Newly Discovered Mechanisms of Antibiotic Self-Resistance with Multiple Enzymes Acting at Different Locations and Stages
Abstract
1. Introduction
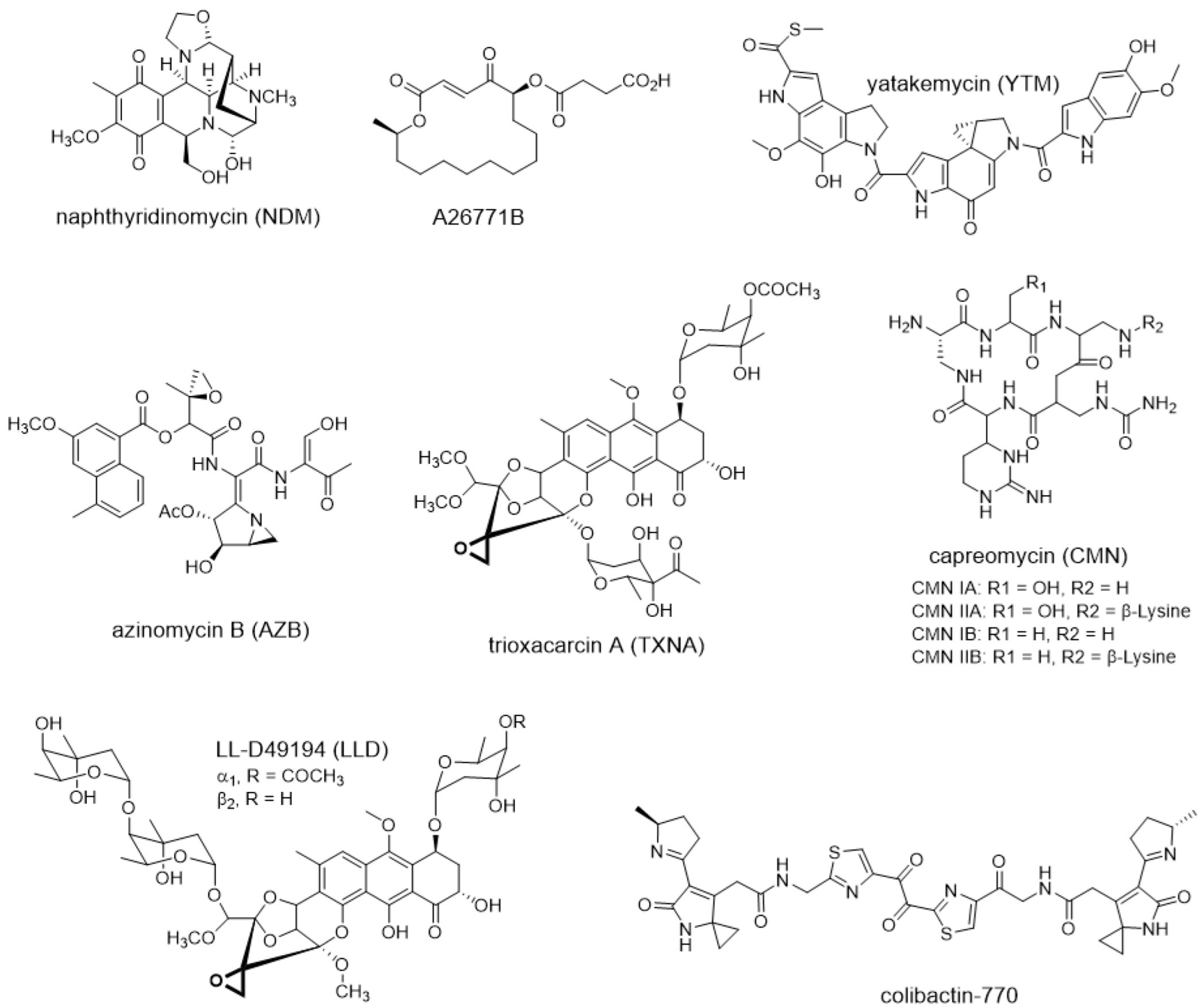
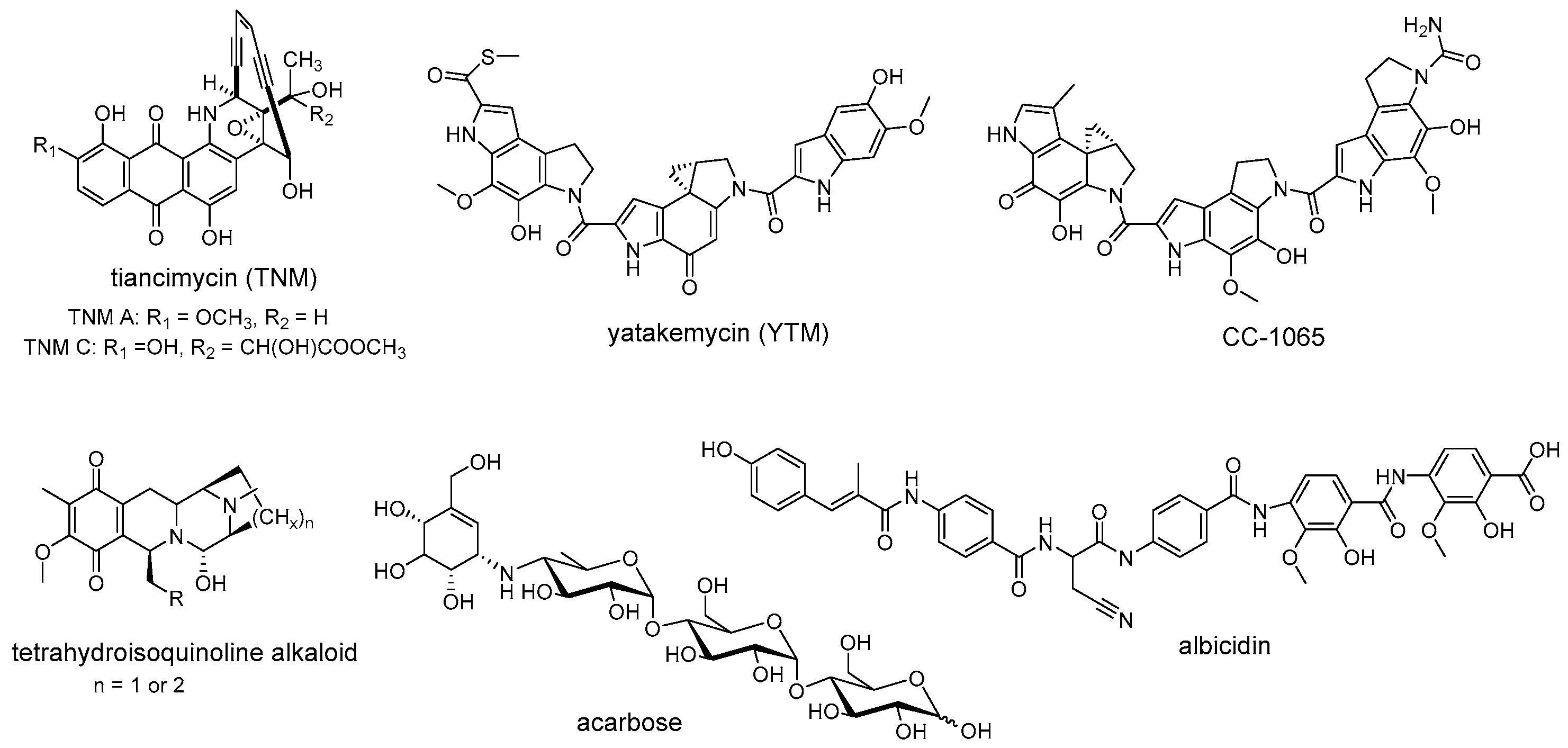
2. Temporal-Spatial Shielding Resistance
3. Intracellular Multi-Level Resistance
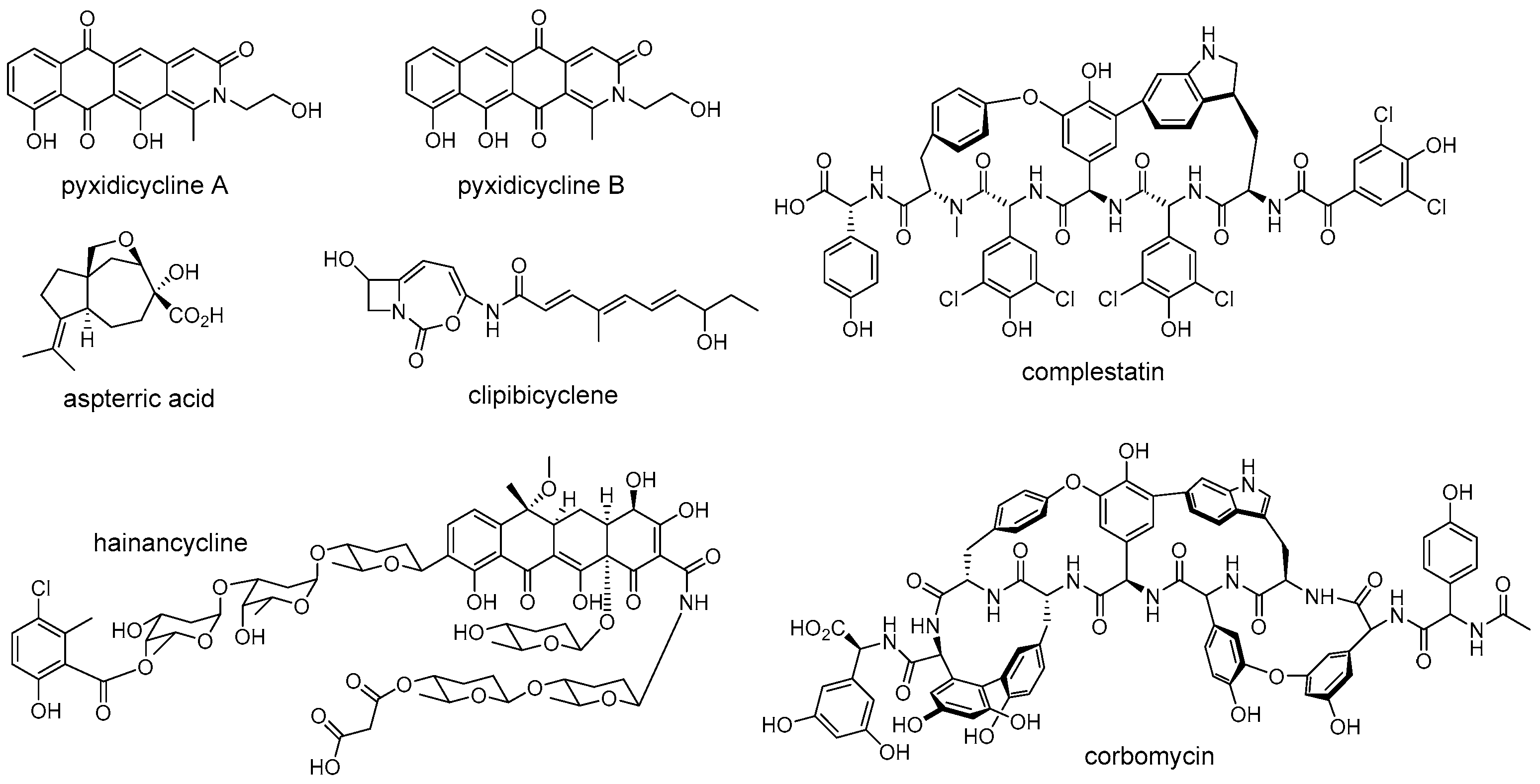
