Rapid Detection of Carbapenemase and Extended-Spectrum β-Lactamase Producing Gram-Negative Bacteria Directly from Positive Blood Cultures Using a Novel Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Processing
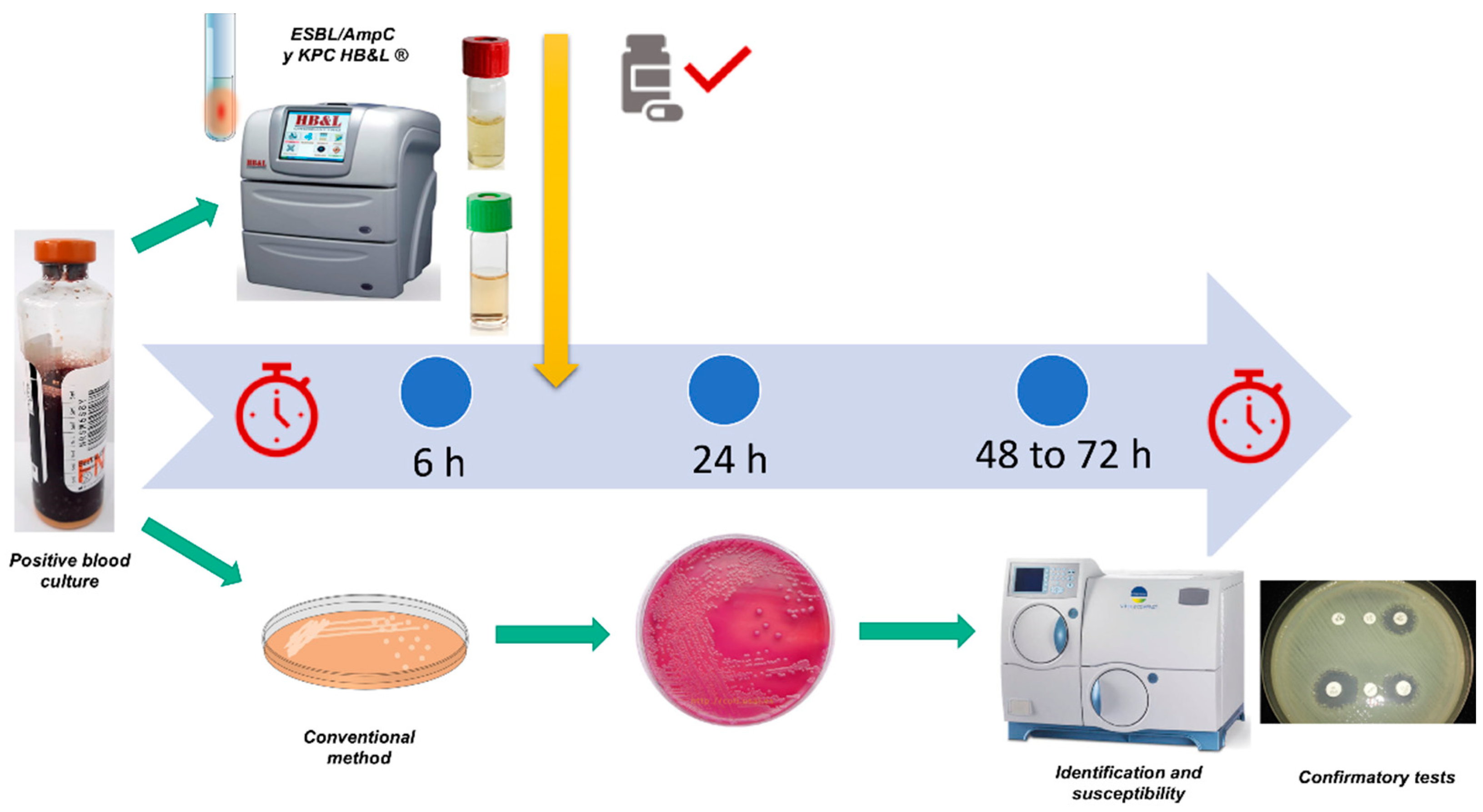
2.1.1. Setting up Blood Cultures for Laser Nephelometry Using HB&L-ESBL/AmpC® and HB&L-Carbapenemase® Kits (New Protocol)
2.1.2. Setting up the Tests through the Conventional Method
2.1.3. Phenotypic Tests for Confirmation of ESBL, AmpC, and Carbapenemase-Producing GNB
2.1.4. Test Controls
2.2. Statistical Analysis
3. Results
3.1. Operational Characteristics of the HB&L-ESBL/AmpC® Test
3.2. Operational Characteristics of the HB&L Carbapenemase® Test
3.3. Time to the Identification of Multi-Drug Resistant Germs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Giske, C.G.; Monnet, D.L.; Cars, O.; Carmeli, Y.; Resistance, R.-A. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob. Agents Chemother. 2008, 52, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef]
- Kang, C.I.; Kim, S.H.; Park, W.B.; Lee, K.-D.; BIN Kim, H.; Kim, E.-C.; Oh, M.-D.; Choe, K.-W. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: Risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob. Agents Chemother. 2005, 49, 760–766. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Tumbarello, M.; Sanguinetti, M.; Montuori, E.; Trecarichi, E.M.; Posteraro, B.; Fiori, B.; Citton, R.; D’Inzeo, T.; Fadda, G.; Cauda, R.; et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteralesceae: The importance of inadequate initial antimicrobial treatment. Antimicrob. Agents Chemother. 2007, 51, 1987–1994. [Google Scholar] [CrossRef]
- Paul, M.; Shani, V.; Muchtar, E.; Kariv, G.; Robenshtok, E.; Leibovici, L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother. 2010, 54, 4851–4863. [Google Scholar] [CrossRef]
