose vs Other Agents for Recurrent Urinary Tract Infection Prevention in Adult Women: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2020, 223, 265.e1–265.e13. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Samonis, G. Bacteriophages in Infectious Diseases and Beyond—A Narrative Review. Antibiotics 2023, 12, 1012. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Kofteridis, D.P. Antimicrobial Peptides in Infectious Diseases and Beyond—A Narrative Review. Life Basel Switz. 2023, 13, 1651. [Google Scholar] [CrossRef]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.-T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against Antimicrobial Resistance: Harnessing the Power of Nanoscale Materials and Technologies. J. Nanobiotechnol. 2022, 20, 375. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Wong, N. Nanotechnology and Its Use in Imaging and Drug Delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Gregoriadis, G. Enzyme Entrapment in Liposomes. Methods Enzymol. 1976, 44, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D. Lipid Bilayers and Biomembranes. Annu. Rev. Biochem. 1972, 41, 753–776. [Google Scholar] [CrossRef] [PubMed]
- Ringsdorf, H. Structure and Properties of Pharmacologically Active Polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Cornu, G.; Michaux, J.L.; Sokal, G.; Trouet, A. Daunorubicin-DNA: Further Clinical Trials in Acute Non-Lymphoblastic Leukemia. Eur. J. Cancer 1974, 10, 695–700. [Google Scholar] [CrossRef]
- Kreuter, J.; Speiser, P.P. In Vitro Studies of Poly(Methyl Methacrylate) Adjuvants. J. Pharm. Sci. 1976, 65, 1624–1627. [Google Scholar] [CrossRef]
- Couvreur, P.; Tulkens, P.; Roland, M.; Trouet, A.; Speiser, P. Nanocapsules: A New Type of Lysosomotropic Carrier. FEBS Lett. 1977, 84, 323–326. [Google Scholar] [CrossRef]
- Hurwitz, E.; Levy, R.; Maron, R.; Wilchek, M.; Arnon, R.; Sela, M. The Covalent Binding of Daunomycin and Adriamycin to Antibodies, with Retention of Both Drug and Antibody Activities. Cancer Res. 1975, 35, 1175–1181. [Google Scholar]
- Trouet, A.; Masquelier, M.; Baurain, R.; Deprez-De Campeneere, D. A Covalent Linkage between Daunorubicin and Proteins That Is Stable in Serum and Reversible by Lysosomal Hydrolases, as Required for a Lysosomotropic Drug-Carrier Conjugate: In Vitro and in Vivo Studies. Proc. Natl. Acad. Sci. USA 1982, 79, 626–629. [Google Scholar] [CrossRef]
- Davis, F.F. The Origin of Pegnology. Adv. Drug Deliv. Rev. 2002, 54, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Hugo, W.B. Antimicrobial Activity and Action of Silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [CrossRef]
- Dequeker, J.; Verdickt, W.; Gevers, G.; Vanschoubroek, K. Longterm Experience with Oral Gold in Rheumatoid Arthritis and Psoriatic Arthritis. Clin. Rheumatol. 1984, 3 (Suppl. S1), 67–74. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Introduction to Nanomedicine. Molecules 2015, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Sheng, J. Introduction to Nanomedicine. In Nanomedicine; Gu, N., Ed.; Micro/Nano Technologies; Springer Nature: Singapore, 2023; pp. 3–16. ISBN 9789811689833. [Google Scholar]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of Silver Nanoparticles by Chemical and Biological Methods and Their Antimicrobial Properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Khan, S.; Mansoor, S.; Rafi, Z.; Kumari, B.; Shoaib, A.; Saeed, M.; Alshehri, S.; Ghoneim, M.M.; Rahamathulla, M.; Hani, U.; et al. A Review on Nanotechnology: Properties, Applications, and Mechanistic Insights of Cellular Uptake Mechanisms. J. Mol. Liq. 2022, 348, 118008. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.-Y.; Forrest, M.L.; Berkland, C.J. Nanoparticles for Biomedical Imaging. Expert Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef]
- Pantarotto, D.; Singh, R.; McCarthy, D.; Erhardt, M.; Briand, J.-P.; Prato, M.; Kostarelos, K.; Bianco, A. Functionalized Carbon Nanotubes for Plasmid DNA Gene Delivery. Angew. Chem. Int. Ed Engl. 2004, 43, 5242–5246. [Google Scholar] [CrossRef]
- Shi Kam, N.W.; Jessop, T.C.; Wender, P.A.; Dai, H. Nanotube Molecular Transporters: Internalization of Carbon Nanotube-Protein Conjugates into Mammalian Cells. J. Am. Chem. Soc. 2004, 126, 6850–6851. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.-P.; Prato, M.; Bianco, A. Translocation of Bioactive Peptides across Cell Membranes by Carbon Nanotubes. Chem. Commun. Camb. Engl. 2004, 16–17. [Google Scholar] [CrossRef]
- Dai, H.; Hafner, J.H.; Rinzler, A.G.; Colbert, D.T.; Smalley, R.E. Nanotubes as Nanoprobes in Scanning Probe Microscopy. Nature 1996, 384, 147–150. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon Nanotube Biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon Nanotubes: Properties, Synthesis, Purification, and Medical Applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Carnahan, M.A.; Finkelstein, S.; Prata, C.A.H.; Degoricija, L.; Lee, S.J.; Grinstaff, M.W. Dendritic Supramolecular Assemblies for Drug Delivery. Chem. Commun. Camb. Engl. 2005, 4309–4311. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Tiriveedhi, V.; Kitchens, K.M.; Nevels, K.J.; Ghandehari, H.; Butko, P. Kinetic Analysis of the Interaction between Poly(Amidoamine) Dendrimers and Model Lipid Membranes. Biochim. Biophys. Acta 2011, 1808, 209–218. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-Based Drug Delivery Systems: History, Challenges, and Latest Developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic Nanoparticles: Technology Overview & Drug Delivery Applications in Oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Kishore, M.; Panat, S.; Upadhyay, N.; Agarwal, N.; Aggarwal, A. Nanotechnology: A Boon in Oral Cancer Diagnosis and Therapeutics. SRM J. Res. Dent. Sci. 2013, 4, 154. [Google Scholar] [CrossRef]
