In Vitro Antifungal Activity of Three Synthetic Peptides against Candida auris and Other Candida Species of Medical Importance
Abstract
1. Introduction
2. Results
2.1. Peptides PNR20, PNR20-1, and 35409 Present Differential Antifungal Activity against Most Candida Species Studied
2.2. Peptides PNR20 and PNR20-1 Inhibit Biofilm Formation in C. albicans and C. auris
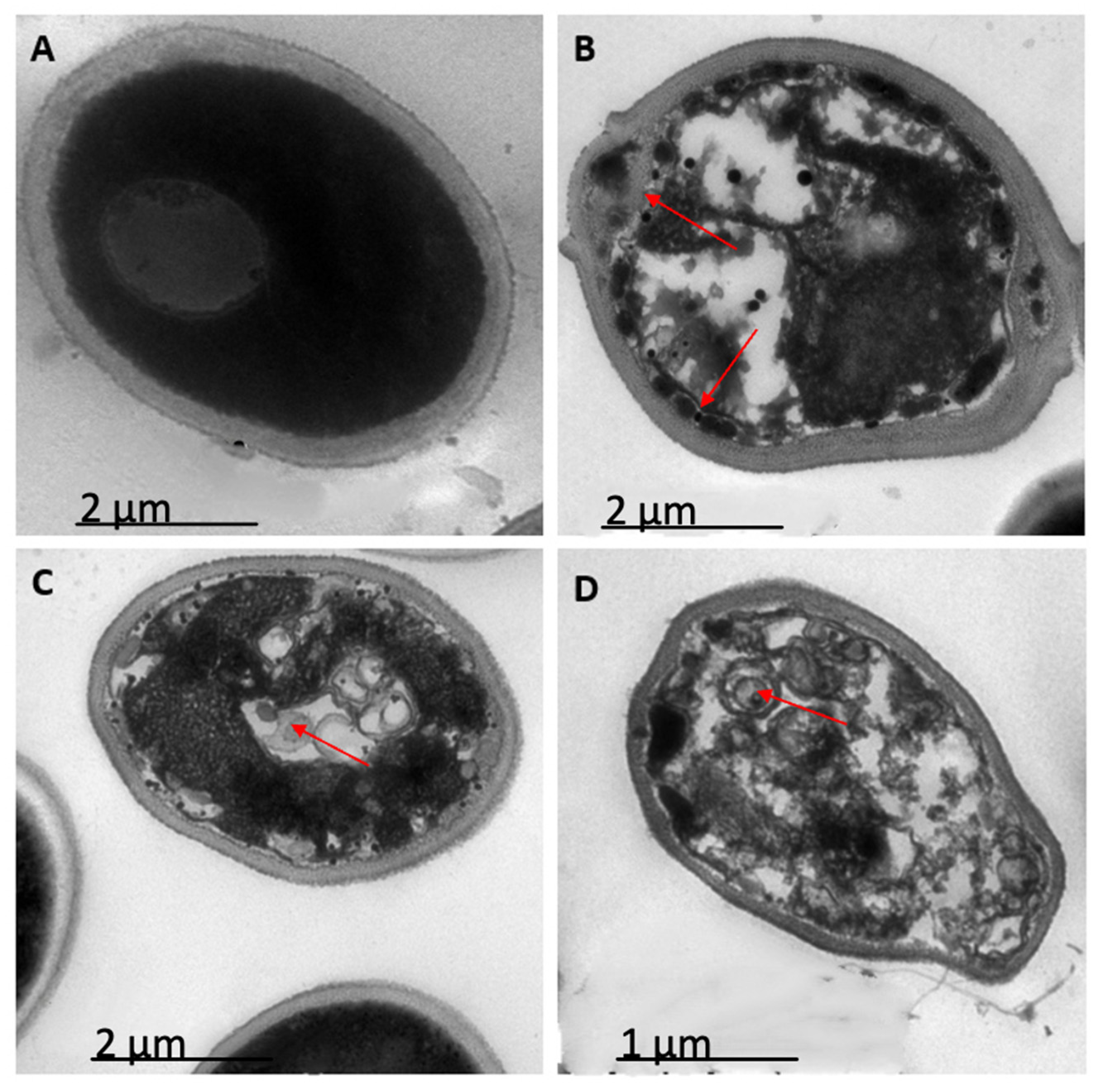
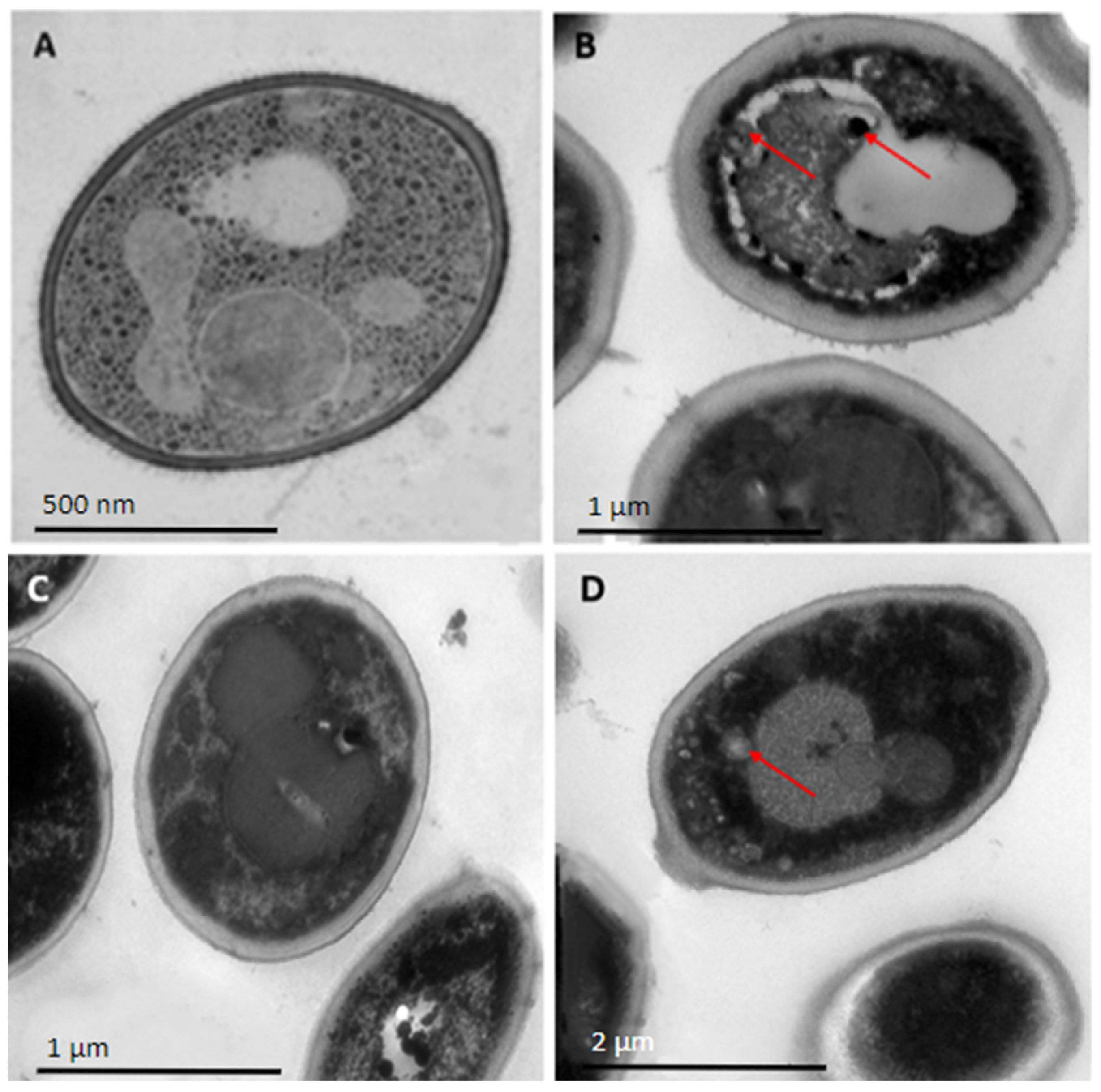
2.3. Peptides PNR20, PNR20-1, and 35409 Affect the Cellular Morphology of C. albicans and C. auris
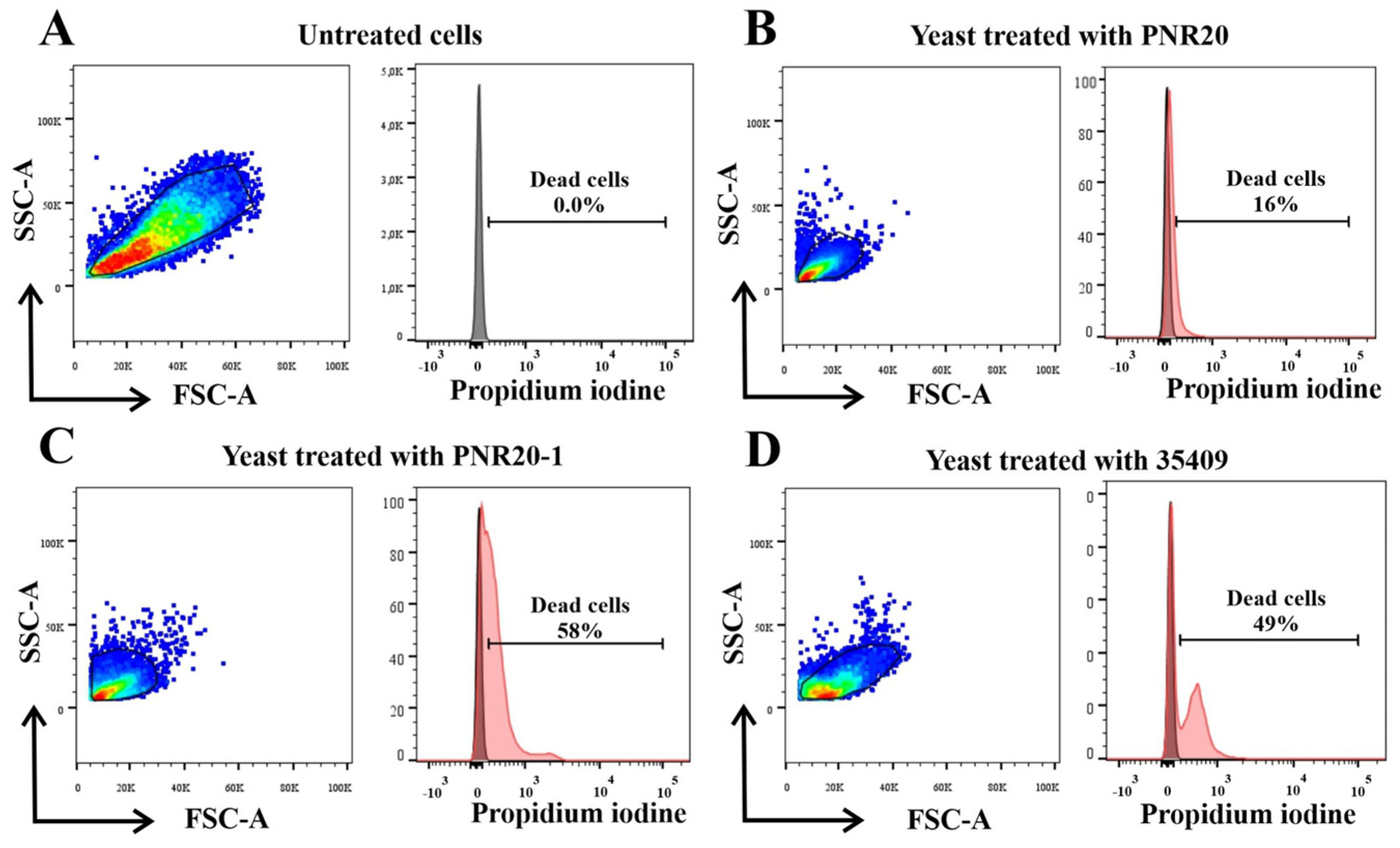
2.4. C. albicans Viability Is Altered after Treatment with Peptides PNR20, PNR20-1, and 35409
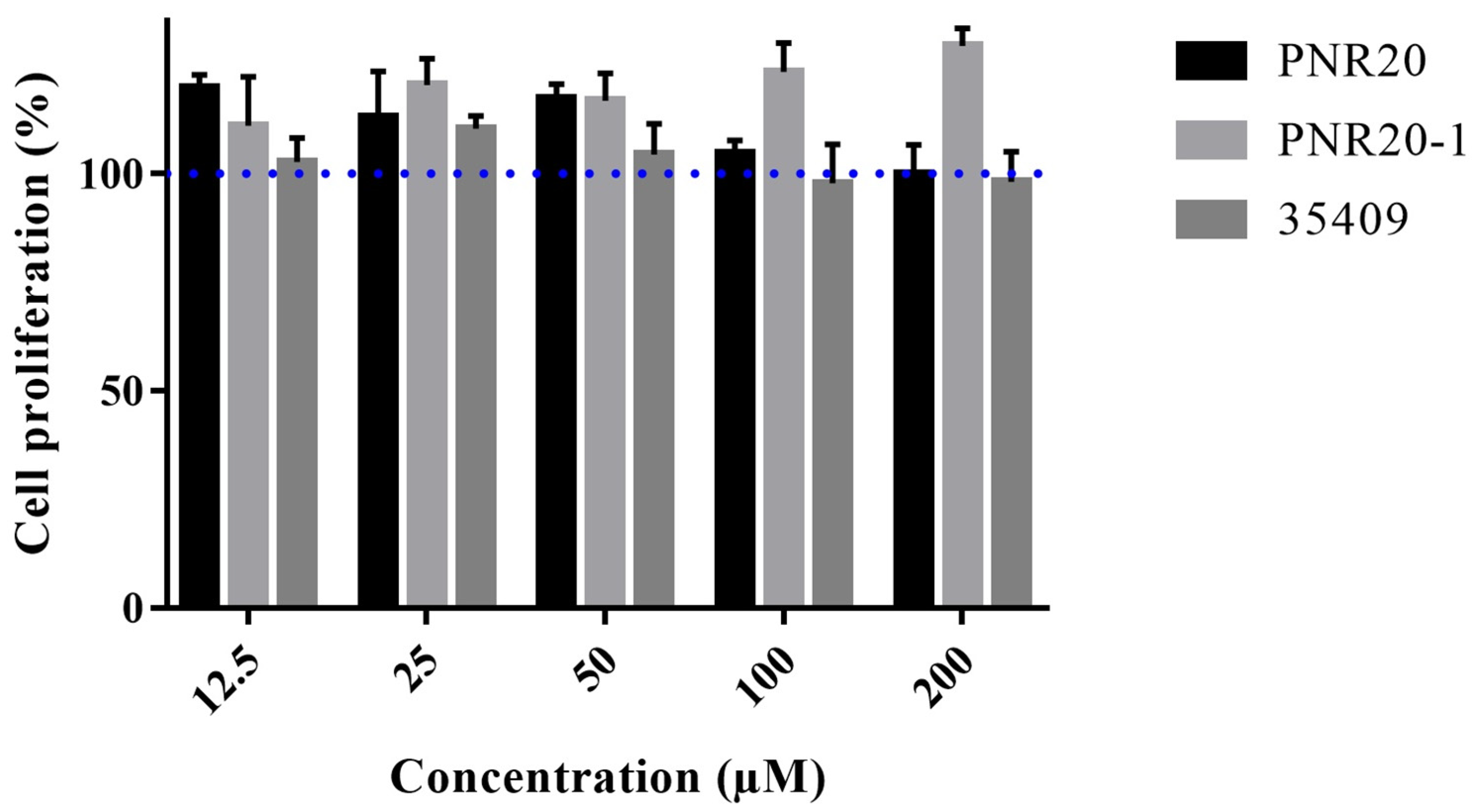
2.5. Peptides PNR20, PNR20-1, and 35409 Are Not Cytotoxic toward Murine Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Synthesis and Purification of Antimicrobial Peptides
4.2. Microorganisms and Cells
4.3. Susceptibility Assay of Planktonic Cells of Candida
4.4. Inhibition of Biofilm Formation Assay
4.5. Evaluation of Cell Morphology by Transmission Electron Microscopy
4.6. Evaluation of Viability by Flow Cytometry
4.7. In Vitro Cytotoxicity Assay with the Murine Cell Line L929
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.A.; Ruiz, J.F.; Melgarejo-Moreno, L.N.; Lemos, E.V. Candidemia in Colombia. Biomedica 2020, 40, 195–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef]
- Escandon, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud. Alerta por Emergencia Global de Infecciones Invasivas Causadas por la Levadura Multirresistente, Candida auris. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/IA/INS/ins-alerta-colombia-candida-auris.pdf (accessed on 24 March 2021).