- Zarkotou, O.; Pournaras, S.; Tselioti, P.; Dragoumanos, V.; Pitiriga, V.; Ranellou, K.; Prekates, A.; Themeli-Digalaki, K.; Tsakris, A. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 2011, 17, 1798–1803. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Salamanca, E.; de Cueto, M.; Hsueh, P.R.; Viale, P.; Paño-Pardo, J.R.; Venditti, M.; Tumbarello, M.; Daikos, G.; Cantón, R.; et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): A retrospective cohort study. Lancet Infect. Dis. 2017, 17, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.P.; Volling, C.; Green, K.; Uleryk, E.M.; Shah, P.S.; McGeer, A. Carbapenem Resistance, Initial Antibiotic Therapy, and Mortality in Klebsiella pneumoniae Bacteremia: A Systematic Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2017, 38, 1319–1328. [Google Scholar] [CrossRef]
- Bush, K.; Fisher, J.F. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M. Multidrug-resistant Gram-negative bacteria: A product of globalisation. J. Hosp. Infect. 2015, 89, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Buergmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Wellington, E.M.; Boxall, A.B.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Escandón-Vargas, K.; Reyes, S.; Gutiérrez, S.; Villegas, M.V. The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev. Anti-Infect. Ther. 2017, 15, 277–297. [Google Scholar] [CrossRef]
- Bonelli, R.R.; Moreira, B.M.; Picão, R.C. Antimicrobial resistance among Enterobacteriaceae in South America: History, current dissemination status and associated socioeconomic factors. Drug Resist. Update 2014, 17, 24–36. [Google Scholar] [CrossRef]
- Villegas, M.V.; Lolans, K.; Correa, A.; Suarez, C.J.; Lopez, J.A.; Vallejo, M.; Quinn, J.P. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob. Agents Chemother. 2006, 50, 2880–2882. [Google Scholar] [CrossRef]
- Villegas, M.V.; Lolans, K.; Correa, A.; Kattan, J.N.; Lopez, J.A.; Quinn, J.P. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolysing beta-lactamase. Antimicrob. Agents Chemother. 2007, 51, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Ovalle, M.V.; Saavedra, S.Y.; González, M.N.; Hidalgo, A.M.; Duarte, C.; Beltrán, M. Results of the national surveillance of antimicrobial resistance of Enterobacteriaceae and Gram negative bacilli in health care-associated infections in Colombia, 2012–2014. Biomedica 2017, 37, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gómez, C.; Blanco, V.M.; Motoa, G.; Correa, A.; Vallejo, M.; Villegas, M.V. Evolution