- Amjad, S.; Shaukat, S.; Rahman, H.M.A.U.; Usman, M.; Farooqi, Z.H.; Nazar, M.F. Application of Anionic-Nonionic Mixed Micellar System for Solubilization of Methylene Blue Dye. J. Mol. Liq. 2023, 369, 120958. [Google Scholar] [CrossRef]
- Katsuki, S.; Matoba, T.; Koga, J.-I.; Nakano, K.; Egashira, K. Anti-Inflammatory Nanomedicine for Cardiovascular Disease. Front. Cardiovasc. Med. 2017, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xie, J.; Zhang, X.; Sun, W.; Zhao, H.; Li, Y.; Wang, C. An Overview of Polymeric Nanomicelles in Clinical Trials and on the Market. Chin. Chem. Lett. 2021, 32, 243–257. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Reddy, B.P.K.; Yadav, H.K.S.; Nagesha, D.K.; Raizaday, A.; Karim, A. Polymeric Micelles as Novel Carriers for Poorly Soluble Drugs—A Review. J. Nanosci. Nanotechnol. 2015, 15, 4009–4018. [Google Scholar] [CrossRef]
- Mir, M.; Ishtiaq, S.; Rabia, S.; Khatoon, M.; Zeb, A.; Khan, G.M.; Ur Rehman, A.; Ud Din, F. Nanotechnology: From In Vivo Imaging System to Controlled Drug Delivery. Nanoscale Res. Lett. 2017, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Santos-Martinez, M.J.; Radomski, A.; Corrigan, O.I.; Radomski, M.W. Nanoparticles: Pharmacological and Toxicological Significance. Br. J. Pharmacol. 2007, 150, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum Dots as a Platform for Nanoparticle Drug Delivery Vehicle Design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Younis, M.A.; Alsharidah, M.; Al Rugaie, O.; Tawfeek, H.M. Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential. Int. J. Nanomed. 2022, 17, 1951–1970. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Dendrimers and Dendritic Polymers in Drug Delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-Based Drug and Imaging Conjugates: Design Considerations for Nanomedical Applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Kaurav, M.; Ruhi, S.; Al-Goshae, H.A.; Jeppu, A.K.; Ramachandran, D.; Sahu, R.K.; Sarkar, A.K.; Khan, J.; Ashif Ikbal, A.M. Dendrimer: An Update on Recent Developments and Future Opportunities for the Brain Tumors Diagnosis and Treatment. Front. Pharmacol. 2023, 14, 1159131. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody-Drug Conjugates: Targeted Drug Delivery for Cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef]
- Burgio, S.; Noori, L.; Marino Gammazza, A.; Campanella, C.; Logozzi, M.; Fais, S.; Bucchieri, F.; Cappello, F.; Caruso Bavisotto, C. Extracellular Vesicles-Based Drug Delivery Systems: A New Challenge and the Exemplum of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2020, 21, 5432. [Google Scholar] [CrossRef]
- Martincic, M.; Tobias, G. Filled Carbon Nanotubes in Biomedical Imaging and Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-X.; Zhu, B.-J. The Research and Applications of Quantum Dots as Nano-Carriers for Targeted Drug Delivery and Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 207. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; El Feki, A. Hepatoprotective Role and Antioxidant Capacity of Selenium on Arsenic-Induced Liver Injury in Rats. Exp. Toxicol. Pathol. Off. J. Ges. Toxikol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and Their Role in Phytopathogens Management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, B.K.; Yadav, S.M.; Gupta, A.K. Applications of Nanotechnology in Agricultural and Their Role in Disease Management. Res. J. Nanosci. Nanotechnol. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of Nanomaterials in Agricultural Production and Crop Protection: A Review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Pan, W.; Zheng, X.; Chen, G.; Su, L.; Luo, S.; Wang, W.; Ye, S.; Weng, J.; Min, Y. Nanotechnology’s Application in Type 1 Diabetes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1645. [Google Scholar] [CrossRef] [PubMed]
- Bahman, F.; Greish, K.; Taurin, S. Nanotechnology in Insulin Delivery for Management of Diabetes. Pharm. Nanotechnol. 2019, 7, 113–128. [Google Scholar] [CrossRef]
- Wang, Z.; Gonzalez, K.M.; Cordova, L.E.; Lu, J. Nanotechnology-Empowered Therapeutics Targeting Neurodegenerative Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1907. [Google Scholar] [CrossRef] [PubMed]
