Exploring Disease Management and Control through Pathogen Diagnostics and One Health Initiative: A Concise Review
Abstract
:1. Introduction
2. Methodology
3. Current Landscape of Disease
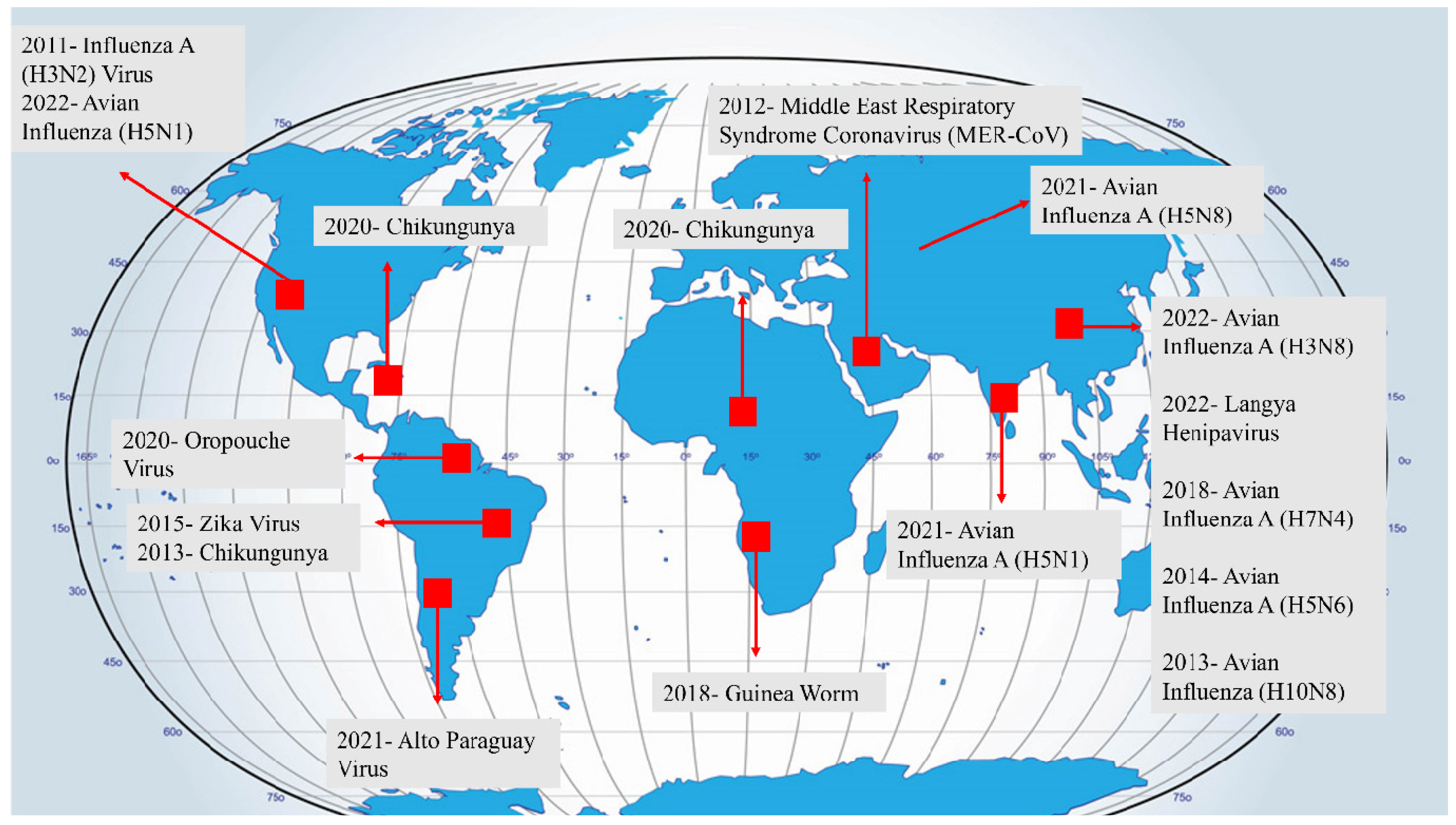
3.1. Zoonotic Viral Pathogens
3.2. Antimicrobial Resistant Pathogens
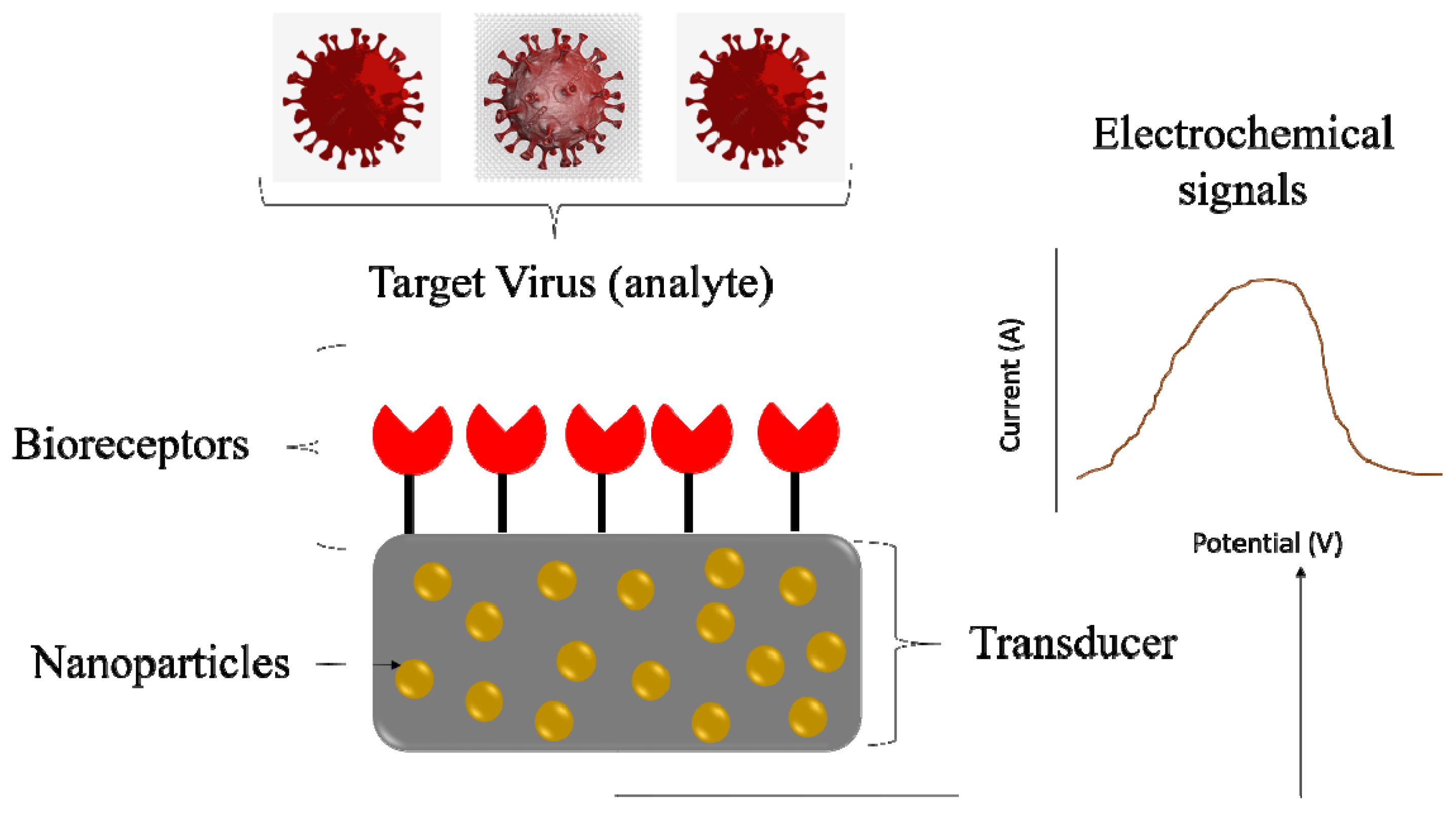
4. Advancements in Diagnostics for Disease Management
Significance of Novel Detection Strategies for Infectious Pathogens
5. One Health Initiatives for Infectious Disease Management and Control
| Intervention | Pathogens Addressed | One Health Policy Approaches | Ref. |
|---|---|---|---|
| Integrated surveillance systems | Bacteria, viruses, parasites | Establishing coordinated surveillance systems that capture human, animal, and environmental data. Integration of data across sectors for a comprehensive view. | [51] |
| Zoonotic disease control Policies | Zoonotic pathogens | Development and implementation of policies focused on controlling and preventing the spread of zoonotic diseases. Includes vaccination programs, biosecurity measures, and regulations on animal trade. | [51,52] |
| Antimicrobial resistance (AMR) policies | Bacteria, fungi | Policies aimed at regulating and promoting responsible use of antimicrobials in human and veterinary medicine. Also addressing the environmental aspects of antimicrobial resistance. | [53] |
| One Health research funding | Various pathogens | Allocating research funds to interdisciplinary studies that investigate the interconnectedness of human, animal, and environmental health. Encouraging collaborative research initiatives. | [53] |
