Abstract
The rapid emergence of antimicrobial resistance is a global concern, and high levels of resistance have been detected in chicken populations worldwide. The purpose of this study was to determine the prevalence of antimicrobial resistance in Escherichia coli and Salmonella spp. isolated from healthy chickens in Timor-Leste. Through a cross-sectional study, cloacal swabs and boot swabs were collected from 25 live bird markets and two layer farms respectively. E. coli and Salmonella spp. from these samples were tested for susceptibility to six antimicrobials using a disk diffusion test, and a subset was tested for susceptibility to 27 antimicrobials using broth-based microdilution. E. coli and Salmonella spp. isolates showed the highest resistance towards either tetracycline or ampicillin on the disk diffusion test. E. coli from layer farms (odds ratio:5.2; 95%CI 2.0–13.1) and broilers (odds ratio:18.1; 95%CI 5.3–61.2) were more likely to be multi-drug resistant than those from local chickens. Based on the broth-based microdilution test, resistance to antimicrobials in the Timor-Leste Antimicrobial Guidelines for humans were low, except for resistance to ciprofloxacin in Salmonella spp. (47.1%). Colistin resistance in E. coli was 6.6%. Although this study shows that antimicrobial resistance in chickens was generally low in Timor-Leste, there should be ongoing monitoring in commercial chickens as industry growth might be accompanied with increased antimicrobial use.
1. Introduction
Antimicrobial resistance (AMR) is a major global challenge, and its rapid emergence is attributed to the inappropriate and excessive use of antimicrobials in humans and animals [1]. Antimicrobials have been used in food-producing animals for improving weight gain, improving feed efficiency and managing diseases [1], with the projected usage expected to rise over the next decade [2]. The impacts of AMR include higher treatment costs, higher treatment failure and reduced animal production [3]. It is estimated that AMR will result in approximately 10 million human deaths per year and a global economic loss around USD 2 trillion per year by 2050 [4], with impacts being more pronounced in low- and middle-income countries due to their weaker public health systems [5].
Timor-Leste is a developing country located in Southeast Asia with a total population in 2022 of 1,340,434 [6]. Animal production is predominantly small-scale [7] and is an integral part of livelihood for most of the rural population [8,9]. Chickens are the most abundant food-producing animals in the country [8], and eggs are one of the most important sources of animal protein [10,11]. Chickens are also important for cultural purposes [12], and most households in Timor-Leste own chickens [8,10]. The chicken population in Timor-Leste can be divided into four types with different husbandry and management practices that might influence antimicrobial usage and hence AMR prevalence—local chickens, fighting cocks, broilers and layers [12]. Local chickens are mostly native chickens that are often raised in backyard settings by smallholder households for egg and meat production [10,13]. Fighting cocks are native roosters that are used for cock fighting. They can have a high market value, receive medical treatment for sustained injuries, and could ultimately be consumed as food [7,12]. Layer and broiler chickens are farmed commercially from imported day-old chicks [12,14], with production increasing in recent years in line with trends in other developing countries [12,15]. There are two large commercial layer farms in the country, but the exact number of broiler farms in the country is uncertain. All four chicken types can be found in live bird markets (LBMs) with varying availability. In LBMs close to the border with Indonesia, live broilers farmed in Indonesia can also be found [12].
All antibiotics in Timor-Leste are imported, and the most commonly imported classes of veterinary antibiotics are tetracyclines, penicillins, aminoglycosides and sulfonamides [14]. The use of antibiotics in animals in Timor-Leste is reportedly low compared to other countries due to the predominately subsistence production system [14]. The largest importer of veterinary antibiotics is the government [14], and these antibiotics are used by government animal health workers who provide free animal health services to smallholder farmers [16,17]. The second largest importers of veterinary antibiotics are commercial layer and broiler farms, where almost all imported antibiotics are meant for oral administration [14].
In the context of chickens, AMR in gastrointestinal bacteria such as E. coli and Salmonella spp. is relevant for both animal health and public health [18,19,20]. E. coli can cause gastrointestinal and extraintestinal infections such as urinary tract infections and blood-stream infections in humans [21]; and avian pathogenic E. coli can cause colibacillosis in chickens [19]. Salmonella spp. can cause gastrointestinal disease in chickens and humans, as well as significant production loss in chickens due to pullorum disease and fowl typhoid [19,22]. Several studies in other countries have shown a high prevalence of AMR in bacteria isolated from chickens, where resistance to tetracycline, penicillin and sulfonamide were often above 80% from bacteria isolated from commercial farms [23,24,25,26]. Even in free-range chickens from rural areas of Bangladesh without recorded antibiotic usage, extended-spectrum β-Lactamase (ESBL) producing E. coli was detected in almost a quarter of the tested faecal samples [27].
In Timor-Leste, there have been some studies on AMR in humans that have shown relatively high rates of carriage of resistant bacteria in humans exhibiting resistance to critically important antimicrobials such as ceftriaxone, ciprofloxacin and gentamicin [28,29]. However, there are no published studies investigating the role of animals in the transmission of antimicrobial resistant bacteria and its potential public health implications. This study focuses on chickens as a potential source of AMR, especially with the expansion of chicken production in the country, which could be accompanied with increase antimicrobial use [30]. This study has the primary objective of estimating the prevalence of AMR in E. coli isolated from cloacal swabs from healthy chickens in LBMs and boot swabs from layer farms in Timor-Leste, and a secondary objective of estimating the prevalence of AMR in Salmonella spp., isolated from the same samples. The study will also provide a baseline for monitoring AMR trends in the future.
2. Materials and Methods
2.1. Study Area and Population
A cross-sectional study was conducted in LBMs and layer farms in all 13 municipalities of Timor-Leste from August 2021 to February 2023.
2.2. Data Collection from Live Bird Markets
The target population for sampling was chickens sold in LBMs. The definition of LBMs is an outdoor space with two or more chicken vendors or traders where live chickens are sold at least once a week [31]. LBMs are typically located in the capital city of municipalities or town centres of sub-municipalities in Timor-Leste. These LBMs typically function as a collection point for chickens produced in nearby areas. Local chickens and fighting cocks are the main types of chickens found at LBMs, although broilers and spent hens can be found occasionally after the end of their production cycles. All chickens are sold live by vendors, and no slaughter occurs at these locations. Customers at these markets include households and food establishments.
In this study, a minimum sample size of 267 E. coli isolates was determined using the Epitools sample size calculator for estimating a proportion using the following parameters assuming there was no clustering: estimated antimicrobial resistance prevalence of 50%, 6% desired precision and 95% confidence level. The minimum sample size for cloacal swabs from chickens from LBMs was adjusted upwards to 334, based on an estimated 80% laboratory recovery rate for E. coli. The initial plan was to allocate samples to municipalities proportionate to the size of the human population. However, the availability of teams to visit municipalities was influenced by research and training activities, resulting in over/under representation of samples from some municipalities. Due to the lack of a central registry of LBMs in the country, a sampling frame was developed in collaboration with the Timor-Leste Ministry of Agriculture, Livestock, Fisheries and Forestry (MALFF) animal health workers working in each municipality. Details of LBM location, size, operating days and hours were collected. The sampling team visited every municipality for a period of up to 3 days, during which they visited the largest LBM that was operational and approximately within 1 hour driving time from the capital city of that municipality. If time permitted, the next largest LBMs that fulfilled the same criteria from each municipality were also visited. This process continued until the target sample size was achieved, with multiple visits to some municipalities.
Visits to most LBMs were with a local government animal health worker who facilitated the initial communication with chicken vendors. Within each LBM, all vendors selling chickens were approached, but only one healthy chicken from each type of chicken (local chicken, fighting cock, broiler and layer) was selected per chicken vendor to reduce the effect of clustering assuming that chickens from the same vendor could have originated from the same farm. The selection of chickens from each vendor was random. When a vendor’s chickens were in an inaccessible cage area, random sampling was only applied to the subgroup of chickens in accessible cage areas. A cloacal sample using an Amies swab (Copan, Italy) was taken from each selected bird, and each swab was labelled. Information on each chicken from which the cloacal sample originated was collected using the REDCap mobile app on Android tablets [32,33]. The sample number, name of chicken vendor, name of the LBM, type of chicken selected (i.e., local chicken, layer, broiler or fighting cock) were recorded.
2.3. Data Collection from Layer Farms
There are two large commercial layer farms in Timor-Leste [14]. Only layer houses that contained adult birds (above 20 weeks of age) within these two farms were targeted for sampling. A minimum sample size of 97 E. coli isolates was determined using the Epitools sample size calculator for estimating a proportion using the following parameters assuming there was no clustering: estimated antimicrobial resistance prevalence of 50%, 10% desired precision and 95% confidence level. Sampling was conducted proportionate to size, according to the total chicken population on each layer farm. This resulted in a minimum of 59 samples and 38 samples collected from the larger and the smaller farms, respectively.