4. Resistance Widespread in Nature
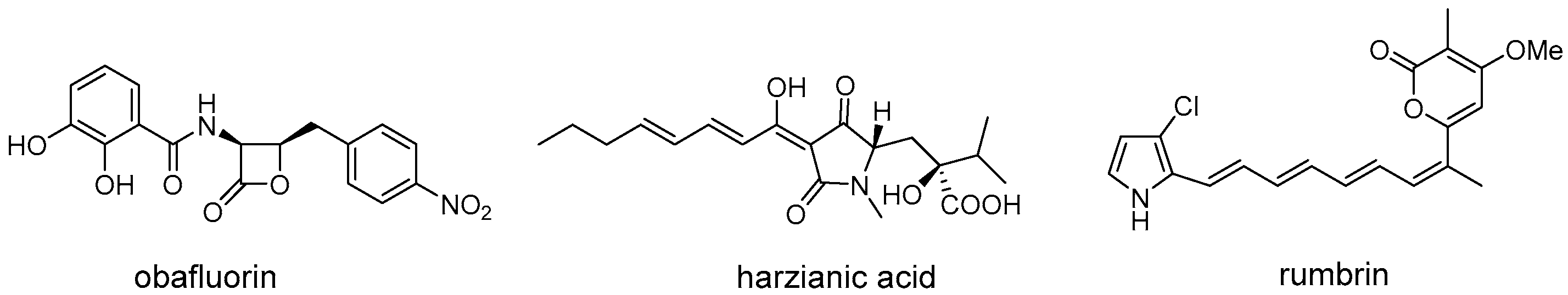
5. Resistance-Guided Natural Products Discovery
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waksman, S.A.; Schatz, A.F.; Reynolds, D.M. Production of antibiotic substances by actinomycetes. Ann. N. Y. Acad. Sci. 2010, 1213, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Beyer, P.; Moorthy, V.; Paulin, S.; Hill, S.R.; Sprenger, M.; Garner, S.; Simão, M.; Guerra, R.; Magrini, N.; Swaminathan, S. The drugs don’t work: WHO’s role in advancing new antibiotics. Lancet 2018, 392, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Neil, J.O. The Review on Antimocronial Resistance. Available online: https://amr-review.org (accessed on 1 October 2022).
