Groin Surgical Site Infection in Vascular Surgery: Systemic Review on Peri-Operative Antibiotic Prophylaxis
Abstract
1. Introduction
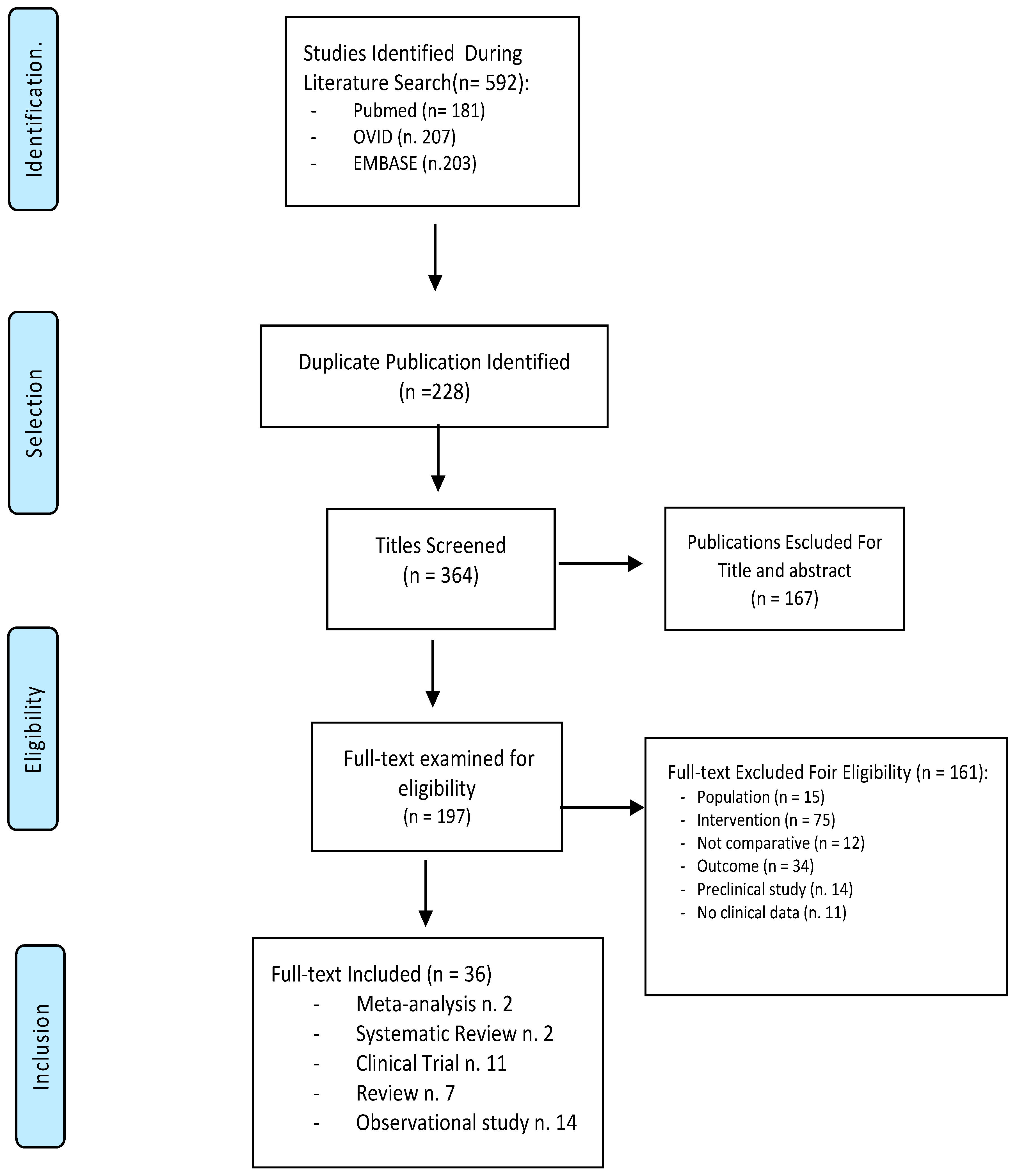
2. Materials and Methods
3. Results
4. Study Descriptions
5. Risk of Bias
6. Pre-Operative Antibiotic Therapy
7. Intraoperative Use of Local Antibiotics
8. Study Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.J.; Podgorny, K.; Berríos-Torres, S.I.; Bratzler, D.W.; Dellinger, E.P.; Greene, L.; Nyquist, A.C.; Saiman, L.; Yokoe, D.S.; Maragakis, L.L.; et al. Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update. Infect. Control Hosp. Epidemiol. 2014, 35, 605–627. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Bandyk, D.F. Vascular surgical site infection: Risk factors and preventive measures. Semin. Vasc. Surg. 2015, 28, 201–207. [Google Scholar] [CrossRef]
- Aicher, B.; Curry, P.; Croal-Abrahams, L.; Hao, S.; Kalsi, R.; Menon, N.; Drucker, C.; Harris, D.; Toursavadkohi, S.; Crawford, R.; et al. Infrainguinal wound infections in vascular surgery: An antiquated challenge without a modern solution. J. Vasc. Nurs. 2017, 35, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.C.; Bartek, S.; Kuntz, K.M.; Anninos, E.; Skillman, J.J. Prospective study of wound complications in continuous infrainguinal incisions after lower limb arterial reconstruction: Incidence, risk factors, and cost. Surgery 1996, 119, 378–383. [Google Scholar] [CrossRef]
- Lee, E.S.; Santilli, S.M.; Olson, M.M.; Kuskowski, M.A.; Lee, J.T. Wound Infection after Infrainguinal Bypass Operations: Multivariate Analysis of Putative Risk Factors. Surg. Infect. 2000, 1, 257–263. [Google Scholar] [CrossRef]
- Vogel, T.R.; Dombrovskiy, V.Y.; Carson, J.L.; Haser, P.B.; Lowry, S.F.; Graham, A.M. Infectious complications after elective vascular surgical procedures. J. Vasc. Surg. 2010, 51, 122–130. [Google Scholar] [CrossRef]
