Abstract
Coagulase-positive staphylococci (CoPS) account for most bacteria-related pyoderma in companion animals. Emergence of methicillin-resistant strains of Staphylococcus pseudintermedius (MRSP), Staphylococcus aureus (MRSA) or Staphylococcus coagulans (MRSC), often with multidrug-resistant (MDR) phenotypes, is a public health concern. The study collection comprised 237 staphylococci (S. pseudintermedius (n = 155), S. aureus (n = 55) and S. coagulans (n = 27)) collected from companion animals, previously characterized regarding resistance patterns and clonal lineages. Biofilm production was detected for 51.0% (79/155), 94.6% (52/55) and 88.9% (24/27) of the S. pseudintermedius, S. aureus and S. coagulans, respectively, and was a frequent trait of the predominant S. pseudintermedius and S. aureus clonal lineages. The production of biofilm varied with NaCl supplementation of the growth media. All S. pseudintermedius and S. aureus strains carried icaADB. Kaplan–Meier survival analysis of Galleria mellonella infected with different CoPS revealed a higher virulence potential of S. aureus when compared with other CoPS. Our study highlights a high frequency of biofilm production by prevalent antimicrobial-resistant clonal lineages of CoPS associated with animal pyoderma, potentially related with a higher virulence potential and persistent or recurrent infections.
1. Introduction
Skin infections, particularly pyoderma, are the main reason for antimicrobial prescription in companion animals [1]. Pyoderma is associated with pain, redness and inflammation of the skin [2]. Coagulase-positive staphylococci (CoPS) are amongst the main bacterial agents of these infections in companion animals [1,2,3]. In dogs, Staphylococcus pseudintermedius is responsible for over 90% of the cases, whereas Staphylococcus aureus and Staphylococcus coagulans, interchangeably recognized as the second or third pathogen most associated with these infections, account for up to 10% of pyoderma episodes [1,4]. The prevalence of pyoderma is lower in cats, ranging from 4% up to 20% [5,6], and is usually caused by S. pseudintermedius, S. aureus or coagulase-negative staphylococci [5]. In other companion animals, such as rabbits and horses, this infection is rare with only a few cases reported [7,8,9].
S. pseudintermedius was first described in 2005 by Devriese et al. and belongs to the Staphylococcus intermedius group (SIG), together with Staphylococcus delphini and Staphylococcus cornubiensis [10,11]. It is a commensal organism that is part of the normal flora of the skin and mucous membranes of dogs, colonizing from 25% up to nearly 70% of healthy dogs [12,13]. Despite its commensal role, S. pseudintermedius can be an opportunistic pathogen, causing, in addition to skin infections, otitis externa and infections in the urinary, respiratory and reproductive tracts [2,14]. Its commensal role in cats, rabbits and horses is not well established. For example, this species presents a low adherence to feline corneocytes and has been found transiently in the skin of healthy cats [15]. S. pseudintermedius has been isolated from circa 10% and 27% of healthy or diseased cats, respectively [16].
Regarding S. aureus, it is also an agent of pyoderma in companion animals, although in a lower frequency. Colonization of companion animals by this species is usually transient and associated with close contact with humans or other animals colonized by S. aureus [1,17]. Studies have reported a frequency of colonization of about 10% of healthy dogs [18] and 25% of healthy cats [16]. Like S. pseudintermedius, infections by S. aureus are mostly of endogenous origin and can affect dogs, cats, horses and rabbits [19,20]. S. coagulans, previously classified as Staphylococcus schleiferi subsp. coagulans [21], is found in the skin of healthy and diseased dogs, in frequencies of 4% and 12% [22], respectively, and is rarely reported in either healthy or diseased cats (<1%) [16]. In immunocompromised dogs, it can cause pyoderma, otitis or urinary tract infections [23,24].
Superficial skin infections are usually treated with topical therapy, based on the use of biocide- or antibiotic-based shampoos, sprays or gels. For severe cases, topical therapy combined with systemic therapy is recommended [1]. Methicillin-resistant strains, which are resistant to all beta-lactam antibiotics, except fifth-generation cephalosporins (e.g., ceftaroline), are often associated with multidrug resistance (MDR) phenotypes [25,26,27]. The increasing report of skin infections caused by such strains makes the treatment of infections more challenging due to restricted therapeutic options [25,26].
CoPS have a range of virulence factors that allow them to evade the host immune system and to establish infection [2,28]. In S. aureus and S. pseudintermedius, several virulence factors have been described including leukocidins, hemolysins, adhesines and enterotoxins [28]. The virulence factors of S. coagulans are still scarcely studied. Yet, the occurrence of staphylococcal enterotoxins (SE) has already been described for this species [29]. Another factor contributing to virulence is the ability to form biofilms, a capacity already described for S. pseudintermedius [30], S. aureus [31] and less extensively for S. coagulans [32]. Biofilms are bacterial communities made up of cells that are reversibly linked together and fixed in a self-producing polymeric matrix [33]. Biofilm formation comprises four phases: attachment, where bacterial cells attach to a biotic or abiotic surface; proliferation/accumulation, when bacteria begin to multiply and accumulate at the primary adhesion site; maturation, when microcolonies evolve into macrocolonies and the biofilm acquires a three-dimensional structure; dispersal, when individual cells detach from the matrix and spread, promoting the dissemination of the bacteria within the host [34]. One of the best know biofilm formation mechanisms is the ica-dependent process in Staphylococcus epidermidis. The ica operon encodes the polysaccharide intercellular adhesin (PIA), which has an important role in the attachment and accumulation phases of biofilm formation. This operon has been detected in other staphylococci such as S. aureus [31]. Biofilms have also an impact on the management of skin infections. Biofilm-associated infections are commonly chronic and more resilient to antibiotherapy; thus, they are associated with higher rates of antimicrobial resistance [35]. Another important virulence factor is the Panton–Valentine leukocidin PVL (S. aureus)/Leukocidin LukI (S. pseudintermedius) [36,37]. These leukocidins promote the formation of a pore in the phospholipid membrane of leukocytes, leading to ion flux, apoptosis and cell death [38]. Several authors have reported a relation between their presence and skin infections [39,40,41], but their role is unclear [42]. Many of these staphylococcal virulence factors are regulated by a quorum sensing system, the agr system. This system is codified by the agrABCD operon that encodes an autoinducing peptide (AIP). The agr system has been described in S. aureus and S. pseudintermedius and, in both species, four types of AIP have been reported, enabling the differentiation of four agr types [43,44].
In this work, we aimed to characterize the virulence potential of a collection of S. pseudintermedius, S. aureus and S. coagulans involved in skin infections in companion animals by assessing their capacity to produce biofilm and relating this capacity with other phenotypic (methicillin/multidrug resistance) and genotypic (agr type, clonal lineage) traits. Representative strains were then evaluated in the Galleria mellonella larvae infection model, which allowed to differentiate the virulence potential of the three CoPS species.
2. Results
2.1. Biofilm Production Is a Frequent Trait of Coagulase-Positive Staphylococci Causing Skin Infections in Companion Animals
The proportion of biofilm producers varied within species (Table 1). Biofilm production was highly frequent in S. aureus and S. coagulans, detected for 52/55 (94.6%) and 24/27 (88.9%) of the isolates, respectively. A lower frequency of biofilm producers was registered for S. pseudintermedius, accounting for about half of the isolates tested (79/155, 51.0%). The proportion of biofilm producers and non-producers according to animal host is detailed for each CoPS in Table 1.