- Almabruk, K.H.; Dinh, L.K.; Philmus, B. Self-resistance of natural product producers: Past, present, and future focusing on self-resistant protein variants. ACS Chem. Biol. 2018, 13, 1426–1437. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, N.; Tang, Y. Recent developments in self-resistance gene directed natural product discovery. Nat. Prod. Rep. 2020, 37, 879–892. [Google Scholar] [CrossRef]
- Wencewicz, T.A. Crossroads of antibiotic resistance and biosynthesis. J. Mol. Biol. 2019, 431, 3370–3399. [Google Scholar] [CrossRef]
- Wright, G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef]
- Cundliffe, E.; Demain, A.L. Avoidance of suicide in antibiotic-producing microbes. J. Ind. Microbiol. Biotechnol. 2010, 37, 643–672. [Google Scholar] [CrossRef]
- Livermore, D. Can better prescribing turn the tide of resistance? Nat. Rev. Microbiol. 2004, 2, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, Q.; Zang, X.; Yuan, S.; Bat-Erdene, U.; Nguyen, C.; Gan, J.; Zhou, J.; Jacobsen, S.E.; Tang, Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature 2018, 559, 415–418. [Google Scholar] [CrossRef]
- Galm, U.; Hager, M.H.; Van Lanen, S.G.; Ju, J.; Thorson, J.S.; Shen, B. Antitumor antibiotics: Bleomycin, enediynes, and mitomycin. Chem. Rev. 2005, 105, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Yan, X.; Crnovcic, I.; Annaval, T.; Chang, C.; Nocek, B.; Rudolf, J.D.; Yang, D.; Hindra; Babnigg, G.; et al. Resistance to enediyne antitumor antibiotics by sequestration. Cell Chem. Biol. 2018, 25, 1075–1085. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, W.H.; Pu, J.Y.; Tang, M.C.; Zhang, L.; Peng, C.; Xu, Y.; Tang, G.L. Extracellularly oxidative activation and inactivation of matured prodrug for cryptic self-resistance in naphthyridinomycin biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, 11232–11237. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.H.; Zhang, Y.; Zhang, Y.Y.; Yu, Q.; Jiang, C.C.; Tang, M.C.; Pu, J.Y.; Wu, L.; Zhao, Y.L.; Shi, T.; et al. Reductive inactivation of the hemiaminal pharmacophore for resistance against tetrahydroisoquinoline antibiotics. Nat. Commun. 2021, 12, 7085. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, J.; Zhang, L.; Zhang, C.; Liu, B.; Hu, Y. Self-resistance in the biosynthesis of fungal macrolides involving cycles of extracellular oxidative activation and intracellular reductive inactivation. Angew. Chem. Int. Ed. 2021, 60, 6639–6645. [Google Scholar] [CrossRef]
- Reimer, D.; Bode, H.B. A natural prodrug activation mechanism in the biosynthesis of nonribosomal peptides. Nat. Prod. Rep. 2014, 31, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chi, H.T.; Wu, L.; Deng, Z.; Yu, Y. Two cryptic self-resistance mechanisms in Streptomyces tenebrarius reveal insights into the biosynthesis of apramycin. Angew. Chem. Int. Ed. 2021, 60, 8990–8996. [Google Scholar] [CrossRef]
- Scott, J.D.; Williams, R.M. Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics. Chem. Rev. 2002, 102, 1669–1730. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-L.; Tang, M.-C.; Song, L.-Q.; Zhang, Y. Biosynthesis of tetrahydroisoquinoline antibiotics. Curr. Top. Med. Chem. 2016, 16, 1717–1726. [Google Scholar] [CrossRef]
- Du, L.; Li, S. Compartmentalized biosynthesis of fungal natural products. Curr. Opin. Biotechnol. 2021, 69, 128–135. [Google Scholar] [CrossRef]
- Zhang, W.; Du, L.; Qu, Z.; Zhang, X.; Li, F.; Li, Z.; Qi, F.; Wang, X.; Jiang, Y.; Men, P.; et al. Compartmentalized biosynthesis of mycophenolic acid. Proc. Natl. Acad. Sci. USA 2019, 116, 13305–13310. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.A.; Stierle, D.B.; Decato, D.; Priestley, N.D.; Alverson, J.B.; Hoody, J.; McGrath, K.; Klepacki, D. The berkeleylactones, antibiotic macrolides from fungal coculture. J. Nat. Prod. 2017, 80, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Mitova, M.I.; Ellis, G.; van der Sar, S.; Phipps, R.K.; Blunt, J.W.; Cummings, N.J.; Cole, A.L.J.; Munro, M.H.G. Bioactivity profiling using HPLC/microtiter-plate analysis: application to a new zealand marine alga-derived fungus, Gliocladium sp. J. Nat. Prod. 2006, 69, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Canova, S.; Lépine, R.; Thys, A.; Baron, A.; Roche, D. Synthesis and biological properties of macrolactam analogs of the natural product macrolide (−)-A26771B. Bioorg. Med. Chem. Lett. 2011, 21, 4768–4772. [Google Scholar] [CrossRef]