Prior to sampling, the number of houses with adult layer birds was determined with the farm manager from each farm. Up to four environmental boot swabs were collected from each house, with the number of boot swabs collected from a house reflecting the size of the chicken population. Commercial boot swabs premoistened with skim milk (Romer Labs, Getzersdorf, Austria) were used for sample collection. Sample collection was performed by walking along the outer faecal lanes underneath each layer house, as the middle faecal lanes were often difficult to access. For houses with cages that were not raised sufficiently high enough for an operator to walk underneath, the boot swab was attached securely to a stick to collect faecal material from at least 10 different locations along the entire length of the faecal lane. Each pair of boot swabs was placed into a sterile plastic bag and labelled. Information on each house from which the boot swab originated from was collected using the REDCap mobile app on Android tablets [32,33]. The house number, source of birds, age of birds, vaccination status and history of antimicrobial use were collected.
2.4. Isolation and Identification of Bacteria
All samples were stored and transported in a cool box with ice packs to the Veterinary Diagnostic Laboratory (VDL) in Dili, the capital of Timor-Leste. The VDL is the only animal health laboratory in the country and is a government laboratory under the MALFF. When samples were not delivered within 24 h of collection, they were stored in refrigerated conditions at the local government office or accommodation. All cloacal swabs and boot swab samples were processed within 7 days upon arrival at VDL and screened for E. coli and Salmonella spp. Antimicrobial susceptibility testing (AST) was performed on the isolates followed by storage in a −80 °C biorepository.
For isolation of E. coli and Salmonella spp., Amies cloacal swabs and boot swabs were pre-enriched in 10 mL and 200 mL of buffered peptone water (BPW), respectively, and incubated at 37 °C for 18–24 h. After incubation, a loopful (10 µL) of overnight BPW culture was streaked onto selective Brilliance E. coli agar (Oxoid, Thermo Fisher, Waltham, MA, USA) and incubated at 37 °C for 18–24 h to isolate E. coli. At the same time, 1 mL of overnight BPW culture was added to 10 mL of Rappaport–Vassiliadis soy broth (RVS broth) (Oxoid, Thermo Fisher) for 18–24 h at 41 °C for enrichment of Salmonella spp. After enrichment, 10 µL of RVS broth was streaked onto Xylose Lysin Deoxycholate agar and incubated at 37 °C for 24 h (Becton Dickinson, East Rutherford, NJ, USA). One suspected E. coli and Salmonella spp. isolate from each sample were propagated on different nutrient agar plates for biochemical identification using Microbact 12A (Oxoid, Thermo Fisher). All isolates identified as Salmonella spp. on Microbact 12A were confirmed using a salmonella latex agglutination test following the manufacturer’s instructions (Oxoid, Thermo Fisher).
2.5. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed using the disk diffusion test (DDT) on all isolated E. coli and Salmonella spp. according to the Clinical and Laboratory Standard Institute (CLSI) testing methodology for DDT [34].
For each isolate, a standardised inoculum with turbidity of 0.5 McFarland was streaked onto Mueller–Hinton agar (Becton Dickinson, USA). The six antimicrobial disks used were ampicillin (AMP; 10 µg), streptomycin (STR; 10 µg), tetracycline (TET; 30 µg), trimethoprim/sulfamethoxazole (SXT; 1.25/23.75 µg), sulfisoxazole (SOX; 250 µg) and enrofloxacin (ENR; 5 µg). These six antimicrobial disks were selected based on commonly used antimicrobials in animals in Timor-Leste [14] and cover five classes of antimicrobials—namely penicillins, aminoglycosides, tetracyclines, fluoroquinolones and sulfonamides. Trimethoprim/sulfamethoxazole and sulfixosazole were considered to be within the same sulfonamide antimicrobial class. The DDT petri dishes were incubated for 18 to 24 h at 37 °C. The zone of inhibition in millimetres was measured under a magnification glass and recorded on the WHONET 5.6 software. The observed inhibition zones were interpreted according to CLSI interpretative criteria for Enterobacterales [34]. Internal quality control for the DDT was performed using E. coli ATCC 25922. External quality assurance was conducted through a program called EQAsia, which is supported by the Fleming Fund.
To understand the resistance profile of isolates to antimicrobials relevant for human health, a subset of isolates was transferred from the VDL to the National Health Laboratory (NHL) in Timor-Leste for AST using a broth-based microdilution method. The NHL is a government human health laboratory that provides reference and clinical microbiological services [35]. These isolates were tested with a panel of 27 antimicrobials on the NMIC-502 (Becton Dickinson, East Rutherford, NJ, USA) using the BD Phoenix M50 instrument at the NHL. This also consisted of the Phoenix ESBL test (BD Diagnostic Systems, Sparks, Md) and Phoenix CPO detect test (BD Diagnostic Systems, Sparks, Md). The Phoenix ESBL test determines whether an isolated is an ESBL based on phenotypic response to a panel of cephalosporins, which are alone or in combination with clavulanic acid, while the Phoenix CPO detect test identifies carbapenemase-producing bacteria of classes A, B and D. The raw minimum inhibitory concentration (MIC) data were entered into the WHONET software. The results were interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints version 13.0 [36], except for nitrofurantoin breakpoints in Salmonella spp., which were interpreted according to CLSI M100 interpretative criteria for Enterobacterales [37] because none were available in EUCAST.
2.6. Data Analysis
All results were validated and checked for missing entries and entry errors. The bacterial recovery rate was determined by the number of samples from which each target bacteria was isolated divided by the total number of tested samples. Descriptive statistics were used to calculate the proportion of resistance to different antimicrobials amongst the E. coli and Salmonella spp. isolates tested with the DDT and broth-based microdilution method. Data were presented based on the origin of the isolates, namely LBM or layer farm. For the DDT results, the percentage of resistance was estimated with 95% confidence intervals (CI) adjusted for clustering by municipality and house, respectively. Multi-drug resistance (MDR) was determined based on the DDT and broth-based microdilution results. An isolate was classified as MDR if it was resistant to three or more antimicrobial classes. The phenotypic resistance profiles of E. coli and Salmonella spp. isolates were described.
The calculation of MIC50 and MIC90, which is the MIC value able to inhibit ≥ 50% and ≥90% of isolates, respectively [38], was performed on the E. coli isolates that underwent broth-based microdilution. Isolates that were resistant to ceftriaxone (MIC > 1 mg/L) and ceftazidime (MIC > 1 mg/L) were considered as potential ESBL producers [39]. Isolates that had meropenem MIC > 0.125 mg/L were considered as potential carbapenemase producers [39].
Mixed-effect logistic regression models were used to assess the crude associations between the source of E. coli isolates (i.e., local chicken, fighting cock, broiler chicken and layer farm) and resistance to each antimicrobial using both the DDT and broth-based microdilution results. The outcome variable was resistant status versus susceptible and intermediate. The source of the isolate (i.e., municipality or layer farm) was fitted as the random effect to account for clustering. Data analyses were performed using Stata 17.0 [40].
3. Results
3.1. Origin of Samples and Recovery of Isolates
In total, 345 cloacal swab samples were collected from 25 LBMs located in 13 municipalities, comprising samples from 254 local chickens, 72 fighting cocks, 17 broiler chickens and 2 layer chickens. During the sampling period, broiler and layer chickens were only found in LBMs located in Bobonaro or Dili municipalities. For LBM sampling, allocation to municipalities was generally proportionate to size. However, Bobonaro municipality was over-represented, and Dili municipality was under-represented. The number of cloacal swab samples collected from each municipality is shown in Figure 1. A detailed breakdown of the number of LBMs, number of cloacal swabs collected, and type of chicken from which cloacal swabs samples originated can be found in Supplementary Table S1. A total of 87 boot swab samples were collected from two large commercial layer farms, with 57 samples from the larger farm and 30 samples from the smaller farm. At the time of sampling, the estimated total number of birds reported by the larger and smaller farms were 113,836 and 80,000, respectively. Both layer farms vaccinated chickens against Newcastle disease. Only one farm reported using antibiotics, specifically an oxytetracycline injectable antibiotic used only in sick birds.
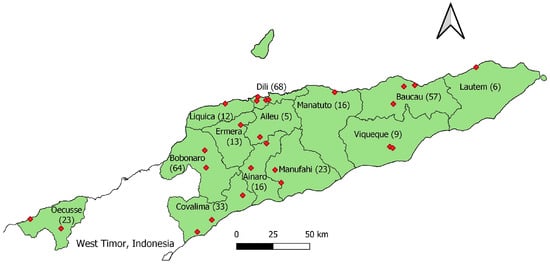
Figure 1.
Map of Timor-Leste showing the 13 municipalities from which cloacal swab samples were collected. Numbers in brackets after each municipality name indicate the number of cloacal swab samples collected in each location. The red diamonds represent individual live bird markets where samples were collected.