Table 1.
Distribution of biofilm production phenotype for the S. pseudintermedius (SP), S. aureus (SA) and S. coagulans (SC) strains in the different growth conditions tested. The proportion of biofilm producers and non-producers according to animal host is also detailed for each CoPS. A strain was considered a biofilm producer if it showed weak, moderate or strong production of biofilm in either one or both conditions tested. A strain was considered a non-producer of biofilm if categorized as non-producer in the two conditions tested.
Biofilm production was highly affected by NaCl (Figure 1), and this effect was more pronounced for S. coagulans, for which 14 out of the 20 strains classified as non-producers in TSB + 1% glucose showed moderate or strong biofilm production upon supplementation of the growth medium with 3% NaCl. A distinct effect was observed for S. aureus and S. pseudintermedius, for which a small increase in the number of non-producers was registered upon increase of NaCl in the growth medium. A change to the biofilm-producing phenotype with increasing NaCl supplementation was also detected for these species but only for 4/12 and 9/88 strains, respectively.
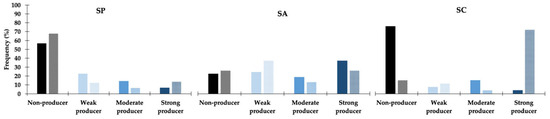
Figure 1.
Effect of NaCl supplementation in biofilm production for the S. pseudintermedius (SP, n = 155), S. aureus (SA, n = 55) and S. coagulans (SC, n = 27) strains. Full-colored columns—TSB supplemented with 1% glucose (SA and SC) and 1% NaCl (SP); partially filled columns—TSB supplemented with 1% glucose and 3% NaCl (all species).
2.2. Relation between Biofilm Phenotypes, Agr Types and Antimicrobial Resistance
The relation between different characteristics of the strains was evaluated using the chi-square test. For this analysis, we only considered moderate or strong biofilm producers. For S. aureus, the biofilm production phenotype was not statistically associated with any of the other traits tested, namely, methicillin resistance (X2 = 3.186; p = 0.074), multidrug resistance (X2 = 0.535; p = 0.734) or agr type (X2 = 3.291; p = 0.462).
For S. pseudintermedius, there was a statistically significant association between biofilm production and agr type (X2 = 9.674; p = 0.021). Comparing moderate/strong producers with weak or non-producers, strains presenting agrIII were more frequently categorized as moderate/strong biofilm producers, whereas strains presenting agrI were mainly weak/non-producers (Figure 2). The relation between biofilm production and methicillin resistance (X2 = 0.914; p = 0.334) or multidrug resistance (X2 = 0.109; p = 0.741) was not statistically significant.
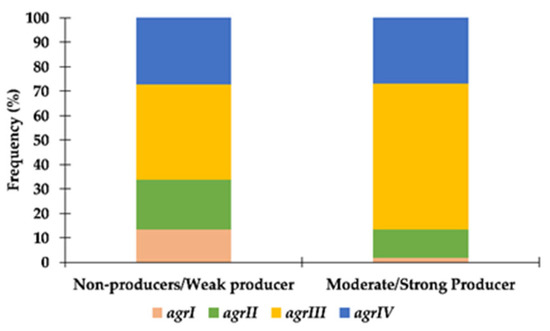
Figure 2.
Distribution of S. pseudintermedius strains according to their agr type and capacity to produce biofilm.
2.3. Relation between Biofilm Phenotypes and Clonal Lineages
The most common S. pseudintermedius and S. aureus lineages include a high frequency of biofilm-producing strains. Nearly half of the S. aureus collection studied, gathered over a 19 year period, comprised ST22-agrI-MRSA isolates [45], 88.0% of which were biofilm producers. The second and third S. aureus predominant lineages, ST5-agrII-MRSA/MSSA (12.7%) and ST398-agrI-MRSA/MSSA (9.1%), respectively, only included biofilm producers.
For S. pseudintermedius, the ST71-agrIII-MRSP-MDR was the most common lineage among the study collection [46] and included 70.8% (17/24) of biofilm-producing strains. On the other hand, all strains (4/4) of ST157-agrIV-MRSP lineage, the second most common lineage, were non-biofilm producers.
Because no MLST scheme is available for S. coagulans, the 27 isolates were previously typed by PGFE, leading to the identification of a predominant clone (PFGE type A) that accounted for 51.9% of the collection [47]. Most S. coagulans of this predominant clone were strong biofilm producers (92.9%, 13/14). However, the only two MRSC strains did not produce biofilm.
These analyses suggest that biofilm production is a frequent trait of the prevalent staphylococcal clonal lineages circulating among companion animals in Portugal, most of which are already related to a high burden of antimicrobial resistance.
2.4. Analysis of Ica and Leukocidin-Encoding Genes across CoPS
We screened by PCR the presence of the icaADB genes, that are part of the ica operon associated with PIA production [48]. All S. aureus and S. pseudintermedius strains carried these genes, indicating a 100% frequency of icaADB genes in both species.
We also screened the lukF-PV/lukS-PV genes (S. aureus) and lukF gene (S. pseudintermedius), encoding leukocidins PVL and LukI, respectively. Similarly to ica, all S. pseudintermedius carried the lukF gene. However, only one S. aureus presented the lukF-PV/lukS-PV genes. This strain was collected from a rabbit and was the only representative of the clonal lineage ST121-agrIV in the study collection [45].
To complement our analysis, we carried out an in silico search of the ica operon genes in all the complete genomes available at the GenBank database (up to July 2022) for the three CoPS species (Supplementary material S1). The presence of the four ica genes (icaA, icaB, icaC and icaD) was detected for 106 out of the 107 S. pseudintermedius complete genomes. A similar search for ica genes against all S. aureus complete genomes (>800 genomes) also revealed their ubiquitous presence in S. aureus. In opposition, no ica genes were detected in the two S. coagulans genomes available.
We carried out a similar approach for the search of leukocidin-encoding genes lukF-lukS in S. pseudintermedius and S. coagulans. We conclude that for both species, these genes were encountered in all tested genomes (one-hundred and seven and two, respectively).
2.5. Virulence Potential of Representative CoPS Strains in the G. mellonella Infection Model
The S. pseudintermedius clinical strains tested were BIOS-V64 and BIOS-V262, both MRSP-MDR, representing ST71-agrIII and ST118-agrII, respectively. The Kaplan–Meier survival curves and mean survival times are presented in Figure 3A and Table 2. Overall, these results show that the virulence potential varied according to the S. pseudintermedius infecting strain, as follows: BIOS-V262 > DSM 21284T > BIOS-V64.
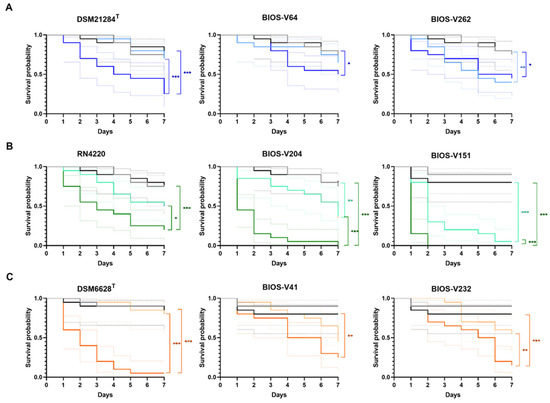
Figure 3.
