Effects of the Carbohydrate Sources Nectar, Sucrose and Invert Sugar on Antibacterial Activity of Honey and Bee-Processed Syrups
Abstract
:1. Introduction
2. Results
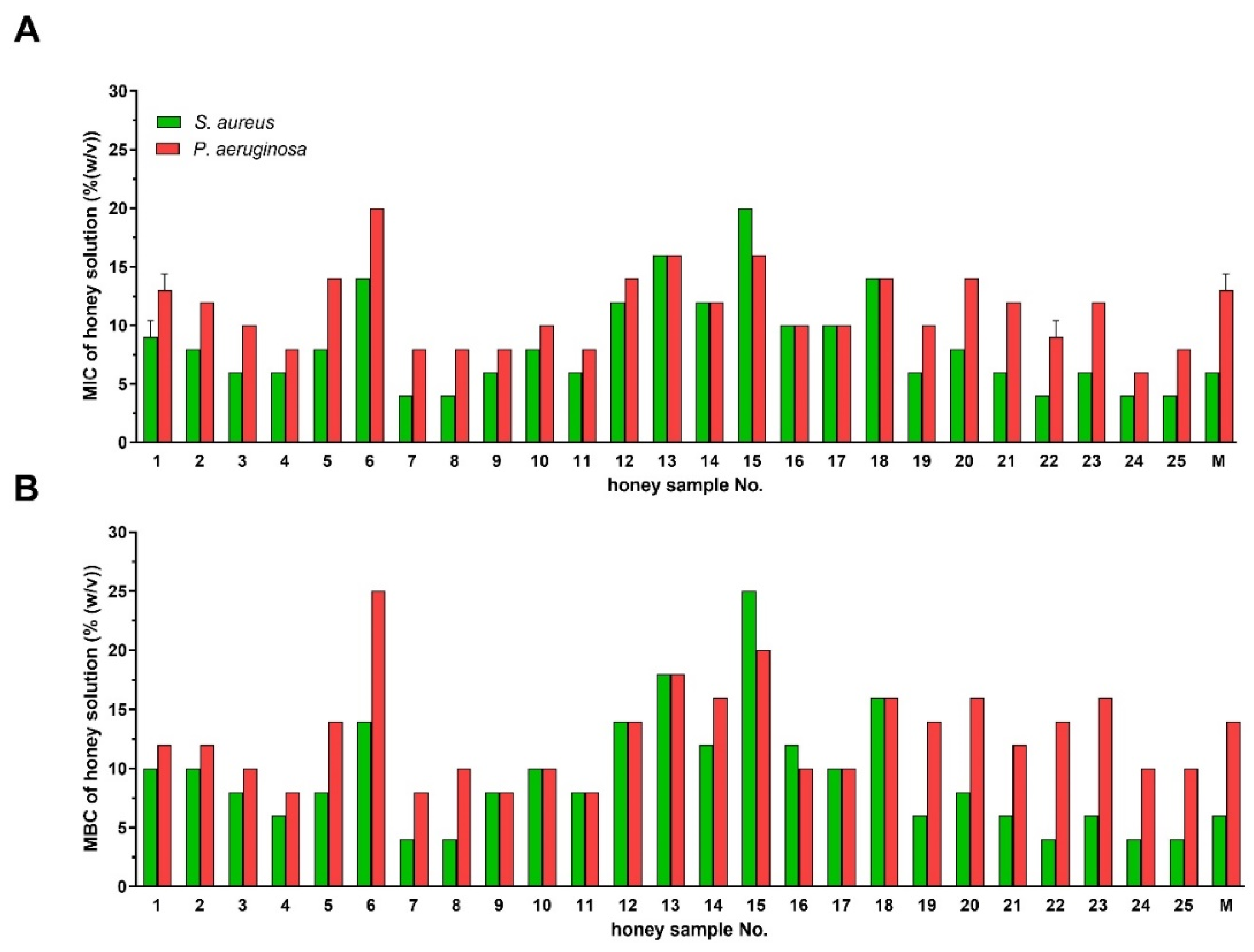
2.1. Antibacterial Activity of Styrian Honey Samples
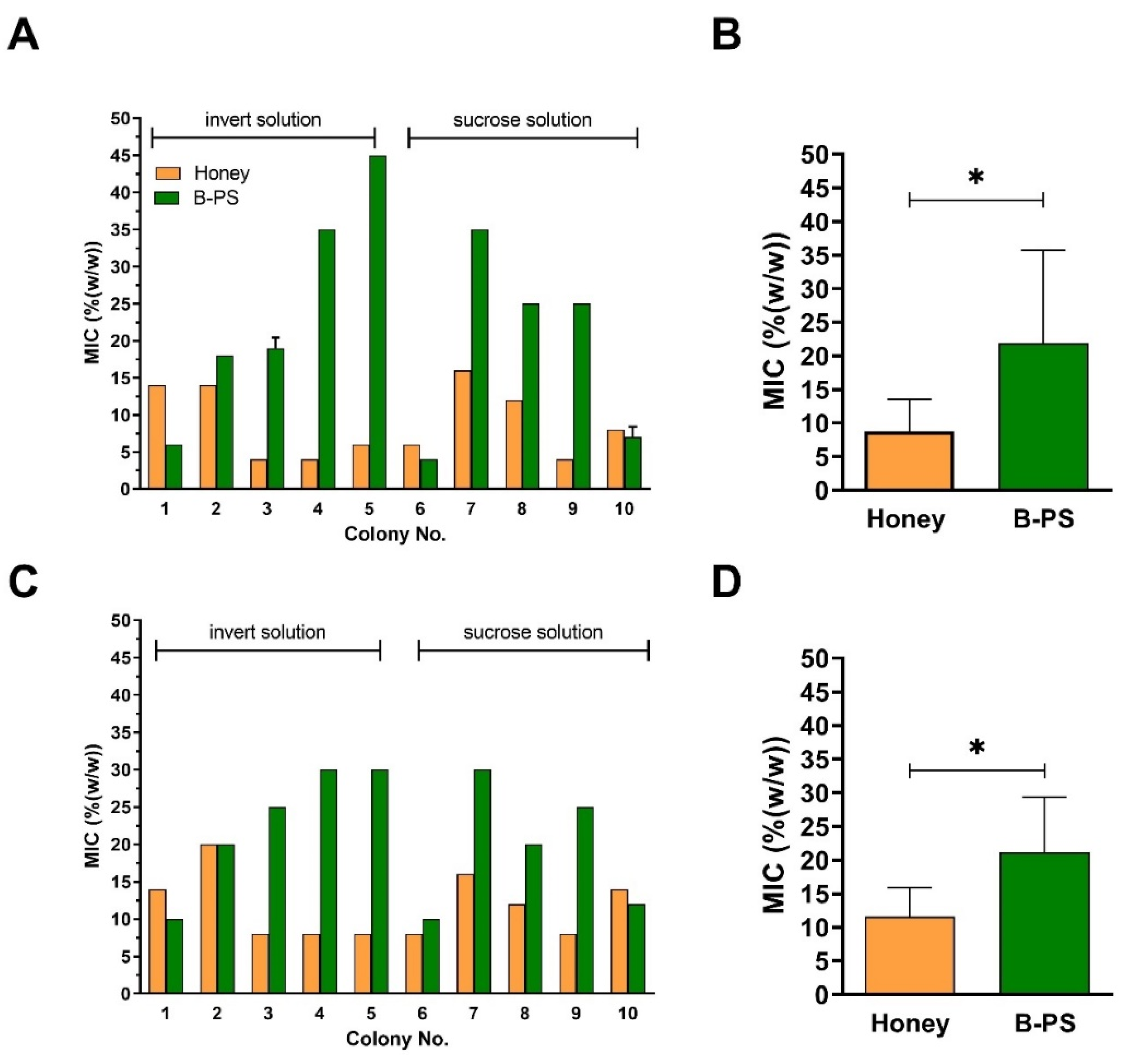
2.2. Comparison of Antibacterial Activities of Natural Honeys and Bee-Processed Syrups
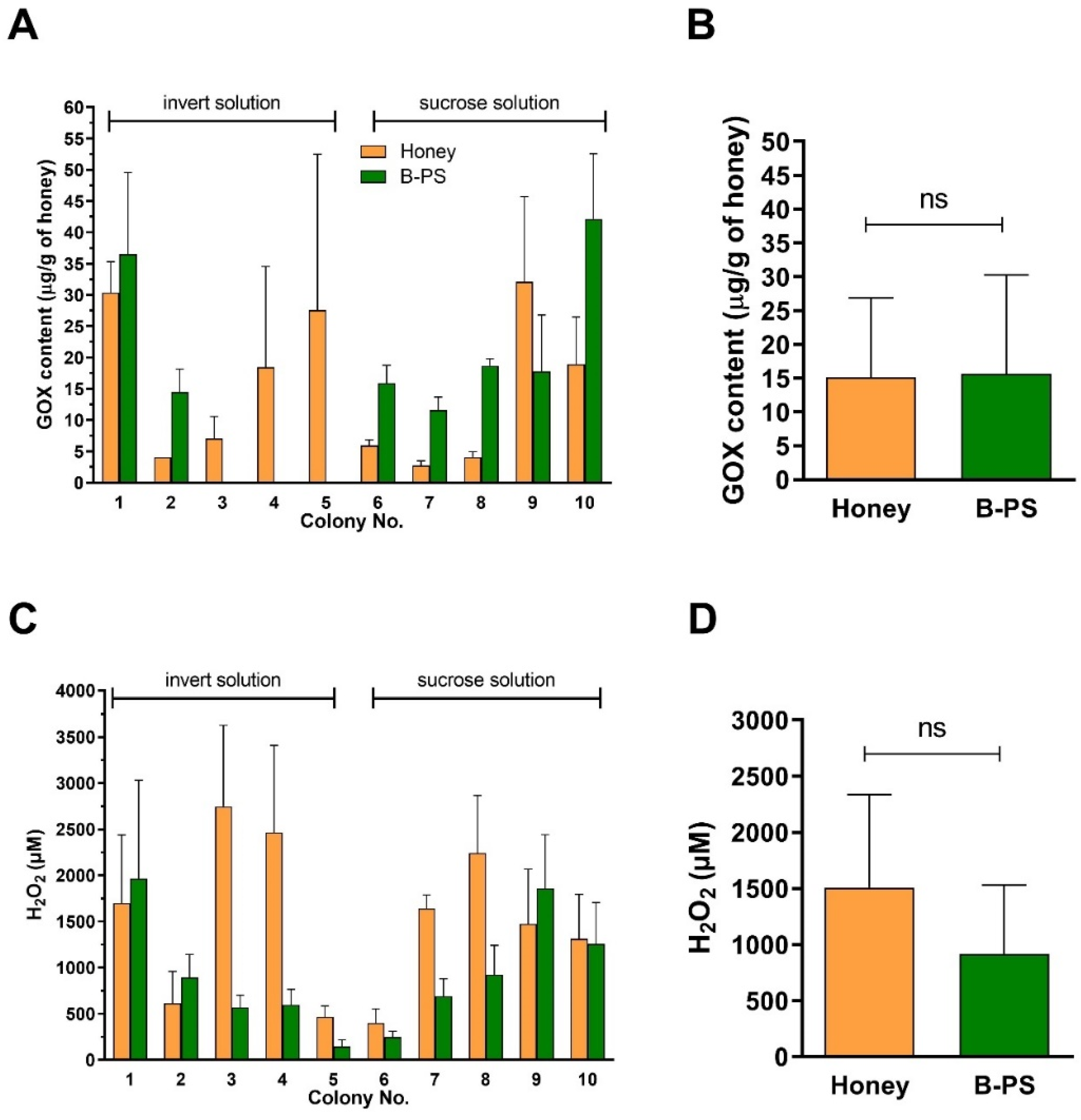
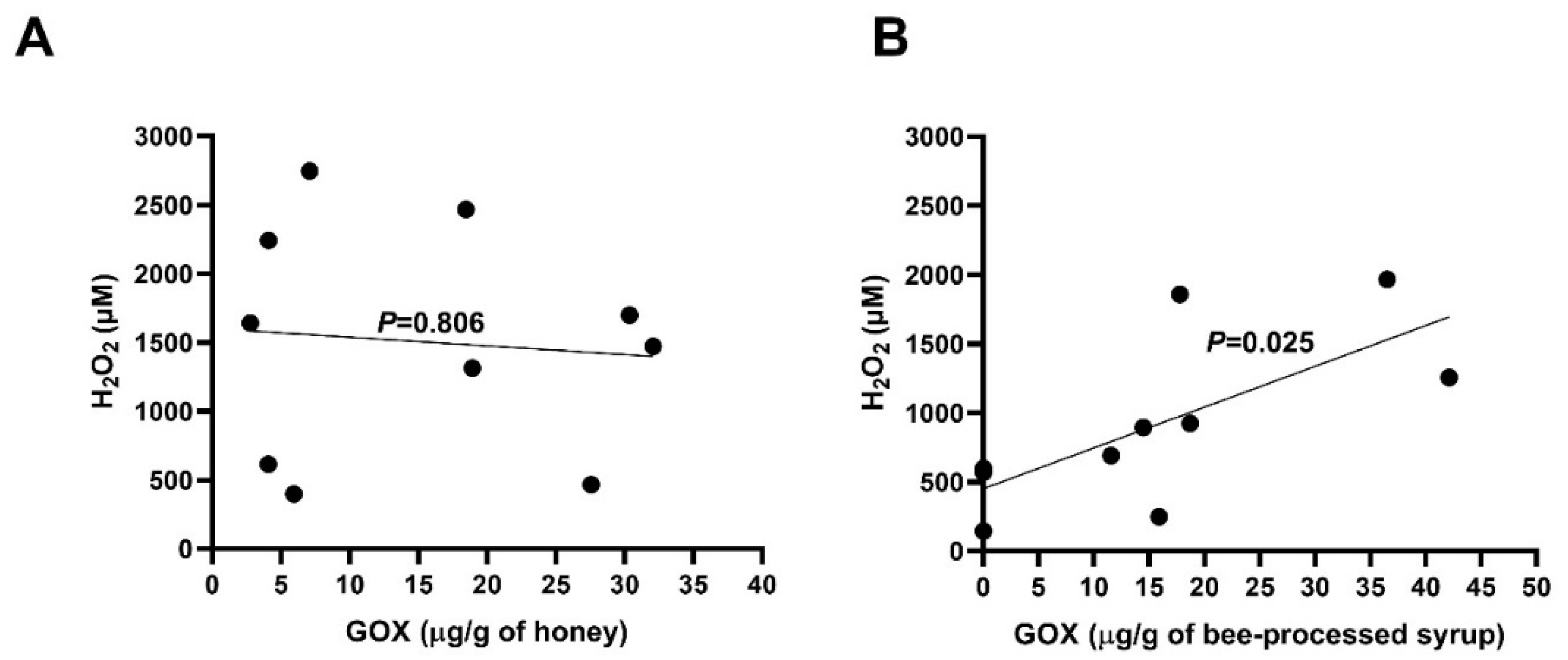
2.3. Content of GOX and Generation of H2O2 in Honeys and Bee-Processed Syrups
2.4. Comparison of Antibacterial Activity and Contents of GOX and Proteins between Sucrose and Inverted Sugar Feeds after Their Processing by Honey Bees
2.5. Colour Analysis of Honey and Bee-Processed Syrups
3. Discussion
4. Materials and Methods
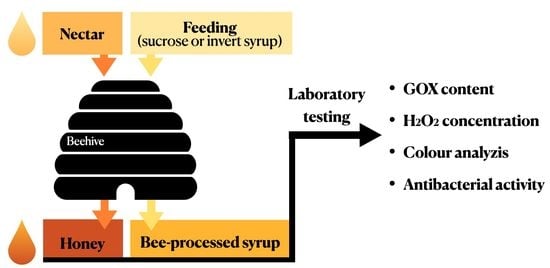
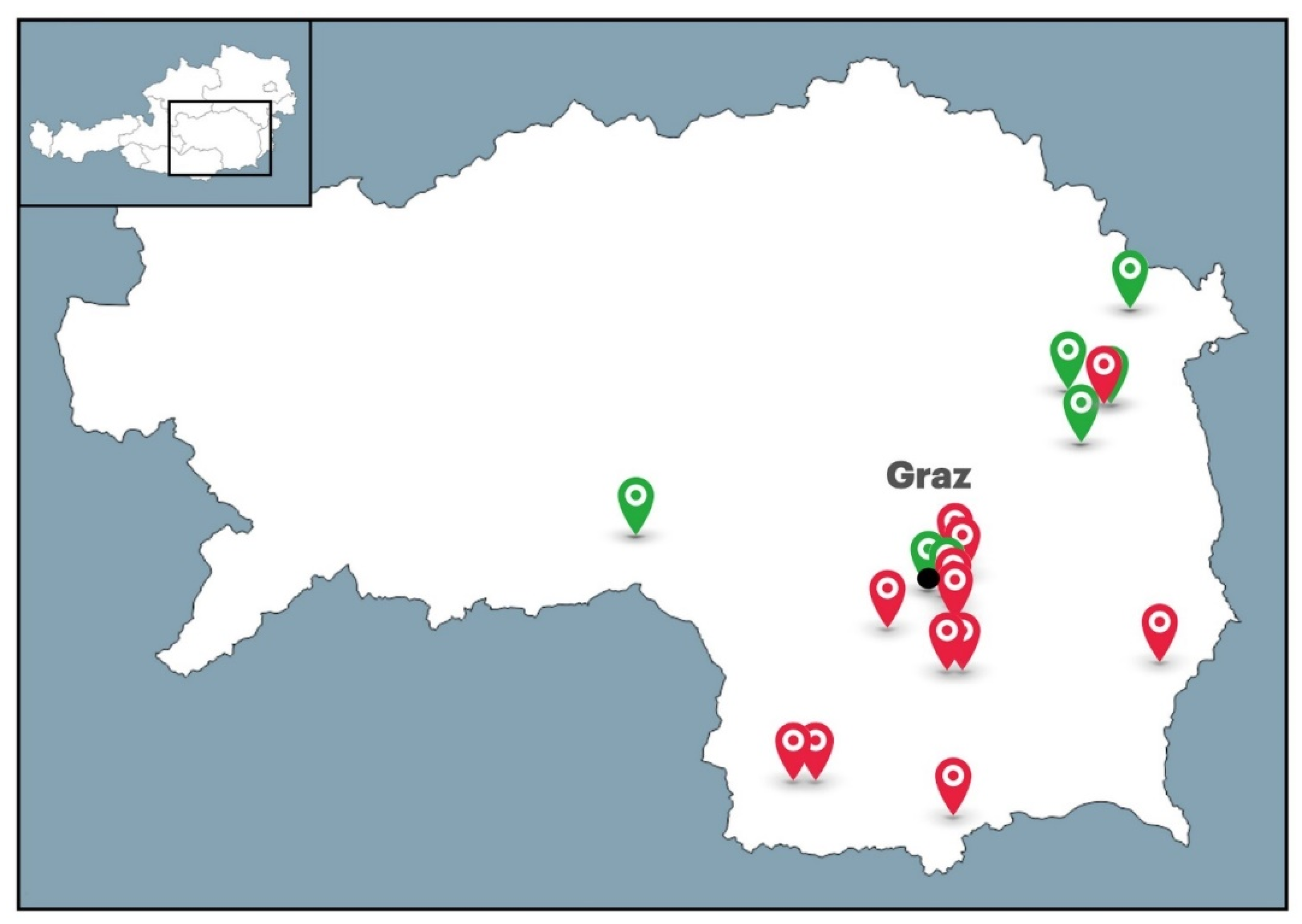
4.1. Sampling of Honeys and Bee-Processed Syrups
4.2. Microorganisms
4.3. Honey Antibacterial Inhibition Assays
4.4. Determination of the GOX Content
4.5. Determination of H2O2 Concentration
4.6. Determination of Colour according to the Pfund Scale
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otero, M.C.B.; Bernolo, L. Honey as Functional Food and Prospects in Natural Honey Production. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 197–210. [Google Scholar] [CrossRef]