- Igarashi, Y.; Futamata, K.; Fujita, T.; Sekine, A.; Furumai, T. Yatakemycin, a novel antifungal antibiotic produced by Streptomyces sp. TP-A0356. J. Antibiot. 2003, 56, 107–113. [Google Scholar] [CrossRef]
- Parrish, J.P.; Kastrinsky, D.B.; Wolkenberg, S.E.; Igarashi, Y.; Boger, D.L. DNA alkylation properties of yatakemycin. J. Am. Chem. Soc. 2003, 125, 10971–10976. [Google Scholar] [CrossRef]
- Jin, W.; Trzupek, J.D.; Rayl, T.J.; Broward, M.A.; Vielhauer, G.A.; Weir, S.J.; Hwang, I.; Boger, D.L. A unique class of duocarmycin and CC-1065 analogues subject to reductive activation. J. Am. Chem. Soc. 2007, 129, 15391–15397. [Google Scholar] [CrossRef]
- MacMillan, K.S.; Boger, D.L. Fundamental relationships between structure, reactivity, and biological activity for the duocarmycins and CC-1065. J. Med. Chem. 2009, 52, 5771–5780. [Google Scholar] [CrossRef] [PubMed]
- Mullins, E.A.; Shi, R.; Eichman, B.F. Toxicity and repair of DNA adducts produced by the natural product yatakemycin. Nat. Chem. Biol. 2017, 13, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jakobi, K.; Welzel, K.; Hertweck, C. Biosynthesis of the antitumor agent chartreusin involves the oxidative rearrangement of an anthracyclic polyketide. Chem. Biol. 2005, 12, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, J.; Cai, Y.; Wu, S.; Yang, K.; Chan, H.C.S.; Huang, W.; Jin, W.-B.; Li, Y.; Yin, Y.; et al. GyrI-like proteins catalyze cyclopropanoid hydrolysis to confer cellular protection. Nat. Commun. 2017, 8, 1485. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.; He, Q.-L.; Zhao, Z.-X.; Zhang, F.; Wang, R.; Kang, J.; Tang, G.-L. Self-resistance to an antitumor antibiotic: A DNA glycosylase triggers the base-excision repair system in yatakemycin biosynthesis. Angew. Chem. Int. Ed. 2012, 51, 10532–10536. [Google Scholar] [CrossRef]
- Mullins, E.A.; Dorival, J.; Tang, G.-L.; Boger, D.L.; Eichman, B.F. Structural evolution of a DNA repair self-resistance mechanism targeting genotoxic secondary metabolites. Nat. Commun. 2021, 12, 6942. [Google Scholar] [CrossRef]
- Mullins, E.A.; Shi, R.; Parsons, Z.D.; Yuen, P.K.; David, S.S.; Igarashi, Y.; Eichman, B.F. The DNA glycosylase AlkD uses a non-base-flipping mechanism to excise bulky lesions. Nature 2015, 527, 254–258. [Google Scholar] [CrossRef][Green Version]
- Schärer, O.D. Chemistry and biology of DNA repair. Angew. Chem. Int. Ed. 2003, 42, 2946–2974. [Google Scholar] [CrossRef]
- Stivers, J.T.; Jiang, Y.L. A mechanistic perspective on the chemistry of DNA repair glycosylases. Chem. Rev. 2003, 103, 2729–2760. [Google Scholar] [CrossRef]
- Coleman, R.S.; Perez, R.J.; Burk, C.H.; Navarro, A. Studies on the mechanism of action of azinomycin B: definition of regioselectivity and sequence selectivity of DNA cross-link formation and clarification of the role of the naphthoate. J. Am. Chem. Soc. 2002, 124, 13008–13017. [Google Scholar] [CrossRef]
- Foulke-Abel, J.; Kelly, G.T.; Zhang, H.; Watanabe, C. Characterization of AziR, a resistance protein of the DNA cross-linking agent azinomycin B. Mol. Biosyst. 2011, 7, 2563–2570. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Xiao, L.; Yang, L.; Li, H.; Zhang, F.; Lei, L.; Li, S.; Feng, X.; Li, A.; et al. Characterization of a novel DNA glycosylase from S. sahachiroi involved in the reduction and repair of azinomycin B induced DNA damage. Nucleic Acids Res. 2016, 44, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Mullins, E.A.; Warren, G.M.; Bradley, N.P.; Eichman, B.F. Structure of a DNA glycosylase that unhooks interstrand cross-links. Proc. Natl. Acad. Sci. USA 2017, 114, 4400–4405. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Y.; Wang, S.; Ying, K.; Xiao, L.; Liu, K.; Zuo, X.; He, J. Identification of a novel structure-specific endonuclease AziN that contributes to the repair of azinomycin B-mediated DNA interstrand crosslinks. Nucleic Acids Res. 2020, 48, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Maskey, R.P.; Helmke, E.; Kayser, O.; Fiebig, H.H.; Maier, A.; Busche, A.; Laatsch, H. Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine Streptomycete and their absolute stereochemistry. J. Antibiot. 2004, 57, 771–779. [Google Scholar] [CrossRef]
