Curcumin: Biological Activities and Modern Pharmaceutical Forms
Abstract
1. Introduction
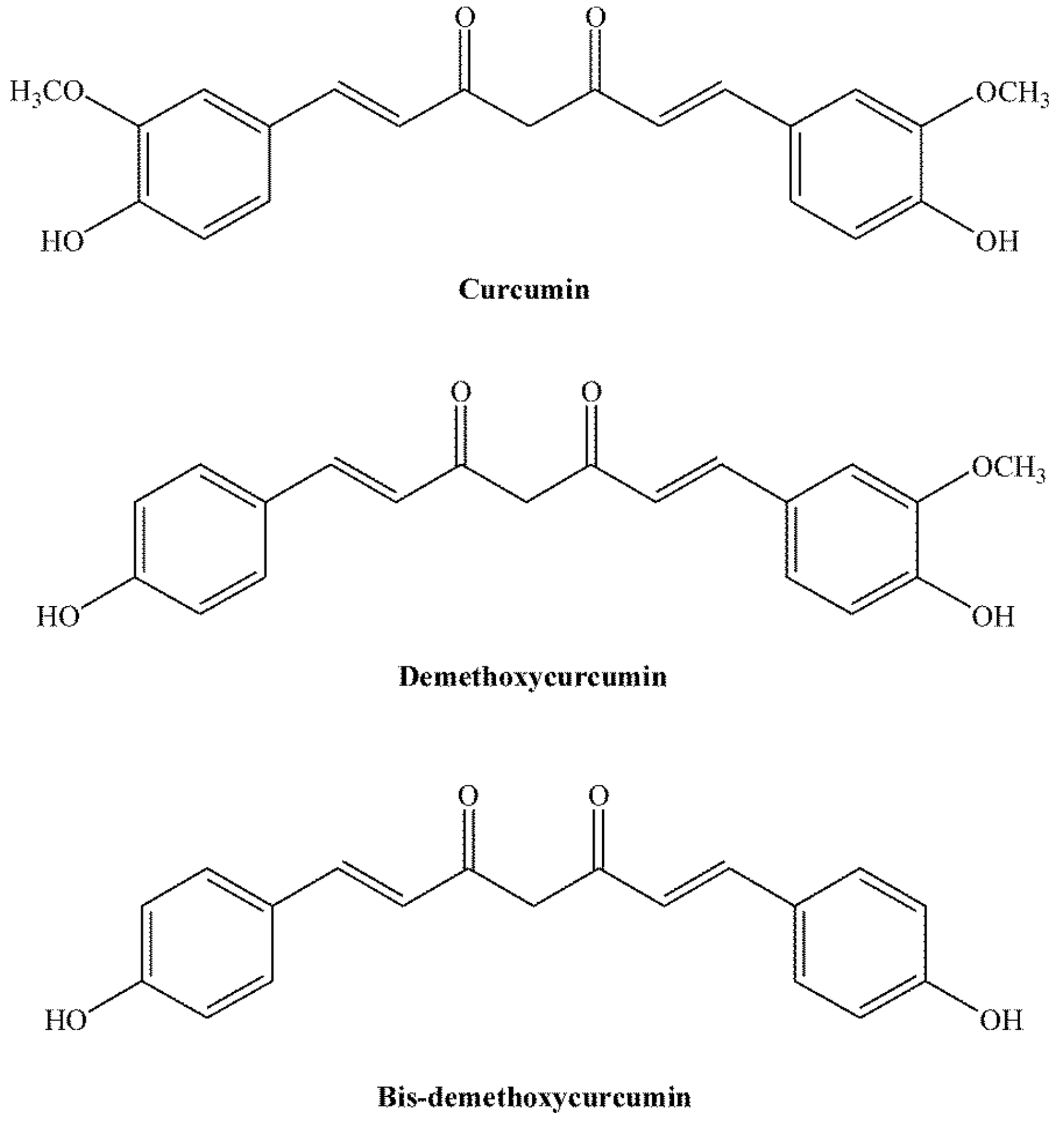
2. Curcumin: Background
3. Isolation of Curcumin from Turmeric Rhizome and Methods of Identification
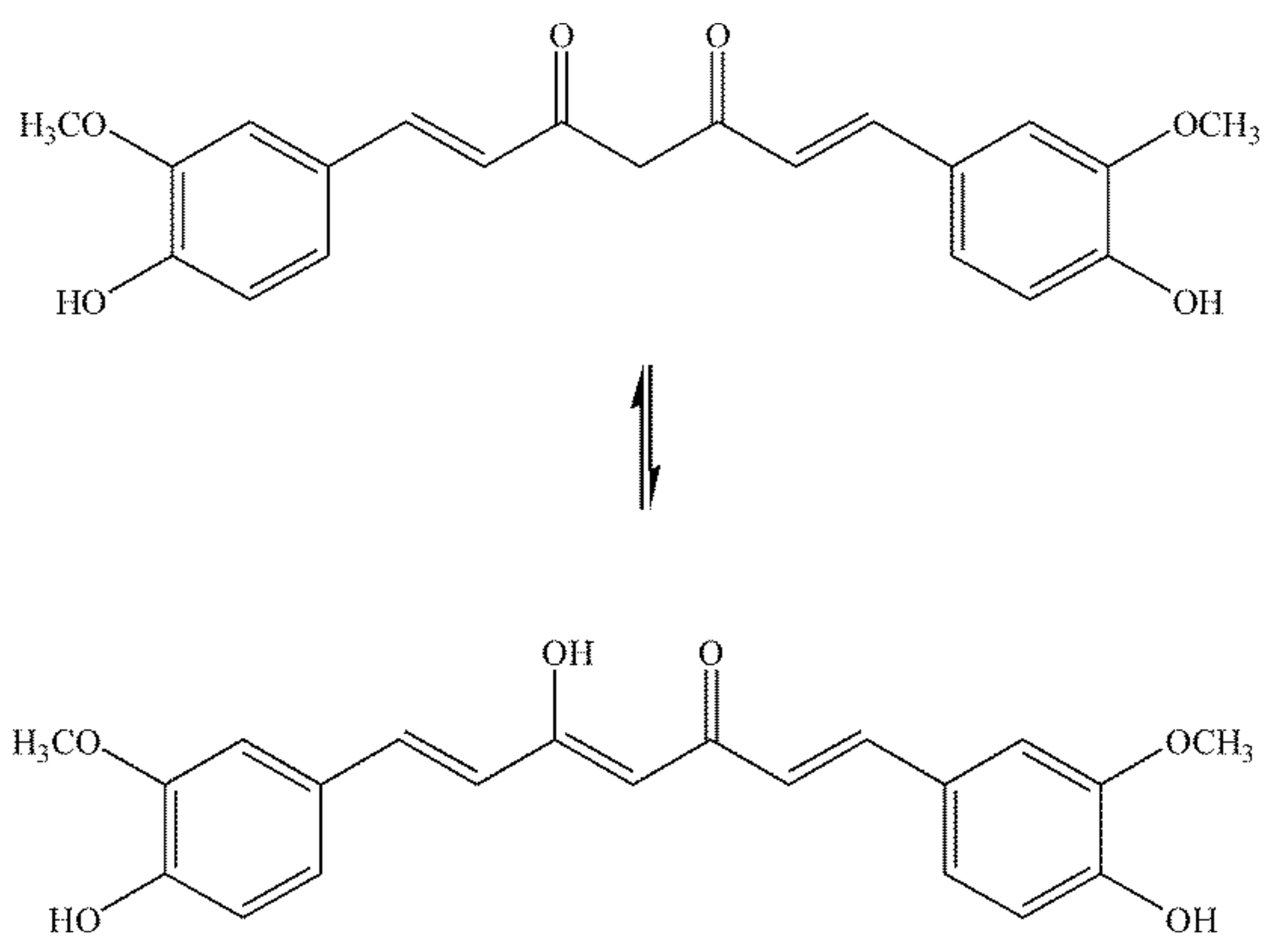
4. Physico-Chemical Properties of Curcumin
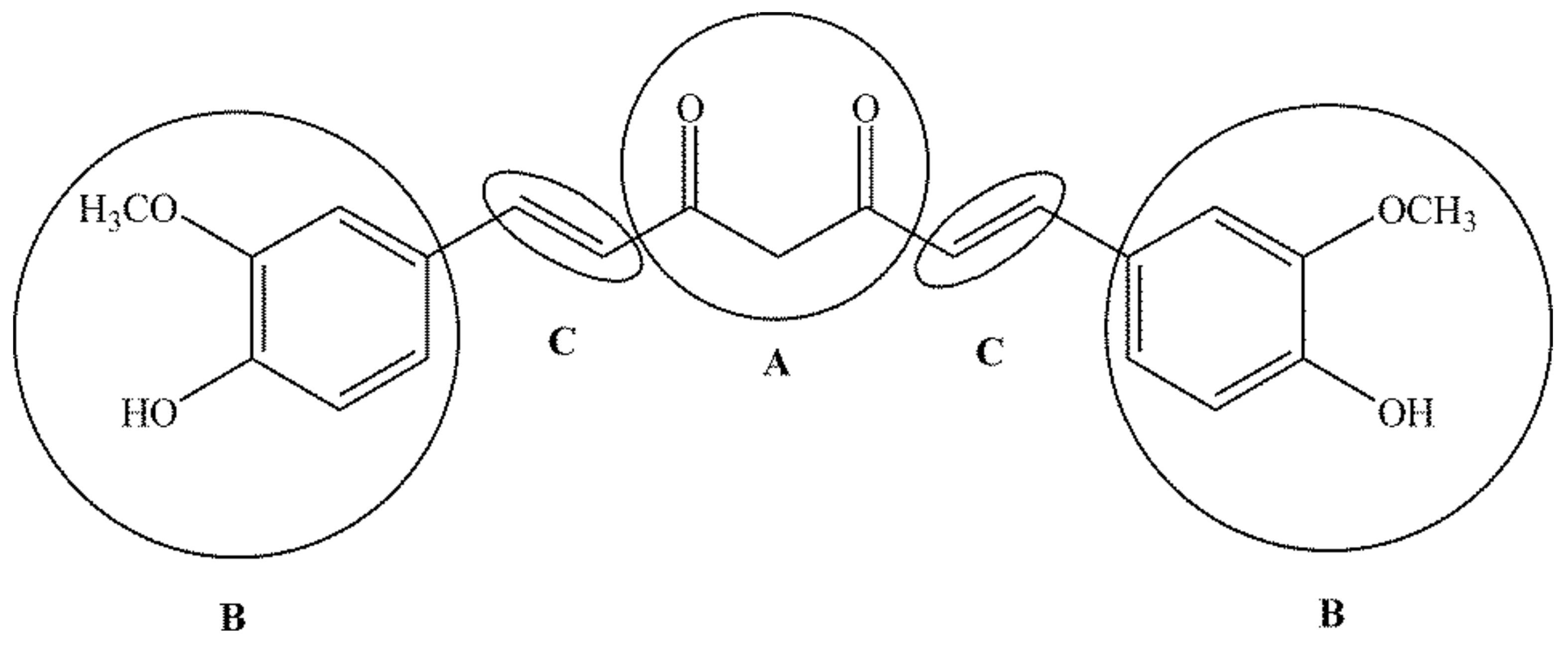
5. Structure, Bioavailability, and Safety of the Application of Curcumin
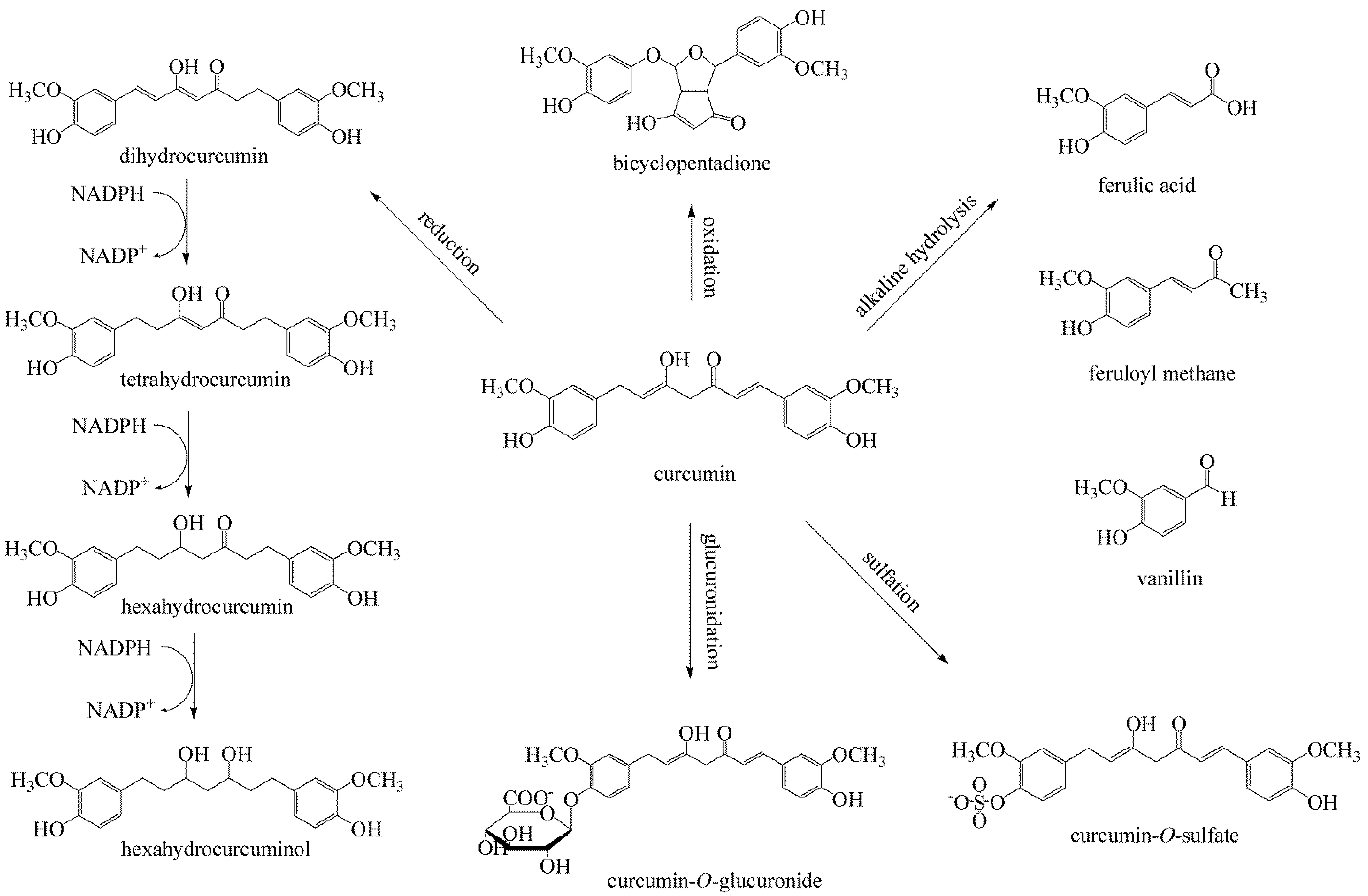
6. The Metabolism of Curcumin
7. Characterization of Curcumin
8. Formulations
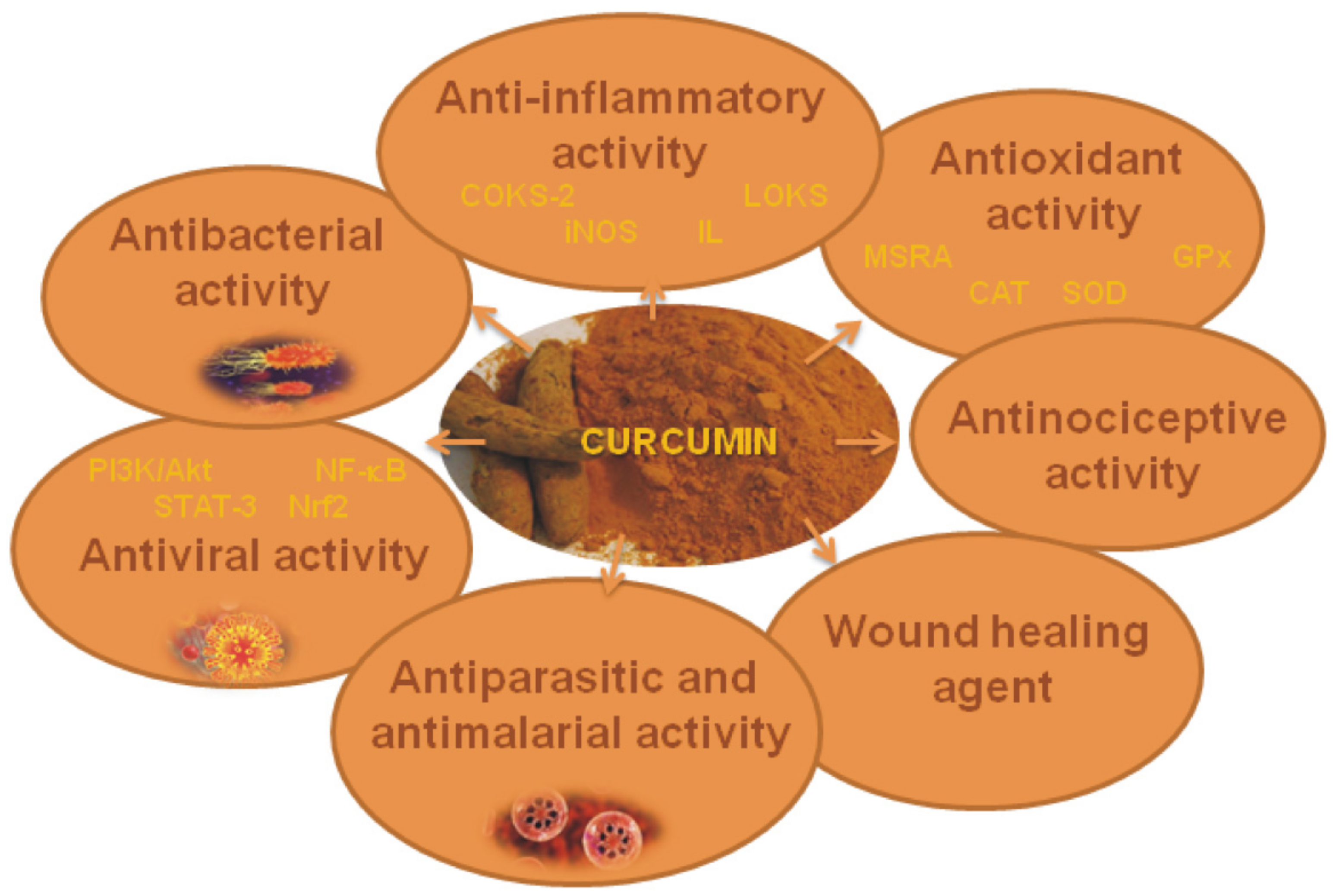
9. Biological Activities of Curcumin
9.1. Antimicrobial Activity
9.1.1. Antibacterial Activity
9.1.2. Antiviral Activity
9.1.3. Antiparasitic and Antimalarial Activity
9.2. Anti-Inflammatory Activity
9.3. Antioxidant Activity
9.4. Antinociceptive Activity
9.5. Wound Healing Agent
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Goel, A. The Holy Grail of Curcumin and its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern? J. Restor. Med. 2017, 6, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin II: Evidence from In Vivo Studies. Nutrients 2020, 12, 58. [Google Scholar] [CrossRef]
- Arshad, L.; Haque, M.A.; Abbas Bukhari, S.N.; Jantan, I. An overview of structure–activity relationship studies of curcumin analogs as antioxidant and anti-inflammatory agents. Future Med. Chem. 2017, 9, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Rehman, M.S.-u.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Heng, M. Phosphorylase Kinase Inhibition Therapy in Burns and Scalds. BioDiscovery 2017, 20, e11207. [Google Scholar] [CrossRef][Green Version]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 316, 359–380. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Almeida, H.H.; Barros, L.; Barreira, J.C.; Calhelha, R.C.; Heleno, S.A.; Sayer, C.; Miranda, C.G.; Leimann, F.V.; Barreiro, M.F.; Ferreira, I.C. Bioactive evaluation and application of different formulations of the natural colorant curcumin (E100) in a hydrophilic matrix (yogurt). Food Chem. 2018, 261, 224–232. [Google Scholar] [CrossRef]
- Vogel, H.; Pelletier, J. Curcumin-biological and medicinal properties. J. Pharma 1815, 2, 24–29. [Google Scholar]
- Vogel, A. Memoire sur la Curcumine. J. Pharm. Chim. 1842, 3, 20–27. [Google Scholar]
- Milobedzka, J.; Kostanecki, S.; Lampe, V. Zur Kenntnis des Curcumins. Ber. Deut. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar] [CrossRef]
- Lampe, V.; Milobedzka, J. Studien über Curcumin. Ber. Deut. Chem. Ges. 1913, 46, 2235–2240. [Google Scholar] [CrossRef]
- Schraufstätter, E.; Bernt, H. Antibacterial Action of Curcumin and Related Compounds. Nature 1949, 164, 456–457. [Google Scholar] [CrossRef]
- Srinivasan, K.R. A Chromatographic Study of the Curcuminoids in Curcuma Longa, L. Pharm. Pharmacol. 1953, 5, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.N.; Srinivasan, M. Hypocholesteremic effect of curcumin in induced hypercholesteremic rats. Indian J. Exp. Boil. 1971, 9, 167–169. [Google Scholar]
- Srinivasan, M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J. Med. Sci. 1972, 26, 269–270. [Google Scholar] [PubMed]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976, 25, 1811–1812. [Google Scholar] [CrossRef]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M.C. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985, 29, 197–202. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, B.B. Activation of Transcription Factor NF-κB Is Suppressed by Curcumin (Diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef]
- Guimarães, A.F.; Vinhas, A.C.A.; Gomes, A.F.; Souza, L.H.; Krepsky, P.B. Essential Oil of Curcuma longa L. Rhizomes Chemical Composition, Yield Variation and Stability. Quím. Nova 2020, 43, 909–913. [Google Scholar] [CrossRef]
- Pawar, H.A.; Gavasane, A.J.; Choudhary, P.D. A Novel and Simple Approach for Extraction and Isolation of Curcuminoids from Turmeric Rhizomes. Nat. Prod. Chem. Res. 2018, 6, 1–4. [Google Scholar] [CrossRef]
- Tripathy, S.; Verma, D.K.; Thakur, M.; Patel, A.R.; Srivastav, P.P.; Singh, S.; Gupta, A.K.; Chávez-González, M.L.; Aguilar, C.N.; Chakravorty, N.; et al. Curcumin Extraction, Isolation, Quantification and Its Application in Functional Foods: A Review with a Focus on Immune Enhancement Activities and COVID-19. Front. Nutr. 2021, 8, 675. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Extraction of bioactive compound curcumin from turmeric (Curcuma longa L.) via different routes: A comparative study. Pak. J. Biotechnol. 2016, 13, 173–180. [Google Scholar]
- Monton, C.; Settharaksa, S.; Luprasong, C.; Songsak, T. An optimization approach of dynamic maceration of Centella asiatica to obtain the highest content of four centelloids by response surface methodology. Rev. Bras. Farmacogn. 2019, 29, 254–261. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Synergistic Effect of Ultrasound and Three Phase Partitioning for the Extraction of Curcuminoids from Curcuma longa and its Bioactivity Profile. Process Biochem. 2020, 93, 85–93. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Ind. Crops Prod. 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Liang, H.; Wang, W.; Xu, J.; Zhang, Q.; Shen, Z.; Zeng, Z.; Li, Q. Optimization of ionic liquid-based microwave-assisted extraction technique for curcuminoids from Curcuma longa L. Food Bioprod. Process. 2017, 104, 57–65. [Google Scholar] [CrossRef]
- Nagavekar, N.; Singhal, R.S. Supercritical fluid extraction of Curcuma longa and Curcuma amada oleoresin: Optimization of extraction conditions, extract profiling, and comparison of bioactivities. Ind. Crops Prod. 2019, 134, 134–145. [Google Scholar] [CrossRef]
- Chao, I.-C.; Wang, C.-M.; Marcotullio, M.C.; Lin, L.-G.; Ye, W.-C.; Zhang, Q.-W. Simultaneous Quantification of Three Curcuminoids and Three Volatile Components of Curcuma longa Using Pressurized Liquid Extraction and High-Performance Liquid Chromatography. Molecules 2018, 23, 1568. [Google Scholar] [CrossRef]
- Nurjanah, N.; Saepudin, E. Curcumin isolation, synthesis and characterization of curcumin isoxazole derivative compound. AIP Conf. Proc. 2019, 2168, 020065. [Google Scholar] [CrossRef]
- Muthukumar, V.P.; Vaishnavi, M.; Theepapriys, S.; Saravanaraj, A. Process Development for the Effective Extraction of Curcumin from Curcuma Longa L (Turmeric). Int. J. Eng. Technol. 2018, 7, 151–155. [Google Scholar] [CrossRef][Green Version]
- Yadav, D.K.; Sharma, K.; Dutta, A.; Kundu, A.; Awasthi, A.; Goon, A.; Banerjee, K.; Saha, S. Purity Evaluation of Curcuminoids in the Turmeric Extract Obtained by Accelerated Solvent Extraction. J. AOAC Int. 2017, 100, 586–591. [Google Scholar] [CrossRef]