For LBMs, the recovery rates for E. coli and Salmonella spp. from 345 chicken cloacal swabs were 85.5% (295 isolates) and 2.3% (8 isolates), respectively. For layer farms, the recovery rate of E. coli and Salmonella spp. from 87 boot swabs were 85.1% (74 isolates) and 33.3% (29 isolates), respectively. The number of isolates of E. coli and Salmonella spp. from LBMs by type of chicken and layer farms along with the recovery rate can be found in Table 1.

Table 1.
Number of isolates and recovery rates of E. coli and Salmonella spp. collected from different chicken types in live bird markets and layer farms in Timor-Leste from August 2021 to February 2023.
3.2. Disk Diffusion Results
The prevalence of antimicrobial resistance in E. coli and Salmonella spp. isolates with 95% confidence intervals based on the location from which the samples were collected (LBMs and layer farms) can be found in Table 2. The E. coli isolates from chickens in LBMs showed the highest resistance levels towards ampicillin and tetracycline. The E. coli isolates from layer farms showed the highest resistance levels towards tetracycline and sulfisoxazole. The E. coli isolates from both LBMs and layer farms had the lowest resistance to enrofloxacin compared to other antimicrobials. Multi-drug resistance was present in 7.8% (95%CI: 5.3–11.4) of E. coli isolates from LBMs and 20.3% (95%CI: 9.7–37.5) of E. coli isolates from layer farms. The prevalence of AMR in E. coli based on origin is shown in Figure 2 and Supplementary Table S2. For each tested antimicrobial, the prevalence of resistance in E. coli isolates generally increased across local chickens, fighting cocks, layer farms and broilers. The Salmonella spp. isolates originating from both LBMs and layer farms showed the highest resistance to tetracycline. A total of 7 out of 37 Salmonella spp. isolates (18.9%) were identified as MDR.

Table 2.
Prevalence of AMR for E. coli and Salmonella spp. isolates from disk diffusion results based on location of collection in live bird markets (LBMs) or layer farms (LFs).
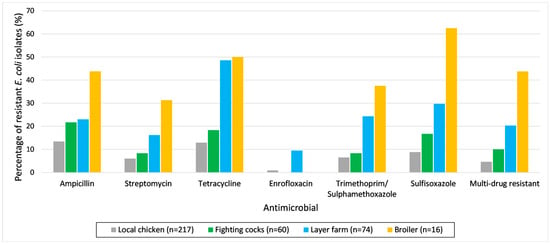
Figure 2.
Percentage of E. coli isolates resistant to different antimicrobials from disk diffusion and multi-drug resistance based on the origin from which they were collected. Local chicken, fighting cock and broiler chicken samples were collected from live bird markets (LBMs). The percentage of resistance of E. coli isolates originating from layer chickens from LBMs is not shown because there were only two isolates.
The phenotypic resistance profile of all E. coli and Salmonella spp. isolates are shown in Table 3. The proportion of E. coli and Salmonella spp. Isolates that were not resistant to any of the tested antimicrobials on the DDT were 72.2% (213/295 isolates) and 25.0% (2/8 isolates), respectively. None of the E. coli isolates were resistant to all six antimicrobials, 3.3% (12/369 isolates) were resistant to five antimicrobials, 3.8% (14/369 isolates) to four antimicrobials, and 6.8% (25/369 isolates) to three antimicrobials. The most common phenotypes with resistance to at least one antimicrobial was TET (17/369 isolates) and AMP-TET (17/369 isolates). The predominant phenotypic resistance profiles of E. coli isolates resistant to three or more antimicrobials were TET-SXT-SOX (10/369 isolates), AMP-STR-TET-SXT-SOX (8/369 isolates) and AMP-TET-SXT-SOX (6/369 isolates). For Salmonella spp. isolates, 5.4% (2/37 isolates) were resistant to all tested antimicrobials, 5.4% (2/37 isolates) were resistant to five antimicrobials, and 8.1% (3/37 isolates) were resistant to four antimicrobials. The predominant phenotypic resistance profile of Salmonella spp. isolates resistant to three or more antimicrobials was AMP-STR-TET-SOX (3/37 isolates).

Table 3.
Phenotypic resistance profiles of E. coli (n = 369) and Salmonella (n = 37) isolated from live bird markets (LBMs) and layer farms (LFs) in Timor-Leste, to tested antimicrobials using disk diffusion.
3.3. Broth-Based Microdilution Results
A subset of 212 E. coli and 17 Salmonella spp. isolates were tested for resistance to an extended panel of antimicrobials using broth-based microdilution. The percentage of resistance, MIC50 and MIC90 to various antimicrobials for the 212 E. coli isolates sent for broth-based microdilution is presented in Table 4. The results for tigecycline and ceftazidime-avibactam using the BD Phoenix M50 instrument has not been validated for use by the NHL and was excluded from analysis. The E. coli isolates tested using broth-based microdilution showed elevated resistance levels to ampicillin (22.2%), amoxicillin-clavulanic acid (20.3%), piperacillin (17.0%) and temocillin (10.4%), which belongs to the penicillin or β-lactam/β-lactam inhibitor combination class of antimicrobials. Resistance to all other antimicrobials was below 10%. Resistance to colistin was 6.6%. Except for ampicillin, amoxicillin-clavulanic acid and trimethoprim-sulfamethoxazole, all antimicrobials included in the Timor-Leste Antimicrobial Guidelines for humans [41] have resistance levels below 6%. There was no resistance to amikacin, meropenem and ertapenem. Eight E. coli isolates (3.8%) were determined to be potential ESBL-producers based on EUCAST guidelines [39], but none were considered as ESBL-positive isolates based on the Phoenix ESBL test. A total of 21 E. coli isolates (9.9%) were potential carbapenemase producers based on EUCAST guidelines [39], but none were considered carbapenemase-producing organisms based on the Phoenix CPO test. Multi-drug resistance was present in 13.7% (29/212) of E. coli isolates that underwent broth-based microdilution.

Table 4.
Antimicrobial susceptibility results of E. coli isolates from live bird markets (LBMs) (n = 169) and layer farms (LFs) (n = 43) using broth-based microdilution. Antimicrobials currently included in the Timor-Leste Antimicrobial Guidelines for humans are marked with an asterisk (*).
The percentages of resistance to various antimicrobials for the 17 Salmonella spp. isolates sent for broth-based microdilution is presented in Table 5. All but 1 of the 17 isolates originated from layer farms. The highest resistance levels were observed in fluoroquinolones, such as ciprofloxacin (47.1%), and penicillins, such as temocillin (41.2%). No or very low resistance was observed to all other classes of antimicrobials. Multi-drug resistance was present in 5.9% (1/17) of the Salmonella spp. isolates that underwent broth-based microdilution, and this isolate originated from a local chicken in a live bird market located in a municipality along the border with Indonesia.

Table 5.
Antimicrobial susceptibility results of Salmonella spp. isolates (n = 17) from live bird markets (LBMs) and layer farms (LFs) using broth-based microdilution. Antimicrobials currently included in the Timor-Leste Antimicrobial Guidelines for humans are marked with an asterisk (*).
The proportion of E. coli and Salmonella spp. isolates that were not resistant to any antimicrobials based on broth-based microdilution were 59.4% (126/212 isolates) and 29.4% (5/17 isolates), respectively. The phenotypic resistance profiles to different antimicrobial classes for multi-drug resistant E. coli and Salmonella spp. based on broth-based microdilution can be found in Supplementary Table S3. The majority of MDR isolates were resistant to both penicillins and β-lactam/β-lactam inhibitor combination of antimicrobial classes. The predominant phenotypic resistance profiles of E. coli isolates resistant to three or more antimicrobials were ampicillin-piperacillin-ampicillin/clavulanic acid (8/212 isolates) and ampicillin-piperacillin-mecillinam-ampicillin/clavulanic acid (4/212 isolates). The most common phenotypic resistance profile of Salmonella spp. isolates resistant to three or more antimicrobials was ampicillin-piperacillin-temocillin-ciprofloxacin (2/17 isolates).
3.4. Comparison of Antimicrobial Resistance in Different Chicken Populations
The logistic regression results to determine the crude associations between the origin of E. coli isolates and resistance to each antimicrobial on the DDT are presented in Table 6. The E. coli isolates originating from layer farms (odds ratio [OR]: 5.2; 95%CI 2.0–13.1) and broilers (OR: 18.1; 95%CI 5.3–61.2) were more likely than those from local chickens to be MDR. E. coli isolates originating from layer farms were more likely than those from local chickens to have resistance to all tested antimicrobials on the DDT except for ampicillin. The highest odds ratios were observed for enrofloxacin (OR: 11.2; 95%CI: 2.3–55.4) and tetracycline (OR: 6.4; 95%CI 3.5–11.7). E. coli isolates from broilers were more likely than those from local chickens to have resistance against all tested antimicrobials on the DDT except for enrofloxacin. The highest odds ratios were observed for sulfisoxazole (OR: 17.4; 95%CI 5.7–53.0), trimethoprim/sulfomethoxazole (OR: 8.7; 95%CI 2.8–27.4) and streptomycin (OR: 8.2, 95%CI 2.2–30.1). Point estimates suggested that E. coli isolates from fighting cocks were more likely than those from local chickens to have resistance against all tested antimicrobials except enrofloxacin, but estimates were imprecise so no definitive conclusion could be reached.