- Prajnamitra, R.P.; Chen, H.-C.; Lin, C.-J.; Chen, L.-L.; Hsieh, P.C.-H. Nanotechnology Approaches in Tackling Cardiovascular Diseases. Molecules 2019, 24, 2017. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Woodrow, K.A. Nanotechnology Approaches to Eradicating HIV Reservoirs. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgem. Pharm. Verfahrenstech. EV 2019, 138, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-C.; Lin, J.-C.; Tsai, H.-H.; Hsu, C.-Y.; Shih, V.; Hu, C.-M.J. Nanotechnology Advances in Pathogen- and Host-Targeted Antiviral Delivery: Multipronged Therapeutic Intervention for Pandemic Control. Drug Deliv. Transl. Res. 2021, 11, 1420–1437. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, M. Combating Malaria with Nanotechnology-Based Targeted and Combinatorial Drug Delivery Strategies. Drug Deliv. Transl. Res. 2016, 6, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, D.C.D.S.; Cavalcanti, I.D.L.; Medeiros, S.M.d.F.R.D.S.; Souza, J.B.d.; Lira Nogueira, M.C.d.B.; Cavalcanti, I.M.F. Nanotechnology and Tuberculosis: An Old Disease with New Treatment Strategies. Tuberc. Edinb. Scotl. 2022, 135, 102208. [Google Scholar] [CrossRef]
- Herrmann, I.K. How Nanotechnology-Enabled Concepts Could Contribute to the Prevention, Diagnosis and Therapy of Bacterial Infections. Crit. Care Lond. Engl. 2015, 19, 239. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, B.; Lin, N.; Gao, J. Recent Nanotechnology-Based Strategies for Interfering with the Life Cycle of Bacterial Biofilms. Biomater. Sci. 2023, 11, 1648–1664. [Google Scholar] [CrossRef]
- Tobin, E.; Brenner, S. Nanotechnology Fundamentals Applied to Clinical Infectious Diseases and Public Health. Open Forum Infect. Dis. 2021, 8, ofab583. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Kaittanis, C.; Santra, S.; Perez, J.M. Emerging Nanotechnology-Based Strategies for the Identification of Microbial Pathogenesis. Adv. Drug Deliv. Rev. 2010, 62, 408–423. [Google Scholar] [CrossRef]
- Hauck, T.S.; Giri, S.; Gao, Y.; Chan, W.C.W. Nanotechnology Diagnostics for Infectious Diseases Prevalent in Developing Countries. Adv. Drug Deliv. Rev. 2010, 62, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better Tests, Better Care: Improved Diagnostics for Infectious Diseases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57 (Suppl. S3), S139–S170. [Google Scholar] [CrossRef] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral Flow (Immuno)Assay: Its Strengths, Weaknesses, Opportunities and Threats. A Literature Survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, S.; Liu, Y.; Liu, C.; Sun, J. Nanosensors for Diagnosis of Infectious Diseases. ACS Appl. Bio Mater. 2021, 4, 3863–3879. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Wan Abas, W.A.B.; Pingguan-Murphy, B.; et al. An Integrated Paper-Based Sample-to-Answer Biosensor for Nucleic Acid Testing at the Point of Care. Lab Chip 2016, 16, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.-P.; Ma, W.; Long, Y.-T. Alcohol Dehydrogenase-Catalyzed Gold Nanoparticle Seed-Mediated Growth Allows Reliable Detection of Disease Biomarkers with the Naked Eye. Anal. Chem. 2015, 87, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- de la Rica, R.; Stevens, M.M. Plasmonic ELISA for the Ultrasensitive Detection of Disease Biomarkers with the Naked Eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef]
- Qu, W.; Liu, Y.; Liu, D.; Wang, Z.; Jiang, X. Copper-Mediated Amplification Allows Readout of Immunoassays by the Naked Eye. Angew. Chem. Int. Ed. Engl. 2011, 50, 3442–3445. [Google Scholar] [CrossRef]
- Singh, P.; Kakkar, S.; Bharti; Kumar, R.; Bhalla, V. Rapid and Sensitive Colorimetric Detection of Pathogens Based on Silver-Urease Interactions. Chem. Commun. Camb. Engl. 2019, 55, 4765–4768. [Google Scholar] [CrossRef]
- Yen, C.-W.; de Puig, H.; Tam, J.O.; Gómez-Márquez, J.; Bosch, I.; Hamad-Schifferli, K.; Gehrke, L. Multicolored Silver Nanoparticles for Multiplexed Disease Diagnostics: Distinguishing Dengue, Yellow Fever, and Ebola Viruses. Lab Chip 2015, 15, 1638–1641. [Google Scholar] [CrossRef]
- Mancuso, M.; Jiang, L.; Cesarman, E.; Erickson, D. Multiplexed Colorimetric Detection of Kaposi’s Sarcoma Associated Herpesvirus and Bartonella DNA Using Gold and Silver Nanoparticles. Nanoscale 2013, 5, 1678–1686. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, W.; Yang, L.; Tan, Y.; Xie, Q.; Yao, S. Copper-Based Metal-Organic Framework Nanoparticles with Peroxidase-Like Activity for Sensitive Colorimetric Detection of Staphylococcus Aureus. ACS Appl. Mater. Interfaces 2017, 9, 24440–24445. [Google Scholar] [CrossRef]
- Brandão, D.; Liébana, S.; Campoy, S.; Cortés, M.P.; Alegret, S.; Pividori, M.I. Simultaneous Electrochemical Magneto Genosensing of Foodborne Bacteria Based on Triple-Tagging Multiplex Amplification. Biosens. Bioelectron. 2015, 74, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, C.H.; Mayuri, P.; Sankaran, K.; Kumar, A.S. An Electrochemical Immunosensor for Efficient Detection of Uropathogenic E. coli Based on Thionine Dye Immobilized Chitosan/Functionalized-MWCNT Modified Electrode. Biosens. Bioelectron. 2016, 82, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An Ultrasensitive Electrochemical DNA Biosensor Based on Graphene/Au Nanorod/Polythionine for Human Papillomavirus DNA Detection. Biosens. Bioelectron. 2015, 68, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Vallés, C.; Benito, A.M.; Maser, W.K.; Rius, F.X.; Riu, J. Graphene-Based Potentiometric Biosensor for the Immediate Detection of Living Bacteria. Biosens. Bioelectron. 2014, 54, 553–557. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Probing the Interactions of Chitosan Capped CdS Quantum Dots with Pathogenic Bacteria and Their Biosensing Application. J. Mater. Chem. B 2013, 1, 6094–6106. [Google Scholar] [CrossRef]
