Application of Heating on the Antioxidant and Antibacterial Properties of Malaysian and Australian Stingless Bee Honey
Abstract
:1. Introduction
2. Results and Discussion
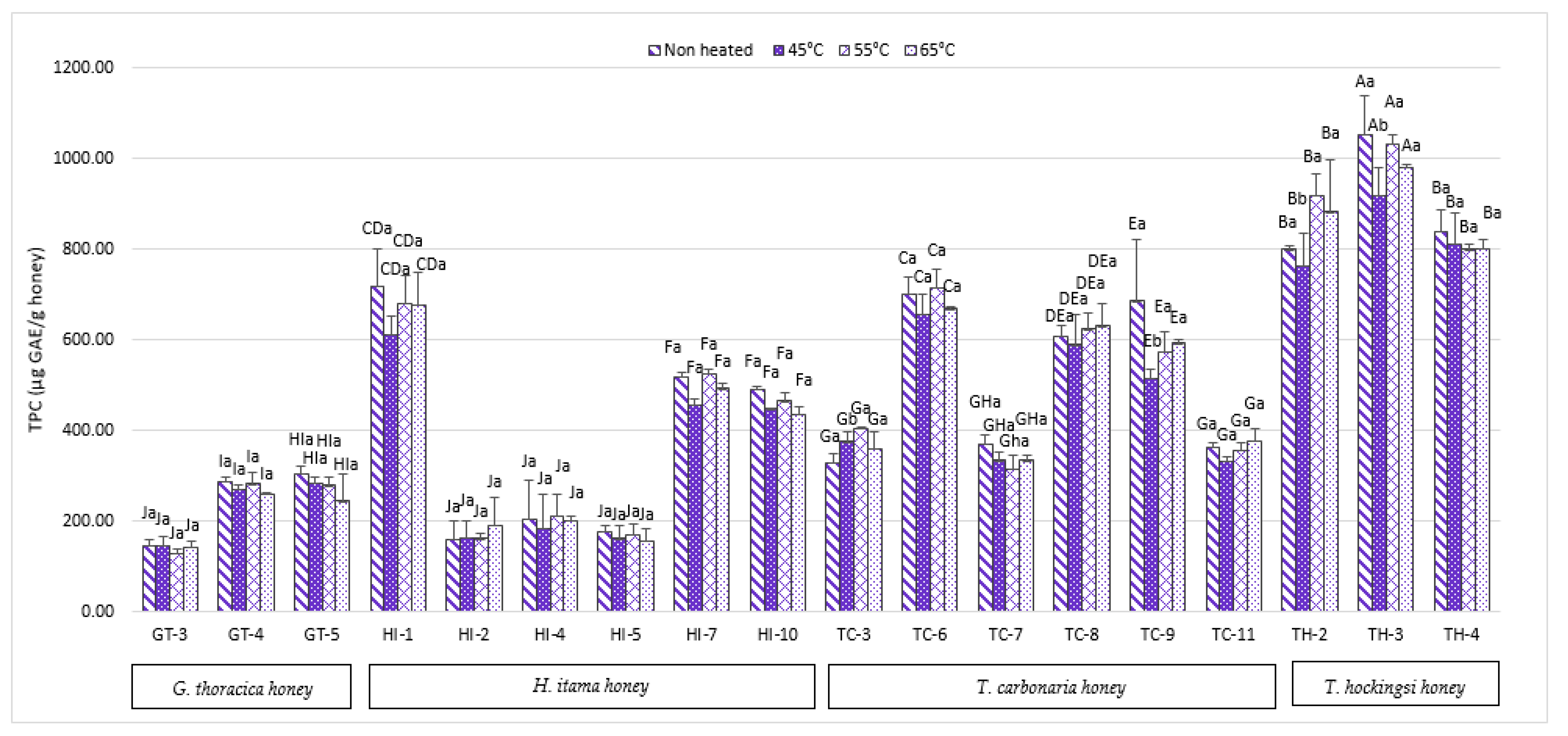
2.1. Total Phenolic Content
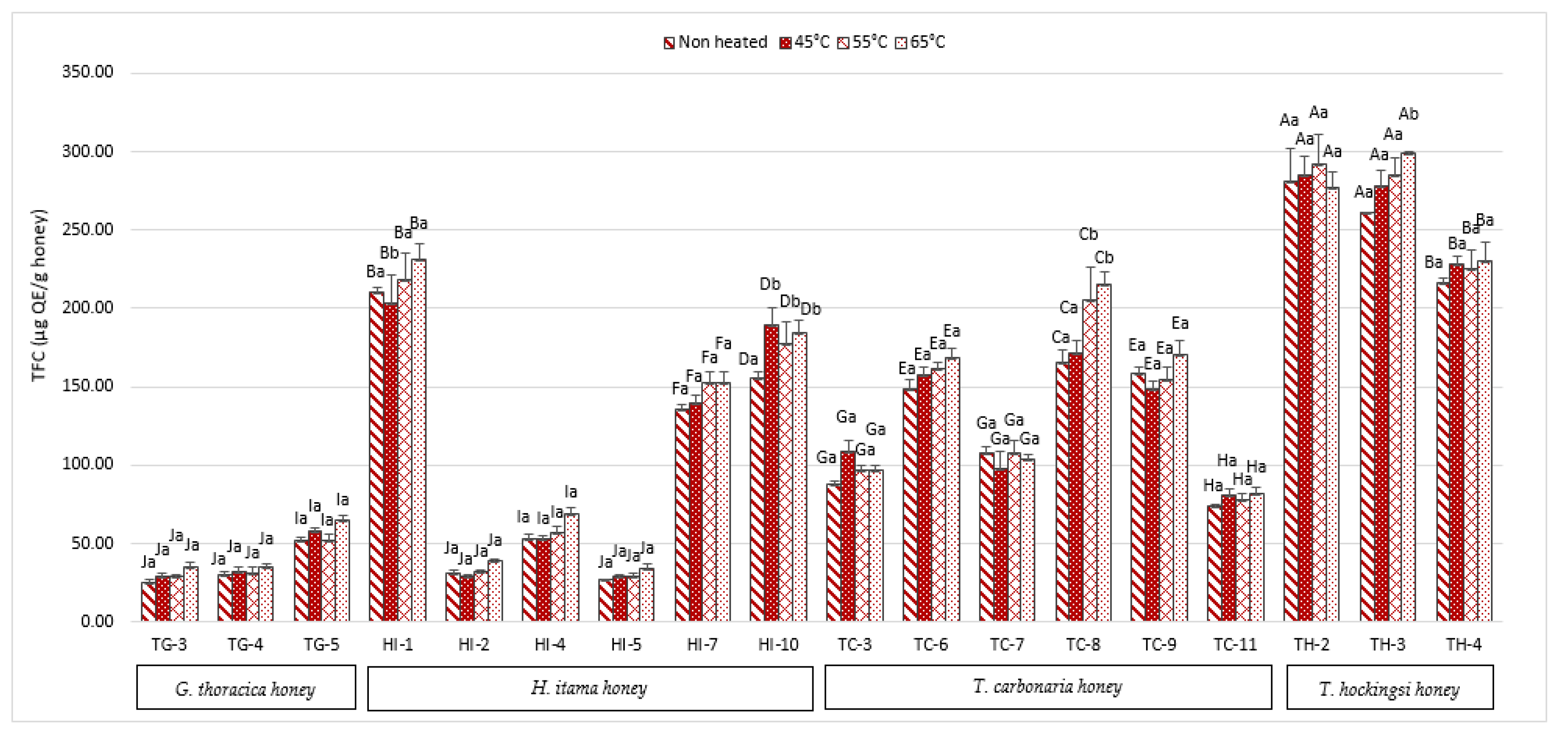