Table 6.
Crude associations between origin of E. coli isolates and antibiotic resistances from disk diffusion using mixed effect logistic regression models. Of the 367 E. coli isolates, 217 were from local chickens, 60 from fighting cocks, 16 from broilers and 74 from layer farms.
The logistic regression for determining the crude associations between the origin of E. coli isolates and resistance to each antimicrobial based on broth-based microdilution are presented in Supplementary Table S4. Broilers were not included in the analysis because there were only six isolates, of which all were susceptible to all antimicrobials tested except for one isolate that was resistant to gentamicin. The E. coli isolates from layer farms were much more likely to be resistant to ciprofloxacin (OR: 12.7; 95%CI: 1.4–117.2), cephalexin (OR: 5.4; 95%CI: 1.2–23.4) and trimethoprim-sulfamethoxazole (OR: 6.4; 95%CI: 2.0–20.2) compared with those from local chickens in LBMs. Although point estimates of the association for ampicillin, piperacillin, cefuroxime and levofloxacin were far from the null (OR > 2), the estimates were imprecise so no definitive conclusion can be reached. No definite conclusion could be reached on whether E. coli isolates from fighting cocks were more likely than those from local chickens to have resistance to all tested antimicrobials based on broth-based microdilution, although point estimates of the association for cefepime, cefixime, ceftazidime, ceftriaxone, cefuroxime, cephalexin, colistin and tobramycin were far from the null (OR > 2).
4. Discussion
4.1. Strength of the Study and Key Findings
This is the first study describing antimicrobial resistance in Enterobacteriaceae originating from chickens in Timor-Leste, and one of the few studies conducted in the context of a small developing country with limited resources. E. coli and Salmonella spp. isolates from chickens showed higher resistance levels to tetracycline and penicillin classes of antimicrobials compared to most other classes. These two classes of antimicrobials were imported in the highest quantities into Timor-Leste for use in animals based on import data between 2016 and 2019 [14] and are the two most commonly used classes of antimicrobials in chickens reported by government animal health workers between 2021 and 2022 [16]. These antimicrobials appear to be the main drivers of MDR bacteria in Timor-Leste.
4.2. Recovery Rate of Isolates
Commensal bacteria like E. coli can be found in the intestinal tract of all chickens [42]. Therefore, the Food and Agriculture Organization Regional Antimicrobial Resistance Monitoring and Surveillance Guidelines states that a 100% recovery rate for E. coli could be expected [18]. In this study, a relatively high E. coli recovery rate from cloacal swabs was achieved, likely due to the use of refrigerated sample transport and good bacterial isolation methods. This E. coli recovery rate was similar to the recovery rate from another study collecting cloacal swabs from broilers in China (81.6%) [43] and was much higher than other studies in Qatar (52.3%) [44] and Malaysia (51.8%) [25] where cloacal swabs were also collected.
In this study, there was a difference in the recovery rates for Salmonella spp. between samples originating from LBMs (2.3%) compared to layer farms (33.3%). The recovery rate for Salmonella spp. from cloacal swabs originating from LBMs is similar to those of other studies targeting village or backyard chickens in Malaysia (2.5%) [45], Paraguay (3.5%) [46] and Iran (5.8%) [47]. The recovery rate for Salmonella spp. from boot swabs from layer farms was similar to those of other studies [48,49,50]. The higher recovery rate for Salmonella spp. from layer farms compared to those from fighting cocks and local chickens from LBMs could be due to a higher prevalence of Salmonella spp. In commercial farms compared to backyard farming, where a higher population density can increase Salmonella spp. Transmission [51]. It could also be due to the use of boot swabs for sample collection on layer farms, which has been shown to yield a higher recovery rate for Salmonella spp. Compared to cloacal swabs [52,53], especially since shedding from the cloaca can be intermittent [54].
4.3. Antimicrobial Resistance in Different Chicken Populations
Antimicrobial resistance based on the DDT results for E. coli isolates from local chickens was lower than reported in other countries focusing on a similar target population of backyard chickens [55,56,57]. This could be due to the lack of access to government veterinary services in Timor-Leste [58], which is a common source of antimicrobials for most backyard farmers [17]. Similarly, antimicrobial resistance based on the DDT results for E. coli and Salmonella spp. isolates from layers and broilers was also lower than those reported in other Southeast Asian countries [25,59]. The lower antimicrobial resistance levels in commercial chickens in Timor-Leste compared to those of other countries could be due to the low availability of veterinary antimicrobials in the country, as there is no local manufacture, and all antimicrobials have to be imported [14]. The comparison of antimicrobial resistance results based on the DDT for Salmonella spp. to other studies should be undertaken cautiously given the small sample size and thus very wide 95% confidence intervals for resistance estimates.
In this study, the percentage of E. coli isolates with MDR based on the DDT results was significantly higher in broiler chickens and layer farms than in local chickens. Specifically, the E. coli isolates originating from broiler chickens and layer farms were more likely to have higher resistance to several antimicrobials, such as tetracyclines, penicillins, fluoroquinolones and sulfonamides, compared to local chickens. For broilers, this may be due to the routine use of these antimicrobials during commercial farming, which has been reported to be common in neighbouring countries such as Indonesia [60,61]. The import of oral tetracyclines, penicillins and fluoroquinolones by broiler farms in Timor-Leste between 2016 and 2019 [14] provides some evidence of this practice, although actual data on antimicrobial usage by broiler farms was not available in this study because sampling of broilers occurred at LBMs. Some broilers collected from LBMs may have also originated from Indonesia through informal trade across the border [12] and reflect the higher resistance profiles found in the country of origin. One study on broiler chickens in Indonesia showed that E. coli resistance to tetracycline, ampicillin and ciprofloxacin were all above 80% [62]. To understand the antimicrobial resistance profiles of only broilers produced in Timor-Leste, future studies could consider sample collection directly from broiler farms, which would also allow the history of antimicrobial usage in broilers to be collected.
It has been well documented that day-old chicks can act as a source of antimicrobial resistant bacteria for chicken farms [63,64,65,66]. Hence, another possible source of resistance for broilers and layers could be from day-old chicks, which are imported from Indonesia or Malaysia where antimicrobial resistance in commercial chickens is known to be high [25,59,62,67]. This may explain the resistance observed in the isolates from layer farms in this study, especially since there was no reported antimicrobial use in these farms except for injectable oxytetracycline in sick chickens. Therefore, the antimicrobial resistance profile of isolates from imported day-old chicks could be investigated through the sampling of day-old chicks at their point of entry into Timor-Leste.
4.4. Public Health Implications
Resistance to gentamicin and ciprofloxacin in E. coli isolates based on broth-based microdilution from this study was lower than that reported in E. coli isolates from the stools of healthy school children from Timor-Leste [29], although a direct comparison cannot be made because the screening of E. coli isolates in the latter study involved the use of selective ESBL agar [29]. No E. coli or Salmonella spp. isolates tested as ESBL positive or carbapenemase-producing organisms in this study. E. coli and Salmonella spp. resistance to antimicrobials listed in the Timor-Leste Antimicrobial Guidelines for human health [41] were generally below 25%, except for resistance to temocillin and ciprofloxacin in Salmonella spp. It is unlikely that the high rates of AMR carriage in humans in Timor-Leste [28,29] is driven by transmission from household chickens.
For colistin, a warning on the accuracy of several commercial systems for MIC results was previously issued by EUCAST, although it did not specifically evaluate the BD Phoenix M50 [68,69]. Recent studies indicate that the BD Phoenix M50 can produce accurate MIC results for colistin [70,71,72], and this has led to its inclusion in this study. The resistance to colistin in E. coli from chickens (6.6%) in this study was higher than that reported in many European countries where resistance is usually less than 1% [73,74,75], but resistance was lower than that reported in other parts of Asia [76]. Although colistin is not currently used in humans in Timor-Leste, resistance is still a concern because it is a drug of last resort against MDR Gram-negative bacteria [77]. It is also classified as a highest priority critically important antimicrobial by the World Health Organization [78], and a highly important antimicrobial agent by the World Organisation for Animal Health [79]. Oral colistin for use in chickens in Timor-Leste contributed to 4.8% of total antimicrobial imports between 2016 and 2019 [14] and could have contributed to the observed resistance. Antibiotic use guidelines for the commercial poultry industry can be developed to reduce AMR emergence to highest priority critically important antimicrobials for humans, such as colistin. For example, the usage of colistin could be limited for definitive treatment only when no other alternatives are available [80].