- Majtan, J. Honey: An immunomodulator in wound healing. Wound Repair Regen. 2014, 22, 187–192. [Google Scholar] [CrossRef]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Hosney-Based Medicinal Formulations: A Critical Review. Appl. Sci. 2021, 11, 5159. [Google Scholar] [CrossRef]
- Papadopoulou, D.; Dabrowska, A.; Harries, P.G.; Webb, J.S.; Allan, R.N.; Salib, R.J. Evaluation of a Bioengineered Honey and Its Synthetic Equivalent as Novel Staphylococcus aureus Biofilm-Targeted Topical Therapies in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2020, 34, 80–86. [Google Scholar] [CrossRef]
- Matoke Holdings Ltd. Antimicrobial Compositions; Matoke Holdings Ltd.: Southmoor, Abingdon, UK, 2018. [Google Scholar]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial activity of different blossom honeys: New findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkasovska, J.; Bugarova, V.; Godocikova, J.; Majtan, V.; Majtan, J. The role of hydrogen peroxide in the antibacterial activity of different floral honeys. Eur. Food Res. Technol. 2019, 245, 2739–2744. [Google Scholar] [CrossRef]
- Roshan, N.; Rippers, T.; Locher, C.; Hammer, K.A. Antibacterial activity and chemical characteristics of several Western Australian honeys compared to manuka honey and pasture honey. Arch. Microbiol. 2017, 199, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [Green Version]