- Johnson, J.A.; Cogbill, T.H.; Strutt, P.J.; Gundersen, A.L. Wound Complications after Infrainguinal Bypass: Classification, Predisposing Factors, and Management. Arch. Surg. 1988, 123, 859–862. [Google Scholar] [CrossRef]
- Giles, K.A.; Hamdan, A.D.; Pomposelli, F.B.; Wyers, M.C.; Siracuse, J.J.; Schermerhorn, M.L. Body Mass Index: Surgical Site Infections and Mortality after Lower Extremity Bypass from the National Surgical Quality Improvement Program 2005–2007. Ann. Vasc. Surg. 2010, 24, 48. [Google Scholar] [CrossRef]
- Siracuse, J.J.; Nandivada, P.; Giles, K.A.; Hamdan, A.D.; Wyers, M.C.; Chaikof, E.L.; Pomposelli, F.B.; Schermerhorn, M.L. Ten Year Experience with Prosthetic Graft Infections Involving the Femoral Artery. J. Vasc. Surg. 2013, 57, 700–705. [Google Scholar] [CrossRef]
- Wiseman, J.T.; Fernandes-Taylor, S.; Barnes, M.L.; Saunders, R.S.; Saha, S.; Havlena, J.; Rathouz, P.J.; Kent, K.C. Predictors of surgical site infection after hospital discharge in patients undergoing major vascular surgery. J. Vasc. Surg. 2015, 62, 1023–1031.e5. [Google Scholar] [CrossRef]
- Davis, F.M.; Sutzko, D.C.; Grey, S.F.; Mansour, M.A.; Jain, K.M.; Nypaver, T.J.; Gaborek, G.; Henke, P.K. Predictors of surgical site infection after open lower extremity revascularization. J. Vasc. Surg. 2017, 65, 1769–1778.e3. [Google Scholar] [CrossRef]
- Bandyk, D.F.; Berni, G.A.; Thiele, B.L.; Towne, J.B. Aortofemoral graft infection due to Staphylococcus epidermidis. Arch. Surg. 1984, 119, 102–108. [Google Scholar] [CrossRef]
- Salzmann, G. Perioperative infection prophylaxis in vascular surgery—A randomized prospective study. Thorac. Cardiovasc. Surg. 1983, 31, 239–242. [Google Scholar] [CrossRef]
- Harris, K.A. Graft infections. J. Vasc. Nurs. Off. Publ. Soc. Peripher. Vasc. Nurs. 1992, 10, 13–17. [Google Scholar]