- Institut Nacional de Salud. Circular Externa 1000-0025. Available online: https://www.ins.gov.co/buscador-eventos/Informacin%20de%20laboratorio/Circular-0025-2017-Fortalecimiento-Acciones-Vigilanica-Candia-auris.pdf (accessed on 24 March 2021).
- Escandon, P. Novel Environmental Niches for Candida auris: Isolation from a Coastal Habitat in Colombia. J. Fungi 2022, 8, 748. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Carvajal-Valencia, S.K.; Lizarazo, D.; Duarte, C.; Escandón, P. Identificación de aislamientos de Candida auris recuperados a través de la vigilancia por laboratorio en Colombia: Un reto para el diagnóstico. Infectio 2020, 24, 224–228. [Google Scholar] [CrossRef]
- Zuluaga-Rodriguez, A. Candida auris: Estrategias y retos para prevenir un brote. Biomedica 2020, 40, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Tanabe, K.; Niimi, M.; Monk, B.C. Candida albicans drug resistance another way to cope with stress. Microbiology 2007, 153, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Castano, G.P.; Rosenau, F.; Standker, L.; Firacative, C. Antimicrobial Peptides: Avant-Garde Antifungal Agents to Fight against Medically Important Candida Species. Pharmaceutics 2023, 15, 789. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Barreto-Santamaria, A.; Curtidor, H.; Arevalo-Pinzon, G.; Herrera, C.; Suarez, D.; Perez, W.H.; Patarroyo, M.E. A New Synthetic Peptide Having Two Target of Antibacterial Action in E. coli ML35. Front. Microbiol. 2016, 7, 2006. [Google Scholar] [CrossRef]