of antimicrobial resistance in Gram negative bacilli from intensive care units in Colombia. Biomedica 2014, 34 (Suppl. S1), 91–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banerjee, R.; Humphries, R. Clinical and laboratory considerations for the rapid detection of carbapenem-resistant Enterobacteriaceae. Virulence 2017, 8, 427–439. [Google Scholar] [CrossRef]
- Findlay, J.; Hopkins, K.L.; Meunier, D.; Woodford, N. Evaluation of three commercial assays for rapid detection of genes encoding clinically relevant carbapenemases in cultured bacteria. J. Antimicrob. Chemother. 2015, 70, 1338–1342. [Google Scholar] [CrossRef]
- Rodríguez-Lucas, C.; Rodicio, M.R.; Costales, I.; Boga, J.A.; Vazquez, F.; Fernández, J. Evaluation of Sepsis Flow Chip for identification of Gram-negative bacilli and detection of antimicrobial resistance genes directly from positive blood cultures. Diagn. Microbiol. Infect. Dis. 2018, 91, 205–209. [Google Scholar] [CrossRef]
- March-Rosselló, G.A. Rapid methods for detection of bacterial resistance to antibiotics. Enferm. Infecc. Microbiol. Clin. 2017, 35, 182–188. [Google Scholar] [CrossRef]
- Perez, K.K.; Olsen, R.J.; Musick, W.L.; Cernoch, P.L.; Davis, J.R.; Peterson, L.E.; Musser, J.M. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J. Infect. 2014, 69, 216–225. [Google Scholar] [CrossRef]
- Josa, D.F.; Bustos, G.; Torres, I.C.; Esparza, S.G. Evaluation of three screening methods for detection of carbapenemase-producing Enterobacteriaceae in rectal swabs. Rev. Chil. Infectol. 2018, 35, 253–261. [Google Scholar] [CrossRef]
- Arena, F.; Giani, T.; Antonelli, A.; Colavecchio, O.L.; Pecile, P.; Viaggi, B.; Favilli, R.; Rossolini, G.M. A new selective broth enrichment automated method for detection of carbapenem-resistant Enterobacteriaceae from rectal swabs. J. Microbiol. Methods 2018, 147, 66–68. [Google Scholar] [CrossRef]
- Marani, S.; Pedna, M.F. Evaluation of the HB&L carbapenemase and extended spectrum beta lactamase-AmpC automated screening kits for the rapid detection of resistant Enterobacteriaceae in rectal swabs. Microbiol. Med. 2017, 32, 6709. [Google Scholar]