- Naksuriya, O.; Van Steenbergen, M.J.; Toraño, J.S.; Okonogi, S.; Hennink, W.E. A Kinetic Degradation Study of Curcumin in Its Free Form and Loaded in Polymeric Micelles. AAPS J. 2016, 18, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Fu, R.; Zhang, L.; Wang, D.; Wang, S. Enhanced extraction of natural pigments from Curcuma longa L. using natural deep eutectic solvents. Ind. Crop. Prod. 2019, 140, 111620. [Google Scholar] [CrossRef]
- Nair, D.S.; Krishnakumar, K.; Krishnan, B. Pharmacological profile of curcumin: A review. J. Biol. Innov. 2017, 6, 533–541. [Google Scholar]
- Rathore, S.; Mukim, M.; Sharma, P.; Devi, S.; Nagar, J.C.; Khalid, M. Curcumin: A Review for Health Benefits. Int. J. Res. Rev. 2020, 7, 273–290. [Google Scholar]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Angelini, G.; Pasc, A.; Gasbarri, C. Curcumin in silver nanoparticles aqueous solution: Kinetics of keto-enol tautomerism and effects on AgNPs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125235. [Google Scholar] [CrossRef]
- Girardon, M.; Parant, S.; Monari, A.; Dehez, F.; Chipot, C.; Rogalska, E.; Canilho, N.; Pasc, A. Triggering Tautomerization of Curcumin by Confinement into Liposomes. ChemPhotoChem 2019, 3, 1034–1041. [Google Scholar] [CrossRef]
- Rege, S.A.; Megha, A.; Momin, S.A. Mini review on Keto-Enol ratio of curcuminoids. Ukr. J. Food Sci. 2019, 7, 27–32. [Google Scholar] [CrossRef]
- Manolova, Y.; Deneva, V.; Antonov, L.; Drakalska, E.; Momekova, D.; Lambov, N. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 815–820. [Google Scholar] [CrossRef]
- Kawano, S.-I.; Inohana, Y.; Hashi, Y.; Lin, J.-M. Analysis of keto-enol tautomers of curcumin by liquid chromatography/mass spectrometry. Chin. Chem. Lett. 2013, 24, 685–687. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Yang, H.; Du, Z.; Wang, W.; Song, M.; Sanidad, K.; Sukamtoh, E.; Zheng, J.; Tian, L.; Xiao, H.; Liu, Z.; et al. Structure–Activity Relationship of Curcumin: Role of the Methoxy Group in Anti-inflammatory and Anticolitis Effects of Curcumin. J. Agric. Food Chem. 2017, 65, 4509–4515. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; Van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.N.; Luis, P.B.; Sintim, H.O.; Schneider, C. Unraveling Curcumin Degradation. J. Biol. Chem. 2015, 290, 4817–4828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sanidad, K.Z.; Sukamtoh, E.; Zhang, G. Potential roles of chemical degradation in the biological activities of curcumin. Food Funct. 2017, 8, 907–914. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.-R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1059–1064. [Google Scholar]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules 2015, 20, 185–205. [Google Scholar] [CrossRef]
- Zaghary, W.; Hanna, E.; Zanoun, M.; Abdallah, N.; Sakr, T. Curcumin: Analysis and Stability. J. Adv. Pharm. Res. 2019, 3, 47–58. [Google Scholar] [CrossRef]
- Kotra, V.S.R.; Satyabanta, L.; Goswami, T.K. A critical review of analytical methods for determination of curcuminoids in turmeric. J. Food Sci. Technol. 2019, 56, 5153–5166. [Google Scholar] [CrossRef]
- Kushwaha, P.; Shukla, B.; Dwivedi, J.; Saxena, S. Validated high-performance thin-layer chromatographic analysis of curcumin in the methanolic fraction of Curcuma longa L. rhizomes. Futur. J. Pharm. Sci. 2021, 7, 178. [Google Scholar] [CrossRef]
- Jadhav, B.-K.; Mahadik, K.-R.; Paradkar, A.-R. Development and Validation of Improved Reversed Phase-HPLC Method for Simultaneous Determination of Curcumin, Demethoxycurcumin and Bis-Demethoxycurcumin. Chromatographia 2007, 65, 483–488. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Jongaroonngamsang, N.; Pummangura, S.; Rojsitthisak, P. A simple isocratic HPLC method for the simultaneous determination of curcuminoids in commercial turmeric extracts. Phytochem. Anal. 2009, 20, 314–319. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Improved HPLC Method for the Determination of Curcumin, Demethoxycurcumin, and Bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef]
- Thorat, B.; Jangle, R. Reversed-phase High-performance Liquid Chromatography Method for Analysis of Curcuminoids and Curcuminoid-loaded Liposome Formulation. Indian J. Pharm. Sci. 2013, 75, 60–66. [Google Scholar] [CrossRef]
- Radha, A.; Ragavendran, P.; Thomas, A.; Kumar, D.S. A cost effective hplc method for the analysis of curcuminoids. Hygeia J. Drugs Med. 2016, 8, 1–15. [Google Scholar] [CrossRef]
- Nugroho, A.; Lukitaning, E.; Rakhmawati, N.; Rohman, A. Analysis of Curcumin in Ethanolic Extract of Curcuma longa Linn. and Curcuma xanthorriza Roxb. Using High Performance Liquid Chromatography with UV-Detection. Res. J. Phytochem. 2015, 9, 188–194. [Google Scholar] [CrossRef][Green Version]
- Hwang, K.-W.; Son, D.; Jo, H.-W.; Kim, C.H.; Seong, K.C.; Moon, J.-K. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. J. Appl. Biol. Chem. 2016, 59, 209–215. [Google Scholar] [CrossRef]
- Na Bhuket, P.R.; Niwattisaiwong, N.; Limpikirati, P.; Khemawoot, P.; Towiwat, P.; Ongpipattanakul, B.; Rojsitthisak, P. Simultaneous determination of curcumin diethyl disuccinate and its active metabolite curcumin in rat plasma by LC–MS/MS: Application of esterase inhibitors in the stabilization of an ester-containing prodrug. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033–1034, 301–310. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J.; Guo, Q.; Tu, P. Simultaneous determination of doxorubicin and curcumin in rat plasma by LC–MS/MS and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 111, 215–221. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. A validated LC-MS/MS method for quantitative analysis of curcumin in mouse plasma and brain tissue and its application in pharmacokinetic and brain distribution studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 969, 101–108. [Google Scholar] [CrossRef]
- Ashraf, K.; Mujeeb, M.; Ahmad, A.; Ahmad, N.; Amir, M. Determination of Curcuminoids in Curcuma longa Linn. by UPLC/Q-TOF–MS: An Application in Turmeric Cultivation. J. Chromatogr. Sci. 2015, 53, 1346–1352. [Google Scholar] [CrossRef]
- Van Nong, H.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Van Vu, L.; Dung, P.T.; Van Trung, T.; Nga, P.T. Fabrication and vibration characterization of curcumin extracted from turmeric (Curcuma longa) rhizomes of the northern Vietnam. SpringerPlus 2016, 5, 1147. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Crystal Engineering of Curcumin with Salicylic Acid and Hydroxyquinol as Coformers. Cryst. Growth Des. 2017, 17, 3974–3988. [Google Scholar] [CrossRef]