- Motoa, G.; Munoz, J.S.; Onate, J.; Pallares, C.J.; Hernandez, C.; Villegas, M.V. Epidemiology of Candida isolates from Intensive Care Units in Colombia from 2010 to 2013. Rev. Iberoam. Micol. 2017, 34, 17–22. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Gomez Cde, B.; Restrepo, C.A.; Parra, H.H.; Arteaga, M.A.; Moreno, A.R.; Marin, A.G. Susceptibility to fluconazole and voriconazole of Candida species isolated from intensive care units patients in Medellin, Colombia (2001–2007). Rev. Iberoam. Micol. 2010, 27, 125–129. [Google Scholar] [CrossRef]
- Maldonado, N.A.; Cano, L.E.; De Bedout, C.; Arbelaez, C.A.; Roncancio, G.; Tabares, A.M.; Robledo, C.G.; Robledo, J.; Grupo, G. Association of clinical and demographic factors in invasive candidiasis caused by fluconazole-resistant Candida species: A study in 15 hospitals, Medellin, Colombia 2010–2011. Diagn. Microbiol. Infect. Dis. 2014, 79, 280–286. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Li, R.; Chen, C.; Zhu, S.; Wang, X.; Yang, Y.; Shi, W.; Chen, S.; Wang, C.; Yan, L.; Shi, J. CGA-N9, an antimicrobial peptide derived from chromogranin A: Direct cell penetration of and endocytosis by Candida tropicalis. Biochem. J. 2019, 476, 483–497. [Google Scholar] [CrossRef]
- de Alteriis, E.; Maselli, V.; Falanga, A.; Galdiero, S.; Di Lella, F.M.; Gesuele, R.; Guida, M.; Galdiero, E. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018, 11, 915–925. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. The in vitro, in vivo antifungal activity and the action mode of Jelleine-I against Candida species. Amino Acids 2018, 50, 229–239. [Google Scholar] [CrossRef]