Ciprofloxacin is classified as a highest priority critically important antimicrobial by the World Health Organization [78] and is among the more commonly used antimicrobials in the health system in Timor-Leste [81]. Although the number of isolates tested was very small, a ciprofloxacin resistance of 50% among the 16 Salmonella spp. isolates originating from layer farms tested using broth-based microdilution was concerning. High ciprofloxacin resistance in Salmonella spp. from chickens has been reported to be an issue in other countries such as China [82,83], Bangladesh [84] and India [85] as well. Ciprofloxacin-resistant serovars, such as the Salmonella enterica serotype Kentucky from poultry is also a global concern [86,87,88], as they can also exhibit resistance to carbapenems and extended-spectrum cephalosporins [89]. However, none of the ciprofloxacin-resistant Salmonella spp. isolates from this study showed resistance to carbapenems and cephalosporins or were considered to be MDR. While there have been no imports of ciprofloxacin for use in animals into Timor-Leste, there have been imports of enrofloxacin, a veterinary fluroquinolone antimicrobial intended for use in commercial chicken production, between 2016 and 2019 [14]. This may have contributed to the elevated resistance to ciprofloxacin, as the use of enrofloxacin has been associated with increasing resistance to ciprofloxacin [24,90]. As the country continues to expand commercial chicken farming [14], the use of antimicrobials may potentially increase particularly if alternative disease control options are lacking for some poultry bacterial pathogens [30]. Therefore, ongoing antimicrobial resistance monitoring in the commercial chicken farming sector is recommended to detect emerging resistance patterns.
4.5. Origin of Samples
The sample collection at LBMs was motivated by the recognition that these locations throughout Timor-Leste play an important role in the supply chain of chickens and in the potential dissemination of bacteria carrying resistance genes to consumers. Sampling from LBMs also means that swabs originate mostly from adult chickens after potential exposure to antimicrobials used during their production. Due to the convergence of chickens from multiple sources to a single point, sampling from LBMs is also known to be more practical and cost-effective especially in low-resource settings [91]. However, the downside of sample collection at LBMs is that it is often not possible to determine the farm-of-origin of the chickens and interact with farmers to understand antimicrobial use in their chickens. Taking these trade-offs into consideration, it is proposed that periodic LBM sampling could be used as the primary method for monitoring AMR trends in chickens in Timor-Leste due to its ability to collect useful data at a low cost, but this could be supplemented with additional sampling from commercial chicken farms and day-old chicks to investigate specific questions of interest.
4.6. Capacity Building and One Health
The animal health sector in Timor-Leste began investigating AMR in animals in 2019 as part of a capacity building programme that included side-by-side mentoring of local Timorese staff in study design, sample collection, sample transport, laboratory testing and data analysis. Rapid capacity development was achieved especially in the VDL through the permanent placement of experienced laboratory scientists to work alongside local Timorese staff over the past four years. Staff from the VDL also benefited from work placements in other government laboratories that routinely performed antimicrobial susceptibility testing in Australia (Berrimah Veterinary Laboratory) and Timor-Leste (NHL). Initial challenges related to limited access to consumables at the VDL was addressed through resource sharing with the NHL. A One Health approach was also utilised in strengthening antimicrobial resistance monitoring [92], especially in the areas of AMR data collection, analysis and communication for human and animal health. This includes the standardisation of AMR testing methods by using the same broth-based microdilution test to improve the comparability of information produced and promoting data sharing and integration through regular scientific meetings.
4.7. Future Work
In this study, AMR monitoring in chickens focused on E. coli as an indicator bacteria and Salmonella spp. as a foodborne zoonotic pathogen. Further characterisation of Salmonella spp., especially those found in commercial poultry production could be considered. This would help determine the diversity of serotypes with zoonotic significance, such as S. enterica serovar Typhimurium and S. enterica serovar Enteriditis [93], and identify serotypes that can cause severe production loss in chickens such as S. pullorum [94]. This would also provide a deeper understanding on the epidemiology of salmonellosis in commercial chicken farms in Timor-Leste, including possible transmission pathways [95]. In addition, AMR monitoring could expand to more animal species, such as cattle, pigs and buffalo, as government animal health workers in Timor-Leste have reported administering antibiotics to these species most frequently [16].
Through further laboratory capacity building, AMR monitoring in animals has been expanding to include other bacteria of public health significance such as Enterococcus spp. and Campylobacter spp. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), which is available at the NHL, could also be utilised for rapid bacterial identification and the further characterisation of isolates [96,97]. The panel of antimicrobials used in this study could be refined to closely harmonise with international guidelines for AMR testing in healthy animals [18], supplemented with the molecular characterization of isolates to identify potential emerging AMR threats.
5. Conclusions
This is the first study investigating AMR in animals in Timor-Leste that focused on E. coli and Salmonella spp. originating from chickens. Among the panel of antimicrobials tested, the highest levels of resistance were observed in antimicrobials belonging to the tetracycline and penicillin classes of antimicrobials, which is consistent with them being the most imported veterinary antimicrobials in the country. The isolates from local chickens had low levels of resistance, and this level of resistance was even lower than what was observed in backyard chickens from other countries. The isolates from commercial layer farms and broilers exhibited a higher level of resistance to several antimicrobials compared to local chickens. The source of resistance in commercial chickens could be attributed to on-farm antimicrobial use or imported day-old chicks. This should be further investigated through sampling from chickens directly at broiler farms, which would allow the concurrent collection of information on antimicrobial usage and sampling from imported day-old chicks to determine the carriage of antimicrobial resistant bacteria. Due to the expected continual growth of commercial chicken production and accompanying potential for increased antimicrobial use, there should be ongoing AMR and antimicrobial use monitoring in commercial chickens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13020120/s1, Table S1: Number of live bird markets (LBM), number of cloacal swabs collected and type of chickens from which cloacal swab samples were collected in 13 municipalities. Table S2: Prevalence of AMR in E. coli isolates from disk diffusion results, based on the origin they were collected from. Layer chickens from live bird markets are excluded from this table because there were only two isolates. Table S3: Phenotypic resistance profile by antimicrobial classes for multi-drug resistant E. coli (n = 29) and Salmonella spp. (n = 1) based on broth-based microdilution. Table S4: Crude associations between origin of E. coli isolates and antimicrobial resistances from broth-based microdilution using mixed effect logistic regression models. Of the 205 E. coli isolates included in the analysis, 125 were from local chickens, 37 from fighting cocks, and 43 from layer farms. Broilers were not included because there were only six isolates.
Author Contributions
Conceptualization, S.T., J.B.d.C.J., F.d.C., S.D., J.R.F., J.Y. and A.P.; Methodology, S.T., A.P., A.A., P.G.V.d.S., C.D.S., S.D., H.E.S. and T.O.; Software, S.T., T.S.B., S.D., H.E.S. and T.O.; Investigation and data curation, A.P., S.D., H.E.S., S.T., A.A., P.G.V.d.S., C.D.S., N.d.J.F., A.X., J.N., I.d.R.F. and I.L., Formal analysis, S.T., A.P., H.E.S., S.D. and T.S.B.; Writing—original draft preparation, S.T., A.P. and H.E.S.; Writing—review and editing, S.T., A.P., T.S.B., S.D., T.O., J.Y., J.R.F., H.E.S., P.G.V.d.S., A.A., C.D.S., J.B.d.C.J., F.d.C., N.d.J.F., A.X., J.N., I.d.R.F., I.L. and J.M.; Visualization, S.T., H.E.S. and S.D.; Supervision, S.T.; A.P., J.Y., J.R.F., S.D., H.E.S., J.B.d.C.J. and F.d.C.; Project administration, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This project is funded by the Department of Health and Social Care’s Fleming Fund using UK aid (FF/87/493). The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health and Social Care or its Management Agent, Mott MacDonald. The Department of Health and Social Care’s Fleming Fund is a UK aid programme supporting up to 25 countries across Africa and Asia to tackle antimicrobial resistance (AMR), a leading contributor to deaths from infectious diseases worldwide.
Institutional Review Board Statement
The animal study protocol and procedures was approved by the Animal Ethics Committee of Charles Darwin University (A20015; 5 May 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Material.
Acknowledgments
We acknowledge the staff from the Ministry of Agriculture, Livestock, Fisheries and Forestry and Ministry of Health of Timor-Leste for their support in this study. We thank Karen Champlin (Menzies School of Health Research) for the overall project coordination of the Fleming Fund work, Agnes Agunos (Public Health Agency of Canada) for critically reviewing the manuscript and Suresh Benedict (Berrimah Veterinary Laboratory, Darwin, Australia) for his advice and assistance.