Kaplan–Meier survival analysis of G. mellonella infected with reference strains and biofilm-producing S. pseudintermedius (A), S. aureus (B) and S. coagulans (C) strains representative of the main clonal lineages causing animal pyoderma. Grey line: “no manipulation” control group; black line: “PBS” control group; light colors: 1 × 105 CFU/larva; dark colors: 1 × 107 CFU/larva. The colored dotted lines indicate the 95% confidence interval for the corresponding survival curve. Statistical differences are highlighted as follows: * p < 0.05; ** p < 0.01; *** p < 0.001.

Table 2.
Mean survival time of G. mellonella larvae infected with representative biofilm-producing CoPS strains.
For S. aureus, the clinical strains chosen were BIOS-V204 (MRSA-ST22-agrI) and BIOS-V151 (MRSA-MDR-ST398-agrI). Overall, S. aureus strains showed higher virulence than S. pseudintermedius. Comparing the virulence potential of the three S. aureus strains, we could rank them as follows: BIOS-V151 > BIOS-V204 > RN4220 (Figure 3B, Table 2).
The S. coagulans clinical strains presented a behavior similar to S. pseudintermedius against G. mellonella (Figure 3C and Table 2). Albeit the reference strain DSM 6628T displayed the highest virulence potential, a comparison between the two clinical strains showed that BIOS-V41 (MSSC-PFGE type A) presented a higher virulence potential; overall, the S. coagulans strains ranked as DSM 6628T > BIOS-V41 > BIOS-V232.
Regardless of the infecting staphylococci or strain, the killing effect upon G. mellonella increased with the bacterial inoculum, as indicated by the mean larvae survival time (Table 2). The joint analysis of this parameter with the Kaplan–Meier survival curves also suggest that S. aureus is the species with the highest virulence potential, followed by S. coagulans and S. pseudintermedius.
3. Discussion
CoPs are the main cause of pyoderma in companion animals [49]. Among these, S. pseudintermedius is the predominant pathogen, particularly in dogs, followed by S. aureus and S. coagulans. Methicillin resistance has been increasing for these three species, causing concerns regarding the management of these infections, which often present a recurrent nature, with animals being subjected to several rounds of antibiotherapy [49].
Besides antimicrobial resistance, virulence factors allow these bacteria to be more successful on promoting infection [28]. One of the most important virulence factors in staphylococci is the production of biofilm, which is often associated with antimicrobial resistance and chronic infections [50]. Although biofilm production by animal-associated CoPS has been studied in a lesser extent than for human-associated staphylococci, the capacity to produce biofilms has already been reported for S. pseudintermedius and S. coagulans [44,51]. The molecular mechanisms of biofilm formation are described in more detailed for S. aureus, and it is thought that these mechanisms are similar to the ones present in other staphylococci [44,51].
In this study, we demonstrated that biofilm formation is a frequent trait in CoPS causing pyoderma in companion animals, with 65.4% (155/237) of the bacterial collection producing biofilms. Of the three species under study, S. aureus showed the highest capacity to form biofilms with nearly 95% producers; this is similar to other reports, which described between 90% to 100% producers [52,53,54,55,56]. S. coagulans was the second species for which biofilm production was more frequent, a trait detected in 88.9% (24/27) of the strains. Finally, S. pseudintermedius showed the lowest frequency of biofilm production, detected in half (51.0%, 79/155) of the strains. Data available in literature indicate higher frequencies of biofilm production, ranging from 90% to 100%, for S. coagulans [29,51] and for S. pseudintermedius [30,44,57,58,59,60,61]. The divergence between these results may rely on the methodology used to assess biofilm production. The crystal violet adhesion method is an effective and low-cost method that allows high-throughput detection of biofilms. However, it shows high intra- and inter-assay variation, which can be exacerbated when comparing inter-laboratorial data due to differences in the criteria used to categorize the biofilm phenotype, and other factors, such as growth media and incubation conditions [62]. In fact, our study highlights that supplementation of the growth media with a high saline concentration impacts significantly on biofilm formation. Our data also indicate that NaCl can act either as an inhibitor or inducer of biofilm production, being more notorious for biofilm induction in S. coagulans and biofilm inhibition in S. aureus. NaCl has been reported to induce the expression of the ica operon in S. aureus [63,64]. However, our observations for S. aureus and S. pseudintermedius indicate that despite a small increase in the biofilm-producing strains, the frequency of moderate/strong biofilm producers diminishes with NaCl supplementation. The ica operon was detected amongst all S. aureus and S. pseudintermedius strains, in accordance with results obtained by other authors [44,57,59,65,66]. Moreover, the in silico analysis corroborated the wide presence of the operon in both species. These apparently contradictory findings may suggest that production of biofilm in these strains may occur partially via an ica-independent mechanism that is affect by higher osmolarity environments [67]. Growth conditions have also been demonstrated to affect the chemical properties of the biofilm matrix [68], such as an increase in biofilm hydrophobicity in the presence of lower NaCl concentrations, which impacts biofilm adherence properties [69]. Further functional studies should highlight the similarities and differences of the process of biofilm formation in CoPS.
No statistical associations could be established between the biofilm phenotype and antimicrobial resistance phenotypes (methicillin resistance and multidrug resistance) or with clonal lineage (ST, agr type) for S. aureus. In opposition, for S. pseudintermedius, an association was found between biofilm production and agr type, namely, a higher frequency of agrIII among biofilm-producing strains and a lower frequency of agrI among non-producing strains. These results differ from the findings of Little and colleagues, which reported a relation between agrIII and agrII and the non-biofilm-producing phenotype [44]. No associations were encountered between biofilm production and antimicrobial resistance phenotypes. These statistical analyses were not extended to S. coagulans, but noteworthily, the only two MRSC strains do not form biofilms.
Pore-forming leukocidins are major virulence factors in staphylococci. In S. aureus and S. coagulans, this leukocidin is encoded by lukF-PV/lukS-PV genes [36,70] and, in S. pseudintermedius, by lukF- lukS genes [37]. Some authors associate these toxins to skin infections, although this relation is not always well established [39,40,41]. All S. pseudintermedius strains carried the gene lukF, suggesting that this gene may be part of the core genome of this species since the studied collection is genetically diverse [46]. This observation is further supported by the in silico detection of both lukF and lukS genes in all available S. pseudintermedius complete genomes. For S. aureus, only one strain isolated from a rabbit presented the lukF-PV/lukS-PV genes. This strain belongs to ST121-agrI, a clonal lineage frequently associated with rabbits [71].