- Van Engelsdorp, D.; Hayes, J.; Underwood, R.M.; Pettis, J.S. A survey of honey bee colony losses in the United States, fall 2008 to spring 2009. J. Apic. Res. 2010, 49, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Pirk, C.W.; Human, H.; Crewe, R.M.; van Engelsdorp, D. A survey of managed honey bee colony losses in the Republic of South Africa—2009 to 2011. J. Apic. Res. 2014, 53, 35–42. [Google Scholar] [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Cornelissen, B.; da Amaro Costa, C.; et al. Honey bee colony winter loss rates for 35 countries participating in the COLOSS survey for winter 2018–2019, and the effects of a new queen on the risk of colony winter loss. J. Apic. Res. 2020, 59, 744–751. [Google Scholar] [CrossRef]
- Kulhanek, K.; Steinhauer, N.; Wilkes, J.; Wilson, M.; Spivak, M.; Sagili, R.R.; Tarpy, D.R.; McDermott, E.; Garavito, A.; Rennich, K.; et al. Survey-derived best management practices for backyard beekeepers improve colony health and reduce mortality. PLoS ONE 2021, 16, e0245490. [Google Scholar] [CrossRef] [PubMed]
- Popovska Stojanov, D.; Dimitrov, L.; Danihlík, J.; Uzunov, A.; Golubovski, M.; Andonov, S.; Brodschneider, R. Direct Economic Impact Assessment of Winter Honeybee Colony Losses in Three European Countries. Agriculture 2021, 11, 398. [Google Scholar] [CrossRef]