Conflicts of Interest
Author Tamsin S. Barnes is a working director of Epivet Pty. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Morel, C.M.; Alm, R.A.; Årdal, C.; Bandera, A.; Bruno, G.M.; Carrara, E.; Colombo, G.L.; de Kraker, M.E.A.; Essack, S.; Frost, I.; et al. A one health framework to estimate the cost of antimicrobial resistance. Antimicrob. Resist. Infect. Control 2020, 9, 187. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- General Directorate of Statistics—Ministry of Finance (Timor Leste). Timor-Leste Population and Housing Census 2022—Preliminary Results. Available online: https://timor-leste.unfpa.org/sites/default/files/pub-pdf/censuspreliminaryresults2022_4.pdf (accessed on 12 December 2023).
- Bettencourt, E.; Tilman, M.; Narciso, V.; Da Silva Carvalho, M.L.; Henriques, P. The Livestock Roles in the Wellbeing of Rural Communities of Timor-Leste. Rev. Econ. Sociol. Rural. 2015, 53, 63–80. [Google Scholar] [CrossRef]
- General Directorate of Statistics—Ministry of Finance (Timor Leste); Ministry of Agriculture and Fisheries. Timor-Leste Agriculture Census 2019: National Report on Final Census Results. Available online: https://inetl-ip.gov.tl/2023/03/16/2241/ (accessed on 12 December 2023).
- Lundahl, M.; Sjöholm, F. Improving the lot of the farmer: Development challenges in Timor-Leste during the second decade of independence. Asian Econ. Pap. 2013, 12, 71–96. [Google Scholar] [CrossRef]
- Wong, J.T.; Bagnol, B.; Grieve, H.; da Costa Jong, J.B.; Li, M.; Alders, R.G. Factors influencing animal-source food consumption in Timor-Leste. Food Secur. 2018, 10, 741–762. [Google Scholar] [CrossRef]
- World Food Programme. Fill the Nutrient Gap—Timor-Leste. Available online: https://www.wfp.org/publications/fill-nutrient-gap-timor-leste (accessed on 12 December 2023).
- Smith, D.; Cooper, T. Evaluating the Opportunities for Smallholder Livestock Keepers in Timor-Leste. Available online: https://www.aciar.gov.au/sites/default/files/2021-12/LS-2017-035-final-report.pdf (accessed on 20 September 2022).
- Da Cruz, C.J. Livestock Development in East Timor. Available online: https://www.aciar.gov.au/sites/default/files/legacy/node/512/pr113.pdf#page=16 (accessed on 12 December 2023).
- Ting, S.; Pereira, A.; Alves, A.d.J.; Fernandes, S.; Soares, C.d.C.; Soares, F.J.; Henrique, O.d.C.; Davis, S.; Yan, J.; Francis, J.R.; et al. Antimicrobial use in animals in Timor-Leste based on veterinary antimicrobial imports between 2016 and 2019. Antibiotics 2021, 10, 426. [Google Scholar] [CrossRef]
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Ting, S.; Pereira, A.; Alves, A.; Vong da Silva, P.G.; Dos Santos, C.; Davis, S.; Sidjabat, H.E.; Yan, J.; Francis, J.R.; Bendita da Costa Jong, J.; et al. Knowledge, attitudes and practices of government animal health workers on antibiotic use and antibiotic resistance in Timor-Leste. Front. Vet. Sci. 2022, 9, 1063530. [Google Scholar] [CrossRef]
- Ting, S.; Pereira, A.; Davis, S.; Vong da Silva, P.G.; Alves, A.; Dos Santos, C.; Toribio, J.-A.L.M.L.; Morais, O.; da Costa Jong, J.B.; Barnes, T.S. Knowledge and practices on antibiotic use and antibiotic resistance among smallholder pig farmers in Timor-Leste. Front. Vet. Sci. 2022, 8, 819643. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation. Monitoring and Surveillance of Antimicrobial Resistance in Bacteria from Healthy Food Animals Intended for Consumption. Available online: https://www.fao.org/documents/card/en?details=ca6897en/ (accessed on 10 December 2023).
- de Mesquita Souza Saraiva, M.; Lim, K.; do Monte, D.F.M.; Givisiez, P.E.N.; Alves, L.B.R.; de Freitas Neto, O.C.; Kariuki, S.; Júnior, A.B.; de Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W. Distinguishing Pathovars from Nonpathovars: Escherichia coli. Microbiol. Spectr. 2020, 8, AME-0014-2020. [Google Scholar] [CrossRef]
- Parvej, M.S.; Nazir, K.H.; Rahman, M.B.; Jahan, M.; Khan, M.F.; Rahman, M. Prevalence and characterization of multi-drug resistant Salmonella Enterica serovar Gallinarum biovar Pullorum and Gallinarum from chicken. Vet. World 2016, 9, 65–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miles, T.D.; McLaughlin, W.; Brown, P.D. Antimicrobial resistance of Escherichia coli isolates from broiler chickens and humans. BMC Vet. Res. 2006, 2, 7. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Carrique-Mas, J.J.; Ngo, T.H.; Ho, H.M.; Ha, T.T.; Campbell, J.I.; Nguyen, T.N.; Hoang, N.N.; Pham, V.M.; Wagenaar, J.A.; et al. Prevalence and risk factors for carriage of antimicrobial-resistant Escherichia coli on household and small-scale chicken farms in the Mekong Delta of Vietnam. J. Antimicrob. Chemother. 2015, 70, 2144–2152. [Google Scholar] [CrossRef]
- Ibrahim, S.; Wei Hoong, L.; Lai Siong, Y.; Mustapha, Z.; Zalati, C.W.S.; Aklilu, E.; Mohamad, M.; Kamaruzzaman, N.F. Prevalence of Antimicrobial Resistance (AMR) Salmonella spp. and Escherichia coli Isolated from Broilers in the East Coast of Peninsular Malaysia. Antibiotics 2021, 10, 579. [Google Scholar] [CrossRef]
- Baker, S.; Bryant, J.E.; Campbell, J.; Carrique-Mas, J.J.; Cuong, N.V.; Duy, D.T.; Hien, V.B.; Hoa, N.T.; Hoang, N.V.M.; Kiet, B.T.; et al. High levels of contamination and antimicrobial-resistant non-typhoidal Salmonella serovars on pig and poultry farms in the Mekong Delta of Vietnam. Epidemiol. Infect. 2015, 143, 3074–3086. [Google Scholar] [CrossRef]
- Hasan, B.; Swedberg, G. Molecular Characterization of Clinically Relevant Extended-Spectrum β-Lactamases bla(CTX-M-15)-Producing Enterobacteriaceae Isolated from Free-Range Chicken from Households in Bangladesh. Microb. Drug Resist. 2022, 28, 780–786. [Google Scholar] [CrossRef]
- Marr, I.; Sarmento, N.; O’Brien, M.; Lee, K.; Gusmao, C.; de Castro, G.; Janson, S.; Tong, S.Y.C.; Baird, R.W.; Francis, J.R. Antimicrobial resistance in urine and skin isolates in Timor-Leste. J. Glob. Antimicrob. Resist. 2018, 13, 135–138. [Google Scholar] [CrossRef]
- Oakley, T.; Le, B.; da Conceicao, V.; Marr, I.; Maia, C.; Soares, M.; Belo, J.C.; Sarmento, N.; da Silva, E.; Amaral, S.; et al. Gastrointestinal Carriage of Antimicrobial Resistance in School-Aged Children in Three Municipalities of Timor-Leste. Antibiotics 2022, 11, 1262. [Google Scholar] [CrossRef]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef]
- Kim, Y.; Biswas, P.K.; Giasuddin, M.; Hasan, M.; Mahmud, R.; Chang, Y.M.; Essen, S.; Samad, M.A.; Lewis, N.S.; Brown, I.H.; et al. Prevalence of Avian Influenza A(H5) and A(H9) Viruses in Live Bird Markets, Bangladesh. Emerg. Infect. Dis. 2018, 24, 2309–2316. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- CLSI. Perfromance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 4th ed.; CLSI Standard VET08; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Sarmento, N.; Oakley, T.; Da Silva, E.S.; Tilman, A.; Monteiro, M.; Alves, L.; Barreto, I.; Marr, I.; Draper, A.D.; de Castro Hall, G. Strong relationships between the Northern Territory of Australia and Timor-Leste. Microbiol. Aust. 2022, 43, 125–129. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.0. 2023. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.0_Breakpoint_Tables.pdf (accessed on 20 November 2023).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Standard M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Editorial: Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 2010, 65, 601–604. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Version 2.0. 2017. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 21 November 2023).
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC.: College Station, TX, USA, 2021. [Google Scholar]
- Ministry of Health. Timor-Leste Antimicrobial Guidelines. 2022. Available online: https://apps.ms.gov.tl/moh5/anti_e/handbook_eng.pdf (accessed on 10 September 2023).