The use of animal models in scientific research is essential to understand the interaction between the pathogen and its host [72]. The animal model G. mellonella has been increasingly used in recent years [73]. This model has several advantages for scientific research purposes, namely: (i) the larva can be kept at 37 °C; (ii) the innate immune system of the larva shows similarities with the innate immune system of mammals; (iii) does not require major adaptations at the laboratory level; (iv) does not require ethical approval [74]. One limitation in the use of G. mellonella is the lack of centers specializing in the supply of larvae for research purposes. Thus, the larvae used in many studies are purchased from suppliers specialized in breeding this species for pet food, which potentiates genotypic differences among larvae. The other limiting factor is uncontrolled breeding and maintenance conditions, which can influence the G. mellonella susceptibility to infections [73,75]. Nevertheless, this animal model has already been proved valuable to study the virulence of several bacteria, including S. aureus [76,77,78,79], and to a lesser extent, S. pseudintermedius and S. coagulans [80]. The infection assays conducted in these studies varied in several parameters such as the time of larvae follow-up, ranging from three up to 10 days [76,77,78,79,80,81,82], and bacterial inoculum, which ranged from 2 × 104 CFU/larva [76], 5 × 106 CFU/larva [77] to 2 × 107 CFU/larva [80]. In this study, we followed larvae survival for 7 days and used two inoculums: 1 × 105 and 1 × 107 CFU/larva. In these conditions, we were able to monitor and differentiate larvae mortality between strains or CoPS species.
The three CoPS species presented different virulence potential in the G. mellonella infection model. Overall, S. aureus strains showed higher virulence potential than the S. pseudintermedius and S. coagulans strains, as observed in the lower survival probabilities and lower mean survival times (Figure 3 and Table 2). A report by Canovas and colleagues also demonstrated a higher virulence potential of S. aureus when compared with S. coagulans [80]. In particular, the two S. aureus clinical strains studied demonstrated a statistically significant higher killing activity than the reference strain. Both clinical strains are MRSA and strong biofilm producers and belong to clonal lineages that are prevalent in our study collection. Strain BIOS-V151 belongs to ST398-agrI [45], a lineage frequently associated with food-producing or companion animals [19]. On the other hand, BIOS-V204 belongs to ST22-agrI, the predominant lineage in our pyoderma-related S. aureus collection as well as in S. aureus from skin infections in humans in the community [83], highlighting concerns on the sharing of such strains between animals and humans.
S. pseudintermedius and S. coagulans presented similar virulence potential. For both species, the biofilm-producing clinical strains only showed increased virulence potential at the higher inoculum tested, 107 CFU/larva. This observation is of relevance considering that skin infections are characterized by a high bacterial burden at the site of infection. The strains tested represent bacteria with a high capacity to produce biofilms from relevant circulating clonal lineages. S. coagulans BIOS-V41 belongs to the most common lineage found amongst the 19-year timespan of our collection [47]. S. pseudintermedius BIOS-V64 is a moderate biofilm producer belonging to MRSP-MDR-ST71-agrIII, the predominant lineage in the S. pseudintermedius collection and one of the most prevalent causing canine infections in several European countries [46,84]. An increased virulence potential at both inoculums tested was only observed for S. pseudintermedius BIOS-V262, a moderate biofilm producer that belongs to MRSP-MDR-ST118-agrII. This lineage is a double locus variant of ST258, a clonal lineage that is replacing ST71 in some European countries [85], raising concerns on the dissemination of ST258 and its variants and their potential higher virulence potential associated with skin infections.
4. Materials and Methods
4.1. Bacterial Isolates
The study included 155 S. pseudintermedius (isolated from 141 dogs, 3 cats and one rabbit), 55 S. aureus (isolated from 27 dogs, 18 cats, four rabbits, one horse and one unknown host) and 27 S. coagulans (from 26 dogs) strains associated with skin infections in companion animals collected over a period of 19 years (1999–2018) at a veterinary research laboratory providing diagnostic services for a veterinary teaching hospital and private veterinary clinics in the Lisbon area (1999–2018) and at a private veterinary diagnostic clinic (2017–2018). A few strains were isolated from the same animal host, as follows: 12 S. pseudintermedius strains were isolated from six dogs (two strains/dog) and six other S. pseudintermedius strains were isolated from two dogs (three strains/dog); eight S. aureus strains were isolated from two dogs, one cat and one rabbit (two strains/animal); two S. coagulans were isolated from the same dog. All strains have been previously characterized regarding their antimicrobial susceptibility profiles, and their clonal lineages were determined by PFGE and/or MLST [45,46,47]. The main strain characteristics are summarized in Supplementary Material S2. All isolates were grown in tryptone soya broth or agar (TSB/TSA, Thermo Scientific™ Oxoid™, Basingstoke, UK) at 37 °C (for broth cultures). Bacterial stocks were kept at −80 °C in TSB supplemented with 10% (v/v) glycerol (Sigma-Aldrich, Saint Louis, MO, USA). The type strains S. pseudintermedius DSM 21284T and S. coagulans DSM 6628T, and the reference strains S. aureus RN4220, S. epidermidis ATCC®12228™ and ATCC®35984™ (S. epidermidis RP62a) were included in the study as controls.
4.2. Assessment of Biofilm Formation
The capacity of the strains to produce biofilm was assessed by the crystal violet adhesion method in 96-well flat-bottom tissue culture plates (Orange Scientific, Braine-l’Alleud, Belgium) [62,86]. Briefly, strains were cultured in TSB for 24 h at 37 °C with no agitation. A cellular suspension adjusted to 5 × 107–1 × 108 CFU/mL in TSB was prepared, diluted in 1:100 in supplemented TSB and 0.2 mL aliquots distributed in quadruplicates in the microtiter plates. Different growth conditions were evaluated as follows: S. pseudintermedius [TSB + 1% glucose (Sigma-Aldrich, Saint Louis, MO, USA) + 1% or 3% NaCl (Merck, Darmstadt, Germany)]; S. aureus and S. coagulans (TSB + 1% glucose w/wo 3% NaCl). After incubation for 24 h at 37 °C, the wells content was discarded carefully with a multichannel micropipette and washed thrice with 0.1 mL phosphate buffered saline (PBS, Sigma-Aldrich, Saint Louis, MO, USA). Adherent cells were fixed with 0.15 mL methanol 99% (Sigma-Aldrich, Saint Louis, MO, USA) for 20 min and after an air-dry overnight period, the biofilm biomass was dyed with 0.15 mL 0.1% (w/v) crystal violet (Sigma-Aldrich, Saint Louis, MO, USA) for 15 min. Following, the microtiter plates were subjected to two wash cycles in two water baths and air-dried for at least 90 min. The bound crystal violet was solubilized with 0.15 mL of 33% (v/v) acetic acid (Sigma-Aldrich, Saint Louis, MO, USA) for 30 min and the associated optical density was measured at 570 nm (well area mode) in a SynergyHT apparatus (Biotek, Winooski, VT, USA). Each assay included, in quadruplicates, the control strains S. epidermidis ATCC®12228™ (ica -), S. epidermidis ATCC®35984™ (ica +) and the corresponding reference strains (S. pseudintermedius DSM 21284T, S. aureus RN4220, S. coagulans DSM 6628T), as well as a negative control (supplemented TSB) and a blank (33% acetic acid). Biofilm production was categorized according to Stepanović criteria [62,86], which establishes a cut-off value (ODc) defined as the geometric media of OD570 of the negative control (supplemented TSB) + 3x the corresponding standard deviation (SD). Strains were characterized as follows: ODstrain < ODc, biofilm non-producers; ODc < ODstrain < 2x ODc, weak producers; 2x ODc < ODstrain < 4x ODc, moderate producers; ODstrain > 4x OD, strong producers. Each assay was performed, at least, in duplicate and, to minimize intra- and inter-assay variability, an assay was only validated when associated with an SD value <20% of the corresponding geometric mean (either considering an individual assay or duplicates) and assigning the strain to the same category. A strain was considered a biofilm producer if assigned to the weak, moderate or strong phenotypes in, at least, one of the growth conditions tested.