- Pounds, L.L.; Montes-Walters, M.; Mayhall, C.G.; Falk, P.S.; Sanderson, E.; Hunter, G.C.; Killewich, L.A. A Changing Pattern of Infection after Major Vascular Reconstructions. Vasc. Endovasc. Surg. 2005, 39, T1–T7. [Google Scholar] [CrossRef]
- Towne, J.B.; Seabrook, G.R.; Bandyk, D.; Freischlag, J.A.; Edmiston, C.E. In situ replacement of arterial prosthesis infected by bacterial biofilms: Long-term follow-up. J. Vasc. Surg. 1994, 19, 226–235. [Google Scholar] [CrossRef]
- Frei, E.; Hodgkiss-Harlow, K.; Rossi, P.J.; Edmiston, C.E., Jr.; Bandyk, D.F. Microbial Pathogenesis of Bacterial Biofilms: A Causative Factor of Vascular Surgical Site Infection. Vasc. Endovasc. Surg. 2011, 45, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Panlilio, A.L.; Culver, D.H.; Gaynes, R.P.; Banerjee, S.; Henderson, T.S.; Tolson, J.S.; Martone, W.J.; National Nosocomial Infections Surveillance System. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975–1991. Infect. Control Hosp. Epidemiol. 1992, 13, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Hayes, P.D.; Darke, S. A prospective audit of complex wound and graft infections in Great Britain and Ireland: The emergence of MRSA. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2001, 21, 289–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cowie, S.E.; Ma, I.; Lee, S.K.; Smith, R.M.; Hsiang, Y.N. Nosocomial MRSA infection in vascular surgery patients: Impact on patient outcome. Vasc. Endovasc. Surg. 2005, 39, 327–334. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.; Andrew, P.; Batt, M.; Becquemin, J.P. A systematic review and meta-analysis of treatments for aortic graft infection. J. Vasc. Surg. 2006, 44, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H. Comparison of Antibiotic Resistance Mechanisms in Antibiotic-Producing and Pathogenic Bacteria. Molecules 2019, 24, 3430. [Google Scholar] [CrossRef]
- WHO. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018; ISBN 9789241514880.