- Bellavita, R.; Maione, A.; Merlino, F.; Siciliano, A.; Dardano, P.; De Stefano, L.; Galdiero, S.; Galdiero, E.; Grieco, P.; Falanga, A. Antifungal and Antibiofilm Activity of Cyclic Temporin L Peptide Analogues against albicans and Non-albicans Candida Species. Pharmaceutics 2022, 14, 454. [Google Scholar] [CrossRef]
- Roscetto, E.; Contursi, P.; Vollaro, A.; Fusco, S.; Notomista, E.; Catania, M.R. Antifungal and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. clinical isolates. Sci. Rep. 2018, 8, 17570. [Google Scholar] [CrossRef]
- Kocendova, J.; Vankova, E.; Volejnikova, A.; Nesuta, O.; Budesinsky, M.; Socha, O.; Hajek, M.; Hadravova, R.; Cerovsky, V. Antifungal activity of analogues of antimicrobial peptides isolated from bee venoms against vulvovaginal Candida spp. FEMS Yeast Res. 2019, 19, foz013. [Google Scholar] [CrossRef]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Samaranayake, Y.H.; Samaranayake, L.P. Candida krusei: Biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J. Med. Microbiol. 1994, 41, 295–310. [Google Scholar] [CrossRef]
- Dal Mas, C.; Rossato, L.; Shimizu, T.; Oliveira, E.B.; da Silva Junior, P.I.; Meis, J.F.; Colombo, A.L.; Hayashi, M.A.F. Effects of the Natural Peptide Crotamine from a South American Rattlesnake on Candida auris, an Emergent Multidrug Antifungal Resistant Human Pathogen. Biomolecules 2019, 9, 205. [Google Scholar] [CrossRef]
- Dartevelle, P.; Ehlinger, C.; Zaet, A.; Boehler, C.; Rabineau, M.; Westermann, B.; Strub, J.M.; Cianferani, S.; Haikel, Y.; Metz-Boutigue, M.H.; et al. D-Cateslytin: A new antifungal agent for the treatment of oral Candida albicans associated infections. Sci. Rep. 2018, 8, 9235. [Google Scholar] [CrossRef]