- Athamna, A.; Freimann, S. A new rapid method for detecting extended-spectrum beta-lactamase/AmpC-producing Enterobacteriaceae directly from positive blood cultures using the Uro4 HB&L™ system. Braz. J. Microbiol. 2019, 50, 927–933. [Google Scholar] [PubMed]
- Barnini, S.; Brucculeri, V.; Morici, P.; Ghelardi, E.; Florio, W.; Lupetti, A. A new rapid method for direct antimicrobial susceptibility testing of bacteria from positive blood cultures. BMC Microbiol. 2016, 16, 185. [Google Scholar] [CrossRef]
- M100-ED30; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute-CLSI: Wayne, PA, USA, 2020.
- Sakarikou, C.; Altieri, A.; Bossa, M.C.; Minelli, S.; Dolfa, C.; Piperno, M.; Favalli, C. Rapid and cost-effective identification and antimicrobial susceptibility testing in patients with Gram-negative bacteremia directly from blood-culture fluid. J. Microbiol. Methods 2018, 146, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hogan, C.A.; Watz, N.; Budvytiene, I.; Banaei, N. Rapid antimicrobial susceptibility testing by VITEK®2 directly from blood cultures in patients with Gram-negative rod bacteremia. Diagn. Microbiol. Infect. Dis. 2019, 94, 116–121. [Google Scholar] [CrossRef]
- Höring, S.; Massarani, A.S.; Löffler, B.; Rödel, J. Rapid antibiotic susceptibility testing in blood culture diagnostics performed by direct inoculation using the VITEK®-2 and BD Phoenix™ platforms. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 471–478. [Google Scholar] [CrossRef]
- Akerlund, A.; Jonasson, E.; Matuschek, E.; Serrander, L.; Sundqvist, M.; Kahlmeter, G.; RAST Study Group. EUCAST rapid antimicrobial susceptibility testing (RAST) in blood cultures: Validation in 55 European laboratories. J. Antimicrob. Chemother. 2020, 75, 3230–3238. [Google Scholar] [CrossRef]
- Boattini, M.; Bianco, G.; Comini, S.; Iannaccone, M.; Casale, R.; Cavallo, R.; Nordmann, P.; Costa, C. Direct detection of extended-spectrum-β-lactamase-producers in Enterobacterales from blood cultures: A comparative analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 407–413. [Google Scholar] [CrossRef]