- Thangavel, K.; Dhivya, K. Determination of curcumin, starch and moisture content in turmeric by Fourier transform near infrared spectroscopy (FT-NIR). Eng. Agric. Environ. Food 2019, 12, 264–269. [Google Scholar] [CrossRef]
- Pöppler, A.-C.; Lübtow, M.M.; Schlauersbach, J.; Wiest, J.; Meinel, L.; Luxenhofer, R. Loading-Dependent Structural Model of Polymeric Micelles Encapsulating Curcumin by Solid-State NMR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 18540–18546. [Google Scholar] [CrossRef]
- Ali, Z.; Saleem, M.; Atta, B.M.; Khan, S.S.; Hammad, G. Determination of curcuminoid content in turmeric using fluorescence spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 192–198. [Google Scholar] [CrossRef]
- Pandey, K.U.; Dalvi, S.V. Understanding stability relationships among three curcumin polymorphs. Adv. Powder Technol. 2019, 30, 266–276. [Google Scholar] [CrossRef]
- Iravani, S.; Soufi, G.J. Electron paramagnetic resonance (EPR) spectroscopy: Food, biomedical and pharmaceutical analysis. Biomed. Spectrosc. Imaging 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Dudylina, A.L.; Ivanova, M.V.; Shumaev, K.B.; Ruuge, E.K. Superoxide Formation in Cardiac Mitochondria and Effect of Phenolic Antioxidants. Cell Biochem. Biophys. 2018, 77, 99–107. [Google Scholar] [CrossRef]
- Morales, N.P.; Sirijaroonwong, S.; Yamanont, P.; Phisalaphong, C. Electron Paramagnetic Resonance Study of the Free Radical Scavenging Capacity of Curcumin and Its Demethoxy and Hydrogenated Derivatives. Biol. Pharm. Bull. 2015, 38, 1478–1483. [Google Scholar] [CrossRef]
- Nikolić, I.; Mitsou, E.; Damjanović, A.; Papadimitriou, V.; Antić-Stanković, J.; Stanojevic, B.; Xenakis, A.; Savić, S. Curcumin-loaded low-energy nanoemulsions: Linking EPR spectroscopy-analysed microstructure and antioxidant potential with in vitro evaluated biological activity. J. Mol. Liq. 2020, 301, 112479. [Google Scholar] [CrossRef]
- Gopi, S.; Ac, K.V.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.; George, R.; Sreeraj, T.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Baspinar, Y.; Üstündas, M.; Bayraktar, O.; Sezgin, C. Curcumin and piperine loaded zein-chitosan nanoparticles: Development and in-vitro characterisation. Saudi Pharm. J. 2018, 26, 323–334. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.Y. Chondroprotective effect of curcumin and lecithin complex in human chondrocytes stimulated by IL-1β via an anti-inflammatory mechanism. Food Sci. Biotechnol. 2019, 28, 547–553. [Google Scholar] [CrossRef]
- Henriques, M.C.; Faustino, M.A.F.; Braga, S.S. Curcumin Innovative Delivery Forms: Paving the “Yellow Brick Road” of Antitumoral Phytotherapy. Appl. Sci. 2020, 10, 8990. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Kasapoglu-Calik, M.; Ozdemir, M. Synthesis and controlled release of curcumin-β-cyclodextrin inclusion complex from nanocomposite poly(N-isopropylacrylamide/sodium alginate) hydrogels. J. Appl. Polym. Sci. 2019, 136, 47554. [Google Scholar] [CrossRef]
- Kongkaneramit, L.; Aiemsumang, P.; Kewsuwan, P. Development of curcumin liposome formulations using polyol dilution method. Songklanakarin J. Sci. Technol. 2016, 38, 605–610. [Google Scholar]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Yuan, F. Stability and release performance of curcumin-loaded liposomes with varying content of hydrogenated phospholipids. Food Chem. 2020, 326, 126973. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B Biointerfaces 2018, 168, 29–34. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Guerrero, S.; Inostroza-Riquelme, M.; Contreras-Orellana, P.; Diaz-Garcia, V.; Lara, P.; Vivanco-Palma, A.; Cárdenas, A.; Miranda, V.; Robert, P.; Leyton, L.; et al. Curcumin-loaded nanoemulsion: A new safe and effective formulation to prevent tumor reincidence and metastasis. Nanoscale 2018, 10, 22612–22622. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J.-L. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef]
- Saber-Moghaddam, N.; Salari, S.; Hejazi, S.; Amini, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; Elyasi, S. Oral nano-curcumin formulation efficacy in management of mild to moderate 28 hospitalized coronavirus disease -19 patients: An open label nonrandomized clinical trial. Phytother. Res. 2021, 35, 2616–2623. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Hou, X.; Yan, F.; Jiang, Z.; Shi, J.; Xie, X.; Shen, J.; Fan, Q.; Wang, Z.; et al. Hybrid curcumin–phospholipid complex-near-infrared dye oral drug delivery system to inhibit lung metastasis of breast cancer. Int. J. Nanomed. 2019, 14, 3311–3330. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhang, L.; He, D.; Ju, J.; Li, W. Studies on the curcumin phospholipid complex solidified with Soluplus®. J. Pharm. Pharmacol. 2018, 70, 242–249. [Google Scholar] [CrossRef]
- Gupta, A.; Costa, A.P.; Xu, X.; Lee, S.-L.; Cruz, C.N.; Bao, Q.; Burgess, D.J. Formulation and characterization of curcumin loaded polymeric micelles produced via continuous processing. Int. J. Pharm. 2020, 583, 119340. [Google Scholar] [CrossRef]
- Karavasili, C.; Andreadis, D.A.; Katsamenis, O.L.; Panteris, E.; Anastasiadou, P.; Kakazanis, Z.; Zoumpourlis, V.; Markopoulou, C.K.; Koutsopoulos, S.; Vizirianakis, I.S.; et al. Synergistic Antitumor Potency of a Self-Assembling Peptide Hydrogel for the Local Co-delivery of Doxorubicin and Curcumin in the Treatment of Head and Neck Cancer. Mol. Pharm. 2019, 16, 2326–2341. [Google Scholar] [CrossRef]
- Liu, K.; Huang, R.-L.; Zha, X.-Q.; Li, Q.-M.; Pan, L.-H.; Luo, J.-P. Encapsulation and sustained release of curcumin by a composite hydrogel of lotus root amylopectin and chitosan. Carbohydr. Polym. 2020, 232, 115810. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a nonionic surfactant on curcumin delivery of nanocellulose reinforced chitosan hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: Development, optimization, in vitro and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 55–64. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Er, B.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Padigaru, M.; Morde, A.; Rai, D. Protective Effect of a Novel Highly Bioavailable Formulation of Curcumin in Experimentally Induced Osteoarthritis Rat Model. Curr. Dev. Nutr. 2020, 4 (Suppl. 2), 1765. [Google Scholar] [CrossRef]
- Fakhri, S.; Shakeryan, S.; Alizadeh, A.; Shahryari, A. Effect of 6 Weeks of High Intensity Interval Training with Nano curcumin Supplement on Antioxidant Defense and Lipid Peroxidation in Overweight Girls- Clinical Trial. Iran. J. Diabetes Obes. 2020, 11, 173–180. [Google Scholar] [CrossRef]