- Ciociola, T.; Pertinhez, T.A.; De Simone, T.; Magliani, W.; Ferrari, E.; Belletti, S.; D’Adda, T.; Conti, S.; Giovati, L. In vitro and in vivo Anti-Candida Activity and Structural Analysis of Killer Peptide (KP)-Derivatives. J. Fungi 2021, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, G.; Coronado, Y.T.; Chaves, G.; Munoz, L.; Navarrete, J.; Salazar, L.M.; Taborda, C.P.; Munoz, J.E. In vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp. J. Fungi 2022, 8, 1173. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Dias, J.; de Souza Silva, C.; de Araujo, A.R.; Souza, J.M.T.; de Holanda Veloso Junior, P.H.; Cabral, W.F.; da Gloria da Silva, M.; Eaton, P.; de Souza de Almeida Leite, J.R.; Nicola, A.M.; et al. Mechanisms of action of antimicrobial peptides ToAP2 and NDBP-5.7 against Candida albicans planktonic and biofilm cells. Sci. Rep. 2020, 10, 10327. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Munoz, J.E.; Ramirez, L.M.; Dias, L.D.S.; Rivas, L.A.; Ramos, L.S.; Santos, A.L.S.; Taborda, C.P.; Parra-Giraldo, C.M. Pathogenicity Levels of Colombian Strains of Candida auris and Brazilian Strains of Candida haemulonii Species Complex in Both Murine and Galleria mellonella Experimental Models. J. Fungi 2020, 6, 104. [Google Scholar] [CrossRef]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, Q.; Hu, F.; Li, H.; Yang, B.; Duan, Z.; Zhang, K.; Wu, J.; Li, W.; Luo, Z. Antifungal activity of MAF-1A peptide against Candida albicans. Int. Microbiol. 2021, 24, 233–242. [Google Scholar] [CrossRef]