- Morales-Narváez, E.; Naghdi, T.; Zor, E.; Merkoçi, A. Photoluminescent Lateral-Flow Immunoassay Revealed by Graphene Oxide: Highly Sensitive Paper-Based Pathogen Detection. Anal. Chem. 2015, 87, 8573–8577. [Google Scholar] [CrossRef]
- Zhong, D.; Zhuo, Y.; Feng, Y.; Yang, X. Employing Carbon Dots Modified with Vancomycin for Assaying Gram-Positive Bacteria like Staphylococcus Aureus. Biosens. Bioelectron. 2015, 74, 546–553. [Google Scholar] [CrossRef]
- Lai, I.P.-J.; Harroun, S.G.; Chen, S.-Y.; Unnikrishnan, B.; Li, Y.-J.; Huang, C.-C. Solid-State Synthesis of Self-Functional Carbon Quantum Dots for Detection of Bacteria and Tumor Cells. Sens. Actuators B Chem. 2016, 228, 465–470. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Aizen, R.; Yehezkeli, O.; Willner, I. Graphene Oxide/Nucleic-Acid-Stabilized Silver Nanoclusters: Functional Hybrid Materials for Optical Aptamer Sensing and Multiplexed Analysis of Pathogenic DNAs. J. Am. Chem. Soc. 2013, 135, 11832–11839. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold Nanoparticles Enhanced SERS Aptasensor for the Simultaneous Detection of Salmonella Typhimurium and Staphylococcus Aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, H.; Hasan, D.; Ruoff, R.S.; Wang, A.X.; Fan, D.L. Near-Field Enhanced Plasmonic-Magnetic Bifunctional Nanotubes for Single Cell Bioanalysis. Adv. Funct. Mater. 2013, 23, 4332–4338. [Google Scholar] [CrossRef]
- Blackie, E.J.; Le Ru, E.C.; Etchegoin, P.G. Single-Molecule Surface-Enhanced Raman Spectroscopy of Nonresonant Molecules. J. Am. Chem. Soc. 2009, 131, 14466–14472. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, Z.; Yu, J.; Choo, P.; Chen, L.; Choo, J. A SERS-Based Lateral Flow Assay Biosensor for Highly Sensitive Detection of HIV-1 DNA. Biosens. Bioelectron. 2016, 78, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Choi, N.; Cheng, Z.; Ko, J.; Chen, L.; Choo, J. Simultaneous Detection of Dual Nucleic Acids Using a SERS-Based Lateral Flow Assay Biosensor. Anal. Chem. 2017, 89, 1163–1169. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, Y. Using Commercially Available Personal Glucose Meters for Portable Quantification of DNA. Anal. Chem. 2012, 84, 1975–1980. [Google Scholar] [CrossRef]
- Lin, Q.; Wen, D.; Wu, J.; Liu, L.; Wu, W.; Fang, X.; Kong, J. Microfluidic Immunoassays for Sensitive and Simultaneous Detection of IgG/IgM/Antigen of SARS-CoV-2 within 15 Min. Anal. Chem. 2020, 92, 9454–9458. [Google Scholar] [CrossRef]
- Qin, P.; Park, M.; Alfson, K.J.; Tamhankar, M.; Carrion, R.; Patterson, J.L.; Griffiths, A.; He, Q.; Yildiz, A.; Mathies, R.; et al. Rapid and Fully Microfluidic Ebola Virus Detection with CRISPR-Cas13a. ACS Sens. 2019, 4, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Scherr, T.F.; Gupta, S.; Wright, D.W.; Haselton, F.R. An Embedded Barcode for “Connected” Malaria Rapid Diagnostic Tests. Lab Chip 2017, 17, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; Cortazar, B.; Tseng, D.; Ozkan, H.; Feng, S.; Wei, Q.; Chan, R.Y.-L.; Burbano, J.; Farooqui, Q.; Lewinski, M.; et al. Cellphone-Based Hand-Held Microplate Reader for Point-of-Care Testing of Enzyme-Linked Immunosorbent Assays. ACS Nano 2015, 9, 7857–7866. [Google Scholar] [CrossRef] [PubMed]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Multiplex Paper-Based Colorimetric DNA Sensor Using Pyrrolidinyl Peptide Nucleic Acid-Induced AgNPs Aggregation for Detecting MERS-CoV, MTB, and HPV Oligonucleotides. Anal. Chem. 2017, 89, 5428–5435. [Google Scholar] [CrossRef] [PubMed]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef]
- Jin, B.; Wang, S.; Lin, M.; Jin, Y.; Zhang, S.; Cui, X.; Gong, Y.; Li, A.; Xu, F.; Lu, T.J. Upconversion Nanoparticles Based FRET Aptasensor for Rapid and Ultrasenstive Bacteria Detection. Biosens. Bioelectron. 2017, 90, 525–533. [Google Scholar] [CrossRef]
- Cho, I.-H.; Bhandari, P.; Patel, P.; Irudayaraj, J. Membrane Filter-Assisted Surface Enhanced Raman Spectroscopy for the Rapid Detection of E. coli O157:H7 in Ground Beef. Biosens. Bioelectron. 2015, 64, 171–176. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.; Yu, B.; Liu, Y.; You, T. A Novel Electrochemiluminescence Immunosensor for the Analysis of HIV-1 P24 Antigen Based on P-RGO@Au@Ru-SiO₂ Composite. ACS Appl. Mater. Interfaces 2015, 7, 24438–24445. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Xiao, R.; Tang, L.; Huang, J.; Wu, D.; Liu, S.; Wang, Y.; Zhang, D.; Wang, S.; et al. Sensitive and Specific Detection of Clinical Bacteria via Vancomycin-Modified Fe3O4@Au Nanoparticles and Aptamer-Functionalized SERS Tags. J. Mater. Chem. B 2018, 6, 3751–3761. [Google Scholar] [CrossRef]
- Thwala, L.N.; Ndlovu, S.C.; Mpofu, K.T.; Lugongolo, M.Y.; Mthunzi-Kufa, P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials 2023, 13, 1247. [Google Scholar] [CrossRef]
- Singh, J.A. Artificial Intelligence and Global Health: Opportunities and Challenges. Emerg. Top. Life Sci. 2019, 3, 741–746. [Google Scholar] [CrossRef]