- Barker, R.J.; Lehner, Y. Laboratory comparision of high fructose corn syrup, grape syrup, honey, and scurose syrup as maintenance food for caged honey bees. Apidologie 1978, 9, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Sammataro, D.; Weiss, M. Comparison of productivity of colonies of honey bees, Apis mellifera, supplemented with sucrose or high fructose corn syrup. J. Insect Sci. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, M.M.; Robinson, G.E. Diet-dependent gene expression in honey bees: Honey vs. sucrose or high fructose corn syrup. Sci. Rep. 2014, 4, 5276. [Google Scholar] [CrossRef] [Green Version]
- Frizzera, D.; Del Fabbro, S.; Ortis, G.; Zanni, V.; Bortolomeazzi, R.; Nazzi, F.; Annoscia, D. Possible side effects of sugar supplementary nutrition on honey bee health. Apidologie 2020, 51, 594–608. [Google Scholar] [CrossRef]
- Tawfik, A.I.; Ahmed, Z.H.; Abdel-Rahman, M.F.; Moustafa, A.M. Influence of winter feeding on colony development and the antioxidant system of the honey bee, Apis mellifera. J. Apic. Res. 2020, 59, 752–763. [Google Scholar] [CrossRef]
- Szczesna, T.; Was, E.; Semkiw, P.; Skubida, P.; Jaskiewicz, K.; Witek, M. Changes of physicochemical properties of starch syrups recommended for winter feeding of honeybees during storage. Agriculture 2021, 11, 374. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Calla, B. Honey as a functional food for Apis mellifera. Annu. Rev. Entomol. 2021, 66, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Godocikova, J.; Bugarova, V.; Kast, C.; Majtan, V.; Majtan, J. Antibacterial potential of Swiss honeys and characterisation of their bee-derived bioactive compounds. J. Sci. Food Agric. 