- Bratzler, D.W.; Houck, P.M.; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: An advisory statement from the National Surgical Infection Prevention Project. Am. J. Surg. 2005, 189, 395–404. [Google Scholar] [CrossRef]
- McDonald, M.; Grabsch, E.; Marshall, C.; Forbes, A. Single-versus multiple-dose antimicrobial prophylaxis for major surgery: A systematic review. Aust. N. Z. J. Surg. 1998, 68, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.; James, C.; Ali, A.; Lawson, S.; Mary, E.; Modak, A. Vascular Surgical Antibiotic Prophylaxis Study (VSAPS). Vasc. Endovasc. Surg. 2010, 44, 521–528. [Google Scholar] [CrossRef]
- Stone, P.A.; AbuRahma, A.F.; Campbell, J.R.; Hass, S.M.; Mousa, A.Y.; Nanjundappa, A.; Srivastiva, M.; Modak, A.; Emmett, M. Prospective Randomized Double blinded Trial Comparing 2 Anti-MRSA Agents with Supplemental Coverage of Cefazolin before Lower Extremity Revascularization. Ann. Surg. 2015, 262, 495–501. [Google Scholar] [CrossRef]
- Classen, D.C.; Evans, R.S.; Pestotnik, S.L.; Horn, S.D.; Menlove, R.L.; Burke, J.P. The Timing of Prophylactic Administration of Antibiotics and the Risk of Surgical-Wound Infection. N. Engl. J. Med. 1992, 326, 281–286. [Google Scholar] [CrossRef]
- Argyriou, C.; Georgiadis, G.S.; Lazarides, M.K.; Georgakarakos, E.; Antoniou, G.A. Endograft Infection after Endovascular Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2017, 24, 688–697. [Google Scholar] [CrossRef]
- Harbarth, S.; Fankhauser, C.; Schrenzel, J.; Christenson, J.; Gervaz, P.; Bandiera-Clerc, C.; Renzi, G.; Vernaz, N.; Sax, H.; Pittet, D. Universal Screening for Methicillin-Resistant Staphylococcus aureus at Hospital Admission and Nosocomial Infection in Surgical Patients. JAMA 2008, 299, 1149–1157. [Google Scholar] [CrossRef]
- Friberg, O.; Svedjeholm, R.; Söderquist, B.; Granfeldt, H.; Vikerfors, T.; Källman, J. Local gentamicin reduces sternal wound infections after cardiac surgery: A randomized controlled trial. Ann. Thorac. Surg. 2005, 79, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Eklund, A.M. Prevention of sternal wound infections with locally administered gentamicin. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2007, 115, 1022–1024. [Google Scholar] [CrossRef]
- Raja, S.G. Local application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection following cardiac surgery. Int. J. Surg. 2012, 10, S10–S14. [Google Scholar] [CrossRef]
- Chang, W.K.; Srinivasa, S.; MacCormick, A.D.F.; Hill, A.G.F. Gentamicin-Collagen Implants to Reduce Surgical Site Infection: Systematic Review and Meta-Analysis of Randomized Trials. Ann. Surg. 2013, 258, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Costa Almeida, C.E.P.; Reis, L.; Carvalho, L.; Costa Almeida, C.M. Collagen implant with gentamicin sulphate reduces surgical site infection in vascular surgery: A prospective cohort study. Int. J. Surg. 2014, 12, 1100–1104. [Google Scholar] [CrossRef]
- Holdsworth, J. Treatment of infective and potentially infective complications of vascular bypass grafting using gentamicin with collagen sponge. Ann. R. Coll. Surg. Engl. 1999, 81, 166–170. [Google Scholar] [PubMed]
- Johnson, J.H. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. AORN J. 1992, 56, 347–348. [Google Scholar] [CrossRef]
- Stewart, A.H.; Eyers, P.S.; Earnshaw, J.J. Prevention of infection in peripheral arterial reconstruction: A systematic review and meta-analysis. J. Vasc. Surg. 2007, 46, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Pitt, H.A.; Postier, R.G.; MacGowan, A.W.; Frank, L.W.; Surmak, A.J.; Sitzman, J.V.; Bouchier-Hayes, D.A.V.I.D. Prophylactic antibiotics in vascular surgery. Topical, systemic, or both? Ann. Surg. 1980, 192, 356–364. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef]