- Bateni, Z.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Goudarzi, R.; Sohrab, G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: A randomized, double-blind clinical trial. Phytother. Res. 2021, 35, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Sarraf, P.; Javanbakht, M.H.; Honarvar, N.M.; Hatami, M.; Soveyd, N.; Tafakhori, A.; Sedighiyan, M.; Djalali, M.; Jafarieh, A.; et al. A Novel Combination of ω-3 Fatty Acids and Nano-Curcumin Modulates Interleukin-6 Gene Expression and High Sensitivity C-reactive Protein Serum Levels in Patients with Migraine: A Randomized Clinical Trial Study. CNS Neurol. Disord. Drug Targets 2018, 17, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Afshar, G.V.; Rasmi, Y.; Yagmayee, P.; Khadem-Ansari, M.-H.; Makhdomii, K.; Rasooli, J. The Effects of Nano-curcumin Supplementation on Serum Level of hs-CRP, Adhesion Molecules, and Lipid Profiles in Hemodialysis Patients, A Randomized Controlled Clinical Trial. Iran. J. Kidney Dis. 2020, 14, 52–61. [Google Scholar]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: A randomized placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef]
- Pawar, K.S.; Mastud, R.N.; Pawar, S.K.; Pawar, S.S.; Bhoite, R.R.; Bhoite, R.R.; Kulkarni, M.V.; Deshpande, A.R. Oral Curcumin with Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Front. Pharmacol. 2021, 12, 669362. [Google Scholar] [CrossRef]
- Kedia, S.; Bhatia, V.; Thareja, S.; Garg, S.; Mouli, V.P.; Bopanna, S.; Tiwari, V.; Makharia, G.; Ahuja, V. Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 147–154. [Google Scholar] [CrossRef]
- Sadeghi, N.; Mansoori, A.; Shayesteh, A.; Hashemi, S.J. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytother. Res. 2019, 34, 1123–1133. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Guo, Y.-J.; Han, W.-X.; Yang, M.-Q.; Wen, L.-P.; Wang, K.-Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef]
- Hamam, F.; Nasr, A. Curcumin-loaded mesoporous silica particles as wound-healing agent: An In vivo study. Saudi J. Med. Med. Sci. 2020, 8, 17–24. [Google Scholar] [CrossRef]
- Peng, K.-T.; Chiang, Y.-C.; Huang, T.-Y.; Chen, P.-C.; Chang, P.-J.; Lee, C.-W. Curcumin nanoparticles are a promising anti-bacterial and anti-inflammatory agent for treating periprosthetic joint infections. Int. J. Nanomed. 2019, 14, 469–481. [Google Scholar] [CrossRef]
- Doukas, S.G.; Doukas, P.G.; Sasaki, C.T.; Vageli, D. The in vivo preventive and therapeutic properties of curcumin in bile reflux-related oncogenesis of the hypopharynx. J. Cell. Mol. Med. 2020, 24, 10311–10321. [Google Scholar] [CrossRef]
- Paolillo, F.R.; Rodrigues, P.G.S.; Bagnato, V.S.; Alves, F.; Pires, L.; Corazza, A.V. The effect of combined curcumin-mediated photodynamic therapy and artificial skin on Staphylococcus aureus–infected wounds in rats. Lasers Med. Sci. 2020, 36, 1219–1226. [Google Scholar] [CrossRef]
- Guorgui, J.; Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin’s lymphoma in mice. Arch. Biochem. Biophys. 2018, 648, 12–19. [Google Scholar] [CrossRef]
- Sherin, S.; Balachandran, S.; Abraham, A. Curcumin incorporated titanium dioxide nanoparticles as MRI contrasting agent for early diagnosis of atherosclerosis- rat model. Veter. Anim. Sci. 2020, 10, 100090. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, R.; Gaspar, J.M.; Sargsyan, D.; Su, Z.-Y.; Zhang, C.; Gao, L.; Cheng, D.; Li, W.; Wang, C.; et al. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis 2018, 39, 669–680. [Google Scholar] [CrossRef]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A dual synergistic of curcumin and gelatin on thermal-responsive hydrogel based on Chitosan-P123 in wound healing application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.S.; Arpana, C.; Balashanmugam, P.; Venkatasubbu, G.D. Enhanced wound healing by PVA/Chitosan/Curcumin patches: In vitro and in vivo study. Colloids Surf. B Biointerfaces 2019, 182, 110339. [Google Scholar] [CrossRef]
- Panda, S.K.; Parachur, V.A.; Mohanty, N.; Swain, T.; Sahu, S. A Comparative Pharmacokinetic Evaluation of a Bioavailable Curcumin Formulation Curene® with Curcumin Formulation Containing Turmeric Volatile Oil and Standard Curcuminoids 95% in Healthy Human Subjects. Funct. Foods Health Dis. 2019, 9, 134–144. [Google Scholar] [CrossRef]
- Ullah, F.; Asgarov, R.; Venigalla, M.; Liang, H.; Niedermayer, G.; Münch, G.; Gyengesi, E. Effects of a solid lipid curcumin particle formulation on chronic activation of microglia and astroglia in the GFAP-IL6 mouse model. Sci. Rep. 2020, 10, 100090. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Sharahi, J.Y.; Ahovan, Z.; Maleki, D.T.; Rad, Z.R.; Rad, Z.R.; Goudarzi, M.; Shariati, A.; Bostanghadiri, N.; Abbasi, E.; Hashemi, A. In vitro antibacterial activity of curcumin-meropenem combination against extensively drug-resistant (XDR) bacteria isolated from burn wound infections. Avicenna J. Phytomedicine 2020, 10, 3–10. [Google Scholar] [CrossRef]
- Mathew, D.; Hsu, W.-L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of dengue virus by curcuminoids. Antivir. Res. 2019, 162, 71–78. [Google Scholar] [CrossRef]
- Jeong, E.-H.; Vaidya, B.; Cho, S.-Y.; Park, M.-A.; Kaewintajuk, K.; Kim, S.R.; Oh, M.-J.; Choi, J.-S.; Kwon, J.; Kim, D. Identification of regulators of the early stage of viral hemorrhagic septicemia virus infection during curcumin treatment. Fish Shellfish. Immunol. 2015, 45, 184–193. [Google Scholar] [CrossRef]
- Ferreira, V.H.; Nazli, A.; Dizzell, S.E.; Mueller, K.; Kaushic, C. The Anti-Inflammatory Activity of Curcumin Protects the Genital Mucosal Epithelial Barrier from Disruption and Blocks Replication of HIV-1 and HSV-2. PLoS ONE 2015, 10, e0124903. [Google Scholar] [CrossRef]
- Li, H.; Zhong, C.; Wang, Q.; Chen, W.; Yuan, Y. Curcumin is an APE1 redox inhibitor and exhibits an antiviral activity against KSHV replication and pathogenesis. Antivir. Res. 2019, 167, 98–103. [Google Scholar] [CrossRef]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef]
- Teymouri, M.; Pirro, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: A review of chemistry, cellular, molecular, and preclinical features. BioFactors 2017, 43, 331–346. [Google Scholar] [CrossRef]
- Babaei, F.; Nassiri-Asl, M.; Hosseinzadeh, H. Curcumin (a constituent of turmeric): New treatment option against COVID-19. Food Sci. Nutr. 2020, 8, 5215–5227. [Google Scholar] [CrossRef]
- Soni, V.K.; Mehta, A.; Ratre, Y.K.; Tiwari, A.K.; Amit, A.; Singh, R.P.; Sonkar, S.C.; Chaturvedi, N.; Shukla, D.; Vishvakarma, N.K. Curcumin, a traditional spice component, can hold the promise against COVID-19? Eur. J. Pharmacol. 2020, 886, 173551. [Google Scholar] [CrossRef]