2020, 100, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Grecka, K.; Kuś, P.M.; Worobo, R.W.; Szweda, P. Study of the anti-staphylococcal potential of honeys produced in Northern Poland. Molecules 2018, 23, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthimidou, E.; Mossialos, D. Antibacterial activity of Greek and Cypriot honeys against Staphylococcus aureus and Pseudomonas aeruginosa in comparison to manuka honey. J. Med. Food 2013, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, K.; Natori, S.; Kubo, T. Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honeybee (Apis mellifera L.). Eur. J. Biochem. 1999, 265, 127–133. [Google Scholar] [CrossRef]
- Santos, K.S.; Dos Delazari Santos, L.; Anita Mendes, M.; De Monson Souza, B.; Malaspina, O.; Palma, M.S. Profiling the proteome complement of the secretion from hypopharyngeal gland of Africanized nurse-honeybees (Apis mellifera L.). Insect Biochem. Mol. Biol. 2005, 35, 85–91. [Google Scholar] [CrossRef]
- Li, J.; Feng, M.; Zhang, Z.; Pan, Y. Identification of the proteome complement of hypopharyngeal glands from two strains of honeybees (Apis mellifera). Apidologie 2008, 39, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase—Its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef]
- Lewkowski, O.; Mureșan, C.I.; Dobritzsch, D.; Fuszard, M.; Erler, S. The Effect of Diet on the Composition and Stability of Proteins Secreted by Honey Bees in Honey. Insects 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- de Brito Sanchez, M.G. Taste Perception in Honey Bees. Chem. Senses 2011, 36, 675–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenberg-Kraag, B. Evidence for correlation between invertase activity and sucrose content during the ripening process of honey. J. Apic. Res. 2014, 53, 364–373. [Google Scholar] [CrossRef]
- Papežíková, I.; Palíková, M.; Syrová, E.; Zachová, A.; Somerlíková, K.; Kováčová, V.; Pecková, L. Effect of Feeding Honey Bee (Apis mellifera Hymenoptera: Apidae) Colonies with Honey, Sugar Solution, Inverted Sugar, and Wheat Starch Syrup on Nosematosis Prevalence and Intensity. J. Econ. Entomol. 2019, 113, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Krainer, S.; Brodschneider, R.; Vollmann, J.; Crailsheim, K.; Riessberger-Gallé, U. Effect of hydroxymethylfurfural (HMF) on mortality of artificially reared honey bee larvae (Apis mellifera carnica). Ecotoxicology 2016, 25, 320–328. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, B.W.; Eggleston, G.; Sammataro, D.; Cornett, C.; Dufault, R.; Deeby, T.; St. Cyr, E. Formation of hydroxymethylfurfural in domestic high-fructose corn syrup and its toxicity to the honey bee (Apis mellifera). J. Agric. Food Chem. 2009, 57, 7369–7376. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef] [Green Version]