| Education and capacity building | Various pathogens | Implementing educational programs to raise awareness about One Health principles. Building capacity among professionals in human and veterinary medicine, as well as environmental sciences. | [54] |
| Environmental conservation policies | Pathogens in the environment | Policies focusing on habitat conservation, sustainable land use, and water management to reduce the risk of disease transmission from wildlife to humans and domestic animals. | [55] |
6. Discussion
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salyer, S.J.; Silver, R.; Simone, K.; Behravesh, C.B. Prioritizing zoonoses for global health capacity building—Themes from One Health zoonotic disease workshops in 7 countries, 2014–2016. Emerg. Infect. Dis. 2017, 23 (Suppl. S1), S55. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Bouamra-Mechemache, Z.; Réquillart, V.; Treich, N. Viewpoint: Regulating meat consumption to improve health, the environment and animal welfare. Food Policy 2020, 97, 101847. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug Resistant Infections Globally; Final Report and Recommendations—The Review on Antimicrobial Resistance; Government of the United Kingdom: London, UK, 2016.
- World Health Organization (WHO). Food Safety 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 19 May 2022).
- Lammie, S.L.; Hughes, J.M. Antimicrobial resistance, food safety, and one health: The need for convergence. Annu. Rev. Food Sci. Technol. 2016, 7, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.N.; Osburn, B.I.; Jay-Russell, M.T. One Health for Food Safety, Food Security, and Sustainable Food Production. Front. Sustain. Food Syst. 2020, 4, 1. [Google Scholar] [CrossRef]