- Ramalingam, M.; Jaisankar, A.; Cheng, L.; Krishnan, S.; Lan, L.; Hassan, A.; Sasmazel, H.T.; Kaji, H.; Deigner, H.-P.; Pedraz, J.L.; et al. Impact of Nanotechnology on Conventional and Artificial Intelligence-Based Biosensing Strategies for the Detection of Viruses. Discov. Nano 2023, 18, 58. [Google Scholar] [CrossRef]
- Martinez, J.L. General Principles of Antibiotic Resistance in Bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-Resistant Gram-Negative Bacteria: A Systematic Review of Current Epidemiology, Prognosis and Treatment Options. J. Antimicrob. Chemother. 2020, 75, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr, J.F. Past, Present, and Future of Antibacterial Economics: Increasing Bacterial Resistance, Limited Antibiotic Pipeline, and Societal Implications. Pharmacotherapy 2017, 37, 71–84. [Google Scholar] [CrossRef]
- Kadouri, D.E.; To, K.; Shanks, R.M.Q.; Doi, Y. Predatory Bacteria: A Potential Ally against Multidrug-Resistant Gram-Negative Pathogens. PLoS ONE 2013, 8, e63397. [Google Scholar] [CrossRef] [PubMed]
- Wiegers, C.; van de Burgwal, L.H.M.; Larsen, O.F.A. Probiotics for the Management of Infectious Diseases: Reviewing the State of the Art. Front. Microbiol. 2022, 13, 877142. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Ricci, E.; Fedele, F.; Chiaffarino, F.; Esposito, G.; Cipriani, S. Systematic Review of the Effect of D-Mannose with or without Other Drugs in the Treatment of Symptoms of Urinary Tract Infections/Cystitis (Review). Biomed. Rep. 2022, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Manohar, A.; Ahuja, J.; Crane, J.K. Immunotherapy for Infectious Diseases: Past, Present, and Future. Immunol. Investig. 2015, 44, 731–737. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The Role of Vaccines in Combatting Antimicrobial Resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, A.; Silletti, M.F.; Faraone, I.; Milella, L. Nanoparticulate Antibiotic Systems as Antibacterial Agents and Antibiotic Delivery Platforms to Fight Infections. J. Nanomater. 2020, 2020, 6905631. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Stalikas, C.D. Qualitative Alterations of Bacterial Metabolome after Exposure to Metal Nanoparticles with Bactericidal Properties: A Comprehensive Workflow Based on (1)H NMR, UHPLC-HRMS, and Metabolic Databases. J. Proteome Res. 2016, 15, 3322–3330. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Liew, K.B.; Janakiraman, A.K.; Sundarapandian, R.; Khalid, S.H.; Razzaq, F.A.; Ming, L.C.; Khan, A.; Kalusalingam, A.; Ng, P.W. A Review and Revisit of Nanoparticles for Antimicrobial Drug Delivery. J. Med. Life 2022, 15, 328–335. [Google Scholar] [CrossRef]
- Skipper, C.P.; Atukunda, M.; Stadelman, A.; Engen, N.W.; Bangdiwala, A.S.; Hullsiek, K.H.; Abassi, M.; Rhein, J.; Nicol, M.R.; Laker, E.; et al. Phase I EnACT Trial of the Safety and Tolerability of a Novel Oral Formulation of Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, e00838-20. [Google Scholar] [CrossRef]
- Aigner, M.; Lass-Flörl, C. Encochleated Amphotericin B: Is the Oral Availability of Amphotericin B Finally Reached? J. Fungi 2020, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D. Surrogate Cells or Trojan Horses. The Discovery of Liposomes. BioEssays News Rev. Mol. Cell. Dev. Biol. 1995, 17, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Ranghar, S.; Sirohi, P.; Verma, P.; Agarwal, V. Nanoparticle-Based Drug Delivery Systems: Promising Approaches against Infections. Braz. Arch. Biol. Technol. 2013, 57, 209–222. [Google Scholar] [CrossRef]
- Kinman, L.; Brodie, S.J.; Tsai, C.C.; Bui, T.; Larsen, K.; Schmidt, A.; Anderson, D.; Morton, W.R.; Hu, S.-L.; Ho, R.J.Y. Lipid-Drug Association Enhanced HIV-1 Protease Inhibitor Indinavir Localization in Lymphoid Tissues and Viral Load Reduction: A Proof of Concept Study in HIV-2287-Infected Macaques. J. Acquir. Immune Defic. Syndr. 2003, 34, 387–397. [Google Scholar] [CrossRef]
- Pereira de Oliveira, M.; Garcion, E.; Venisse, N.; Benoit, J.-P.; Couet, W.; Olivier, J.-C. Tissue Distribution of Indinavir Administered as Solid Lipid Nanocapsule Formulation in Mdr1a (+/+) and Mdr1a (−/−) CF-1 Mice. Pharm. Res. 2005, 22, 1898–1905. [Google Scholar] [CrossRef]
- Jin, S.X.; Bi, D.Z.; Wang, J.; Wang, Y.Z.; Hu, H.G.; Deng, Y.H. Pharmacokinetics and Tissue Distribution of Zidovudine in Rats Following Intravenous Administration of Zidovudine Myristate Loaded Liposomes. Pharmazie 2005, 60, 840–843. [Google Scholar]
- Qasim, M.; Lim, D.-J.; Park, H.; Na, D. Nanotechnology for Diagnosis and Treatment of Infectious Diseases. J. Nanosci. Nanotechnol. 2014, 14, 7374–7387. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of Ketoconazole and PAMAM Dendrimers: Formulation and Antifungal Activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Agashe, H.B.; Garg, M.; Balakrishnan, P.; Kabra, M.; Jain, N.K. Poly (Propyleneimine) Dendrimer Based Nanocontainers for Targeting of Efavirenz to Human Monocytes/Macrophages in Vitro. J. Drug Target. 2007, 15, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Ren, G. Potential Impact of Nanotechnology on the Control of Infectious Diseases. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1–2. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Nino, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, Characterization, and Evaluation of Antimicrobial and Cytotoxic Effect of Silver and Titanium Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 681–688. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Sioss, J.A.; Stoermer, R.L.; Sha, M.Y.; Keating, C.D. Silica-Coated, Au/Ag Striped Nanowires for Bioanalysis. Langmuir ACS J. Surf. Colloids 2007, 23, 11334–11341. [Google Scholar] [CrossRef]