- Dourado, D.; Freire, D.T.; Pereira, D.T.; Amaral-Machado, L.; Alencar, N.; de Barros, A.L.B.; Egito, E.S.T. Will curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials? Biomed. Pharmacother. 2021, 139, 111578. [Google Scholar] [CrossRef]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID -19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Subhan, F.; Khalil, A.A.K.; Zeeshan, M.; Haider, A.; Tauseef, I.; Haleem, S.K.; Ibrahim, A.S. Curcumin: From Ancient Spice to Modern Anti-Viral Drug in COVID-19 Pandemic. Life Sci. 2020, 1, 69–73. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Gencer, M.Z.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef]
- Khanra, S.; Kumar, Y.P.; Dash, J.; Banerjee, R. In vitro screening of known drugs identified by scaffold hopping techniques shows promising leishmanicidal activity for suramin and netilmicin. BMC Res. Notes 2018, 51, 990–997. [Google Scholar] [CrossRef]
- Bafghi, A.F.; Haghirosadat, B.F.; Yazdian, F.; Mirzaei, F.; Pourmadadi, M.; Pournasir, F.; Hemati, M.; Pournasir, S. A novel delivery of curcumin by the efficient nanoliposomal approach against Leishmania major. Prep. Biochem. Biotechnol. 2021, 51, 990–997. [Google Scholar] [CrossRef]
- Mallo, N.; Lamas, J.; Sueiro, R.A.; Leiro, J.M. Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis. Molecules 2020, 25, 5321. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Castañeda, I.A.; Hernández-Hernández, J.M.; Pérez-Rangel, A.; González-Pozos, S.; Carranza-Rosales, P.; Charles-Niño, C.L.; Tapia-Pastrana, G.; Ramírez-Herrera, M.A.; Castillo-Romero, A. Amoebicidal activity of curcumin on Entamoeba histolytica trophozoites. J. Pharm. Pharmacol. 2018, 70, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gutiérrez, F.; Palomo-Ligas, L.; Hernández-Hernández, J.M.; Pérez-Rangel, A.; Aguayo-Ortiz, R.; Hernández-Campos, A.; Castillo, R.; González-Pozos, S.; Cortés-Zárate, R.; Ramírez-Herrera, M.A.; et al. Curcumin alters the cytoskeleton and microtubule organization on trophozoites of Giardia lamblia. Acta Trop. 2017, 172, 113–121. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, A.A.M.; Hegab, M.H.A.; Seliem, M.M.E.; Barakat, A.M.A.; Mostafa, N.E.; Abdel-Maksoud, H.A.; Abdelhameed, R.M. Curcumin@metal organic frameworks nano-composite for treatment of chronic toxoplasmosis. J. Mater. Sci. Mater. Med. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Qian, W.; Wang, H.; Shan, D.; Li, B.; Liu, J.; Liu, Q. Activity of several kinds of drugs against Neospora caninum. Parasitol. Int. 2015, 64, 597–602. [Google Scholar] [CrossRef]
- Bazh, E.K.A.; El-Bahy, N.M. In vitro and in vivo screening of anthelmintic activity of ginger and curcumin on Ascaridia galli. Parasitol. Res. 2013, 112, 3679–3686. [Google Scholar] [CrossRef]
- El-Bahy, N.M.; Bazh, E.K.A. Anthelmintic activity of ginger, curcumin, and praziquentel against Raillietina cesticillus (in vitro and in vivo). Parasitol. Res. 2015, 114, 2427–2434. [Google Scholar] [CrossRef]
- Novaes, R.D.; Sartini, M.V.P.; Rodrigues, J.P.F.; Gonçalves, R.V.; Santos, E.C.; Souza, R.L.M.; Caldas, I.S. Curcumin Enhances the Anti-Trypanosoma cruzi Activity of Benznidazole-Based Chemotherapy in Acute Experimental Chagas Disease. Antimicrob. Agents Chemother. 2016, 60, 3355–3364. [Google Scholar] [CrossRef]
- Busari, Z.A.; Dauda, K.A.; Morenikeji, O.A.; Afolayan, F.; Oyeyemi, O.T.; Meena, J.; Sahu, D.; Panda, A.K. Antiplasmodial Activity and Toxicological Assessment of Curcumin PLGA-Encapsulated Nanoparticles. Front. Pharmacol. 2017, 8, 622. [Google Scholar] [CrossRef]
- Naseri, S.; Darroudi, M.; Aryan, E.; Gholoobi, A.; Rahimi, H.R.; Ketabi, K.; Movaqar, A.; Abdoli, M.; Gouklani, H.; Tei-mourpour, R. The antiviral effects of curcumin nanomicelles on the attachment and entry of hepatitis C virus. Iran. J. Virol. 2017, 11, 29–35. [Google Scholar]
- Ahmed, J.; Tan, Y.; Ambegaokar, S. Effects of Curcumin on Vesicular Stomatitis Virus (VSV) Infection and Dicer-1 Expression. FASEB J. 2017, 31, 622.11. [Google Scholar] [CrossRef]
- Sharma, R.K.; Cwiklinski, K.; Aalinkeel, R.; Reynolds, J.L.; Sykes, D.E.; Quaye, E.; Oh, J.; Mahajan, S.D.; Schwartz, S.A. Immunomodulatory activities of curcumin-stabilized silver nanoparticles: Efficacy as an antiretroviral therapeutic. Immunol. Investig. 2017, 46, 833–846. [Google Scholar] [CrossRef]
- Huang, H.-I.; Chio, C.-C.; Lin, J.-Y. Inhibition of EV71 by curcumin in intestinal epithelial cells. PLoS ONE 2018, 13, e0191617. [Google Scholar] [CrossRef]
- Poursina, Z.; Mohammadi, A.; Yazdi, S.Z.; Humpson, I.; Vakili, V.; Boostani, R.; Rafatpanah, H. Curcumin increased the expression of c-FLIP in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. J. Cell. Biochem. 2019, 120, 15740–15745. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yadav, A.; Gupta, N.; Sharma, S.; Kakkar, R.; Cwiklinski, K.; Quaye, E.; Mahajan, S.D.; Schwartz, S.A.; Sharma, R.K. Multifunctional mesoporous curcumin encapsulated iron-phenanthroline nanocluster: A new Anti-HIV agent. Colloids Surf. B Biointerfaces 2019, 180, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Nabila, N.; Suada, N.K.; Denis, D.; Yohan, B.; Adi, A.C.; Veterini, A.S.; Anindya, A.L.; Sasmono, R.T.; Rachmawati, H. Antiviral Action of Curcumin Encapsulated in Nanoemulsion against Four Serotypes of Dengue Virus. Pharm. Nanotechnol. 2020, 8, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.; Zang, G.; Chen, T.; Lu, N.; Wang, S.; Zhang, G. Curcumin Inhibits Replication of Human Parainfluenza Virus Type 3 by Affecting Viral Inclusion Body Formation. BioMed Res. Int. 2021, 2021, 13. [Google Scholar] [CrossRef]
- Thongsri, P.; Pewkliang, Y.; Borwornpinyo, S.; Wongkajornsilp, A.; Hongeng, S.; Sa-Ngiamsuntorn, K. Curcumin inhibited hepatitis B viral entry through NTCP binding. Sci. Rep. 2021, 11, 19125. [Google Scholar] [CrossRef]
- Tiwari, B.; Pahuja, R.; Kumar, P.; Rath, S.K.; Gupta, K.C.; Goyal, N. Nanotized Curcumin and Miltefosine, a Potential Combination for Treatment of Experimental Visceral Leishmaniasis. Antimicrob. Agents Chemother. 2017, 61, e01169–16. [Google Scholar] [CrossRef]
- Ullah, R.; Rehman, A.; Zafeer, M.F.; Rehman, L.; Khan, Y.A.; Khan, M.A.H.; Khan, S.N.; Khan, A.U.; Abidi, S.M.A. Anthelmintic Potential of Thymoquinone and Curcumin on Fasciola gigantica. PLoS ONE 2017, 12, e0171267. [Google Scholar] [CrossRef]
- Asadpour, M.; Namazi, F.; Razavi, S.M.; Nazifi, S. Comparative efficacy of curcumin and paromomycin against Cryptosporidium parvum infection in a BALB/c model. Veter. Parasitol. 2018, 250, 7–14. [Google Scholar] [CrossRef]