- Welburn, S. One Health: The 21st century challenge. Vet. Record. 2011, 168, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.; Fouchier, R.; Rimmelzwaan, G.; van den Brand, J.; van Riel, D.; Osterhaus, A. Pigs, poultry, and pandemic influenza: How zoonotic pathogens threaten human health. Adv. Exp. Med. Biol. 2011, 719, 59–66. [Google Scholar] [PubMed]
- Espinosa, R.; Tago, D.; Trench, N. Infectious Diseases and Meat Production. Environ. Resour. Econ. 2020, 76, 1019–1044. [Google Scholar] [CrossRef]
- Addis, M.; Sisay, D. A review on major food borne bacterial illnesses. J. Trop. Dis. Public Health 2015, 3, 1–7. [Google Scholar]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Loh, E.H.; Zambrana-Torrelio, C.; Olival, K.J.; Bogich, T.L.; Johnson, C.K.; Mazet, J.A.; Karesh, W.; Daszak, P. Targeting Transmission Pathways for Emerging Zoonotic Disease Surveillance and Control. Vector Borne Zoonotic Dis. 2015, 15, 432–437. [Google Scholar] [CrossRef]
- Fidler, D.P. Health as foreign policy: Harnessing globalization for health. Health Promot. Int. 2006, 21 (Suppl. S1), 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.W.; Parmley, E.J.; Avery, B.P.; Irwin, R.J.; Reid-Smith, R.J.; Deckert, A.E.; Finley, R.L.; Daignault, D.; Alexander, D.C.; Allen, V. A One-Health Genomic Investigation of Gentamicin Resistance in Salmonella from Human and Chicken Sources in Canada, 2014 to 2017. Antimicrob. Agents Chemother. 2021, 65, e00966-21. [Google Scholar] [CrossRef] [PubMed]
- Emerging Infections: How and Why They Arise. (5 January 2023b). GOV.UK. Available online: https://www.gov.uk/government/publications/emerging-infections-characteristics-epidemiology-and-global-distribution/emerging-infections-how-and-why-they-arise#figure-1 (accessed on 19 December 2023).