- Comini, S.; Bianco, G.; Boattini, M.; Banche, G.; Ricciardelli, G.; Allizond, V.; Cavallo, R.; Costa, C. Evaluation of a diagnostic algorithm for rapid identification of Gram-negative species and detection of extended-spectrum β-lactamase and carbapenemase directly from blood cultures. J. Antimicrob. Chemother. 2022, 77, 2632–2641. [Google Scholar] [CrossRef]
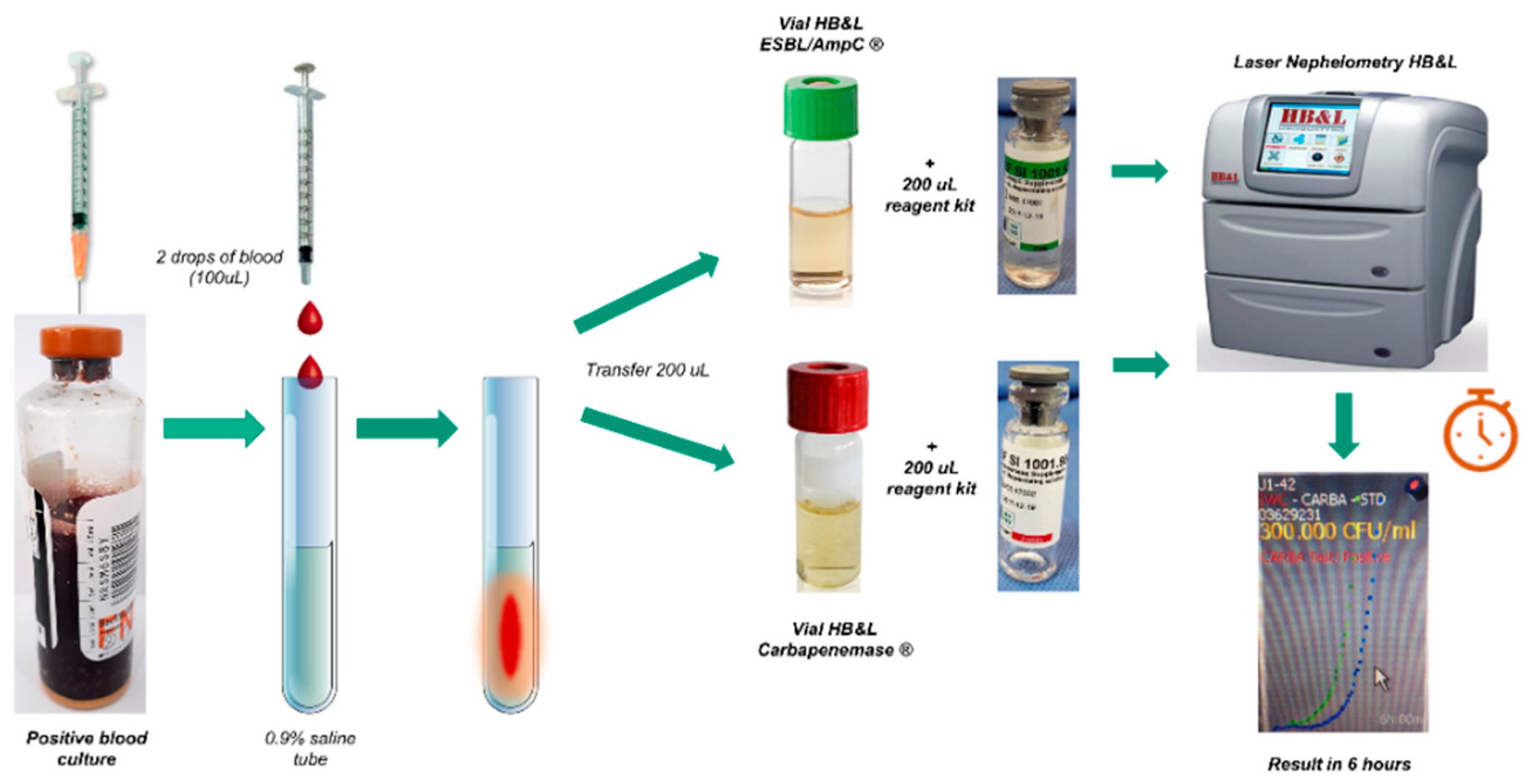
| Characteristic | Total (n = 96) |
|---|---|
| Sex, n (%) | |
| Men | 43 (44.8) |
| Women | 53 (55.2) |
| Age, median (IQR) | 73 (59, 80) |
| Comorbidities, n (%) | |
| Arterial hypertension | 53 (55.2) |
| Coronary disease | 16 (16.7) |
| COPD | 18 (18.8) |
| OSAHS | 8 (8.3) |
| Diabetes Mellitus | 33 (33.4) |
| Obesity | 12 (12.5) |
| Chronic kidney disease | 28 (29.2) |
| Severity, median (IQR) | |
| APACHE II | 12.5 (8, 20.75) |
| SOFA | 4 (2.25, 6.75) |
| Origin of samples, n (%) | |
| Hospitalization | 48 (45.8) |
| Intensive care unit | 40 (41.7) |
| Emergencies | 11 (11.5) |
| Source of infection, n (%) | |
| Community-acquired | 61 (63.5) |
| Nosocomial infection | 33 (34.4) |
| Bacteremia, n (%) | |
| Primary | 7 (7.3) |
| Secondary | 89 (92.7) |
| Origin, n (%) | |
| Urine | 45 (46.9) |
| Abdomen | 12 (12.5) |
| Bile duct | 10 (10.4) |
| Lung | 8 (8.3) |
| Time, hours (median [IQR]) | |
| Bact/Alert 3D® positivity | 13 (12, 16) |
| HB&L® positivity | 19 (18, 22) |
| Conventional culture® | 61 (60, 64) |
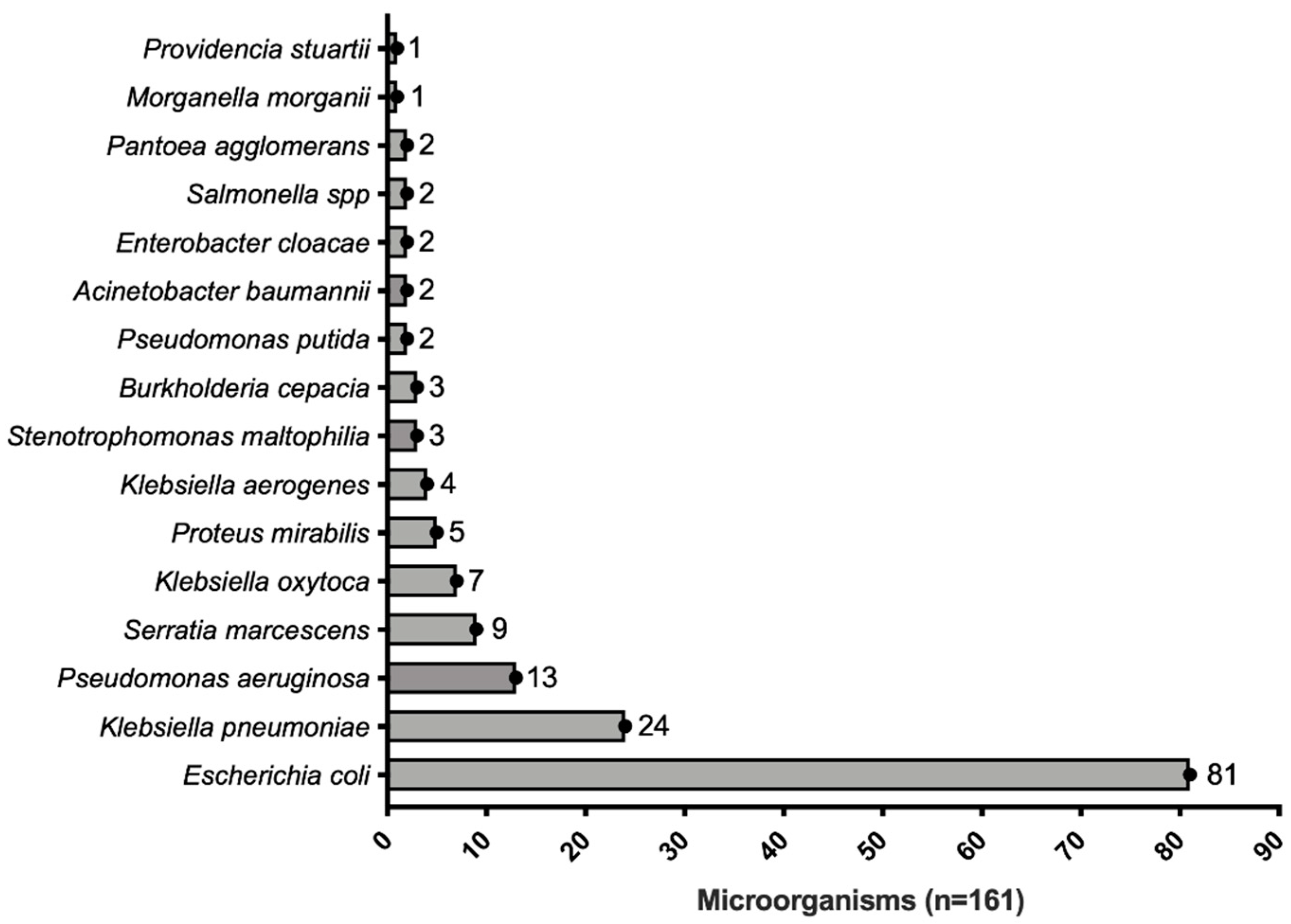
| Microorganism | (n) | Conventional Method/HB&L Method | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitive | IRT | ASBL | ESBL | Repressed AmpC | Unrepressed AmpC | Resistance to Carbapenems | |||
| Enterobacterials | E. coli | 81 | 34/34 | 13/13 | 15/15 | 17/17 | 2/2 | ||
| K. pneumoniae | 24 | 6/6 | 1/1 | 2/2 | 15/15 | ||||
| S. marcescens | 9 | 4/4 | 5/5 | ||||||
| K. oxytoca | 7 | 4/4 | 2/2 | 1/1 | |||||
| P. mirabilis | 5 | 5/5 | |||||||
| K. aerogenes | 4 | 1/1 | 1/1 | 2/2 | |||||
| E. cloacae | 2 | 2/2 | |||||||
| Salmonella spp. | 2 | 1/1 | 1/1 | ||||||
| P. agglomerans | 2 | 2/2 | |||||||
| M. morganii | 1 | 1/1 | |||||||
| P. stuartii | 1 | 1/1 | |||||||
| NF GNB | P. putida | 2 | 2/2 | ||||||