- Maiese, W.M.; Labeda, D.P.; Korshalla, J.; Kuck, N.; Fantini, A.A.; Wildey, M.J.; Thomas, J.; Greenstein, M. LL-D49194 antibiotics, a novel family of antitumor agents: Taxonomy, fermentation and biological properties. J. Antibiot. 2006, 43, 253–258. [Google Scholar] [CrossRef]
- Fitzner, A.; Frauendorf, H.; Laatsch, H.; Diederichsen, U. Formation of gutingimycin: Analytical investigation of trioxacarcin A-mediated alkylation of dsDNA. Anal. Bioanal. Chem. 2008, 390, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Pfoh, R.; Laatsch, H.; Sheldrick, G.M. Crystal structure of trioxacarcin A covalently bound to DNA. Nucleic Acids Res. 2008, 36, 3508–3514. [Google Scholar] [CrossRef]
- Mullins, E.A.; Rubinson, E.H.; Pereira, K.N.; Calcutt, M.W.; Christov, P.P.; Eichman, B.F. An HPLC–tandem mass spectrometry method for simultaneous detection of alkylated base excision repair products. Methods. 2013, 64, 59–66. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Hou, X.-F.; Qi, L.-H.; Yin, Y.; Li, Q.; Pan, H.-X.; Chen, X.-Y.; Tang, G.-L. Biosynthesis of trioxacarcin revealing a different starter unit and complex tailoring steps for type II polyketide synthase. Chem. Sci. 2015, 6, 3440–3447. [Google Scholar] [CrossRef]
- Yang, K.; Qi, L.-H.; Zhang, M.; Hou, X.-F.; Pan, H.-X.; Tang, G.-L.; Wang, W.; Yuan, H. The SARP family regulator Txn9 and two-component response regulator Txn11 are key activators for trioxacarcin biosynthesis in Streptomyces bottropensis. Curr. Microbiol. 2015, 71, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Shen, Y.; Hou, X.F.; Li, W.J.; Tang, G.L. Discovery of druggability-improved analogues by investigation of the LL-D49194alpha1 biosynthetic pathway. Org. Lett. 2019, 21, 2322–2325. [Google Scholar] [CrossRef]
- Chen, X.; Bradley, N.P.; Lu, W.; Wahl, K.L.; Zhang, M.; Yuan, H.; Hou, X.F.; Eichman, B.F.; Tang, G.L. Base excision repair system targeting DNA adducts of trioxacarcin/LL-D49194 antibiotics for self-resistance. Nucleic Acids Res. 2022, 50, 2417–2430. [Google Scholar] [CrossRef] [PubMed]
- Heifets, L.; Simon J Fau-Pham, V.; Pham, V. Capreomycin is active against non-replicating M. tuberculosis. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 6. [Google Scholar] [CrossRef][Green Version]
- Shi, R.; Itagaki N Fau-Sugawara, I.; Sugawara, I. Overview of anti-tuberculosis (TB) drugs and their resistance mechanisms. Mini-Rev. Med. Chem. 2007, 7, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Stanley, R.E.; Blaha, G.; Grodzicki, R.L.; Strickler, M.D.; Steitz, T.A. The structures of the anti-tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome. Nat. Struct. Mol. Biol. 2010, 17, 289–293. [Google Scholar] [CrossRef]
- Felnagle, E.A.; Rondon, M.R.; Berti, A.D.; Crosby, H.A.; Thomas, M.G. Identification of the biosynthetic gene cluster and an additional gene for resistance to the antituberculosis drug capreomycin. Appl. Environ. Microbiol. 2007, 73, 4162–4170. [Google Scholar] [CrossRef]
- Pan, Y.C.; Wang, Y.L.; Toh, S.I.; Hsu, N.S.; Lin, K.H.; Xu, Z.; Huang, S.C.; Wu, T.K.; Li, T.L.; Chang, C.Y. Dual-mechanism confers self-resistance to the antituberculosis antibiotic capreomycin. ACS Chem. Biol. 2022, 17, 138–146. [Google Scholar] [CrossRef]