- Malik, M.A.; Lone, S.A.; Wani, M.Y.; Talukdar, M.I.A.; Dar, O.A.; Ahmad, A.; Hashmi, A.A. S-benzyldithiocarbazate imine coordinated metal complexes kill Candida albicans by causing cellular apoptosis and necrosis. Bioorg. Chem. 2020, 98, 103771. [Google Scholar] [CrossRef]
- Ramamourthy, G.; Park, J.; Seo, C.; Vogel, J.H.; Park, Y. Antifungal and Antibiofilm Activities and the Mechanism of Action of Repeating Lysine-Tryptophan Peptides against Candida albicans. Microorganisms 2020, 8, 758. [Google Scholar] [CrossRef]
- CDC. Antifungal Susceptibility Testing and Interpretation. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 1 June 2023).
- CLSI supplement M27M44S; Performance Standards for Antifungal Susceptibility Testing of Yeasts. CLSI: Wayne, PA, USA, 2022.
- Munoz, J.E.; Rossi, D.C.P.; Ishida, K.; Spadari, C.C.; Melhem, M.S.C.; Garcia, D.M.; Caires, A.C.F.; Taborda, C.P.; Rodrigues, E.G. Antifungal Activity of the Biphosphinic Cyclopalladate C7a against Candida albicans Yeast Forms in vitro and in vivo. Front. Microbiol. 2017, 8, 771. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Danihelova, M.; Veverka, M.; Sturdik, E.; Jantova, S. Antioxidant action and cytotoxicity on HeLa and NIH-3T3 cells of new quercetin derivatives. Interdiscip. Toxicol. 2013, 6, 209–216. [Google Scholar] [CrossRef]
| Strain | Peptide | MIC | IC90 | IC50 |
|---|---|---|---|---|
| C. albicans ATCC 10231 | PNR20 | 100 µM | 50 µM | 6.25 µM |
| PNR20-1 | 100 µM | ND | 6.25 µM | |
| 35409 | >100 µM | >100 µM | 25 µM | |
| C. parapsilosis ATCC 22019 | PNR20 | 100 µM | 25 µM | 6.25 µM |
| PNR20-1 | >100 µM | 50 µM | 6.25 µM | |
| 35409 | >100 µM | 100 µM | 1.56 µM | |