- Brandenberger, C.; Rothen-Rutishauser, B.; Mühlfeld, C.; Schmid, O.; Ferron, G.A.; Maier, K.L.; Gehr, P.; Lenz, A.-G. Effects and Uptake of Gold Nanoparticles Deposited at the Air-Liquid Interface of a Human Epithelial Airway Model. Toxicol. Appl. Pharmacol. 2010, 242, 56–65. [Google Scholar] [CrossRef]
- Mihu, M.R.; Sandkovsky, U.; Han, G.; Friedman, J.M.; Nosanchuk, J.D.; Martinez, L.R. The Use of Nitric Oxide Releasing Nanoparticles as a Treatment against Acinetobacter Baumannii in Wound Infections. Virulence 2010, 1, 62–67. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release Off. J. Control. Release Soc. 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Losasso, C.; Belluco, S.; Cibin, V.; Zavagnin, P.; Mičetić, I.; Gallocchio, F.; Zanella, M.; Bregoli, L.; Biancotto, G.; Ricci, A. Antibacterial Activity of Silver Nanoparticles: Sensitivity of Different Salmonella Serovars. Front. Microbiol. 2014, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Vig Slenters, T.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver Coordination Polymers for Prevention of Implant Infection: Thiol Interaction, Impact on Respiratory Chain Enzymes, and Hydroxyl Radical Induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Pei, X.; Xie, W.; Chen, J.; Li, Y.; Wang, J.; Gao, H.; Wan, Q. pH-Triggered Size-Tunable Silver Nanoparticles: Targeted Aggregation for Effective Bacterial Infection Therapy. Small Weinh. Bergstr. Ger. 2022, 18, e2200915. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-Y.; Hoon Byeon, J.; Park, J.-H.; Hwang, J. Susceptibility Constants of Escherichia Coli and Bacillus Subtilis to Silver and Copper Nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Moniri Javadhesari, S.; Alipour, S.; Mohammadnejad, S.; Akbarpour, M.R. Antibacterial Activity of Ultra-Small Copper Oxide (II) Nanoparticles Synthesized by Mechanochemical Processing against S. Aureus and E. Coli. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110011. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhang, B.; Xing, X.; Zhao, Y.; Cai, R.; Wang, W.; Gu, Q. Biosynthesis of Copper Nanoparticles Using Shewanella Loihica PV-4 with Antibacterial Activity: Novel Approach and Mechanisms Investigation. J. Hazard. Mater. 2018, 347, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kareem, M.M.; Zohri, A.A. Extracellular Mycosynthesis of Gold Nanoparticles Using Trichoderma Hamatum: Optimization, Characterization and Antimicrobial Activity. Lett. Appl. Microbiol. 2018, 67, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, M.; Kalpana, V.N.; Devi Rajeswari, V.; Panneerselvam, A. Biosynthesis, Characterization, and Evaluation of Bioactivities of Leaf Extract-Mediated Biocompatible Gold Nanoparticles from Alternanthera Bettzickiana. Biotechnol. Rep. Amst. Neth. 2018, 19, e00268. [Google Scholar] [CrossRef]
- Arockiya Aarthi Rajathi, F.; Parthiban, C.; Ganesh Kumar, V.; Anantharaman, P. Biosynthesis of Antibacterial Gold Nanoparticles Using Brown Alga, Stoechospermum Marginatum (Kützing). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 166–173. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Krishnakumar, C.; Arulmozhi, P.; Mahadevan, S.; Parameswari, N. Biosynthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from Leaf Extract of Glycosmis Pentaphylla (Retz.) DC. Microb. Pathog. 2018, 116, 44–48. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Deepak Nallaswamy, V. Phyto-Assisted Synthesis of Zinc Oxide Nanoparticles Using Cassia Alata and Its Antibacterial Activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Saha, R.K.; Debanath, M.K.; Paul, B.; Medhi, S.; Saikia, E. Antibacterial and Nonlinear Dynamical Analysis of Flower and Hexagon-Shaped ZnO Microstructures. Sci. Rep. 2020, 10, 2598. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial Activities of Zinc Oxide Nanoparticles against Escherichia Coli O157:H7: Antibacterial ZnO Nanoparticles. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Studies on Antibacterial Activity of ZnO Nanoparticles by ROS Induced Lipid Peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.R.; Naghiha, R.; Kheirizadeh, M.; Sadatfaraji, H.; Mirzaei, A.; Alvand, Z.M. Microwave Assisted Extraction as an Efficient Approach for Biosynthesis of Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biological Properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1109–1118. [Google Scholar] [CrossRef]
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. [Google Scholar] [CrossRef]
- Courtney, C.M.; Goodman, S.M.; McDaniel, J.A.; Madinger, N.E.; Chatterjee, A.; Nagpal, P. Photoexcited Quantum Dots for Killing Multidrug-Resistant Bacteria. Nat. Mater. 2016, 15, 529–534. [Google Scholar] [CrossRef]
- Hao, X.; Huang, L.; Zhao, C.; Chen, S.; Lin, W.; Lin, Y.; Zhang, L.; Sun, A.; Miao, C.; Lin, X.; et al. Antibacterial Activity of Positively Charged Carbon Quantum Dots without Detectable Resistance for Wound Healing with Mixed Bacteria Infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111971. [Google Scholar] [CrossRef]
