Anti-Inflammatory Activity of Glyceryl 1,3-Distearate Identified from Clinacanthus nutans Extract against Bovine Mastitis Pathogens
Abstract
1. Introduction
2. Results
2.1. LPS-Induced Cell Death and Inflammation in Bovine Endothelial Cells
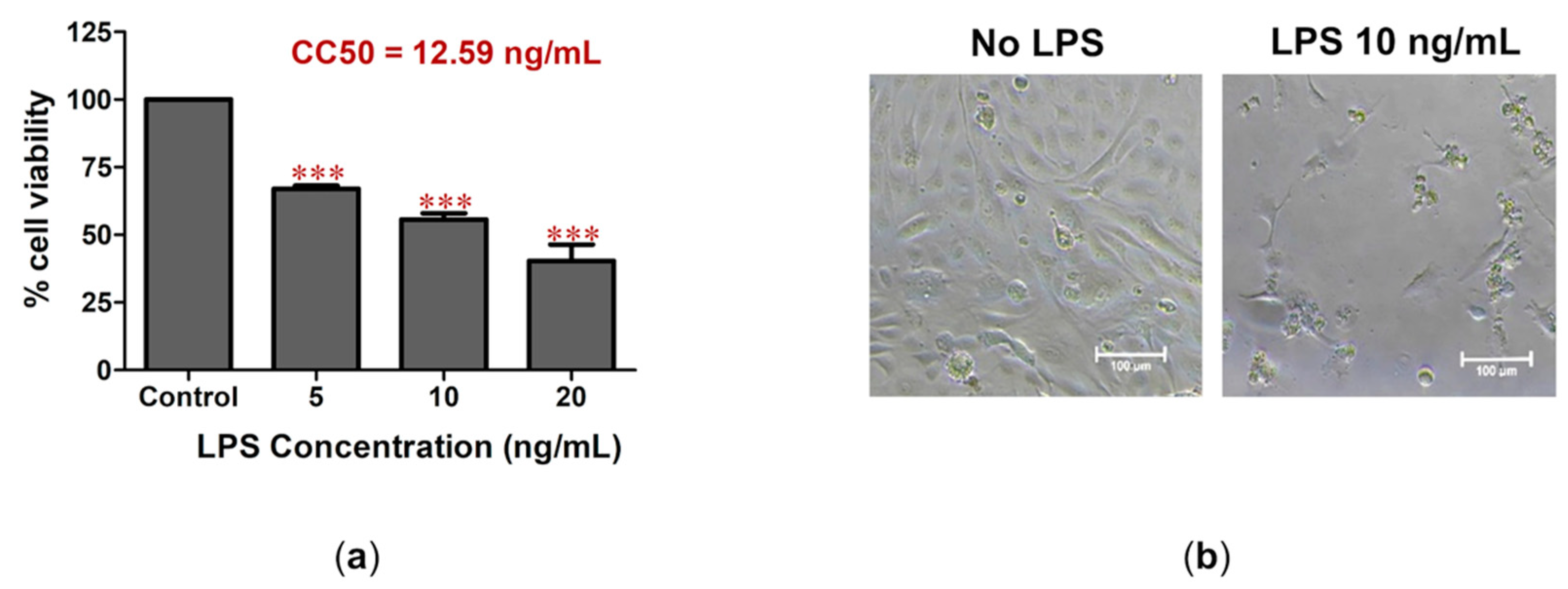
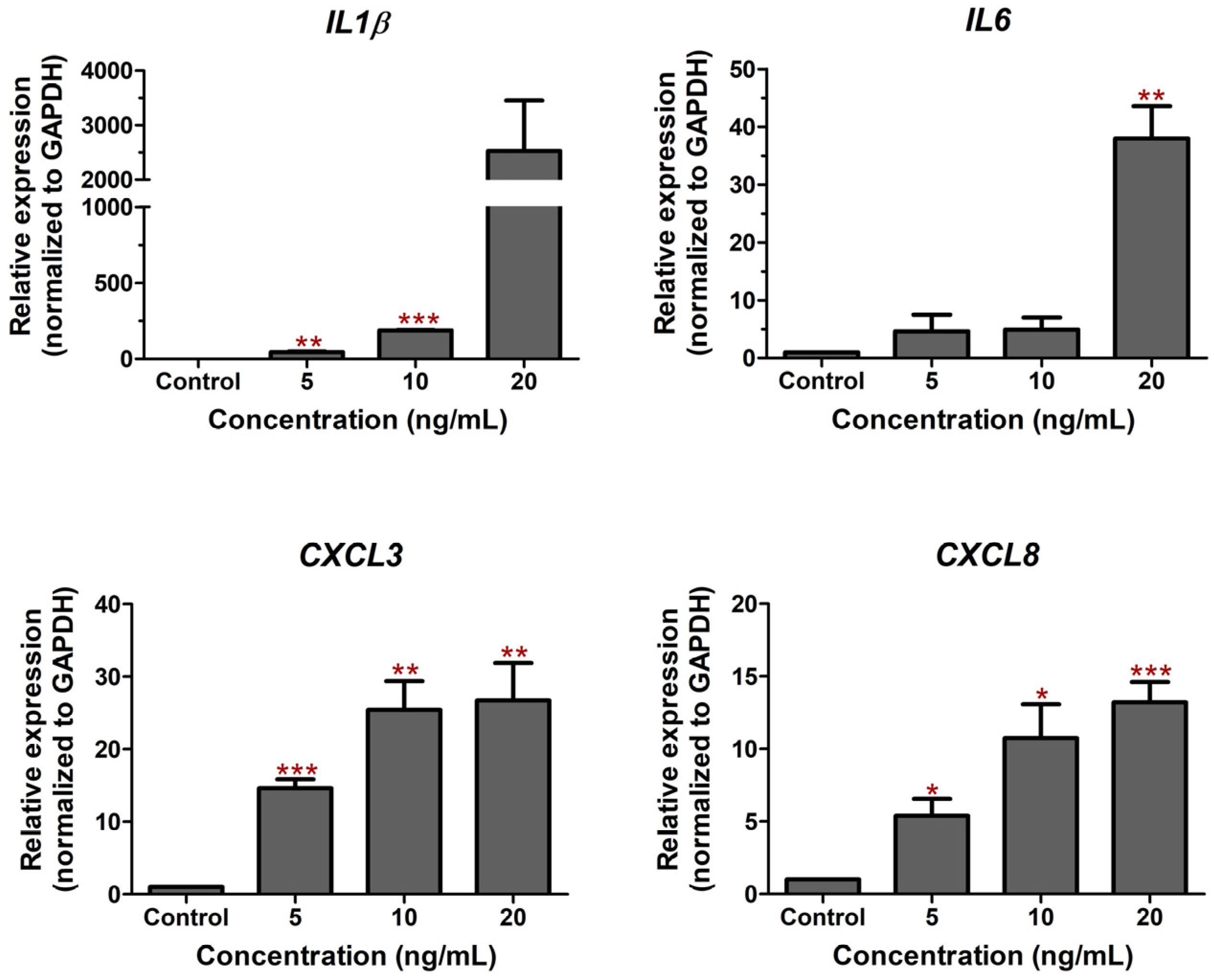
2.2. C. nutans Extract Protected against Cell Death and Lowered Inflammation Response
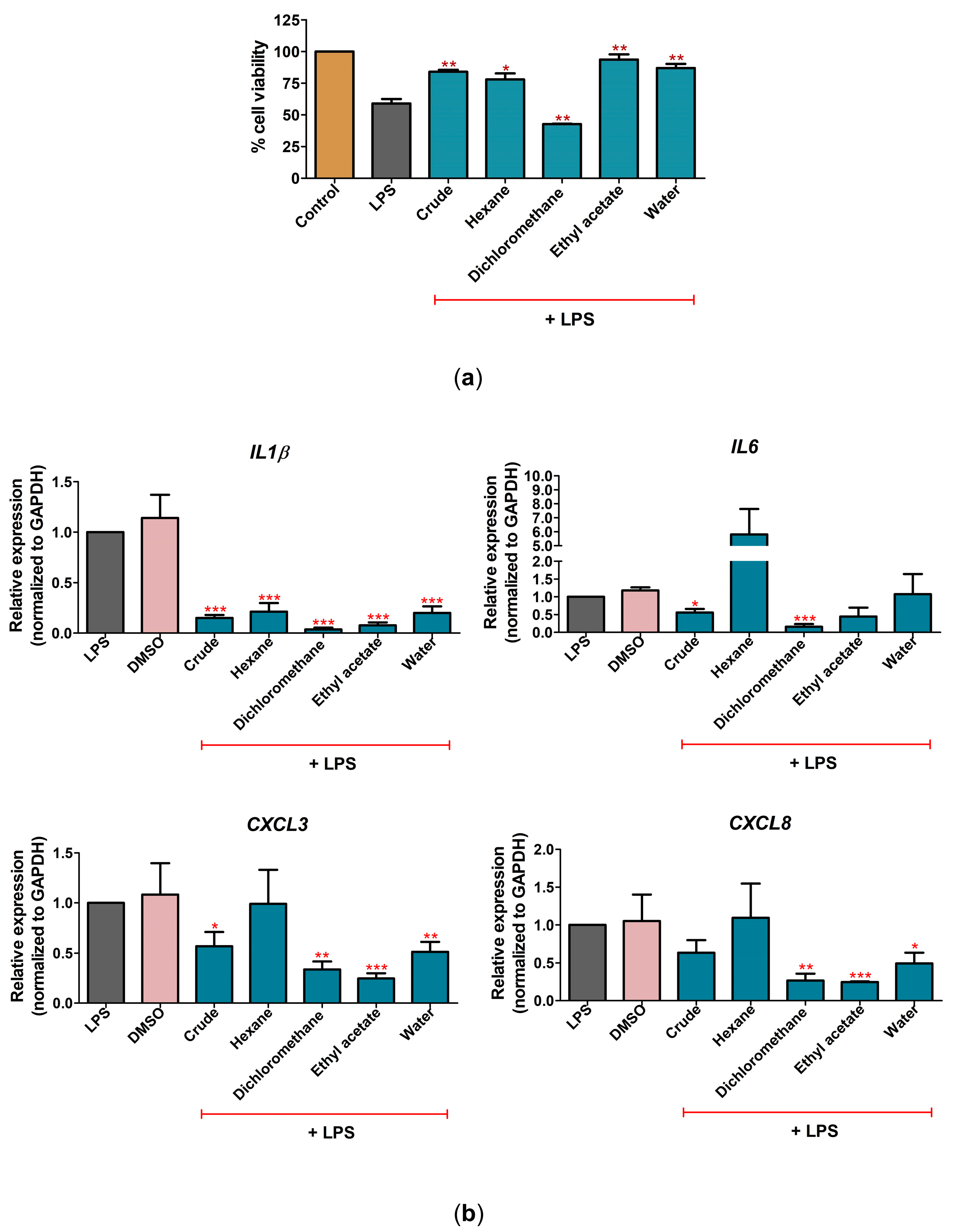
2.3. C. nutans Extract Fractions Exerted Anti-Cell-Death and Anti-Inflammation Activity
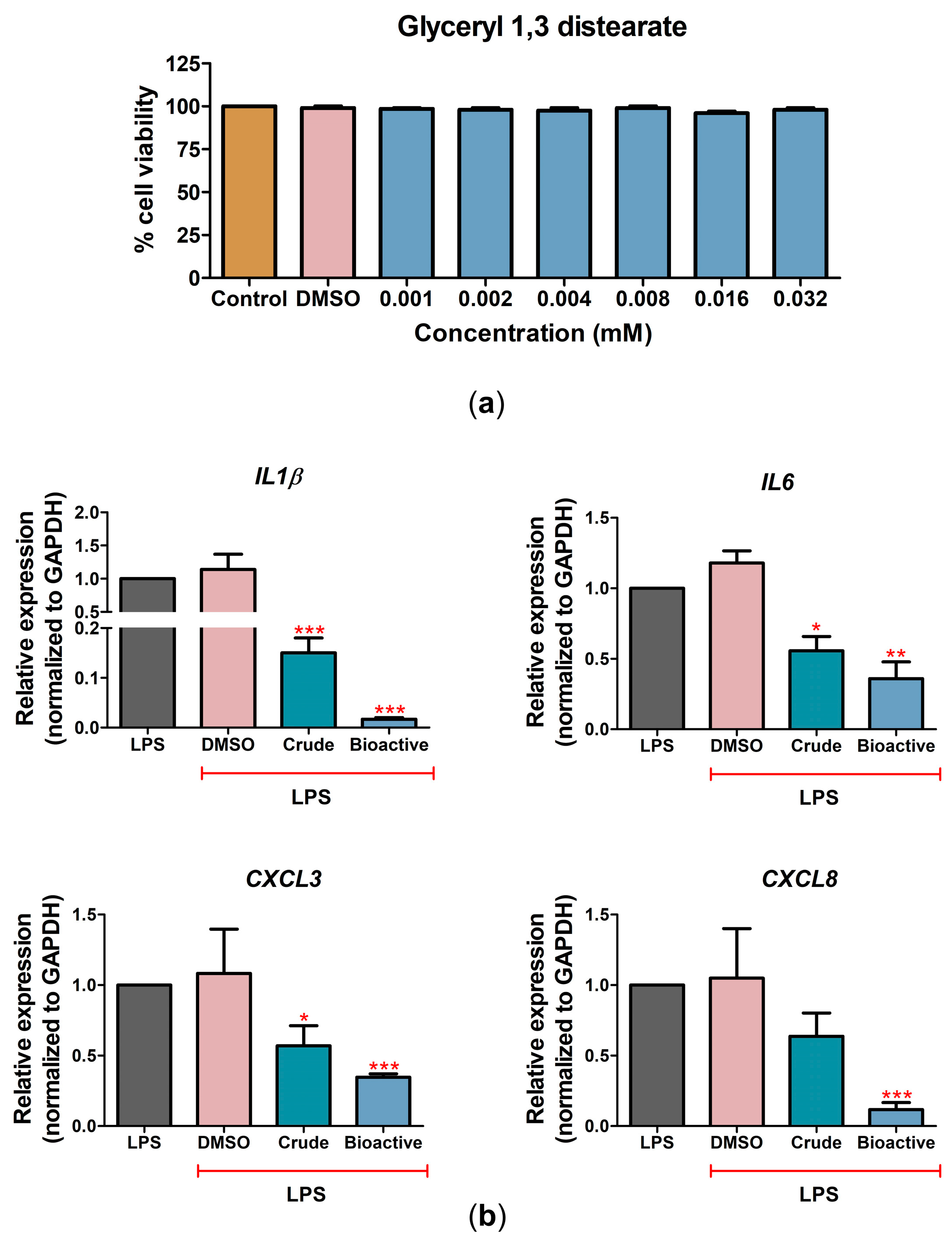
2.4. The Bioactive Compound Glyceryl 1,3-Distearate Promoted Anti-Inflammation Activity
3. Discussion
4. Materials and Methods
4.1. Herb Extracts
4.2. Fractionation of Herb Extracts
4.3. Cell Lines and Reagents
4.4. Cell Viability
4.5. Real-Time PCR
4.6. Thin-Layer Chromatography (TLC) Analysis
4.7. Gas Chromatography-Mass Spectrometry/Mass Spectrometry (GC-MS/MS) Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaiprateep, E.-O.; Thavornwat, C. Optimization of Clinacanthus nutans biodegradable analgesic patch. Nat. Prod. Commun. 2018, 13, 1934578X1801301201. [Google Scholar] [CrossRef]
- Alam, A.; Ferdosh, S.; Ghafoor, K.; Hakim, A.; Juraimi, A.S.; Khatib, A.; Sarker, Z.I. Clinacanthus nutans: A review of the medicinal uses, pharmacology and phytochemistry. Asian Pac. J. Trop. Med. 2016, 9, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.Z.; Arsad, H.; Samian, M.R.; Ab Majid, A.H.; Hamdan, M.R. Evaluation of genetic diversity of Clinacanthus nutans (Acanthaceaea) using RAPD, ISSR and RAMP markers. Physiol. Mol. Biol. Plants 2016, 22, 523–534. [Google Scholar] [CrossRef]
- Zulkipli, I.N.; Rajabalaya, R.; Idris, A.; Sulaiman, N.A.; David, S.R. Clinacanthus nutans: A review on ethnomedicinal uses, chemical constituents and pharmacological properties. Pharm. Biol. 2017, 55, 1093–1113. [Google Scholar] [CrossRef] [PubMed]
- Kongkaew, C.; Chaiyakunapruk, N. Efficacy of Clinacanthus nutans extracts in patients with herpes infection: Systematic review and meta-analysis of randomised clinical trials. Complement. Ther. Med. 2011, 19, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sangkitporn, S.; Chaiwat, S.; Balachandra, K.; Na-Ayudhaya, T.D.; Bunjob, M.; Jayavasu, C. Treatment of herpes zoster with Clinacanthus nutans (bi phaya yaw) extract. J. Med. Assoc. Thail. Chotmaihet Thangphaet 1995, 78, 624–627. [Google Scholar]
- Wanikiat, P.; Panthong, A.; Sujayanon, P.; Yoosook, C.; Rossi, A.G.; Reutrakul, V. The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans extracts. J. Ethnopharmacol. 2008, 116, 234–244. [Google Scholar] [CrossRef]
- Arullappan, S.; Rajamanickam, P.; Thevar, N.; Kodimani, C.C. In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop. J. Pharm. Res. 2014, 13, 1455–1461. [Google Scholar] [CrossRef]