- Mohammed, S.; Pisimisis, G.T.; Daram, S.P.; Bechara, C.F.; Barshes, N.R.; Lin, P.H.; Kougias, P. Impact of intraoperative administration of local vancomycin on inguinal wound complications. J. Vasc. Surg. 2013, 57, 1079–1083. [Google Scholar] [CrossRef]
- Cernohorsky, P.; Reijnen, M.M.P.J.; Tielliu, I.F.J.; van Sterkenburg, S.M.M.; 586 van den Dungen, J.J.A.M.; Zeebregts, C.J. The relevance of aortic endograft prosthetic infection. J. Vasc. Surg. 2011, 54, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Chehab, M.A.; Thakor, A.; Tulin-Silver, S.; Connolly, B.L.; Cahill, A.M.; Ward, T.J.; Padia, S.A.; Kohi, M.P.; Midia, M.; Brody, L.; et al. Adult and Pediatric Antibiotic Prophylaxis during Vascular and IR Procedures: A Society of Interventional Radiology Practice Parameter Update Endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J. Vasc. Interv. Radiol. 2018, 29, 1483–1501.e2. [Google Scholar] [CrossRef]
- Diekema, D.J.; Edmond, M.B. Look before you leap: Active surveillance for multidrug resistant organisms. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 44, 1101–1107. [Google Scholar] [CrossRef]
- Malde, D.J.; Abidia, A.; McCollum, C.; Welch, M. The success of routine MRSA screening in vascular surgery: A nine year review. Int. Angiol. 2006, 25, 204–208. [Google Scholar] [PubMed]
- Hussain, S.T. Local application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection following vascular surgery. Int. J. Surg. 2012, 10, S5–S9. [Google Scholar] [CrossRef]
- Ruszczak, Z.; Friess, W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv. Drug Deliv. Rev. 2003, 55, 1679–1698. [Google Scholar] [CrossRef] [PubMed]
- Modarai, B.; Dasgupta, P.; Taylor, J.; Koffman, G.; Khan, M.S. Follow-up of polytetrafluoroethylene arteriovenous fistulae for haemodialysis. Int. J. Clin. Pract. 2005, 59, 1005–1007. [Google Scholar] [CrossRef]
- Rasheed, H.; Diab, K.; Singh, T.; Chauhan, Y.; Haddad, P.; Zubair, M.M.; Vowels, T.; Androas, E.; Rojo, M.; Auyang, P.; et al. Contemporary Review to Reduce Groin Surgical Site Infections in Vascular Surgery. Ann. Vasc. Surg. 2021, 72, 578–588. [Google Scholar] [CrossRef]
| Study | Random Sequence Generation (Selction Bias) | Allocation Concealment (Selection Bias) | Blinding (Performance Bias and Detection Bias) | Selective Reporting (Reporting Bias) | Other Bias | Jadad Score |
|---|---|---|---|---|---|---|
| Bratzler DW et al., 2005 [25] | High | High | High | Low | Low | 2 |
| McDonald M et al., 1998 [26] | Low | Low | High | Low | Low | 3 |
| Patrick S et al., 2010 [27] | Low | Low | High | Low | Low | 3 |
| Stone PA et al., 2015 [28] | High | High | High | Low | Low | 2 |
| Classen DC et al., 1992 [29] | Low | Low | High | Low | Low | 3 |
| Argyriou C et al., 2017 [30] | High | High | High | Low | Low | 2 |
| Harbarth S et al., 2008 [31] | High | High | High | Low | Low | 2 |
| Friberg O et al., 2005 [32] | Low | Low | High | Low | Low | 3 |
| Eklund AM et al., 2007 [33] | Low | Low | High | Low | Low | 3 |
| Raja SG et al., 2012 [34] | High | High | High | Low | Low | 2 |
| Chang WK et al., 2013 [35] | High | High | High | Low | Low | 2 |
| Costa Almeida CEP et al., 2014 [36] | High | Low | High | Low | Low | 3 |
| Holdsworth et al., 1999 [37] | Low | High | Low | Low | Low | 3 |
| References | Methods | Partecipants | Intervention | Outcomes | Primary Findings |
|---|---|---|---|---|---|
| Johnson JH et al., 1992 [38] | Single blinded, randomized controlled trial | 2847 patients undergoing elective clean or “clean-contaminated” surgical procedures | Administration of antibiotics pre- v/s peri- and post-operatively | Surgical-wound infections | SSI rate of 0.6% for pre- vs. 1.4% peri- and 3.3% post-operative antibiotic administration (p less than 0.0001; relative risk, 5.8; 95 percent confidence interval, 2.6 to 12.3) |