- Koopmans, M. Food-Borne Viruses from a Global Perspective. Institute of Medicine (US). In Improving Food Safety Through a One Health Approach: Workshop Summary; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Vidic, J.; Manzano, M.; Chang, C.-M.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Brown, I.H. History of highly pathogenic avian influenza. Rev. Sci. Tech. 2009, 28, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Capua, I.; Alexander, D.J. Avian influenza infections in birds—A moving target. Influenza Other Respir Viruses 2007, 1, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lycett, S.J.; Duchatel, F.; Digard, P. A brief history of bird flu. Philos. Trans. R Soc. Lond. B Biol. Sci. 2019, 374, 20180257. [Google Scholar] [CrossRef]
- Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
- Nagy, A.; Černíková, L.; Kunteová, K.; Dirbáková, Z.; Thomas, S.S.; Slomka, M.J.; Dán, Á.; Varga, T.; Máté, M.; Jiřincová, H.; et al. A universal RT-qPCR assay for “One Health” detection of influenza A viruses. PLoS ONE 2021, 16, e0244669. [Google Scholar] [CrossRef]
- Velavan, T.P.; Pallerla, S.R.; Johne, R.; Todt, D.; Steinmann, E.; Schemmerer, M.; Wenzel, J.J.; Hofmann, J.; Shih, J.W.K.; Wedemeyer, H. Hepatitis E: An update on One Health and clinical medicine. Liver Int. 2021, 41, 1462–1473. [Google Scholar] [CrossRef]
- Harrison, L.C.; DiCaprio, E. Hepatitis E Virus: An Emerging Foodborne Pathogen. Front. Sustain. Food Syst. 2018, 2, 14. [Google Scholar] [CrossRef]
- Kar, P.; Karna, R. A review of the diagnosis and management of hepatitis E. Curr. Treat. Options Infect. Dis. 2020, 12, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bourret, V. Avian influenza viruses in pigs: An overview. Vet. J. 2018, 239, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Awada, L.; Brown, I.; Chen, H.; Claes, F.; Dauphin, G.; Donis, R.; Culhane, M.; Hamilton, K.; Lewis, N.; et al. Review of influenza A virus in swine worldwide: A call for increased surveillance and research. Zoonoses Public Health 2014, 61, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K. The origin and virulence of the 1918 “Spanish” influenza virus. Proc. Am. Philos. Soc. 2006, 150, 86–112. [Google Scholar] [PubMed]
- Hoffmann, B.; Beer, M.; Reid, S.M.; Mertens, P.; Oura, C.A.; van Rijn, P.A.; Slomka, M.J.; Banks, J.; Brown, I.H.; Alexanderet, D.J.; et al. A review of RT-PCR technologies used in veterinary virology and disease control: Sensitive and specific diagnosis of five livestock diseases notifiable to the World Organisation for Animal Health. Vet. Microbiol. 2009, 139, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vidic, J.; Chaix, C.; Manzano, M.; Heyndrickx, M. Food sensing: Detection of Bacillus cereus spores in dairy products. Biosensors 2020, 10, 15. [Google Scholar] [CrossRef]
- Gunjan Vidic, J.; Manzano, M.; Raj, V.S.; Pandey, R.P.; Chang, C.M. Comparative meta-analysis of antimicrobial resistance from different food sources along with one health approach in Italy and Thailand. One Health 2023, 16, 100477. [Google Scholar] [CrossRef]
- Osterhaus, A.D.M.E.; Vanlangendonck, C.; Barbeschi, M.; Bruschke, C.J.M.; Christensen, R.; Daszak, P.; de Groot, F.; Doherty, P.; Drury, P.; Gmacz, S.; et al. Make science evolve into a One Health approach to improve health and security: A white paper. One Health Outlook 2020, 2, 6. [Google Scholar] [CrossRef]