- Panya, A.; Pundith, H.; Thongyim, S.; Kaewkod, T.; Chitov, T.; Bovonsombut, S.; Tragoolpua, Y. Antibiotic-antiapoptotic dual function of Clinacanthus nutans (Burm. F.) lindau leaf extracts against bovine mastitis. Antibiotics 2020, 9, 429. [Google Scholar] [CrossRef]
- Huang, D.; Guo, W.; Gao, J.; Chen, J.; Olatunji, J.O. Clinacanthus nutans (Burm. f.) Lindau ethanol extract inhibits hepatoma in mice through upregulation of the immune response. Molecules 2015, 20, 17405–17428. [Google Scholar] [CrossRef]
- Azooz, M.; El-Wakeel, S.A.; Yousef, H. Financial and economic analyses of the impact of cattle mastitis on the profitability of Egyptian dairy farms. Vet. World 2020, 13, 1750. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.E.; Heller, E.D.; Jacoby, S.; Krifucks, O.; Leitner, G. Comparison of the immune responses associated with experimental bovine mastitis caused by different strains of Escherichia coli. J. Dairy Res. 2017, 84, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef] [PubMed]
- Farhana, A.; Khan, Y.S. Biochemistry, lipopolysaccharide. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Schukken, Y.H.; Günther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.-J.; Sipka, A. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Willimann, K. Chemokines: Role in inflammation and immune surveillance. Ann. Rheum. Dis. 2004, 63, ii84–ii89. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.R.; Osmani, R.A.; Ghodake, P.P.; Shaikh, S.M.; Chavan, S.R. Mastitis: An intensive crisis in veterinary science. Int. J. Pharma Res. Health Sci. 2014, 2, 96–103. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Khoo, L.W.; Audrey Kow, S.; Lee, M.T.; Tan, C.P.; Shaari, K.; Tham, C.L.; Abas, F. A comprehensive review on phytochemistry and pharmacological activities of Clinacanthus nutans (Burm. f.) Lindau. Evid.-Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef]
- Zhao, X.; Lacasse, P. Mammary tissue damage during bovine mastitis: Causes and control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef]
- Grandel, U.; Grimminger, F. Endothelial responses to bacterial toxins in sepsis. Crit. Rev. Immunol. 2003, 23. [Google Scholar] [CrossRef]
- Kuo, X.; Herr, D.R.; Ong, W.-Y. Anti-inflammatory and cytoprotective effect of Clinacanthus nutans leaf but not stem extracts on 7-ketocholesterol induced brain endothelial cell injury. Neuromolecular Med. 2021, 23, 176–183. [Google Scholar] [CrossRef] [PubMed]

| No. | Classification | IUPAC Name | Name of Compound | Molecular Formula | Peak Area | RT |
|---|---|---|---|---|---|---|
| 1 | Fatty Acids | 1-ethoxybutane | Butane, 1-ethoxy- | C6H14O | 185,779 (15) | 3.496 |
| 2 | propyl acetate | n-Propyl acetate | C5H10O2 | 3,357,413 (04) | 3.733 | |