- Fang, F.; Li, M.; Zhang, J.; Lee, C.-S. Different Strategies for Organic Nanoparticle Preparation in Biomedicine. ACS Mater. Lett. 2020, 2, 531–549. [Google Scholar] [CrossRef]
- Wang, Y. Liposome as a Delivery System for the Treatment of Biofilm-Mediated Infections. J. Appl. Microbiol. 2021, 131, 2626–2639. [Google Scholar] [CrossRef] [PubMed]
- Smiechowicz, E.; Niekraszewicz, B.; Kulpinski, P.; Dzitko, K. Antibacterial Composite Cellulose Fibers Modified with Silver Nanoparticles and Nanosilica. Cellulose 2018, 25, 3499–3517. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H. Polymeric Nanomaterials for Efficient Delivery of Antimicrobial Agents. Pharmaceutics 2021, 13, 2108. [Google Scholar] [CrossRef]
- Lu, R.; Hollingsworth, C.; Qiu, J.; Wang, A.; Hughes, E.; Xin, X.; Konrath, K.M.; Elsegeiny, W.; Park, Y.-D.; Atakulu, L.; et al. Efficacy of Oral Encochleated Amphotericin B in a Mouse Model of Cryptococcal Meningoencephalitis. mBio 2019, 10, e00724-19. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Atukunda, M.; Kagimu, E.; Musubire, A.K.; Akampurira, A.; Tugume, L.; Ssebambulidde, K.; Kasibante, J.; Nsangi, L.; Mugabi, T.; et al. Oral Lipid Nanocrystal Amphotericin B for Cryptococcal Meningitis: A Randomized Clinical Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 77, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Bozbey, I.; Gun, Z.; Karakurt, A. Parenteral Systemic Antifungal Drugs and Their Clinical Drug Informations. Med. Sci. Int. Med. J. 2019, 8, 250–254. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome(®)): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Leenders, A.C.; Daenen, S.; Jansen, R.L.; Hop, W.C.; Lowenberg, B.; Wijermans, P.W.; Cornelissen, J.; Herbrecht, R.; van der Lelie, H.; Hoogsteden, H.C.; et al. Liposomal Amphotericin B Compared with Amphotericin B Deoxycholate in the Treatment of Documented and Suspected Neutropenia-Associated Invasive Fungal Infections. Br. J. Haematol. 1998, 103, 205–212. [Google Scholar] [CrossRef]
- Hamill, R.J.; Sobel, J.D.; El-Sadr, W.; Johnson, P.C.; Graybill, J.R.; Javaly, K.; Barker, D.E. Comparison of 2 Doses of Liposomal Amphotericin B and Conventional Amphotericin B Deoxycholate for Treatment of AIDS-Associated Acute Cryptococcal Meningitis: A Randomized, Double-Blind Clinical Trial of Efficacy and Safety. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 51, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J.; Agarwal, D.; Rai, M.; Murray, H.W. Single-Dose Liposomal Amphotericin B for Visceral Leishmaniasis in India. N. Engl. J. Med. 2010, 362, 504–512. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Q.; Jiang, J.; Gao, L. Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance. Antibiotics 2022, 11, 390. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Pu, F.; Liu, Z.; Liu, X.; Dong, K.; Liu, C.; Ren, J.; Qu, X. Hydrogel-Based Artificial Enzyme for Combating Bacteria and Accelerating Wound Healing. Nano Res. 2020, 13, 496–502. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Bao, X.; Ni, Y.; Teng, Y.; Liu, J.; Sun, X.; Sun, Y.; Li, H.; Zhou, Y. Nanodiamond as Efficient Peroxidase Mimic against Periodontal Bacterial Infection. Carbon 2020, 169, 370–381. [Google Scholar] [CrossRef]
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent Advances in Nanotechnology for Eradicating Bacterial Biofilm. Theranostics 2022, 12, 2383–2405. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Baveye, P.; Neu, T.R.; Stoodley, P.; Szewzyk, U.; Wingender, J.; Wuertz, S. Who Put the Film in Biofilm? The Migration of a Term from Wastewater Engineering to Medicine and Beyond. NPJ Biofilms Microbiomes 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- van Gestel, J.; Vlamakis, H.; Kolter, R. Division of Labor in Biofilms: The Ecology of Cell Differentiation. Microbiol. Spectr. 2015, 3, MB-0002-2014. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should We Stay or Should We Go: Mechanisms and Ecological Consequences for Biofilm Dispersal. Nat. Rev. Microbiol. 2011, 10, 39–50. [Google Scholar] [CrossRef]
- An, D.; Parsek, M.R. The Promise and Peril of Transcriptional Profiling in Biofilm Communities. Curr. Opin. Microbiol. 2007, 10, 292–296. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic Selenium Nanoparticles: Characterization, Antimicrobial Activity and Effects on Human Dendritic Cells and Fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Smitha, M.S.; Singh, S.P. The Role of Nanotechnology in Combating Multi-Drug Resistant Bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Nanomaterials for Alternative Antibacterial Therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as Therapeutic Options for Treating Multidrug-Resistant Bacteria: Research Progress, Challenges, and Prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of Silver Nanoparticles Using Dioscorea Bulbifera Tuber Extract and Evaluation of Its Synergistic Potential in Combination with Antimicrobial Agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar] [CrossRef]