- Ghosh, A.; Banerjee, T. Nanotized curcumin-benzothiophene conjugate: A potential combination for treatment of cerebral malaria. IUBMB Life 2020, 72, 2637–2650. [Google Scholar] [CrossRef]
- Elmi, T.; Ardestani, M.S.; Hajialiani, F.; Motevalian, M.; Mohamadi, M.; Sadeghi, S.; Zamani, Z.; Tabatabaie, F. Novel chloroquine loaded curcumin based anionic linear globular dendrimer G2: A metabolomics study on Plasmodium falciparum in vitro using 1H NMR spectroscopy. Parasitology 2020, 147, 747–759. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Wei, Z.; Zhang, X.; Wang, Y.; Li, F.; Fu, Y.; Liu, B. Inhibition of histone deacetylase reduces lipopolysaccharide-induced-inflammation in primary mammary epithelial cells by regulating ROS-NF-кB signaling pathways. Int. Immunopharmacol. 2018, 56, 230–234. [Google Scholar] [CrossRef]
- Shehzad, A.; Qureshi, M.; Anwar, M.N.; Lee, Y.S. Multifunctional Curcumin Mediate Multitherapeutic Effects. J. Food Sci. 2017, 82, 2006–2015. [Google Scholar] [CrossRef]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98. [Google Scholar] [CrossRef]
- Chai, Y.-S.; Chen, Y.-Q.; Lin, S.-H.; Xie, K.; Wang, C.-J.; Yang, Y.-Z.; Xu, F. Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 2020, 125, 109946. [Google Scholar] [CrossRef]
- Hui, S.; Liu, K.; Zhu, X.; Kang, C.; Mi, M.T. Effect of Curcumin on IL-6 and IL-8: A Meta-analysis and Systematic Review. J. Nutr. Food Sci. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef]
- Castaño, P.R.; Parween, S.; Pandey, A.V. Bioactivity of Curcumin on the Cytochrome P450 Enzymes of the Steroidogenic Pathway. Int. J. Mol. Sci. 2019, 20, 4606. [Google Scholar] [CrossRef]
- Moutinho, M.S.; Aragão, S.; Carmo, D.; Casaca, F.; Silva, S.; Ribeiro, J.; Sousa, H.; Pires, I.; Queiroga, F.; Colaço, B.; et al. Curcumin and Rutin Down-regulate Cyclooxygenase-2 and Reduce Tumor-associated Inflammation in HPV16-Transgenic Mice. Anticancer Res. 2018, 38, 1461–1466. [Google Scholar] [CrossRef]
- Liu, Z.; Ying, Y. The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and Its Potential Use in the Associated Severe Pneumonia. Front. Cell Dev. Biol. 2020, 8, 479. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rajanikant, G.K. Curcumin Stimulates the Antioxidant Mechanisms in Mouse Skin Exposed to Fractionated γ-Irradiation. Antioxidants 2015, 4, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Meshkibaf, M.H.; Maleknia, M.; Noroozi, S. Effect of curcumin on gene expression and protein level of methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund’s adjuvant inflammation-induced male rats. J. Inflamm. Res. 2019, 12, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Haryuna, T.S.H.; Munir, D.; Maria, A.; Bashiruddin, J. The Antioxidant Effect of Curcumin on Cochlear Fibroblasts in Rat Models of Diabetes Mellitus. Iran. J. Otorhinolaryngol. 2017, 29, 197–202. [Google Scholar] [CrossRef]
- Asouri, M.; Ataee, R.; Ahmadi, A.A.; Amini, A.; Moshaei, M.R. Antioxidant and Free Radical Scavenging Activities of Curcumin. Asian J. Chem. 2013, 25, 7593–7595. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS Protection Efficiency and Free Radical-Scavenging Activity of Curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D.; Luo, F.; Wu, Y.; Yan, F.; Weng, Z.; et al. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohydr. Polym. 2020, 228, 115398. [Google Scholar] [CrossRef]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Y.; Yun, X.; Ou, Y.; Zhang, W.; Li, J.-X. Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci. Rep. 2014, 4, 4932. [Google Scholar] [CrossRef]
- Pieretti, S.; Ranjan, A.P.; Di Giannuario, A.; Mukerjee, A.; Marzoli, F.; Di Giovannandrea, R.; Vishwanatha, J.K. Curcumin-loaded Poly (d,l-lactide-co-glycolide) nanovesicles induce antinociceptive effects and reduce pronociceptive cytokine and BDNF release in spinal cord after acute administration in mice. Colloids Surf. B Biointerfaces 2017, 158, 379–386. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Lio, R.M.S.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. Today 2017, 22, 1582–1592. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-incorporated crosslinked sodium alginate-g-poly (N-isopropyl acrylamide) thermo-responsive hydrogel as an in-situ forming injectable dressing for wound healing: In vitro characterization and in vivo evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Li, D.; Xie, S.; Xiao, X.; Velkov, T. Curcumin Attenuates Colistin-Induced Neurotoxicity in N2a Cells via Anti-inflammatory Activity, Suppression of Oxidative Stress, and Apoptosis. Mol. Neurobiol. 2018, 55, 421–434. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, R.; Ma, Y.; Zhang, Z.; Xie, Z. Curcumin Attenuates Airway Inflammation and Airway Remolding by Inhibiting NF-κB Signaling and COX-2 in Cigarette Smoke-Induced COPD Mice. Inflammation 2018, 41, 1804–1814. [Google Scholar] [CrossRef]
- Vasanthkumar, T.; Hanumanthappa, M.; Lakshminarayana, R. Curcumin and capsaicin modulates LPS induced expression of COX-2, IL-6 and TGF-β in human peripheral blood mononuclear cells. Cytotechnology 2019, 71, 963–976. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef]
- Özçelik, M.; Erisir, M.; Guler, O.; Baykara, M.; Kirman, E. The effect of curcumin on lipid peroxidation and selected antioxidants in irradiated rats. Acta Veter. Brno 2019, 87, 379–385. [Google Scholar] [CrossRef]
- Alizadeh, M.; Kheirouri, S. Curcumin reduces malondialdehyde and improves antioxidants in humans with diseased conditions: A comprehensive meta-analysis of randomized controlled trials. BioMedicine 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Shi, Y.; Gong, C.; Liu, T.; Nan, W.; Ma, L.; Wu, Z.; Da, C.; Zhou, K.; Zhang, H. Curcumin Exerts Antinociceptive Effects in Cancer-Induced Bone Pain via an Endogenous Opioid Mechanism. Front. Neurosci. 2021, 15, 696861. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Shin, J.Y.; Yoon, J.J.; Yin, M.; Yoon, M.H. Differential expression of spinal γ-aminobutyric acid and opioid receptors modulates the analgesic effects of intrathecal curcumin on postoperative/inflammatory pain in rats. Anesth. Pain Med. 2018, 13, 82–92. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, D.; Yang, H.; Fu, H. Evidence for the Participation of Acid-Sensing Ion Channels (ASICs) in the Antinociceptive Effect of Curcumin in a Formalin-Induced Orofacial Inflammatory Model. Cell. Mol. Neurobiol. 2016, 37, 635–642. [Google Scholar] [CrossRef]
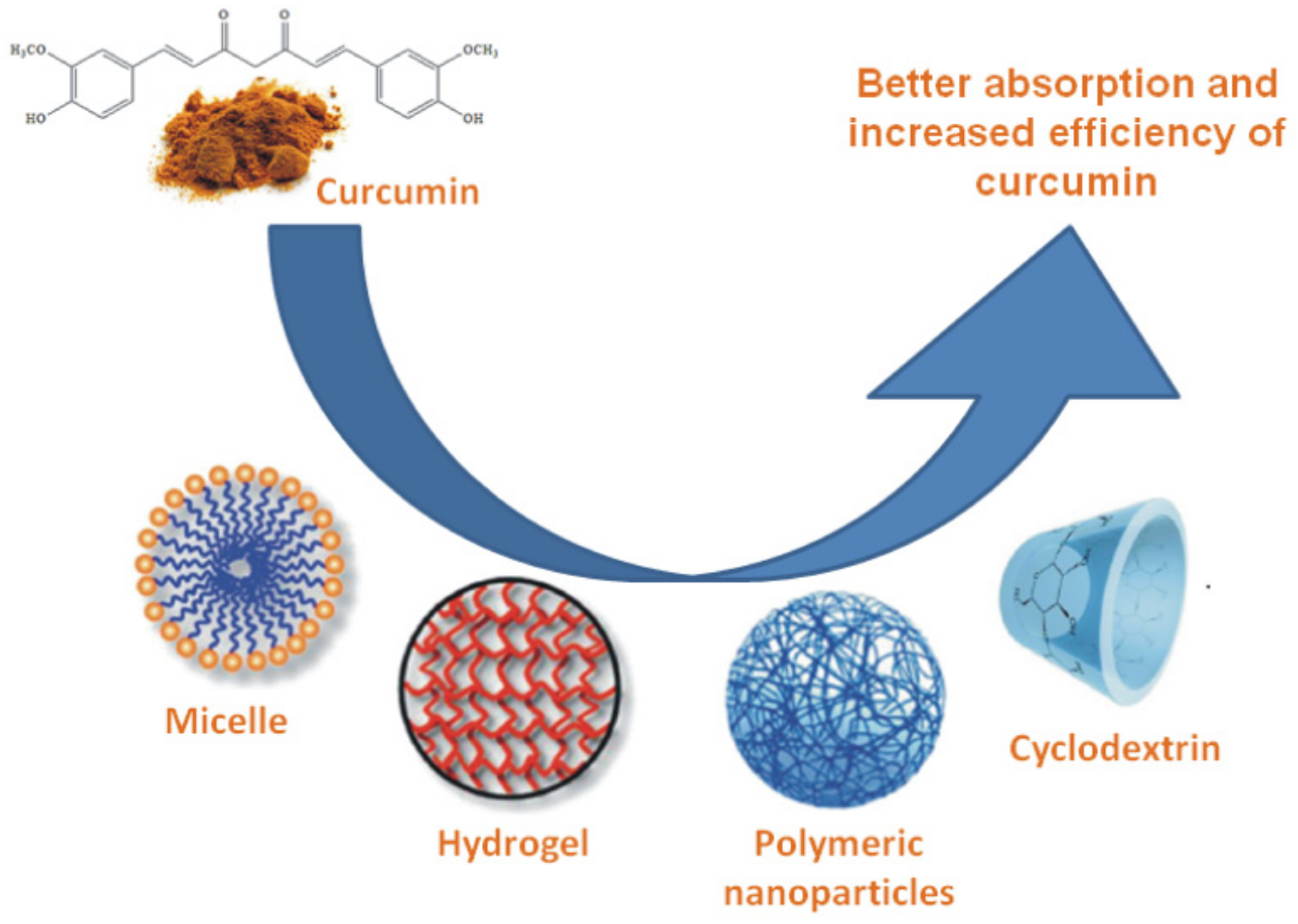
| Year | Discovery | Reference |
|---|---|---|