- Nougayrède, J.-P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Bondarev, V.; Richter, M.; Romano, S.; Piel, J.; Schwedt, A.; Schulz-Vogt, H.N. The genus Pseudovibrio contains metabolically versatile bacteria adapted for symbiosis. Environ. Microbiol. 2013, 15, 2095–2113. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Vizcaino Maria, I.; Crawford Jason, M.; Drake, H.L. Gut symbionts from distinct hosts exhibit genotoxic activity via divergent colibactin biosynthesis pathways. Appl. Environ. Microbiol. 2015, 81, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Stornetta, A.; Villalta, P.W.; Wilson, M.R.; Boudreau, P.D.; Zha, L.; Balbo, S.; Balskus, E.P. Reactivity of an unusual amidase may explain colibactin’s DNA cross-linking activity. J. Am. Chem. Soc. 2019, 141, 11489–11496. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Kim, C.S.; Healy, A.R.; Wernke, K.M.; Wang, Z.; Frischling, M.C.; Shine, E.E.; Wang, W.; Herzon, S.B.; Crawford, J.M. Structure elucidation of colibactin and its DNA cross-links. Science 2019, 365, eaax2685. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Liu, X.; Ye, W.; Li, Z.R.; Qian, P.Y. Biosynthesis and bioactivities of microbial genotoxin colibactins. Nat. Prod. Rep. 2022, 39, 991–1014. [Google Scholar] [CrossRef]
- Bian, X.; Fu, J.; Plaza, A.; Herrmann, J.; Pistorius, D.; Stewart, A.F.; Zhang, Y.; Müller, R. In vivo evidence for a prodrug activation mechanism during colibactin maturation. ChemBioChem 2013, 14, 1194–1197. [Google Scholar] [CrossRef]
- Brotherton, C.A.; Balskus, E.P. A prodrug resistance mechanism is involved in colibactin biosynthesis and cytotoxicity. J. Am. Chem. Soc. 2013, 135, 3359–3362. [Google Scholar] [CrossRef]
- Mousa, J.J.; Newsome, R.C.; Yang, Y.; Jobin, C.; Bruner, S.D. ClbM is a versatile, cation-promiscuous MATE transporter found in the colibactin biosynthetic gene cluster. Biochem. Biophys. Res. Commun. 2017, 482, 1233–1239. [Google Scholar] [CrossRef]
- Krah, A.; Huber, R.G.; Zachariae, U.; Bond, P.J. On the ion coupling mechanism of the MATE transporter ClbM. BBA Biomembr. 2020, 1862, 183137. [Google Scholar] [CrossRef]
- Mousa, J.J.; Yang, Y.; Tomkovich, S.; Shima, A.; Newsome, R.C.; Tripathi, P.; Oswald, E.; Bruner, S.D.; Jobin, C. MATE transport of the E. coli-derived genotoxin colibactin. Nat. Microbiol. 2016, 1, 15009. [Google Scholar] [CrossRef]
- Velilla, J.A.; Volpe, M.R.; Kenney, G.E.; Walsh, R.M., Jr.; Balskus, E.P.; Gaudet, R. Structural basis of colibactin activation by the ClbP peptidase. Nat. Chem. Biol. 2022. [Google Scholar] [CrossRef]
- Tripathi, P.; Shine, E.E.; Healy, A.R.; Kim, C.S.; Herzon, S.B.; Bruner, S.D.; Crawford, J.M. ClbS Is a cyclopropane hydrolase that confers colibactin resistance. J. Am. Chem. Soc. 2017, 139, 17719–17722. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Bruner, S.D. Structural basis for the interactions of the colibactin resistance gene product ClbS with DNA. Biochemistry 2021, 60, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Molan, K.; Podlesek, Z.; Hodnik, V.; Butala, M.; Oswald, E.; Žgur Bertok, D. The Escherichia coli colibactin resistance protein ClbS is a novel DNA binding protein that protects DNA from nucleolytic degradation. DNA Repair. 2019, 79, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ellabaan, M.M.H.; Charusanti, P.; Munck, C.; Blin, K.; Tong, Y.; Weber, T.; Sommer, M.O.A.; Lee, S.Y. Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat. Commun. 2017, 8, 15784. [Google Scholar] [CrossRef]
- Sikandar, A.; Cirnski, K.; Testolin, G.; Volz, C.; Bronstrup, M.; Kalinina, O.V.; Muller, R.; Koehnke, J. Adaptation of a bacterial multidrug resistance system revealed by the structure and function of AlbA. J. Am. Chem. Soc. 2018, 140, 16641–16649. [Google Scholar] [CrossRef]
- Rostock, L.; Driller, R.; Gratz, S.; Kerwat, D.; von Eckardstein, L.; Petras, D.; Kunert, M.; Alings, C.; Schmitt, F.J.; Friedrich, T.; et al. Molecular insights into antibiotic resistance—How a binding protein traps albicidin. Nat. Commun. 2018, 9, 3095. [Google Scholar] [CrossRef]
- Balaich, J.; Estrella, M.; Wu, G.; Jeffrey, P.D.; Biswas, A.; Zhao, L.; Korennykh, A.; Donia, M.S. The human microbiome encodes resistance to the antidiabetic drug acarbose. Nature 2021, 600, 110–115. [Google Scholar] [CrossRef]