- Mukherjee, S.; Honkala, A.; Rolnik, B. Review of the Clinical Trial Failures of Nanomedicines Targeting Cancer. J. Stud. Res. 2022, 11. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Chandrasekhar, D.; PokkaVayalil, V. Cost Minimization Analysis on IV to Oral Conversion of Antimicrobial Agent by the Clinical Pharmacist Intervention. Clin. Epidemiol. Glob. Health 2019, 7, 60–65. [Google Scholar] [CrossRef]
- McCarthy, K.; Avent, M. Oral or Intravenous Antibiotics? Aust. Prescr. 2020, 43, 45–48. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines: Past, Present and Future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B. The Contribution of Vaccination to Global Health: Past, Present and Future. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.R.; Fauci, A.S. Novel Vaccine Technologies for the 21st Century. Nat. Rev. Immunol. 2020, 20, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Matić, Z.; Šantak, M. Current View on Novel Vaccine Technologies to Combat Human Infectious Diseases. Appl. Microbiol. Biotechnol. 2022, 106, 25–56. [Google Scholar] [CrossRef] [PubMed]
- Lugade, A.A.; Bharali, D.J.; Pradhan, V.; Elkin, G.; Mousa, S.A.; Thanavala, Y. Single Low-Dose Un-Adjuvanted HBsAg Nanoparticle Vaccine Elicits Robust, Durable Immunity. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 923–934. [Google Scholar] [CrossRef]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front. Vet. Sci. 2021, 8, 654289. [Google Scholar] [CrossRef]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines against Tumors. J. Immunol. Baltim. Md 1950 2004, 173, 3148–3154. [Google Scholar] [CrossRef]
- Mottram, P.L.; Leong, D.; Crimeen-Irwin, B.; Gloster, S.; Xiang, S.D.; Meanger, J.; Ghildyal, R.; Vardaxis, N.; Plebanski, M. Type 1 and 2 Immunity Following Vaccination Is Influenced by Nanoparticle Size: Formulation of a Model Vaccine for Respiratory Syncytial Virus. Mol. Pharm. 2007, 4, 73–84. [Google Scholar] [CrossRef]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Gomes, A.C.; Cabral-Miranda, G.; Krueger, C.C.; Leoratti, F.M.; Stein, J.V.; Bachmann, M.F. Delivering Adjuvants and Antigens in Separate Nanoparticles Eliminates the Need of Physical Linkage for Effective Vaccination. J. Control. Release Off. J. Control. Release Soc. 2017, 251, 92–100. [Google Scholar] [CrossRef]
- Gomes, A.C.; Mohsen, M.; Bachmann, M.F. Harnessing Nanoparticles for Immunomodulation and Vaccines. Vaccines 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.S.; Endsley, A.N.; Huang, L. Design Considerations for Liposomal Vaccines: Influence of Formulation Parameters on Antibody and Cell-Mediated Immune Responses to Liposome Associated Antigens. Vaccine 2012, 30, 2256–2272. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Zhang, M.; Chen, R.; Niu, R.; Deng, Y. Mannose Derivative and Lipid A Dually Decorated Cationic Liposomes as an Effective Cold Chain Free Oral Mucosal Vaccine Adjuvant-Delivery System. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgem. Pharm. Verfahrenstech. EV 2014, 88, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Badiee, A.; Khamesipour, A.; Samiei, A.; Soroush, D.; Shargh, V.H.; Kheiri, M.T.; Barkhordari, F.; Robert Mc Master, W.; Mahboudi, F.; Jaafari, M.R. The Role of Liposome Size on the Type of Immune Response Induced in BALB/c Mice against Leishmaniasis: Rgp63 as a Model Antigen. Exp. Parasitol. 2012, 132, 403–409. [Google Scholar] [CrossRef]
- Alexyuk, P.G.; Bogoyavlenskiy, A.P.; Alexyuk, M.S.; Turmagambetova, A.S.; Zaitseva, I.A.; Omirtaeva, E.S.; Berezin, V.E. Adjuvant Activity of Multimolecular Complexes Based on Glycyrrhiza Glabra Saponins, Lipids, and Influenza Virus Glycoproteins. Arch. Virol. 2019, 164, 1793–1803. [Google Scholar] [CrossRef]
- Morein, B.; Hu, K.-F.; Abusugra, I. Current Status and Potential Application of ISCOMs in Veterinary Medicine. Adv. Drug Deliv. Rev. 2004, 56, 1367–1382. [Google Scholar] [CrossRef]
- Pourmand, A.; Abdollahi, M. Current Opinion on Nanotoxicology. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2012, 20, 95. [Google Scholar] [CrossRef]
- Petrarca, C.; Clemente, E.; Amato, V.; Pedata, P.; Sabbioni, E.; Bernardini, G.; Iavicoli, I.; Cortese, S.; Niu, Q.; Otsuki, T.; et al. Engineered Metal Based Nanoparticles and Innate Immunity. Clin. Mol. Allergy CMA 2015, 13, 13. [Google Scholar] [CrossRef]
- Diaz-Arévalo, D.; Zeng, M. Nanoparticle-Based Vaccines. In Nanopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–150. ISBN 978-0-12-817778-5. [Google Scholar]
- Boyce, N. Trial Halted after Gene Shows up in Semen. Nature 2001, 414, 677. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; A Ahmed, E.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Sandhiya, S.; Dkhar, S.A.; Surendiran, A. Emerging Trends of Nanomedicine--an Overview. Fundam. Clin. Pharmacol. 2009, 23, 263–269. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Al-Daihan, S. On the Toxicity of Therapeutically Used Nanoparticles: An Overview. J. Toxicol. 2009, 2009, 754810. [Google Scholar] [CrossRef] [PubMed]
- Hagens, W.I.; Oomen, A.G.; de Jong, W.H.; Cassee, F.R.; Sips, A.J.A.M. What Do We (Need to) Know about the Kinetic Properties of Nanoparticles in the Body? Regul. Toxicol. Pharmacol. RTP 2007, 49, 217–229. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug Delivery and Nanoparticles:Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).