Statistical analyses were performed in SPSS v26.0 (IBM®, Armonk, NY, USA) to verify associations between biofilm production and agr type and resistance traits (methicillin resistance and MDR phenotype) using the chi-square test. Statistical significance was considered for p < 0.05.
4.3. Isolation of Total DNA, Agr Typing and Identification of Ica Genes by PCR
Total DNA from each strain was extracted by the boiling method as described by Alexopoulou and colleagues [87].
Agr typing was performed for all S. pseudintermedius and S. aureus. For S. pseudintermedius, the agrD gene was amplified by PCR and sequenced using the primers described in Supplementary Material S2. The nucleotide sequences were then compared with the four reference sequences of the four agr types, as follows: agrI (accession no. EU157336); agrII (accession no. EU157366); agrIII (accession no. EU157334); agrIV (accession no. EU157330) [88]. For S. aureus, agr typing was carried out following a multiplex PCR approach, as described by Lina and colleagues [89].
All strains were screened by PCR for the presence of the icaADB genes associated with biofilm production using primers specific for each species (Supplementary Material S2). The presence of the determinants lukF-PV and lukS-PV encoding PVL or lukF encoding LukI was screened by PCR using the primers also described in Supplementary Material S2 [90]. Primers were designed using Primer-blast [91] based on the genome of S. pseudintermedius HKU10-03 (accession no. CP002439) and S. aureus RN4220 (accession no. CP076105). All primers used were synthesized by Invitrogen (Waltham, MA, USA).
4.4. In Silico Search of the Presence of the Ica Operon and PVL/LukI Determinants in Staphylococcal Genomes
The presence of ica and leukocidin-encoding genes was evaluated in the complete genomes available at GenBank database (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 30 August 2022, National Library of Medicine, Bethesda, MD, USA) for S. pseudintermedius (n = 107), S. aureus (n > 800) and S. coagulans (n = 2, only chromosome sequences available).
Using the Blast tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 30 August 2022, National Library of Medicine, Bethesda, MD, USA), those genomes were queried for the presence of lukF-lukS (S. pseudintermedius, S. coagulans) and the four genes of the ica operon (icaA, icaB, icaC and icaD) (all three species). These searches were carried out using the nucleotide sequences of the respective genes of the representative genomes S. pseudintermedius SP_11304-3A (accession no. NZ_CP065921.1) and S. aureus NCTC 8325 (accession no. NC_007795.1).
4.5. Assessment of Virulence Potential in a G. mellonella Infection Model
The G. mellonella infection model has been applied to evaluate the virulence of different pathogens [81]. In this study, G. mellonella was used to assess the virulence potential of representative strains of each staphylococcal species in study (three strains per species, corresponding to one reference and two clinical strains). The strains tested were biofilm producers and presented additional relevant phenotypic and genotypic characteristics, such as methicillin resistance and belonging to predominant clonal lineages (Table 3). The virulence potential of each strain was tested using two different bacterial inoculums, 105 and 107 CFU/larva.

Table 3.
Main characteristics of the biofilm-producing strains studied on the G. mellonella infection assays [45,46,47].
G. mellonella at the larva stage were acquired from a pet-food supplier (Reptimundo, Faro, Portugal). The larvae were maintained in the dark at room temperature and were used within one week [92]. Prior to each assay, the larvae were acclimatized overnight to 37 °C. The bacterial inoculum was prepared by overnight growth in TSB at 37 °C and 180 rpm; the bacterial cells were collected, washed thrice in PBS, resuspended and adjusted in PBS to 5 × 108 CFU/mL (corresponding to OD600 of 0.25–0.26 (S. aureus) and 0.29–0.31 (S. pseudintermedius and S. coagulans)). The CFU/mL was verified by plating aliquots of the cellular suspension in TSA. An aliquot was further diluted 1:100 in PBS to achieve 5 × 106 CFU/mL. For each cellular suspension, serial dilutions were prepared up to 10−7 and an aliquot of 0.1 mL was plated in TSA (VWR International LLC, Radnor, PA, USA) for bacteria enumeration.
Groups of ten larvae, selected based on similar weight (200–250 mg/larvae) and size (20–25 mm), no melanization and presence of motility [93] were restrained [92] and inoculated with 0.02 mL of (i) PBS (control), (ii) 5 × 108 CFU/mL (corresponding to 1 × 107 CFU/larva) and (iii) 5 × 106 CFU/mL (corresponding to 1 × 105 CFU/larva) using an insulin syringe (Braun Omnican® 100, B. Braun, Melsungen, Germany). A fourth group of ten non-manipulated larvae was included per assay. The larvae were kept at 37 °C with food (bee wax shavings (Reptimundo)) [94], and larvae survival was evaluated each 24 h post-infection for seven days. Dead larvae (full melanization, lack of motility) were removed at each timepoint, transferred to a falcon tube and placed in a secondary containment vessel at −20 °C overnight prior to disposal for incineration. The virulence potential of each staphylococcal strain was evaluated in two independent infection assays. Data were analyzed by Kaplan–Meier survival curves and the mean survival time was determined using GraphPad Prism v 8.0.1 (San Diego, CA, USA). Survival rates between groups were compared with the Log-Rank (Mantel–Cox) test. Statistical significance was considered for p-values <0.05.
5. Conclusions
This study provides evidence on the high prevalence of biofilm production by CoPS strains causing skin infections in companion animals in Portugal. Worrisomely, production of biofilm was detected amongst predominant S. pseudintermedius and S. aureus clonal lineages associated with a high burden of antimicrobial resistance. Additionally, we highlight an increased virulence potential for these antimicrobial-resistant CoPS strains, raising concerns on the future management of skin infection in companion animals and strengthening the need for improved surveillance of these pathogens.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics11101339/s1, Supplementary material S1: In silico search of ica genes and leukocidin determinants in complete genomes of S. pseudintermedius, S. aureus and S. coagulans; Supplementary material S2: Characteristics of the staphylococcal isolates studied and list of the primers used.