- Xu, S.; Campisi, E.; Li, J.; Fischetti, V.A. Decontamination of Escherichia coli O157:H7 on fresh Romaine lettuce using a novel bacteriophage lysin. Int. J. Food Microbiol. 2021, 341, 109068. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2015. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); European Centre for Disease Prevention and Control: Solna, Sweden, 2015. [Google Scholar]
- Nnachi, R.C.; Sui, N.; Ke, B.; Luo, Z.; Bhalla, N.; He, D.; Yang, Z. Recent progress on biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ. Int. 2022, 166, 107357. [Google Scholar] [CrossRef]
- Liu, L.; Moore, M.D. A survey of analytical techniques for noroviruses. Foods 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, X.; Cheng, J.; Zhou, H.; Zhang, Y.; Dai, Y. Advances in the application of molecular diagnostic techniques for the detection of infectious disease pathogens. Mol. Med. Rep. 2023, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Vasala, A.; Hytönen, V.P.; Laitinen, O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell Infect. Microbiol. 2020, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Cormontagne, D.; Rigourd, V.; Vidic, J.; Rizzotto, F.; Bille, E.; Ramarao, N. Bacillus cereus induces severe infections in preterm neonates: Implication at the hospital and human milk bank level. Toxins 2021, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Vizzini, P.; Manzano, M.; Farre, C.; Meylheuc, T.; Chaix, C.; Ramarao, N.; Vidic, J. Highly sensitive detection of Campylobacter spp. In chicken meat using a silica nanoparticle enhanced dot blot DNA biosensor. Biosens. Bioelectron. 2021, 171, 112689. [Google Scholar] [CrossRef]
- Vidic, J.; Vizzini, P.; Manzano, M.; Kavanaugh, D.; Ramarao, N.; Zivkovic, M.; Radonic, V.; Knezevic, N.; Giouroudi, I.; Gadjanski, I. Point-of-need DNA testing for detection of foodborne pathogenic bacteria. Sensors 2019, 19, 1100. [Google Scholar] [CrossRef] [PubMed]
- Reynoso, E.C.; Laschi, S.; Palchetti, I.; Torres, E. Advances in antimicrobial resistance monitoring using sensors and biosensors: A review. Chemosensors 2021, 9, 232. [Google Scholar] [CrossRef]
- Balbinot, S.; Srivastav, A.M.; Vidic, J.; Abdulhalim, I.; Manzano, M. Plasmonic biosensors for food control. Trends Food Sci. Technol. 2021, 111, 128–140. [Google Scholar] [CrossRef]
- Marin, M.; Nikolic, M.V.; Vidic, J. Rapid point-of-need detection of bacteria and their toxins in food using gold nanoparticles. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5880–5900. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.; Radovic, M.; Rizzotto, F.; Vizzini, P.; Jaric, S.; Pavlovic, Z.; Radonic, V.; Nikolic, M.V.; Vidic, J. Advances in nanomaterials-based electrochemical biosensors for foodborne pathogen detection. Nanomaterials 2021, 11, 2700. [Google Scholar] [CrossRef]
- Ghai, R.R.; Wallace, R.M.; Kile, J.C.; Shoemaker, T.R.; Vieira, A.R.; Negron, M.E.; Shadomy, S.V.; Sinclair, J.R.; Goryoka, G.W.; Salyer, S.J.; et al. A generalizable one health framework for the control of zoonotic diseases. Sci. Rep. 2022, 12, 8588. [Google Scholar] [CrossRef] [PubMed]
- Taking a One Health Approach to Tackling Infectious Diseases. IVVN. Available online: https://www.intvetvaccnet.co.uk/blog/taking-a-one-health-approach-to-tackling-infectious-diseases (accessed on 3 November 2022).