| 1815 | Vogel and Pelletier were the first to report the “Orange-yellow Substance” isolated from the rhizome of Curcuma longa and named it curcumin. | [16] |
| 1842 | Vogel Extracted pure preparation of curcumin but did not report its formula. | [17] |
| 1910 | Milobedzka and Lampe identified chemical structure of curcumin as diferuloylmethane, or 1,6-heptadiene-3,5-dione-1,7-bis-(4-hidroxy-3-methoxyphenyl)-(1E, 6E). | [18] |
| 1913 | The synthesis of curcumin was published. | [19] |
| 1949 | Schraufstatter et al. Reported that curcumin is a biologically active compound with antibacterial properties. | [20] |
| 1953 | Srinivasan separated and quantified the components of curcumin using chromatography. | [21] |
| 1971 | It was discovered that curcumin lowers cholesterol | [22] |
| 1972 | It was discovered that curcumin lowers the level of sugar in the blood | [23] |
| 1973 | It was discovered that curcumin has an anti-inflammatory effect | [24] |
| 1976 | It was discovered that curcumin has an antioxidant effect | [25] |
| 1980 | Kuttan et al. demonstrated anticancer activity of curcumin both in vitro and in vivo. | [26] |
| 1995 | Curcumin exhibits anti-inflammatory activity by suppressing the proinflammatory transcription factor, nuclear factor-kappa B (NF-κB) | [27] |
| Matrix Sample | Column | Mobile Phase | λ, nm | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Turmeric Powder | RP C18 | Acetonitrile and 0.1% Trifluro-Acetic Acid (50:50, v/v) | 420 | 27.99 ng/mL | [70] |
| Turmeric Extracts | Alltima C18 column | Acetonitrile and 2% Acetic Acid (40:60, v/v) | 425 | 0.90 μg/mL | [71] |
| Commercial Samples of Turmeric | C18 | Methanol, 2% Acetic acid, and Acetonitrile | 425 | 0.05 µg/mL | [72] |
| Curcuminoids-Loaded Liposome | Zorbax Eclipse XDB C18 (4 × 150mm, 5 µm) | Acetonitrile and 0.1% OrthoPhosphoric Acid (50:50, v/v) | 425 | 0.124 µg/mL | [73] |
| Samples of Turmeric | Zorbax SB-C18 column (4.6 × 250 mm, 5 µm) | Acetonitrile and 0.4% Aqueous Acetic Acid | 430 | 0.31 μg/mL | [37] |
| Extract of Turmeric | C18 (4.6 × 150mm, 5 µm) | Acetonitrile and 2% Acetic Acid (55:45, v/v) | 425 | 0.0738 ppm | [74] |
| Extract of Turmeric | Waters Xterra MS C18 column (4.6 × 250 mm, 5 µm) | Distilled Water and Acetonitrile (65:35, v/v) Containing 1% Acetic Acid | 425 | 1.13 μg/mL | [75] |
| Turmeric Rhizome | Brownlee SPP C18 column (4.6 × 100 mm, 2.7 µm) | Water and Acetonitrile (70:30, v/v) | 420 | 1.0 μg/mL | [76] |
| Disease | Dose | Duration | Patients | Results | Reference |
|---|---|---|---|---|---|
| Overweight | 80 mg/Day | 6 Weeks | 48 Overweight Girl Students | Positive antioxidant effect and prevention of lipid peroxidation in overweight individuals. | [116] |
| Metabolic syndrome(MetS) | 80 mg/Day | 12 Weeks | 50 Patients | Supplementation with Nanomicelle curcumin Significantly improved serum triglyceride in MetS patients. | [117] |
| Diabetic sensorimotor polyneuropathy | 80 mg/Day | 8 Weeks | 80 Diabetic patients | Nanocurcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus. | [118] |
| Migraine | 80 mg/Day | 2 Months | 80 Patients | Combination of omega-3 fatty acids and nanocurcumin modulates interleukin-6 gene Expression and high-sensitivity C-reactive protein serum levels in patients with migraine. | [119] |
| Nonalcoholic fatty liver disease (NAFLD) | 80 mg/Day | 3 Months | 84 Patients | Nanocurcumin improves glucose indices, lipids, inflammation, and nesfatin in overweight and obese patients with nonalcoholic fatty liver disease (NAFLD). | [120] |
| Hemodialysis (HD) | 120 mg/Day | 12 Weeks | 60 Patients | Nanocurcumin shows beneficial effects in lowering inflammation and Hs-CRP levels, as well as adhesion molecules (ICAM-1, VCAM-1), in hemodialysis patients. | [121] |
| Overweight and obesity | 500 mg/Day | 10 Weeks | 60 Adolescent | Ten weeks of curcumin supplementation had beneficial effects on inflammation and oxidative stress markers among postpubescent overweight and obese girl adolescents. | [122] |
| Coronavirus disease-2019 | 1050 mg/Day | 14 Days | 158 Patients | Curcumin is a safe and natural therapeutic option to prevent post-COVID-19 thromboembolic events. | [123] |
| Ulcerative colitis (UC) | 450 mg/Day | 8 Weeks | 41 Patients | Low-dose oral curcumin is not effective in inducing remission in mild-to-moderate ulcerative colitis. | [124] |
| Ulcerative colitis (UC) | 1500 mg/Day | 8 Weeks | 70 Patients | Consumption of the curcumin supplement, along with drug therapy, significant improvement of the clinical outcomes, quality of life, Hs-CRP, and ESR in patients with mild-to-moderate UC. | [125] |
| Curcumin Form | Activity | Animal Model | Reference |
|---|---|---|---|
| Curcumin Nanocurcumin | Antidepressive effect Wound-healing Agent | Sprague–Dawley rats Male Wistar rats | [126] [127] |
| Curcumin, nanoparticles | Antibacterial and anti-inflammatory agent | Male C57BL/6 mice | [128] |
| Curcumin | Inhibitors of NF-κB | Mus musculus, C57BL/6J | [129] |
| Curcumin | Decontaminate and accelerate the Wound contraction | Wistar Rats | [130] |
| Curcumin, Nanoparticles | Adjuvant agent for the treatment of Hodgkin’s Lymphoma | Mice | [131] |
| Curcumin, Nanoparticles | Contrasting agent | Sprague–Dawley rats | [132] |
| Curcumin C3 Complex | Cancer prevention | Male C57BL/6 wild-type mice | [133] |
| Curcumin, Hydrogel | Wound-healing agent | Mus musculus var. albino mice | [134] |
| PVA/Chitosan/Curcumin Patches | Wound-healing agent | Wistar Rats | [135] |
| Isolate | Genes | Curcumin MIC µg/mL | FICI |
|---|---|---|---|
| Klebsiella pneumonie | DHA | 128 | 0.5 |
| Pseudomonas aeruginosa | VEB | 128 | 0.5 |
| Acinetobacter baumanni | OXA-23, OXA-24 | 128 | 0.37 |
| Acinetobacter baumanni | OXA-23, OXA-24 | 128 | 1 |
| Pseudomonas aeruginosa | IMP-1 | 128 | 1 |
| Enterococcus faecalis ATCC 29212 | Type strain | 128 | 0.26 |
| Pseudomonas aeruginosa | GES | 128 | 0.75 |
| Acinetobacter baumanni | OXA-23, OXA-24 | 512 | 0.25 |
| Acinetobacter baumanni ATCC19606 | Type Strain | 512 | 0.5 |
| Acinetobacter baumanni | OXA-23, OXA-24 | 512 | 0.25 |
| Pseudomonas aeruginosa | IMP-1 | 512 | 0.064 |
| Pseudomonas aeruginosa | VIM-1 | 512 | 0.064 |
| Escherichia coli ATCC 25922 | Type Strain | 256 | 0.4 |
| Klebsiella pneumonie ATCC700603 | Type Strain | 256 | 0.5 |
| Klebsiella pneumonie | NDM-6 | 256 | 0.28 |
| Klebsiella pneumonie | NDM-1 | 256 | 0.56 |
| Klebsiella pneumonie | NDM-6 | 256 | 0.56 |
| Pseudomonas aeruginosa | IMP-2 | 256 | 0.56 |
| Activity | Substance | Type of Microorganism | Therapeutic Effect | Reference |
|---|---|---|---|---|
| Antiviral | ||||