Author Contributions
Conceptualization, I.C. and S.S.C.; funding acquisition, S.S.C. and I.C.; investigation, M.A., K.O., C.M. and S.S.C.; methodology, P.A., A.E.R., I.C. and S.S.C.; project administration I.C.; resources, C.P., S.S.C. and I.C.; supervision, I.C. and S.S.C.; validation, P.A., I.C. and S.S.C.; visualization, M.A, K.O. and S.S.C.; writing—original draft, M.A. and S.S.C.; writing—review and editing, M.A., K.O., C.M., P.A., C.P., A.E.R., I.C. and S.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Project BIOSAFE funded by FEDER through the Programa Operacional Factores de Competitividade—COMPETE, by the Fundação para a Ciência e a Tecnologia (FCT, Portugal)—Grant LISBOA-01-0145-FEDER-030713, PTDC/CAL-EST/30713/2017 and by FCT through grant UI/BD/151061/2021 (C.M) and funds to GHTM (UID/04413/2020) and the CIISA Project (UID/CVT/00276/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data have been provided in the paper. Raw data can also be provided by the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hillier, A.; Lloyd, D.H.; Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D.; et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet. Dermatol. 2014, 25, 163-e43. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Helbig, K. The Complex Diseases of Staphylococcus pseudintermedius in Canines: Where to Next? Vet. Sci. 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, A.; Lloyd, D. What has changed in canine pyoderma? A narrative review. Vet. J. 2018, 235, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.J.; Woodward, M.; Hoppers, S.M.; Liu, C.; Pucheu-Haston, C.M.; Mitchell, M.S. Residual antibacterial activity of canine hair treated with five mousse products against Staphylococcus pseudintermedius in vitro. Vet. Dermatol. 2019, 30, 183-e57. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Vogelnest, L.J. Feline superficial pyoderma: A retrospective study of 52 cases (2001–2011). Vet. Dermatol. 2012, 23, 448-e86. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Miller Jr, W.H. Superficial bacterial pyoderma in cats. Vet. Dermatol. 2013, 24, 373. [Google Scholar] [CrossRef]
- Aires-De-Sousa, M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microbiol. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef]
- Benato, L.; Stoeckli, M.R.; Smith, S.H.; Dickson, S.; Thoday, K.L.; Meredith, A. A case of antibacterial-responsive mucocutaneous disease in a seven-year-old dwarf lop rabbit (Oryctolagus cuniculus) resembling mucocutaneous pyoderma of dogs. J. Small Anim. Pract. 2013, 54, 209–212. [Google Scholar] [CrossRef]
- Kadlec, K.; Schwarz, S. Antimicrobial resistance of Staphylococcus pseudintermedius. Vet. Dermatol. 2012, 23, 276-e55. [Google Scholar] [CrossRef]
- Devriese, L.A.; Vancanneyt, M.; Baele, M.; Vaneechoutte, M.; De Graef, E.; Snauwaert, C.; Cleenwerck, I.; Dawyndt, P.; Swings, J.; Decostere, A.; et al. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int. J. Syst. Evol. Microbiol. 2005, 55, 1569–1573. [Google Scholar] [CrossRef]
- Lee, J.; Murray, A.; Bendall, R.; Gaze, W.; Zhang, L.; Vos, M. Improved Detection of Staphylococcus intermedius Group in a Routine Diagnostic Laboratory. J. Clin. Microbiol. 2015, 53, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.E.; Chirino-Trejo, M. Prevalence, Sites of Colonization, and Antimicrobial Resistance Among Staphylococcus pseudintermedius Isolated from Healthy Dogs in Saskatoon, Canada. J. Vet. Diagn. Investig. 2011, 23, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Cuny, C.; Layer-Nicolaou, F.; Weber, R.; Köck, R.; Witte, W. Colonization of Dogs and Their Owners with Staphylococcus aureus and Staphylococcus pseudintermedius in Households, Veterinary Practices, and Healthcare Facilities. Microorganisms 2022, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Bannoehr, J.; Guardabassi, L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012, 23, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Woolley, K.L.; Kelly, R.F.; Fazakerley, J.; Williams, N.; Nuttall, T.J.; McEwan, N.A. Reduced in vitro adherence of Staphylococcus species to feline corneocytes compared to canine and human corneocytes. Vet. Dermatol. 2007, 19, 1–6. [Google Scholar] [CrossRef]
- Bierowiec, K.; Korzeniowska-Kowal, A.; Wzorek, A.; Rypuła, K.; Gamian, A. Prevalence of Staphylococcus Species Colonization in Healthy and Sick Cats. BioMed Res. Int. 2019, 2019, 4360525. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, A.; Lloyd, D.H. Companion animals: A reservoir for methicillin-resistant Staphylococcus aureus in the community? Epidemiol. Infect. 2010, 138, 595–605. [Google Scholar] [CrossRef]
- Duquette, R.A.; Nuttall, T.J. Methicillin-resistant Staphylococcus aureus in dogs and cats: An emerging problem? J. Small Anim. Pract. 2004, 45, 591–597. [Google Scholar] [CrossRef]
- Haag, A.F.; Fitzgerald, J.R.; Penadés, J.R. Staphylococcus aureus in Animals. Microbiol. Spectr. 2019, 7, 11. [Google Scholar] [CrossRef]
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Wirth, J.S.; Saravanan, V.S. Phylogenomic analyses of the Staphylococcaceae family suggest the reclassification of five species within the genus Staphylococcus as heterotypic synonyms, the promotion of five subspecies to novel species, the taxonomic reassignment of five Staphylococcus species to Mammaliicoccus gen. nov., and the formal assignment of Nosocomiicoccus to the family Staphylococcaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 5926–5936. [Google Scholar] [CrossRef] [PubMed]
- Griffeth, G.C.; Morris, D.O.; Abraham, J.L.; Shofer, F.S.; Rankin, S.C. Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet. Dermatol. 2008, 19, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.; Roberts, L.; Jones, M.; Young, V. Staphylococcus schleiferi subspecies coagulans in companion animals. Vet. Rec. 2007, 161, 107. [Google Scholar] [CrossRef]
- Hanselman, B.A.; Kruth, S.; Weese, J.S. Methicillin-resistant staphylococcal colonization in dogs entering a veterinary teaching hospital. Vet. Microbiol. 2008, 126, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; Van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M.; et al. Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Harrison, E.M.; Stanczak-Mrozek, K.; Leggett, B.; Waller, A.; Holmes, M.A.; Lloyd, D.H.; Lindsay, J.A.; Loeffler, A. Genomic insights into the rapid emergence and evolution of MDR in Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 2014, 70, 997–1007. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef]
- González-Martín, M.; Corbera, J.A.; Suárez-Bonnet, A.; Tejedor-Junco, M.T. Virulence factors in coagulase-positive staphylococci of veterinary interest other than Staphylococcus aureus. Vet. Q. 2020, 40, 118–131. [Google Scholar] [CrossRef]
- Lee, G.Y.; Lee, H.-H.; Hwang, S.Y.; Hong, J.; Lyoo, K.-S.; Yang, S.-J. Carriage of Staphylococcus schleiferi from canine otitis externa: Antimicrobial resistance profiles and virulence factors associated with skin infection. J. Vet. Sci. 2019, 20, e6. [Google Scholar] [CrossRef]