| Bratzler DW et al., 2005 [25] | Systematic review and meta-analysis | 22 trials of prophylactic systemic antibiotics | Prophylactic systemic antibiotics administration | Wound infection or early graft infection | Prophylactic systemic antibiotics reduced the risk of wound infection (RR, 0.25; 95% confidence interval [CI], 0.17 to 0.38) |
| Stewart AH et al., 2007 [39] | Guidelines review | Published North American guidelines for antimicrobial prophylaxis until 2002 | Pre- and post-operative antimicrobial prophylaxis | Surgical-wound infections | Infusion of the first antimicrobial dose should begin within 60 min before surgical incision and that prophylactic antimicrobial agents should be discontinued within 24 h of the end of surgery |
| Pitt HA et al., 1980 [40] | Single blinded, randomized controlled trial | 217 patients scheduled for vascular surgery with groin incision | No antibiotic v/s topical cephradine prior to closure v/s 24-h perioperative e.v. cephradine and v/s both topical and intravenous cephradine. | Groin and abdominal incisional infections | - Groin and abdominal incisional infections significantly reduced (p < 0.01) among patients who received prophylactic antibiotics by either the topical, systemic, or combined routes of administration. - No significant differences were noted among the three antibiotic groups. |
| Bratzler DW et al., 2013 [41] | Practice guidelines | Primary literature of Therapeutic Guidelines on Antimicrobial Prophylaxis in Surgery | - Single pre-incision dose of cefazolin or cefuroxime- Continuing prophylaxis. | Primary prophylaxis and eradication of wound infection | Recommendation of a single pre-incision dose of cefazolin or cefuroxime with appropriate intraoperative redosing. No evidence for continuing prophylaxis until all drains and catheters are removed. Clindamycin or vancomycin as alternative in patients with b-lactam allergy. Vancomycin used for prophylaxis in patients known to be colonized with MRSA. (Strength of evidence for prophylaxis = A) |
| McDonald M et al., 1998 [26] | Systematic review | - 28 Clinical Trials - 9478 patients | Antimicrobial single v/s multiple dose in surgical prophylaxis | Post-operative surgical site infections rate prevention | No clear advantage of either single or multiple-dose regimens of antibiotics |
| Stone PA et al., 2015 [28] | Single center prospective double blinded randomized study | 178 patients were evaluated at 90 days for surgical site infection | Vancomycin v/s Vancomycin + Daptomycin pre-operative administration | Post-operative SSI rate prophylaxis | Vancomycin supplemental prophylaxis seems to reduce the incidence of Gram-positive infection compared with adding supplemental Daptomycin prophylaxis (p = 0.11). |
| Patrick S et al., 2010 [27] | Single institution prospective randomized study | 169 low-risk patients undergoing elective vascular procedures | Cefazolin, cefazolin + vancomycin, or cefazolin + daptomycin surgical prophylaxis | Post-operative surgical site infections rate prevention | Significant fewer infectious complications in the cefazolin + daptomycin group |
| Mohammed S et al., 2013 [42] | Retrospective Cohort study | 454 patients who underwent open vascular procedures | Systemic vancomycin v/s systemic + local application of vancomycin powder | Inguinal wound infection and dehiscence over a 30-day period | Addition of intraoperative local vancomycin did not improve the rates of inguinal wound dehiscence or deep infections but had a positive impact on superficial wound infections |
| Classen DC et al., 1992 [29] | Single institution prospective randomized study | 2847 patients undergoing elective clean surgical procedures | Antibiotic administration 2 to 24 h before the surgical incision | Post-operative surgical wound infection rate | Prophylactic administration of antibiotics in the two hours before surgery reduces the risk of wound infection. |