| C. glabrata ATCC 2001 | PNR20 | 100 µM | ND | 6.25 µM |
| PNR20-1 | >100 µM | >100 µM | 3.12 µM | |
| 35409 | >100 µM | >100 µM | 100 µM | |
| C. krusei ATCC 6558 | PNR20 | 25 µM | 12.5 µM | 3.12 µM |
| PNR20-1 | 25 µM | ND | ND | |
| 35409 | 100 µM | 50 µM | 3.12 µM | |
| C. tropicalis ATCC750 | PNR20 | 50 µM | 25 µM | 6.25 µM |
| PNR20-1 | 100 µM | 50 µM | 25 µM | |
| 35409 | 50 µM | ND | ND | |
| C. auris H0059-13-1421 FCZ susceptible | PNR20 | >100 µM | >100 µM | 25 µM |
| PNR20-1 | >100 µM | >100 µM | 6.25 µM | |
| 35409 | >100 µM | >100 µM | 12.5 µM | |
| C. auris H0059-13-2220 FCZ and AMB resistant | PNR20 | >100 µM | >100 µM | 25 µM |
| PNR20-1 | >100 µM | >100 µM | 100 µM | |
| 35409 | >100 µM | >100 µM | >100 µM | |
| C. auris H0059-13-2251 FCZ and AMB resistant | PNR20 | >100 µM | >100 µM | 100 µM |
| PNR20-1 | >100 µM | >100 µM | >100 µM | |
| 35409 | >100 µM | >100 µM | 50 µM | |
| C. auris H0059-13-2276 FCZ and AMB resistant | PNR20 | >100 µM | >100 µM | 100 µM |
| PNR20-1 | >100 µM | >100 µM | 100 µM | |
| 35409 | >100 µM | >100 µM | >100 µM | |
| C. auris H0059-13-2265 FCZ and AMB resistant | PNR20 | >100 µM | >100 µM | 100 µM |
| PNR20-1 | >100 µM | >100 µM | >100 µM | |
| 35409 | >100 µM | >100 µM | >100 µM |
| Time | Species | Peptide | BMIC | IC90 | IC50 |
|---|---|---|---|---|---|
| 24 h | C. albicans ATCC 10231 | PNR20 | 12.5 µM | ND | ND |
| PNR20-1 | 25 µM | 12.5 µM | 3.12 µM | ||
| 35409 | ≥100 µM | ≥100 µM | 3.12 µM | ||
| C. auris H0059-13-1421 | PNR20 | ND | ND | 25 µM | |
| PNR20-1 | ND | ND | 25 µM | ||
| 35409 | ND | ND | 12.5 µM | ||
| 72 h | C. albicans ATCC 10231 | PNR20 | 25 µM | ND | ND |