- British Veterinary Association. (n.d.) BVA Blog—The Importance of Veterinary Vaccines in the One Health Agenda. Available online: https://www.bva.co.uk/news-and-blog/blog-article/the-importance-of-veterinary-vaccines-in-the-one-health-agenda/ (accessed on 13 November 2019).
- El-Zoghby, E.F.; Aly, M.M.; Nasef, S.A.; Hassan, M.K.; Arafa, A.S.; Selim, A.A.; Kholousy, S.G.; Kilany, W.H.; Safwat, M.; Abdelwhab, E.M.; et al. Surveillance on A/H5N1 virus in domestic poultry and wild birds in Egypt. Virol. J. 2013, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Control of Neglected Zoonotic Diseases: A Route to Poverty Alleviation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Pike, J.; Bogich, T.; Elwood, S.; Finnoff, D.C.; Daszak, P. Economic optimization of a global strategy to address the pandemic threat. Proc. Natl. Acad. Sci. USA 2014, 111, 18519–18523. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Dar, O.A.; Hasan, R.; Schlundt, J.; Harbarth, S.; Caleo, G.; Dar, F.K.; Littmann, J.; Rweyemamu, M.; Buckley, E.J.; Shahid, M.; et al. Exploring the evidence base for national and regional policy interventions to combat resistance. Lancet 2016, 387, 285–295. [Google Scholar] [CrossRef]
- Gibbs, E.P. The evolution of One Health: A decade of progress and challenges for the future. Vet. Rec. 2014, 174, 85–91. [Google Scholar] [CrossRef]
- World Bank. Operational Framework for Strengthening Human, Animal and Environmental Public Health Systems at Their Interface; World Bank Group: Washington, DC, USA, 2018. [Google Scholar]
- Rabinowitz, P.M.; Kock, R.; Kachani, M.; Kunkel, R.; Thomas, J.; Gilbert, J.; Wallace, R.; Blackmore, C.; Wong, D.; Karesh, W.; et al. Toward proof of concept of a one health approach to disease prediction and control. Emerg. Infect. Dis. 2013, 19, e130265. [Google Scholar] [CrossRef]
| Pathogen Type | Diagnostic Technique | Limitations | Ref. |
|---|---|---|---|
| Viruses | Serological assays | Limited to past infection/exposure | |
| Viral culture | Slow and requires specific growth conditions | [40] | |
| Antigen-based assays | Sensitivity may vary with the test | ||
| Bacteria | Culture-based techniques | Slow results | |
| Gram-staining | Limited to bacterial cell structure | [40] | |
| Biochemical tests | Species-level identification may be lacking | ||
| Fungi | Culture-based techniques | Slow growth and identification | |
| Microscopic examination | Limited to visual characteristics | [40] | |
| Serological tests | Limited sensitivity and specificity | ||
| Parasites | Microscopic examination | Limited to detecting visible stages | |
| Serological tests | May not detect early infections | [40] | |
| Stool examination | May require multiple samples |
| Pathogen Type | Novel Detection Strategy | One Health Intervention | Importance/Significance | Ref. |
|---|---|---|---|---|
| Bacteria | Molecular diagnostics (PCR) | Early disease surveillance in animals and humans | Rapid and specific detection for timely intervention and understanding genetic factors in disease transmission. | [40,41] |
| Next-generation sequencing | Cross-sector data sharing and collaboration | |||
| Viruses | Metagenomic sequencing | Integrating environmental data | Detecting emerging viruses and understanding reservoirs and potential for point-of care testing and rapid response. | [40,41] |
| CRISPR-based diagnostics | Educating healthcare professionals | |||
| Fungi | DNA barcoding | Monitoring wildlife populations | Identifying fungal pathogens in zoonotic diseases and rapid identification of fungal species. | [40,41] |