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Dai, Y.; Liu, Y.; Song, Y.; Yu, L.; Feng, C.; Liu, M.; Xie, Z.; Shang, Y.; et al. Longitudinal monitoring of multidrug resistance in Escherichia coli on broiler chicken fattening farms in Shandong, China. Poult. Sci. 2021, 100, 100887. [Google Scholar] [CrossRef]
- Eltai, N.O.; Abdfarag, E.A.; Al-Romaihi, H.; Wehedy, E.; Mahmoud, M.H.; Alawad, O.K.; Al-Hajri, M.M.; Al Thani, A.A.; Yassine, H.M. Antibiotic Resistance Profile of Commensal Escherichia coli Isolated from Broiler Chickens in Qatar. J. Food Prot. 2018, 81, 302–307. [Google Scholar] [CrossRef]
- Jajere, S.M.; Hassan, L.; Abdul Aziz, S.; Zakaria, Z.; Abu, J.; Nordin, F.; Faiz, N.M. Salmonella in native “village” chickens (Gallus domesticus): Prevalence and risk factors from farms in South-Central Peninsular Malaysia. Poult. Sci. 2019, 98, 5961–5970. [Google Scholar] [CrossRef]
- Leotta, G.A.; Suzuki, K.; Alvarez, F.; Nuñez, L.; Silva, M.; Castro, L.; Faccioli, M.; Zarate, N.; Weiler, N.; Alvarez, M. Prevalence of Salmonella spp. in backyard chickens in Paraguay. Int. J. Poult. Sci. 2010, 9, 533–536. [Google Scholar] [CrossRef]
- Jafari, R.; Ghorbanpour, M.; Jaideri, A. An investigation into Salmonella infection status in backyard chickens in Iran. Int. J. Poult. Sci. 2007, 6, 227–229. [Google Scholar] [CrossRef]
- Soria, M.C.; Soria, M.A.; Bueno, D.J.; Godano, E.I.; Gómez, S.C.; ViaButron, I.A.; Padin, V.M.; Rogé, A.D. Salmonella spp. contamination in commercial layer hen farms using different types of samples and detection methods. Poult. Sci. 2017, 96, 2820–2830. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Habib, I.; Whiddon, S.; Wang, P.; Mohammed, A.B.; Robertson, I.; Goodchild, S. Occurrence and Characterization of Salmonella Isolated from Table Egg Layer Farming Environments in Western Australia and Insights into Biosecurity and Egg Handling Practices. Pathogens 2020, 9, 56. [Google Scholar] [CrossRef]
- Samper-Cativiela, C.; Prieto, M.E.; Collado, S.; De Frutos, C.; Branscum, A.J.; Saez, J.L.; Alvarez, J. Risk Factors for Salmonella Detection in Commercial Layer Flocks in Spain. Animals 2023, 13, 3181. [Google Scholar] [CrossRef]
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef]
- Pacholewicz, E.; Wisselink, H.J.; Koene, M.G.J.; van der Most, M.; Gonzales, J.L. Environmental Sampling Methods for Detection of Salmonella Infections in Laying Hens: A Systematic Review and Meta-Analysis. Microorganisms 2023, 11, 2100. [Google Scholar] [CrossRef]
- Mueller-Doblies, D.; Sayers, A.R.; Carrique-Mas, J.J.; Davies, R.H. Comparison of sampling methods to detect Salmonella infection of turkey flocks. J. Appl. Microbiol. 2009, 107, 635–645. [Google Scholar] [CrossRef]
- Carrique-Mas, J.J.; Davies, R.H. Sampling and bacteriological detection of Salmonella in poultry and poultry premises: A review. Rev. Sci. Tech. 2008, 27, 665–677. [Google Scholar] [CrossRef]
- Hedman, H.D.; Eisenberg, J.N.S.; Trueba, G.; Rivera, D.L.V.; Herrera, R.A.Z.; Barrazueta, J.V.; Rodriguez, G.I.G.; Krawczyk, E.; Berrocal, V.J.; Zhang, L. Impacts of small-scale chicken farming activity on antimicrobial-resistant Escherichia coli carriage in backyard chickens and children in rural Ecuador. One Health 2019, 8, 100112. [Google Scholar] [CrossRef]
- Kamboh, A.A.; Shoaib, M.; Abro, S.H.; Khan, M.A.; Malhi, K.K.; Yu, S. Antimicrobial Resistance in Enterobacteriaceae Isolated from Liver of Commercial Broilers and Backyard Chickens. J. Appl. Poult. Res. 2018, 27, 627–634. [Google Scholar] [CrossRef]
- Saeed, M.A.; Saqlain, M.; Waheed, U.; Ehtisham-ul-Haque, S.; Khan, A.U.; Rehman, A.u.; Sajid, M.; Atif, F.A.; Neubauer, H.; El-Adawy, H. Cross-Sectional Study for Detection and Risk Factor Analysis of ESBL-Producing Avian Pathogenic Escherichia coli Associated with Backyard Chickens in Pakistan. Antibiotics 2023, 12, 934. [Google Scholar] [CrossRef]
- Millar, J.; Morais, O.; Da Silva, H.; Hick, P.; Foster, A.; Jong, J.B.d.C.; Pereira, A.; Ting, S.; da Conceição, F.; Toribio, J.-A.L.M.L. Community engagement strengthens pig disease knowledge and passive surveillance in Timor-Leste. Front. Vet. Sci. 2023, 9, 1024094. [Google Scholar] [CrossRef]
- Usui, M.; Ozawa, S.; Onozato, H.; Kuge, R.; Obata, Y.; Uemae, T.; Ngoc, P.T.; Heriyanto, A.; Chalemchaikit, T.; Makita, K.; et al. Antimicrobial susceptibility of indicator bacteria isolated from chickens in Southeast Asian countries (Vietnam, Indonesia and Thailand). J. Vet. Med. Sci. 2014, 76, 685–692. [Google Scholar] [CrossRef]
- Coyne, L.; Patrick, I.; Arief, R.; Benigno, C.; Kalpravidh, W.; McGrane, J.; Schoonman, L.; Sukarno, A.H.; Rushton, J. The Costs, Benefits and Human Behaviours for Antimicrobial Use in Small Commercial Broiler Chicken Systems in Indonesia. Antibiotics 2020, 9, 154. [Google Scholar] [CrossRef]
- Zalizar, L.; Relawati, R.; Pancapalaga, W. Usage of Antibiotic on Chicken Poultry in District of Malang, East Java, Indonesia. In Proceedings of the International Seminar “Improving Tropical Production for Food Security”, Kendari, Indonesia, 3–5 November 2015; p. 158. [Google Scholar]
- Hardiati, A.; Safika, S.; Wibawan, I.W.T.; Indrawati, A.; Pasaribu, F.H. Isolation and detection of antibiotics resistance genes of Escherichia coli from broiler farms in Sukabumi, Indonesia. J. Adv. Vet. Anim. Res. 2021, 8, 84–90. [Google Scholar] [CrossRef]
- Moreno, M.A.; García-Soto, S.; Hernández, M.; Bárcena, C.; Rodríguez-Lázaro, D.; Ugarte-Ruíz, M.; Domínguez, L. Day-old chicks are a source of antimicrobial resistant bacteria for laying hen farms. Vet. Microbiol. 2019, 230, 221–227. [Google Scholar] [CrossRef]
- Coppola, N.; Cordeiro, N.F.; Trenchi, G.; Esposito, F.; Fuga, B.; Fuentes-Castillo, D.; Lincopan, N.; Iriarte, A.; Bado, I.; Vignoli, R. Imported One-Day-Old Chicks as Trojan Horses for Multidrug-Resistant Priority Pathogens Harboring mcr-9, rmtG, and Extended-Spectrum β-Lactamase Genes. Appl. Environ. Microbiol. 2022, 88, e0167521. [Google Scholar] [CrossRef]
- Okorafor, O.N.; Anyanwu, M.U.; Nwafor, E.O.; Anosa, G.N.; Udegbunam, R.I. Multidrug-resistant enterobacteria colonize commercial day-old broiler chicks in Nigeria. Vet. World 2019, 12, 418–423. [Google Scholar] [CrossRef]
- Dougnon, P.; Dougnon, V.; Legba, B.; Fabiyi, K.; Soha, A.; Koudokpon, H.; Sintondji, K.; Deguenon, E.; Hounmanou, G.; Quenum, C.; et al. Antibiotic profiling of multidrug resistant pathogens in one-day-old chicks imported from Belgium to benin. BMC Vet. Res. 2023, 19, 17. [Google Scholar] [CrossRef]
- Elmi, S.A.; Simons, D.; Elton, L.; Haider, N.; Abdel Hamid, M.M.; Shuaib, Y.A.; Khan, M.A.; Othman, I.; Kock, R.; Osman, A.Y. Identification of Risk Factors Associated with Resistant Escherichia coli Isolates from Poultry Farms in the East Coast of Peninsular Malaysia: A Cross Sectional Study. Antibiotics 2021, 10, 117. [Google Scholar] [CrossRef]
- Matuschek, E.; Åhman, J.; Webster, C.; Kahlmeter, G. Antimicrobial susceptibility testing of colistin—Evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin. Microbiol. Infect. 2018, 24, 865–870. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Antimicrobial Susceptibility Testing of Colistin—Problems Detected with Several Commercially Available Products. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Warnings/Warnings_docs/Warning_-_colistin_AST.pdf (accessed on 20 October 2023).