| Cernohorsky P et al., 2011 [43] | Multicenter retrospective cohort study | 1431 endovascular procedures | Prophylactic antimicrobial therapy | Incidence of endograft infection and mortality rate | Endograft infection rate below 1%, with a mortality rate of 25%. Antimicrobial therapy helps conservative treatment in selected cases of patients with an infected endograft. |
| Argyriou C et al., 2017 [30] | Meta-Analysis | 12 studies reporting on 362 patients | Endovascular aneurysm repair (EVAR) | Evidence on the outcomes of graft infection after EVAR | Supportive medical antimicrobial treatment without surgical intervention has a significant associated mortality. |
| Chehab MA et al., 2018 [44] | Practice guidelines | Primary literature of Therapeutic Guidelines on Antimicrobial Prophylaxis Vascular and IR Procedures | Prophylactic antimicrobial therapy | SSI antimicrobial prophylaxis | Recommendation 1: intravenous (IV) antibiotic agents must be administered within 1 h of an incision. Recommendation 2: A repeat dose of antibiotic agents should be administered if a period of 2 h has lapsed from the initial dose. |
| Diekema DJ. et al., 2007 [45] | Review | Evidence-based guidelines | Program for detection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci among hospital patients | Steps that should be performed when planning active surveillance cultures for detection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci | Preparing the laboratory and reducing the turnaround time for screening tests; monitoring and optimizing the intervention of instituting contact precautions; monitoring and ameliorating the known adverse effects of contact precautions. |
| Harbarth S et. al., 2008 [31] | Prospective, interventional cohort study. Clinical trial | 754 patients | Compare rapid MRSA screening on admission plus standard infection control measures vs. standard infection control alone | Perioperative antibiotic prophylaxis of MRSA carriers and topical decolonization for 5 days. | A universal, rapid MRSA admission screening strategy did not reduce nosocomial MRSA infection in a surgical department with endemic MRSA prevalence but relatively low rates of MRSA infection |
| Malde DJ et al., 2006 [46] | Retrospective Cohort study | 280 vascular patients | Data analysis of two period groups of MRSA positive vascular patients | Wound infection, major limb amputation and mortality rates | MRSA screening identifies patients at risk of serious complications and is associated with a reduction in these complications following both elective and emergency surgery |
| References | Methods | Partecipants | Intervention | Outcomes | Primary Findings |
|---|---|---|---|---|---|
| Hussain ST et al., 2012 [47] | Review | Five publications on development of SSI in vascular surgery | Prophylactic use of GCCI in fem-pop graft surgery | Reduction in SSI rate incidence | GCCI have a role to play in preventing and treating SSI following vascular reconstruction. |
| Ruszczak Z et al., 2003 [48] | Review | Primary literature on collagen as a biomaterial in drug delivery systems for antibiotics | Treatment and prophylaxis of bone and soft tissue infections | Incidence of SSI | The incidence of SSI was 4.3% in the treatment group and 9.0% in the control group (relative risk 0.47; 95% confidence interval 0.33–0.68; p < 0.001). |
| Friberg O et al., 2005 [32] | RCT | 2000 cardio-vascular surgery patients | Standard prophylaxis combined with CCGI v/s standard alone (control) | Incidence of SSI | The incidence of SSI was 4.3% in the treatment group and 9.0% in the control group (relative risk 0.47; 95% confidence interval 0.33–0.68; p < 0.001). |