- Wehmeier, U.F. The biosynthesis and metabolism of acarbose in Actinoplanes sp. SE 50/110: A progress report. Biocatal. Biotransform. 2003, 21, 279–284. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug. Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Van Lanen, S.G.; Shen, B. Microbial genomics for the improvement of natural product discovery. Curr. Opin. Microbiol. 2006, 9, 252–260. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Fischbach, M.A. Natural products version 2.0: Connecting genes to molecules. J. Am. Chem. Soc. 2010, 132, 2469–2493. [Google Scholar] [CrossRef] [PubMed]
- Thaker, M.N.; Wang, W.; Spanogiannopoulos, P.; Waglechner, N.; King, A.M.; Medina, R.; Wright, G.D. Identifying producers of antibacterial compounds by screening for antibiotic resistance. Nat. Biotechnol. 2013, 31, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Alanjary, M.; Kronmiller, B.; Adamek, M.; Blin, K.; Weber, T.; Huson, D.; Philmus, B.; Ziemert, N. The antibiotic resistant target seeker (ARTS), an exploration engine for antibiotic cluster prioritization and novel drug target discovery. Nucleic Acids Res. 2017, 45, W42–W48. [Google Scholar] [CrossRef]
- Panter, F.; Krug, D.; Baumann, S.; Muller, R. Self-resistance guided genome mining uncovers new topoisomerase inhibitors from myxobacteria. Chem. Sci. 2018, 9, 4898–4908. [Google Scholar] [CrossRef]
- Culp, E.J.; Sychantha, D.; Hobson, C.; Pawlowski, A.C.; Prehna, G.; Wright, G.D. ClpP inhibitors are produced by a widespread family of bacterial gene clusters. Nat. Microbiol. 2022, 7, 451–462. [Google Scholar] [CrossRef]
- Li, L.Y.; Hu, Y.L.; Sun, J.L.; Yu, L.B.; Shi, J.; Wang, Z.R.; Guo, Z.K.; Zhang, B.; Guo, W.; Tan, R.; et al. Resistance and phylogeny guided discovery reveals structural novelty of tetracycline antibiotics. Chem. Sci. 2022, 13, 12892–12898. [Google Scholar] [CrossRef]
- Scott, T.A.; Batey, S.F.D.; Wiencek, P.; Chandra, G.; Alt, S.; Francklyn, C.S.; Wilkinson, B. Immunity-guided identification of threonyl-tRNA synthetase as the molecular target of obafluorin, a beta-lactone antibiotic. ACS Chem. Biol. 2019, 14, 2663–2671. [Google Scholar] [CrossRef]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef]
- Xie, L.; Zang, X.; Cheng, W.; Zhang, Z.; Zhou, J.; Chen, M.; Tang, Y. Harzianic acid from trichoderma afroharzianum is a natural product inhibitor of acetohydroxyacid synthase. J. Am. Chem. Soc. 2021, 143, 9575–9584. [Google Scholar] [CrossRef]
- Zhong, B.; Wan, J.; Shang, C.; Wen, J.; Wang, Y.; Bai, J.; Cen, S.; Hu, Y. Biosynthesis of rumbrins and inspiration for discovery of HIV inhibitors. Acta Pharm. Sin. B. 2022, 12, 4193–4203. [Google Scholar] [CrossRef] [PubMed]
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Van Nieuwenhze, M.S.; Brun, Y.V.; et al. Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature 2020, 578, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.; Chan, A.N.; Wright, G.D. The antibiotic resistome: A guide for the discovery of natural products as antimicrobial agents. Chem. Rev. 2021, 121, 3464–3494. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Pan, H.-X.; Tang, G.-L. Newly Discovered Mechanisms of Antibiotic Self-Resistance with Multiple Enzymes Acting at Different Locations and Stages. Antibiotics 2023, 12, 35. https://doi.org/10.3390/antibiotics12010035
Chen X, Pan H-X, Tang G-L. Newly Discovered Mechanisms of Antibiotic Self-Resistance with Multiple Enzymes Acting at Different Locations and Stages. Antibiotics. 2023; 12(1):35. https://doi.org/10.3390/antibiotics12010035
Chicago/Turabian StyleChen, Xiaorong, Hai-Xue Pan, and Gong-Li Tang. 2023. "Newly Discovered Mechanisms of Antibiotic Self-Resistance with Multiple Enzymes Acting at Different Locations and Stages" Antibiotics 12, no. 1: 35. https://doi.org/10.3390/antibiotics12010035
APA StyleChen, X., Pan, H.-X., & Tang, G.-L. (2023). Newly Discovered Mechanisms of Antibiotic Self-Resistance with Multiple Enzymes Acting at Different Locations and Stages. Antibiotics, 12(1), 35. https://doi.org/10.3390/antibiotics12010035