- Anantharajah, A.; Glupczynski, Y.; Hoebeke, M.; Bogaerts, P.; Declercq, P.; Denis, O.; Descy, J.; Floré, K.; Magerman, K.; Rodriguez-Villalobos, H.; et al. Multicenter study of automated systems for colistin susceptibility testing. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 575–579. [Google Scholar] [CrossRef]
- Bhatia, M.; Shamanna, V.; Nagaraj, G.; Gupta, P.; Omar, B.J.; Diksha; Chaudhary, R.; Ravikumar, K.L. Assessment of in vitro colistin susceptibility of Carbapenem-resistant clinical Gram-negative bacterial isolates using four commercially available systems & Whole-genome sequencing: A diagnostic accuracy study. Diagn. Microbiol. Infect. Dis. 2023, 108, 116155. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, W.; Zhu, Y.; Jing, N.; Wang, S.; Yuan, Y.; Ma, B.; Xu, J.; Chu, Y.; Zhang, J.; et al. Evaluation of Commercial Products for Colistin and Polymyxin B Susceptibility Testing for mcr-Positive and Negative Escherichia coli and Klebsiella pneumoniae in China. Infect. Drug Resist. 2023, 16, 1171–1181. [Google Scholar] [CrossRef]
- Brătfelan, D.O.; Tabaran, A.; Colobatiu, L.; Mihaiu, R.; Mihaiu, M. Prevalence and Antimicrobial Resistance of Escherichia coli Isolates from Chicken Meat in Romania. Animals 2023, 13, 3488. [Google Scholar] [CrossRef]
- Kempf, I.; Fleury, M.A.; Drider, D.; Bruneau, M.; Sanders, P.; Chauvin, C.; Madec, J.-Y.; Jouy, E. What do we know about resistance to colistin in Enterobacteriaceae in avian and pig production in Europe? Int. J. Antimicrob. Agents 2013, 42, 379–383. [Google Scholar] [CrossRef]
- Kempf, I.; Jouy, E.; Chauvin, C. Colistin use and colistin resistance in bacteria from animals. Int. J. Antimicrob. Agents 2016, 48, 598–606. [Google Scholar] [CrossRef]
- Dawadi, P.; Bista, S.; Bista, S. Prevalence of Colistin-Resistant Escherichia coli from Poultry in South Asian Developing Countries. Vet. Med. Int. 2021, 2021, 6398838. [Google Scholar] [CrossRef]
- Apostolakos, I.; Piccirillo, A. A review on the current situation and challenges of colistin resistance in poultry production. Avian Pathol. 2018, 47, 546–558. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine: 6th Revision; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Organisation for Animal Health. OIE List of Antimicrobial Agents of Veterinary Importance (June 2021). Available online: https://www.woah.org/app/uploads/2021/06/a-oie-list-antimicrobials-june2021.pdf (accessed on 19 October 2023).
- Al-Tawfiq, J.A.; Laxminarayan, R.; Mendelson, M. How should we respond to the emergence of plasmid-mediated colistin resistance in humans and animals? Int. J. Infect. Dis. 2017, 54, 77–84. [Google Scholar] [CrossRef]
- Harris, L.; Bongers, A.; Yan, J.; Francis, J.R.; Marr, I.; Lake, S.; Martins, S. Estimates of Antibacterial Consumption in Timor-Leste Using Distribution Data and Variation in Municipality Usage Patterns. Antibiotics 2021, 10, 1468. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, J.; Zhang, X.; Wang, S.; Chen, K.; Lin, Q.; Xu, C.; Qu, X.; Zhang, H.; Liao, M.; et al. Highly prevalent multidrug resistance and QRDR mutations in Salmonella isolated from chicken, pork and duck meat in Southern China, 2018–2019. Int. J. Food Microbiol. 2021, 340, 109055. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.M.; Wu, G.J.; Zhao, H.Y.; He, T.; Cao, X.Y.; Dai, L.; Xia, L.N.; Qin, S.S.; Shen, J.Z. Prevalence of antimicrobial resistance among Salmonella isolates from chicken in China. Foodborne Pathog. Dis. 2011, 8, 45–53. [Google Scholar] [CrossRef]
- Chaudhary, P.; Salam, S.m.A.; Reza, M.A.; Ahaduzzaman, M. High prevalence of ciprofloxacin and ceftriaxone resistance Salmonella in the retail chicken market of Chattogram, Bangladesh. Turk. J. Vet. Res. 2019, 3, 51–55. [Google Scholar]
- Sharma, J.; Kumar, D.; Hussain, S.; Pathak, A.; Shukla, M.; Prasanna Kumar, V.; Anisha, P.N.; Rautela, R.; Upadhyay, A.K.; Singh, S.P. Prevalence, antimicrobial resistance and virulence genes characterization of nontyphoidal Salmonella isolated from retail chicken meat shops in Northern India. Food Control 2019, 102, 104–111. [Google Scholar] [CrossRef]
- Rickert-Hartman, R.; Folster, J.P. Ciprofloxacin-resistant Salmonella enterica serotype Kentucky sequence type 198. Emerg. Infect. Dis. 2014, 20, 910–911. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, S.; Huang, Y.; Gao, Y.; Shen, H.; Chen, Z.; Bai, J.; Zhan, Z.; Wen, J.; Liao, M.; et al. Ciprofloxacin-Resistant Salmonella enterica Serovar Kentucky ST198 in Broiler Chicken Supply Chain and Patients, China, 2010–2016. Microorganisms 2020, 8, 140. [Google Scholar] [CrossRef]
- Vázquez, X.; Fernández, J.; Bances, M.; Lumbreras, P.; Alkorta, M.; Hernáez, S.; Prieto, E.; de la Iglesia, P.; de Toro, M.; Rodicio, M.R.; et al. Genomic Analysis of Ciprofloxacin-Resistant Salmonella enterica Serovar Kentucky ST198 From Spanish Hospitals. Front. Microbiol. 2021, 12, 720449. [Google Scholar] [CrossRef]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef]
- Bui, C.B.; Carrique-Mas, J.; Diep, T.Q.; Do, D.H.; Henry, W.; Hoang, N.V.; Inui, K.; Le, T.D.; Nguyen, T.T.; Phan, M.Q.; et al. Detection of HPAI H5N1 viruses in ducks sampled from live bird markets in Vietnam. Epidemiol. Infect. 2013, 141, 601–611. [Google Scholar] [CrossRef]
- Aenishaenslin, C.; Häsler, B.; Ravel, A.; Parmley, J.; Stärk, K.; Buckeridge, D. Evidence needed for antimicrobial resistance surveillance systems. Bull. World Health Organ. 2019, 97, 283–289. [Google Scholar] [CrossRef]
- Shittu, O.B.; Uzairue, L.I.; Ojo, O.E.; Obuotor, T.M.; Folorunso, J.B.; Raheem-Ademola, R.R.; Olanipekun, G.; Ajose, T.; Medugu, N.; Ebruke, B.; et al. Antimicrobial resistance and virulence genes in Salmonella enterica serovars isolated from droppings of layer chicken in two farms in Nigeria. J. Appl. Microbiol. 2022, 132, 3891–3906. [Google Scholar] [CrossRef] [PubMed]
- Kipper, D.; Mascitti, A.K.; De Carli, S.; Carneiro, A.M.; Streck, A.F.; Fonseca, A.S.K.; Ikuta, N.; Lunge, V.R. Emergence, Dissemination and Antimicrobial Resistance of the Main Poultry-Associated Salmonella Serovars in Brazil. Vet. Sci. 2022, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Florio, W.; Baldeschi, L.; Rizzato, C.; Tavanti, A.; Ghelardi, E.; Lupetti, A. Detection of Antibiotic-Resistance by MALDI-TOF Mass Spectrometry: An Expanding Area. Front. Cell. Infect. Microbiol. 2020, 10, 572909. [Google Scholar] [CrossRef] [PubMed]
- Elabbasy, M.T.; Hussein, M.A.; Algahtani, F.D.; Abd El-Rahman, G.I.; Morshdy, A.E.; Elkafrawy, I.A.; Adeboye, A.A. MALDI-TOF MS Based Typing for Rapid Screening of Multiple Antibiotic Resistance E. coli and Virulent Non-O157 Shiga Toxin-Producing E. coli Isolated from the Slaughterhouse Settings and Beef Carcasses. Foods 2021, 10, 820. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).