- Erler, S.; Moritz, R.F. Pharmacophagy and pharmacophory: Mechanisms of self-medication and disease prevention in the honeybee colony (Apis mellifera). Apidologie 2016, 47, 389–411. [Google Scholar] [CrossRef] [Green Version]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; HS, A. Dietary phytochemicals, honey bee longevity and pathogen tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Eyer, M.; Neumann, P.; Dietemann, V. A look into the cell: Honey storage in honey bees, Apis mellifera. PLoS ONE 2016, 11, e0161059. [Google Scholar] [CrossRef] [Green Version]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef]
- Bugarova, V.; Majtan, J. De jure honey: Even without antibacterial activity. Bee World 2021, 98, 12–13. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Standards for Honey. Codex Stan 12-1981; Codex Alimentarius: Rome, Italy, 2001. [Google Scholar]
- Ferreira, I.C.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]



| Honey Sample | Botanical Origin | Sample Extraction from Colony * |
|---|---|---|
| 1 | polyfloral | >1 |
| 2 | chestnut | >1 |
| 3 | linden | >1 |
| 4 | linden | 1 |
| 5 | linden | 1 |
| 6 | polyfloral | 1 |
| 7 | polyfloral | 1 |
| 8 | polyfloral | 1 |
| 9 | linden | 1 |
| 10 | polyfloral | >1 |
| 11 | linden | 1 |
| 12 | polyfloral | 1 |
| 13 | polyfloral | 1 |
| 14 | polyfloral | 1 |
| 15 | polyfloral | 1 |
| 16 | polyfloral | 1 |