- Jantorn, P.; Heemmamad, H.; Soimala, T.; Indoung, S.; Saising, J.; Chokpaisarn, J.; Wanna, W.; Tipmanee, V.; Saeloh, D. Antibiotic Resistance Profile and Biofilm Production of Staphylococcus pseudintermedius Isolated from Dogs in Thailand. Pharmaceuticals 2021, 14, 592. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.M.; Amer, A.M.; Badr, J.M.; Helmy, N.M.; Elhelw, R.A.; Orabi, A.; Bakry, M.; Saad, A.S.A. Antimicrobial Resistance, Biofilm Formation and mecA Characterization of Methicillin-Susceptible S. aureus and Non-S. aureus of Beef Meat Origin in Egypt. Front. Microbiol. 2016, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, R. Biofilms: Microbial Cities of Scientific Significance. J. Microbiol. Exp. 2014, 1, 00014. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Curr Top Microbiol Immunol 2008, 322, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Parastan, R.; Kargar, M.; Solhjoo, K.; Kafilzadeh, F. Staphylococcus aureus biofilms: Structures, antibiotic resistance, inhibition, and vaccines. Gene Rep. 2020, 20, 100739. [Google Scholar] [CrossRef]
- Shallcross, L.J.; Fragaszy, E.; Johnson, A.M.; Hayward, A.C. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 43–54. [Google Scholar] [CrossRef]
- Abouelkhair, M.A.; Bemis, D.A.; Giannone, R.J.; Frank, L.; Kania, S.A. Characterization of a leukocidin identified in Staphylococcus pseudintermedius. PLoS ONE 2018, 13, e0204450. [Google Scholar] [CrossRef]
- Alonzo, F., 3rd; Torres, V.J. The Bicomponent Pore-Forming Leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2014, 78, 199–230. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Gillet, Y.; Issartel, B.; Vanhems, P.; Fournet, J.-C.; Lina, G.; Bes, M.; Vandenesch, F.; Piémont, Y.; Brousse, N.; Floret, D.; et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002, 359, 753–759. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.-O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine Leukocidin-Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7, 16. [Google Scholar] [CrossRef]
- Xu-Yang, W.; Wang, X.-Y.; Cui, P.; Zhang, Y.-M.; Zhang, W.-H.; Zhang, Y. The Agr Quorum Sensing System Represses Persister Formation through Regulation of Phenol Soluble Modulins in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2189. [Google Scholar] [CrossRef]
- Little, S.V.; Bryan, L.K.; Hillhouse, A.E.; Cohen, N.D.; Lawhon, S.D. Characterization of agr Groups of Staphylococcus pseudintermedius Isolates from Dogs in Texas. mSphere 2019, 4, e00033-19. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Ribeiro, R.; Serrano, M.; Oliveira, K.; Ferreira, C.; Leal, M.; Pomba, C.; Couto, I. Staphylococcus aureus Causing Skin and Soft Tissue Infections in Companion Animals: Antimicrobial Resistance Profiles and Clonal Lineages. Antibiotics 2022, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.; Costa, S.S.; Andrade, M.; Ramos, B.; Leal, M.; Abrantes, P.; Pomba, C.; Couto, I. Clonal lineages of Staphylococcus pseudintermedius associated with skin and soft tissues infections in pets, Portugal. In Proceedings of the 31st European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 9–12 July 2021. [Google Scholar]
- Costa, S.; Oliveira, V.; Serrano, M.; Pomba, C.; Couto, I. Phenotypic and Molecular Traits of Staphylococcus coagulans Associated with Canine Skin Infections in Portugal. Antibiotics 2021, 10, 518. [Google Scholar] [CrossRef]
- Götz, F. Staphylococcus and biofilms. Mol Microbiol 2002, 43, 1367–1378. [Google Scholar] [CrossRef]
- Morris, D.O.; Loeffler, A.; Davis, M.F.; Guardabassi, L.; Weese, J.S. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: Diagnosis, therapeutic considerations and preventative measures. Vet. Dermatol. 2017, 28, 304-e69. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef]
- Souza-Silva, T.; Rossi, C.C.; Andrade-Oliveira, A.L.; Vilar, L.C.; Pereira, M.F.; Penna, B.D.A.; Giambiagi-Demarval, M. Interspecies transfer of plasmid-borne gentamicin resistance between Staphylococcus isolated from domestic dogs to Staphylococcus aureus. Infect. Genet. Evol. 2022, 98, 105230. [Google Scholar] [CrossRef]
- Gaire, U.; Shrestha, U.T.; Adhikari, S.; Adhikari, N.; Bastola, A.; Rijal, K.R.; Ghimire, P.; Banjara, M.R. Antibiotic Susceptibility, Biofilm Production, and Detection of mecA Gene among Staphylococcus aureus Isolates from Different Clinical Specimens. Diseases 2021, 9, 80. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Namvar, A.E. Detection of genes involved in biofilm formation in Staphylococcus aureus isolates. GMS Hyg Infect Control 2016, 11, Doc07. [Google Scholar] [CrossRef] [PubMed]
- Olia, A.H.G.; Ghahremani, M.; Ahmadi, A.; Sharifi, Y. Comparison of biofilm production and virulence gene distribution among community- and hospital-acquired Staphylococcus aureus isolates from northwestern Iran. Infect. Genet. Evol. 2020, 81, 104262. [Google Scholar] [CrossRef] [PubMed]
- Omidi, M.; Firoozeh, F.; Saffari, M.; Sedaghat, H.; Zibaei, M.; Khaledi, A. Ability of biofilm production and molecular analysis of spa and ica genes among clinical isolates of methicillin-resistant Staphylococcus aureus. BMC Res. Notes 2020, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bissong, M.E.A.; Ateba, C.N. Genotypic and Phenotypic Evaluation of Biofilm Production and Antimicrobial Resistance in Staphylococcus aureus Isolated from Milk, North West Province, South Africa. Antibiotics 2020, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Soares Filipe, J.F.; Drago, L.; Martino, P.A. Investigation on Antibiotic-Resistance, Biofilm Formation and Virulence Factors in Multi Drug Resistant and Non Multi Drug Resistant Staphylococcus pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Walker, M.; Rousseau, J.; Weese, J.S. Characterization of the biofilm forming ability of Staphylococcus pseudintermedius from dogs. BMC Vet Res. 2013, 9, 93. [Google Scholar] [CrossRef]
- Proietti, P.C.; Stefanetti, V.; Hyatt, D.R.; Marenzoni, M.L.; Capomaccio, S.; Coletti, M.; Bietta, A.; Franciosini, M.P.; Passamonti, F. Phenotypic and genotypic characterization of canine pyoderma isolates of Staphylococcus pseudintermedius for biofilm formation. J. Vet. Med Sci. 2015, 77, 945–951. [Google Scholar] [CrossRef]
- Bierowiec, K.; Miszczak, M.; Korzeniowska-Kowal, A.; Wzorek, A.; Płókarz, D.; Gamian, A. Epidemiology of Staphylococcus pseudintermedius in cats in Poland. Sci. Rep. 2021, 11, 18898. [Google Scholar] [CrossRef]
- Garbacz, K.; Żarnowska, S.; Piechowicz, L.; Haras, K. Pathogenicity potential of Staphylococcus pseudintermedius strains isolated from canine carriers and from dogs with infection signs. Virulence 2013, 4, 255–259. [Google Scholar] [CrossRef][Green Version]
- Stepanović, S.; Vuković, D.; Hola, V.; DI Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Jain, A. Glucose & sodium chloride induced biofilm production & ica operon in clinical isolates of staphylococci. Indian J. Med Res. 2013, 138, 262–266. [Google Scholar] [PubMed]