| PNR20-1 | 100 µM | ND | 50 µM | ||
| 35409 | ND | ND | 50 µM | ||
| C. auris H0059-13-1421 | PNR20 | ND | ND | 25 µM | |
| PNR20-1 | ND | ND | 12.5 µM | ||
| 35409 | ND | ND | 25 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, R.; Barreto-Santamaría, A.; Arévalo-Pinzón, G.; Firacative, C.; Gómez, B.L.; Escandón, P.; Patarroyo, M.A.; Muñoz, J.E. In Vitro Antifungal Activity of Three Synthetic Peptides against Candida auris and Other Candida Species of Medical Importance. Antibiotics 2023, 12, 1234. https://doi.org/10.3390/antibiotics12081234
Torres R, Barreto-Santamaría A, Arévalo-Pinzón G, Firacative C, Gómez BL, Escandón P, Patarroyo MA, Muñoz JE. In Vitro Antifungal Activity of Three Synthetic Peptides against Candida auris and Other Candida Species of Medical Importance. Antibiotics. 2023; 12(8):1234. https://doi.org/10.3390/antibiotics12081234
Chicago/Turabian StyleTorres, Richar, Adriana Barreto-Santamaría, Gabriela Arévalo-Pinzón, Carolina Firacative, Beatriz L. Gómez, Patricia Escandón, Manuel Alfonso Patarroyo, and Julián E. Muñoz. 2023. "In Vitro Antifungal Activity of Three Synthetic Peptides against Candida auris and Other Candida Species of Medical Importance" Antibiotics 12, no. 8: 1234. https://doi.org/10.3390/antibiotics12081234
APA StyleTorres, R., Barreto-Santamaría, A., Arévalo-Pinzón, G., Firacative, C., Gómez, B. L., Escandón, P., Patarroyo, M. A., & Muñoz, J. E. (2023). In Vitro Antifungal Activity of Three Synthetic Peptides against Candida auris and Other Candida Species of Medical Importance. Antibiotics, 12(8), 1234. https://doi.org/10.3390/antibiotics12081234