| MALDI-TOF mass spectrometry | Promoting hygiene and sanitation in food production | |||
| Parasites | Nucleic acid amplification | Establishing One Health policies | Improved diagnosis of parasitic infections and identifying and tracking zoonotic parasites. | [40,41] |
| Serological tests with antigens | Cross-species surveillance |
| Pathogen Detection Strategy | Subtypes | Advantages | Disadvantages | Pathogens Detected | Antimicrobial Resistance | Ref. |
|---|---|---|---|---|---|---|
| Molecular diagnostics | PCR | Rapid, sensitive, specific, high-throughput, can detect low levels of pathogens. | Expensive, requires trained personnel and specialized equipment. | Bacteria, virus, fungi, parasites | Yes | [41,42] |
| Loop-mediated isothermal amplification | Rapid, sensitive, specific, low-cost. | Limited multiplexing capability, susceptibility to non-specific amplification. | ||||
| Nucleic acid sequence-based amplification | Rapid, sensitive, specific. | Limited multiplexing capability. | ||||
| Biosensors | Optical biosensors | Rapid, portable, real-time detection, high sensitivity, low sample volume required. | Limited multiplexing capability, may require specialized equipment. | Bacteria, virus, fungi, parasites | Yes | [43] |
| Electrochemical sensors | Rapid, portable, real-time detection, high sensitivity, low sample volume. | Limited multiplexing capability. | ||||
| Piezoelectric biosensors | Rapid, sensitive, specific, real-time detection, label-free detection. | Limited multiplexing capability. | ||||
| Next-generation sequencing | Whole-genome sequencing | High-throughput, comprehensive pathogen detection and characterization, can identify new and emerging pathogens. | Expensive, requires specialized equipment and trained personnel. | Bacteria, virus, fungi, parasites | Yes | [44] |
| Metagenomic sequencing (MGS) | High-throughput, comprehensive pathogen detection and characterization, can identify new and emerging pathogens, can detect co-infections and mixed infections. | Expensive, requires specialized equipment and trained personnel. | ||||
| Targeted amplicon sequencing (TAS) | Rapid and sensitive detection of specific pathogens or gene targets, can detect low levels of pathogen, high throughput with multiplexing capability. | Expensive, requires specialized equipment and trained personnel. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, R.; Vidic, J.; Auger, S.; Wen, H.-C.; Pandey, R.P.; Chang, C.-M. Exploring Disease Management and Control through Pathogen Diagnostics and One Health Initiative: A Concise Review. Antibiotics 2024, 13, 17. https://doi.org/10.3390/antibiotics13010017
Mukherjee R, Vidic J, Auger S, Wen H-C, Pandey RP, Chang C-M. Exploring Disease Management and Control through Pathogen Diagnostics and One Health Initiative: A Concise Review. Antibiotics. 2024; 13(1):17. https://doi.org/10.3390/antibiotics13010017
Chicago/Turabian StyleMukherjee, Riya, Jasmina Vidic, Sandrine Auger, Hsiao-Chuan Wen, Ramendra Pati Pandey, and Chung-Ming Chang. 2024. "Exploring Disease Management and Control through Pathogen Diagnostics and One Health Initiative: A Concise Review" Antibiotics 13, no. 1: 17. https://doi.org/10.3390/antibiotics13010017
APA StyleMukherjee, R., Vidic, J., Auger, S., Wen, H.-C., Pandey, R. P., & Chang, C.-M. (2024). Exploring Disease Management and Control through Pathogen Diagnostics and One Health Initiative: A Concise Review. Antibiotics, 13(1), 17. https://doi.org/10.3390/antibiotics13010017