| 3 | butan-2-yl acetate | sec-Butyl acetate | C6H12O2 | 1,647,493 (07) | 4.465 | |
| 4 | 1-butoxybutane | n-Butyl ether | C8H18O | 267,510 (14) | 7.555 | |
| 5 | 2-ethylhexyl hexyl sulfite | Sulfurous acid | C14H30O3S | 168,824 (16) | 20.671 | |
| 6 | octadecanoic acid | Hexadecanoic acid | C18H36O2 | 2,304,434 (05) | 36.235 | |
| 7 | methyl (11E,14E,17E)-icosa-11,14,17-trienoate | 11,14,17-Eicosatrienoic acid | C21H36O2 | 1,199,156 (10) | 38.983 | |
| 8 | ethyl (9Z,12Z,15Z)-octadeca-9,12,15-trienoate | 9,12,15-Octadecatrienoic acid | C20H34O2 | 4,601,038 (02) | 39.635 | |
| 9 | ethyl octadecanoate | Octadecanoic acid | C20H40O2 | 1,615,070 (08) | 40.169 | |
| 10 | dioctyl hexanedioate | Hexanedioic acid | C22H42O4 | 901,322 (12) | 43.862 | |
| 11 | 2,3-dihydroxypropyl hexadecanoate | Hexadecanoic acid | C19H38O4 | 1,532,912 (09) | 45.993 | |
| 12 | (2-hydroxy-3-octadecanoyloxypropyl) octadecanoate | Glyceryl 1,3-distearate | C39H76O5 | 1,954,834 (06) | 49.320 | |
| 13 | Glycosides | (2S,3R,4S,5S,6R)-2-ethoxy-6-(hydroxymethyl) oxane-3,4,5-triol | Ethyl alpha-d glucopyranoside | C8H16O6 | 949,858 (11) | 28.386 |
| 14 | Terpenoids | (E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-ol | Phytol | C20H40O | 4,125,560 (03) | 38.460 |
| 15 | Cyclopentanes | cyclopent-4-ene-1,3-dione | 4-Cyclopentene-1,3-dione | C5H4O2 | 503,713 (13) | 7.986 |