- Lee, S.; Choi, K.-H.; Yoon, Y. Effect of NaCl on Biofilm Formation of the Isolate from Staphylococcus aureus Outbreak Linked to Ham. Korean J. Food Sci. Anim. Resour. 2014, 34, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Fowler Jr, V.G.; Fey, P.D.; Reller, L.B.; Chamis, A.L.; Corey, G.R.; Rupp, M.E. The intercellular adhesin locus ica is present in clinical isolates of Staphylococcus aureus from bacteremic patients with infected and uninfected prosthetic joints. Med. Microbiol. Immunol. 2001, 189, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, J.K.-M.; Horstkotte, M.A.; Rohde, H.; Mack, D. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med. Microbiol. Immunol. 2002, 191, 101–106. [Google Scholar] [CrossRef]
- Panda, S.; Singh, D.V. Biofilm Formation by ica-Negative Ocular Isolates of Staphylococcus haemolyticus. Front. Microbiol. 2018, 9, 2687. [Google Scholar] [CrossRef]
- Kwon, M.; Hussain, M.S.; Oh, D.H. Biofilm formation of Bacillus cereus under food-processing-related conditions. Food Sci. Biotechnol. 2017, 26, 1103–1111. [Google Scholar] [CrossRef]
- Xu, H.; Zou, Y.; Lee, H.-Y.; Ahn, J. Effect of NaCl on the Biofilm Formation by Foodborne Pathogens. J. Food Sci. 2010, 75, M580–M585. [Google Scholar] [CrossRef]
- Roberts, S.; O’Shea, K.; Morris, D.; Robb, A.; Morrison, D.; Rankin, S. A real-time PCR assay to detect the Panton Valentine Leukocidin toxin in staphylococci: Screening Staphylococcus schleiferi subspecies coagulans strains from companion animals. Vet. Microbiol. 2005, 107, 139–144. [Google Scholar] [CrossRef]
- Viana, D.; Selva, L.; Callanan, J.; Guerrero, I.; Ferrian, S.; Corpa, J. Strains of Staphylococcus aureus and pathology associated with chronic suppurative mastitis in rabbits. Vet. J. 2011, 190, 403–407. [Google Scholar] [CrossRef]
- Swearengen, J.R. Choosing the right animal model for infectious disease research. Anim. Model. Exp. Med. 2018, 1, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.F.; Rossi, C.C.; da Silva, G.C.; Rosa, J.N.; Bazzolli, D.M.S. Galleria mellonella as an infection model: An in-depth look at why it works and practical considerations for successful application. Pathog. Dis. 2020, 78, ftaa056. [Google Scholar] [CrossRef]
- Mikulak, E.; Gliniewicz, A.; Przygodzka, M.; Solecka, J. Galleria mellonella L. as model organism used in biomedical and other studies. Przegl Epidemiol. 2018, 72, 57–73. [Google Scholar] [PubMed]
- Sheehan, G.; Dixon, A.; Kavanagh, K. Utilization of Galleria mellonella larvae to characterize the development of Staphylococcus aureus infection. Microbiology 2019, 165, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, T.M.; Upton, M. Synthetic epidermicin NI01 can protect Galleria mellonella larvae from infection with Staphylococcus aureus. J. Antimicrob. Chemother. 2013, 68, 2269–2273. [Google Scholar] [CrossRef]
- Furi, L.; Ciusa, M.L.; Knight, D.; Di Lorenzo, V.; Tocci, N.; Cirasola, D.; Aragones, L.; Coelho, J.R.; Freitas, A.T.; Marchi, E.; et al. Evaluation of Reduced Susceptibility to Quaternary Ammonium Compounds and Bisbiguanides in Clinical Isolates and Laboratory-Generated Mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 3488–3497. [Google Scholar] [CrossRef]
- Taglialegna, A.; Varela, M.C.; Rosato, R.R.; Rosato, A.E. VraSR and Virulence Trait Modulation during Daptomycin Resistance in Methicillin-Resistant Staphylococcus aureus Infection. mSphere 2019, 4, e00557-18. [Google Scholar] [CrossRef]
- Canovas, J.; Baldry, M.; Bojer, M.S.; Andersen, P.S.; Gless, B.H.; Grzeskowiak, P.K.; Stegger, M.; Damborg, P.P.; Olsen, C.A.; Ingmer, H. Cross-Talk between Staphylococcus aureus and Other Staphylococcal Species via the agr Quorum Sensing System. Front. Microbiol. 2016, 7, 1733. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Ghukasyan, G.; Emily, M.; Felden, B.; Donnio, P.-Y. Galleria mellonella Larvae as an Infection Model to Investigate sRNA-Mediated Pathogenesis in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2021, 11, 631710. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Liu, Y.; Xu, S.; Wu, M.; Liu, Z.; Qi, C.; Zhang, G.; Li, J.; Huang, X. Synergistic effect of linezolid with fosfomycin against Staphylococcus aureus in vitro and in an experimental Galleria mellonella model. J. Microbiol. Immunol. Infect. 2018, 53, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Costa, S.; Serrano, M.; Oliveira, K.; Trigueiro, G.; Pomba, C.; Couto, I. Clonal Lineages, Antimicrobial Resistance, and PVL Carriage of Staphylococcus aureus Associated to Skin and Soft-Tissue Infections from Ambulatory Patients in Portugal. Antibiotics 2021, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Oliveira, A.; Manageiro, V.; Caniça, M.; Contente, D.; Capita, R.; Alonso-Calleja, C.; Carvalho, I.; Capelo, J.; Igrejas, G.; et al. Clonal Diversity and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Canine Pyoderma. Microorganisms 2021, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Bergot, M.; Martins-Simoes, P.; Kilian, H.; Châtre, P.; Worthing, K.; Norris, J.; Madec, J.-Y.; Laurent, F.; Haenni, M. Evolution of the Population Structure of Staphylococcus pseudintermedius in France. Front. Microbiol. 2018, 9, 3055. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Alexopoulou, K.; Foka, A.; Petinaki, E.; Jelastopulu, E.; Dimitracopoulos, G.; Spiliopoulou, I. Comparison of two commercial methods with PCR restriction fragment length polymorphism of the tuf gene in the identification of coagulase-negative staphylococci. Lett. Appl. Microbiol. 2006, 43, 450–454. [Google Scholar] [CrossRef]
- Couto, N.; Belas, A.; Oliveira, M.; Almeida, P.; Clemente, C.; Pomba, C. Comparative RNA-seq-Based Transcriptome Analysis of the Virulence Characteristics of Methicillin-Resistant and -Susceptible Staphylococcus pseudintermedius Strains Isolated from Small Animals. Antimicrob. Agents Chemother. 2016, 60, 962–967. [Google Scholar] [CrossRef]
- Lina, G.; Boutite, F.; Tristan, A.; Bes, M.; Etienne, J.; Vandenesch, F. Bacterial Competition for Human Nasal Cavity Colonization: Role of Staphylococcal agr Alleles. Appl. Environ. Microbiol. 2003, 69, 18–23. [Google Scholar] [CrossRef]
- Futagawa-Saito, K.; Sugiyama, T.; Karube, S.; Sakurai, N.; Ba-Thein, W.; Fukuyasu, T. Prevalence and Characterization of Leukotoxin-Producing Staphylococcus intermedius in Isolates from Dogs and Pigeons. J. Clin. Microbiol. 2004, 42, 5324–5326. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Fredericks, L.R.; Lee, M.D.; Roslund, C.R.; Crabtree, A.M.; Allen, P.B.; Rowley, P.A. The design and implementation of restraint devices for the injection of pathogenic microorganisms into Galleria mellonella. PLoS ONE 2020, 15, e0230767. [Google Scholar] [CrossRef] [PubMed]
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Prata, M.C.A.; Jorge, A.O.C.; Junqueira, J.C. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Banville, N.; Browne, N.; Kavanagh, K. Effect of nutrient deprivation on the susceptibility of Galleria mellonella larvae to infection. Virulence 2012, 3, 497–503. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).