| Curcumin, nanomicelles | Hepatitis C virus | The antiviral effects of curcumin nanomicelles on hepatitis C virus. | [167] | |
| Curcumin | Vesicular stomatitis virus | Determination of curcumin effects on vesicular stomatitis virus Dicer-1 expression. | [168] | |
| Curcumin | Chikungunya virus, zika virus | Antiviral activity of curcumin against zika and chikungunya virus. | [147] | |
| Curcumin, nanoparticles | Human immunodeficiency virus 1 (HIV-1) | Immunomodulatory activities of curcumin-stabilized silver nanoparticles on HIV-1. | [169] | |
| Curcumin | Enterovirus 71 (EV71) | Antiviral effects of curcumin on EV71. | [170] | |
| Curcumin | Human T lymphotropic virus 1 (HTLV-1) | Determination of curcumin on the expression of c-FLIP in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. | [171] | |
| Curcumin | Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV8) | Antiviral activity of curcumin against KSHV replication and pathogenesis. | [146] | |
| Curcumin | Human immunodeficiency virus 1 (HIV-1) | Multifunctional mesoporous curcumin encapsulated iron phenanthroline Nanocluster on HIV-1. | [172] | |
| Curcumin | Zika virus | Inhibitory effects of novel natural products against zika virus. | [173] | |
| Curcumin, nanocurcumin | Dengue virus | Antiviral activity of curcumin encapsulated in nanoemulsion against dengue virus serotypes. | [174] | |
| Curcumin | Transmissible gastroenteritis virus | Antiviral effects of curcumin on transmissible gastroenteritis virus. | [175] | |
| Curcumin | Human parainfluenza virus type 3 | Evaluation of curcumin on replication of human parainfluenza virus type 3. | [176] | |
| Curcumin | Hepatitis B virus | Evaluation of curcumin on viral entry of hepatitis B. | [156] | |
| Antiparasitic and Antimalarial | ||||
| Curcumin and netilmicin | Leishmania major, Leishmania donovani | Antileishmanial activity of netilmicin combined with curcumin significantly enhanced compared with when used alone. | [177] | |
| Nanoformulation of curcumin and miltefosine | Leishmania donovani | Combination therapy of curcumin with miltefosine exhibited a synergistic effect on both promastigotes and amastigotes under in vitro conditions. | [166] | |
| Curcumin Encapsulated to PLGA | Plasmodium berghei | Encapsulation of curcumin in PLGA led to increased parasite suppression about 56.8% at 5 mg/kg of nanoformulation, which was higher than in free curcumin (40.5%) at 10 mg/kg. | [178] | |
| Curcumin alone | Giardia lamblia | Curcumin inhibited giardia proliferation disrupted the cytoskeletal structures of trophozoites in the dose-dependent mode. | [160] | |
| Curcumin alone | Fasciola gigantica | A significant decrease was observed in the expression of glutathione-S-transferase and superoxide dismutase. | [179] | |
| Curcumin alone | Cryptosporidium parvum | The anticryptosporidial and antioxidant activity of curcumin against C. parvum were confirmed. | [180] | |
| Nanotized curcumin- benzothiophene conjugate | Plasmodium falciparum | The improved oral bioavailability of the nanotized formulation lowered the dosage at which the pharmacological effect was achieved while avoiding any observable adverse side effects. | [181] | |
| Curcumin, nanocomposite | Plasmodium falciparum | The antiparasitic effect of the nanocomposite on the metabolites of plasmodium falciparum | [182] |
| Activity | Substance | Target | Therapeutic Effect | Reference |
|---|---|---|---|---|
| Anti-inflammatory | ||||
| Curcumin | COX-2 NF-κB p-IκB ROS | Attenuates colistin-induced neurotoxicity in N2a cells via anti-inflammatory activity, suppression of oxidative stress, and a apoptosis. | [206] | |
| Curcumin | NF-κB COX-2 | Attenuates airway inflammation and airway remoulding in cigarette smoke-induced COPD mice. | [207] | |
| Curcumin and rutin | COX-2 | Reduce tumour-associated inflammation in HPV16-transgenic mice. | [189] | |
| Curcumin, curcumin and capsaicin | COX-2 IL-6 TGF-β | Combined curcumin and capsaicin are efficient against the lipopolysaccharide Induced expression of proinflammatory cytokines in peripheral blood mononuclear cells. | [208] | |
| Antioxidant | ||||
| Curcumin-loaded sodium alginate/ZnO hydrogel beads | DPPH Assay | Composite hydrogel beads have protected curcumin from light degradation can therefore prolong its antioxidant activity. | [209] | |
| Curcumin | MDA SOD GSH-Px | Curcumin protects the liver, kidneys and brain from the oxidative damage caused by irradiation. | [210] | |
| Curcumin, curcumin and piperine | MDA SOD Catalase | Curcumin may be used as an adjunct therapy in individuals with oxidative stress. | [211] | |
| Curcumin | MDA total Antioxidant Capacity (TAC) | Pure curcumin reduces MDA concentration and increases total antioxidant capacity. | [212] | |
| Antinociceptive | ||||
| Curcumin | DRG Neurons β-Endorphin and Enkephalin | The curcumin attenuates cancer-induced bone pain | [213] | |
| Curcumin | γ-Aminobutyric Acid (GABA) and Opioid Receptors | Antinociception of curcumin | [214] | |
| Curcumin-loaded PLGA nanovesicles (PLGA-CUR) | Cytokine and BDNF | Antinociceptive effects of PLGA-CUR | [201] | |
| Curcumin | The acid-Sensing Ion Channels (ASICs) | Antinociceptive Effects of Curcumin | [215] | |
| Wound healing agent | ||||
| PVA/chitosan/curcumin patches | Cell Line Studies and MTT Assay | Antibacterial activity of PVA/Chi/Cur against four major bacterial strains commonly found in wound sites and water retainability indicates it to be a perfect material for wound treatment. | [135] | |
| Nanocurcumin | Fibroblast, Collagen, Reepithelization | Curcumin nanoformulation enhanced wound repair by inhibiting the inflammatory response, stimulating angiogenesis, inducing fibroblast proliferation and enhancing reepithelization and synthesis of collagen. | [127] | |
| Curcumin, Hydrogel | L929 Fibroblast Cells | Curcumin incorporation accelerates full-thickness skin wound healing | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. https://doi.org/10.3390/antibiotics11020135
Urošević M, Nikolić L, Gajić I, Nikolić V, Dinić A, Miljković V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics. 2022; 11(2):135. https://doi.org/10.3390/antibiotics11020135
Chicago/Turabian StyleUrošević, Maja, Ljubiša Nikolić, Ivana Gajić, Vesna Nikolić, Ana Dinić, and Vojkan Miljković. 2022. "Curcumin: Biological Activities and Modern Pharmaceutical Forms" Antibiotics 11, no. 2: 135. https://doi.org/10.3390/antibiotics11020135
APA StyleUrošević, M., Nikolić, L., Gajić, I., Nikolić, V., Dinić, A., & Miljković, V. (2022). Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics, 11(2), 135. https://doi.org/10.3390/antibiotics11020135