| 16 | Disulfides | (methyldisulfanyl)methane | Dimethyl disulfide | C2H6S2 | 5,088,889 (01) | 4.298 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongyim, S.; Chiangchin, S.; Pandith, H.; Tragoolpua, Y.; Jangsutthivorawat, S.; Panya, A. Anti-Inflammatory Activity of Glyceryl 1,3-Distearate Identified from Clinacanthus nutans Extract against Bovine Mastitis Pathogens. Antibiotics 2023, 12, 549. https://doi.org/10.3390/antibiotics12030549
Thongyim S, Chiangchin S, Pandith H, Tragoolpua Y, Jangsutthivorawat S, Panya A. Anti-Inflammatory Activity of Glyceryl 1,3-Distearate Identified from Clinacanthus nutans Extract against Bovine Mastitis Pathogens. Antibiotics. 2023; 12(3):549. https://doi.org/10.3390/antibiotics12030549
Chicago/Turabian StyleThongyim, Saruda, Salinee Chiangchin, Hataichanok Pandith, Yingmanee Tragoolpua, Siriphorn Jangsutthivorawat, and Aussara Panya. 2023. "Anti-Inflammatory Activity of Glyceryl 1,3-Distearate Identified from Clinacanthus nutans Extract against Bovine Mastitis Pathogens" Antibiotics 12, no. 3: 549. https://doi.org/10.3390/antibiotics12030549
APA StyleThongyim, S., Chiangchin, S., Pandith, H., Tragoolpua, Y., Jangsutthivorawat, S., & Panya, A. (2023). Anti-Inflammatory Activity of Glyceryl 1,3-Distearate Identified from Clinacanthus nutans Extract against Bovine Mastitis Pathogens. Antibiotics, 12(3), 549. https://doi.org/10.3390/antibiotics12030549