| Eklund AM et al., 2007 [33] | RCT | 557 patients who underwent elective cardio-vascular surgery | Standard prophylaxis combined with CCGI v/s standard alone (control) | Incidence of SSI | Postoperative SSI occurred in 11 of 272 patients (4.0%) in the study group and in 16 of 270 patients (5.9%) in the control group. This difference was not statistically significant (p = 0.20). |
| Raja SG et al., 2012 [34] | RCT | 9 publications on prophylactic use of GCCI in cardiovascular surgery | Adjunctive use of GCCI in prophylaxis of SSI | Morbidity associated with SSI following surgery | The adjunctive use of GCCI is particularly beneficial in high-risk patients and also cost saving. |
| Chang WK et al., 2013 [35] | Meta-analysis | 6979 patients from major medical databases and trial registers for RCTs | Use of GCCI in prophylaxis of SSI | Endpoint of interest was the incidence of SSI | GCCI reduced SSI [OR = 0.51; 95% CI: 0.33–0.77; p = 0.001; number needed to treat (NNT) = 21; I = 75%]. These results were seen in subset analysis of clean-contaminated surgery (OR = 0.43; 95% CI: 0.20–0.93; p = 0.03; NNT = 9) specifically. |
| Modarai B et al., 2005 [49] | Single institution prospective randomized study | Fifty-nine upper limb PTFE grafts in 48 patients | Use of GCCI in prophylaxis of SSI | Incidence of SSI | The use of prosthetic material is associated with a poor overall patency rate and high risk of infective complications. |
| Costa Almeida CEP et al., 2014 [36] | Controlled Clinical Trial | 60 patients with lower limb ischaemia | GCCI in the groin incision adjacent to the prosthesis | SSI rate and in-hospital days | GCCI use decreasing SSI rate and in-hospital days, and also reduce health care costs. |
| Rasheed et al., 2021 [50] | Review | Literature review of preventive strategies for groin SSI | Antimicrobial therapy and CCGI | Post-operative SSI | Collagen gentamicin implants are useful in preventing surgical site infection in the groin after vascular surgical procedures. |
| Holdsworth J et al., 1999 [37] | Single institution prospective randomized clinical trial | 25 patients with infective and potentially infective complications of vascular bypass grafting | Use of GCCI in prevention and treatment of SSI | In situ prevention and treatment of graft infection | 7/11 in situ treatments of a graft infection were successfully aborted; in the other 4 grafts were removed. None of the other 14 patients treated with GCCI subsequently had infective sequelae. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amato, B.; Compagna, R.; De Vivo, S.; Rocca, A.; Carbone, F.; Gentile, M.; Cirocchi, R.; Squizzato, F.; Spertino, A.; Battocchio, P. Groin Surgical Site Infection in Vascular Surgery: Systemic Review on Peri-Operative Antibiotic Prophylaxis. Antibiotics 2022, 11, 134. https://doi.org/10.3390/antibiotics11020134
Amato B, Compagna R, De Vivo S, Rocca A, Carbone F, Gentile M, Cirocchi R, Squizzato F, Spertino A, Battocchio P. Groin Surgical Site Infection in Vascular Surgery: Systemic Review on Peri-Operative Antibiotic Prophylaxis. Antibiotics. 2022; 11(2):134. https://doi.org/10.3390/antibiotics11020134
Chicago/Turabian StyleAmato, Bruno, Rita Compagna, Salvatore De Vivo, Aldo Rocca, Francesca Carbone, Maurizio Gentile, Roberto Cirocchi, Francesco Squizzato, Andrea Spertino, and Piero Battocchio. 2022. "Groin Surgical Site Infection in Vascular Surgery: Systemic Review on Peri-Operative Antibiotic Prophylaxis" Antibiotics 11, no. 2: 134. https://doi.org/10.3390/antibiotics11020134
APA StyleAmato, B., Compagna, R., De Vivo, S., Rocca, A., Carbone, F., Gentile, M., Cirocchi, R., Squizzato, F., Spertino, A., & Battocchio, P. (2022). Groin Surgical Site Infection in Vascular Surgery: Systemic Review on Peri-Operative Antibiotic Prophylaxis. Antibiotics, 11(2), 134. https://doi.org/10.3390/antibiotics11020134