| 17 | polyfloral | 1 |
| 18 | linden | 1 |
| 19 | polyfloral | >1 |
| 20 | chestnut | >1 |
| 21 | linden | >1 |
| 22 | linden/honeydew | >1 |
| 23 | chestnut | >1 |
| 24 | polyfloral | >1 |
| 25 | linden | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugarova, V.; Godocikova, J.; Bucekova, M.; Brodschneider, R.; Majtan, J. Effects of the Carbohydrate Sources Nectar, Sucrose and Invert Sugar on Antibacterial Activity of Honey and Bee-Processed Syrups. Antibiotics 2021, 10, 985. https://doi.org/10.3390/antibiotics10080985
Bugarova V, Godocikova J, Bucekova M, Brodschneider R, Majtan J. Effects of the Carbohydrate Sources Nectar, Sucrose and Invert Sugar on Antibacterial Activity of Honey and Bee-Processed Syrups. Antibiotics. 2021; 10(8):985. https://doi.org/10.3390/antibiotics10080985
Chicago/Turabian StyleBugarova, Veronika, Jana Godocikova, Marcela Bucekova, Robert Brodschneider, and Juraj Majtan. 2021. "Effects of the Carbohydrate Sources Nectar, Sucrose and Invert Sugar on Antibacterial Activity of Honey and Bee-Processed Syrups" Antibiotics 10, no. 8: 985. https://doi.org/10.3390/antibiotics10080985
APA StyleBugarova, V., Godocikova, J., Bucekova, M., Brodschneider, R., & Majtan, J. (2021). Effects of the Carbohydrate Sources Nectar, Sucrose and Invert Sugar on Antibacterial Activity of Honey and Bee-Processed Syrups. Antibiotics, 10(8), 985. https://doi.org/10.3390/antibiotics10080985