| P. aeruginosa | 13 | 5/5 | 3/1 | 5/5 | |||||
| B. cepacia | 3 | 2/2 | 1/1 | ||||||
| S. maltophilia | 3 | 3/3 | |||||||
| A. baumannii | 2 | 2/2 | |||||||
| ESBL/AmpC® Vial | Carbapenemase® Vial | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | Positive | Negative | Total | |||
| Positive conventional culture | 61 | 2 | 63 | S (95%) | 34 | 0 | 34 | S (100%) |
| Negative conventional culture | 0 | 98 | 98 | E (100%) | 0 | 127 | 0 | E (100%) |
| Total | 61 | 100 | 161 | 34 | 127 | 161 | ||
| VPP 100% | VPN 98% | VPP 100% | VPN 100% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Josa, D.F.; Bustos, I.G.; Yusef, S.A.; Crevoisier, S.; Silva, E.; López, N.; Leal, R.; Molina, I.T.; Osorio, J.P.; Arias, G.; et al. Rapid Detection of Carbapenemase and Extended-Spectrum β-Lactamase Producing Gram-Negative Bacteria Directly from Positive Blood Cultures Using a Novel Protocol. Antibiotics 2023, 12, 34. https://doi.org/10.3390/antibiotics12010034
Josa DF, Bustos IG, Yusef SA, Crevoisier S, Silva E, López N, Leal R, Molina IT, Osorio JP, Arias G, et al. Rapid Detection of Carbapenemase and Extended-Spectrum β-Lactamase Producing Gram-Negative Bacteria Directly from Positive Blood Cultures Using a Novel Protocol. Antibiotics. 2023; 12(1):34. https://doi.org/10.3390/antibiotics12010034
Chicago/Turabian StyleJosa, Diego Fernando, Ingrid Gisell Bustos, Soad Amira Yusef, Stephanie Crevoisier, Edwin Silva, Natalia López, Rafael Leal, Isabel Torres Molina, Juan Pablo Osorio, Gerson Arias, and et al. 2023. "Rapid Detection of Carbapenemase and Extended-Spectrum β-Lactamase Producing Gram-Negative Bacteria Directly from Positive Blood Cultures Using a Novel Protocol" Antibiotics 12, no. 1: 34. https://doi.org/10.3390/antibiotics12010034
APA StyleJosa, D. F., Bustos, I. G., Yusef, S. A., Crevoisier, S., Silva, E., López, N., Leal, R., Molina, I. T., Osorio, J. P., Arias, G., Cortés-Muñoz, F., Sánchez, C., & Reyes, L. F. (2023). Rapid Detection of Carbapenemase and Extended-Spectrum β-Lactamase Producing Gram-Negative Bacteria Directly from Positive Blood Cultures Using a Novel Protocol. Antibiotics, 12(1), 34. https://doi.org/10.3390/antibiotics12010034