2.2. Total Flavonoid Content
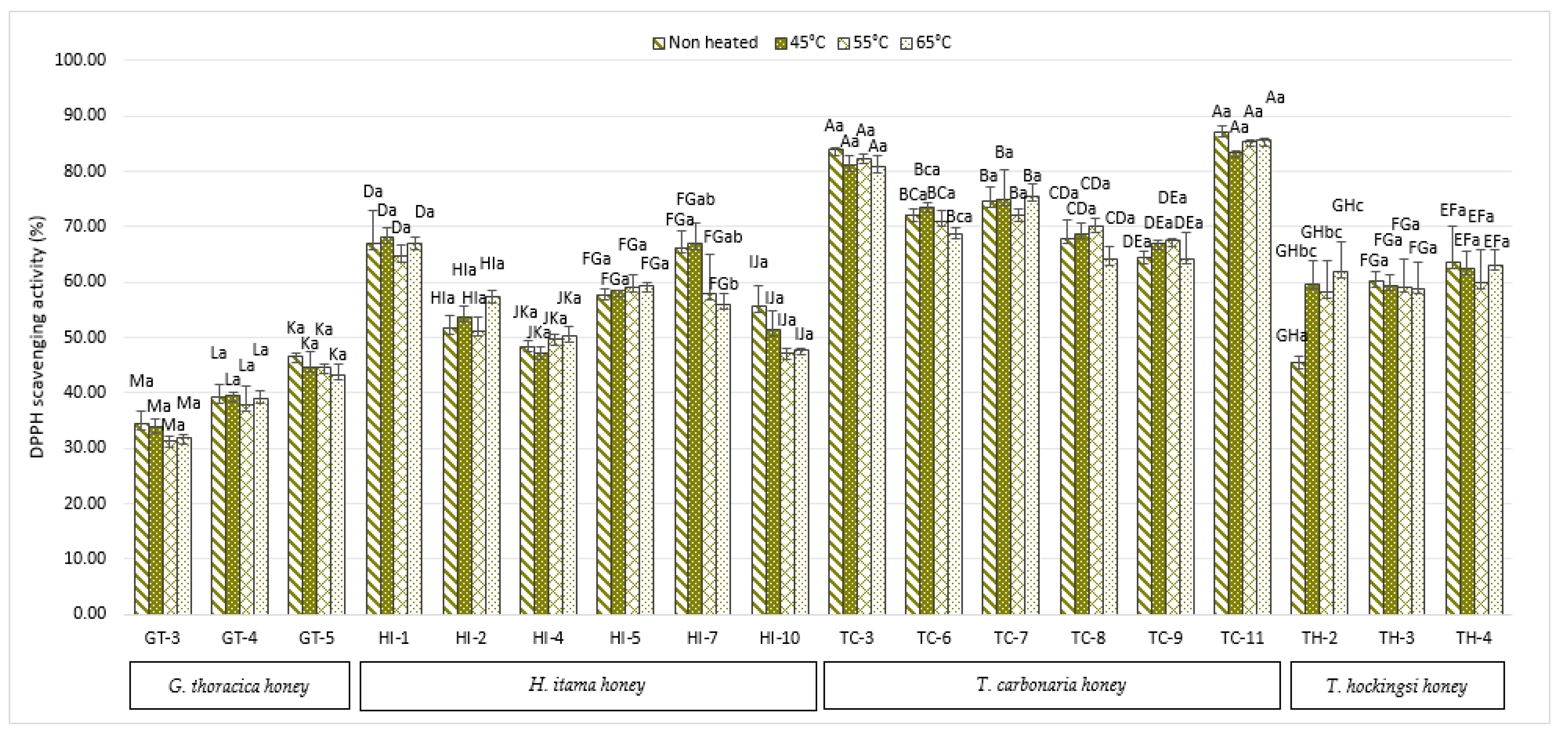
2.3. Inhibition of Free Radicals (DPPH) by Scavenging Activity
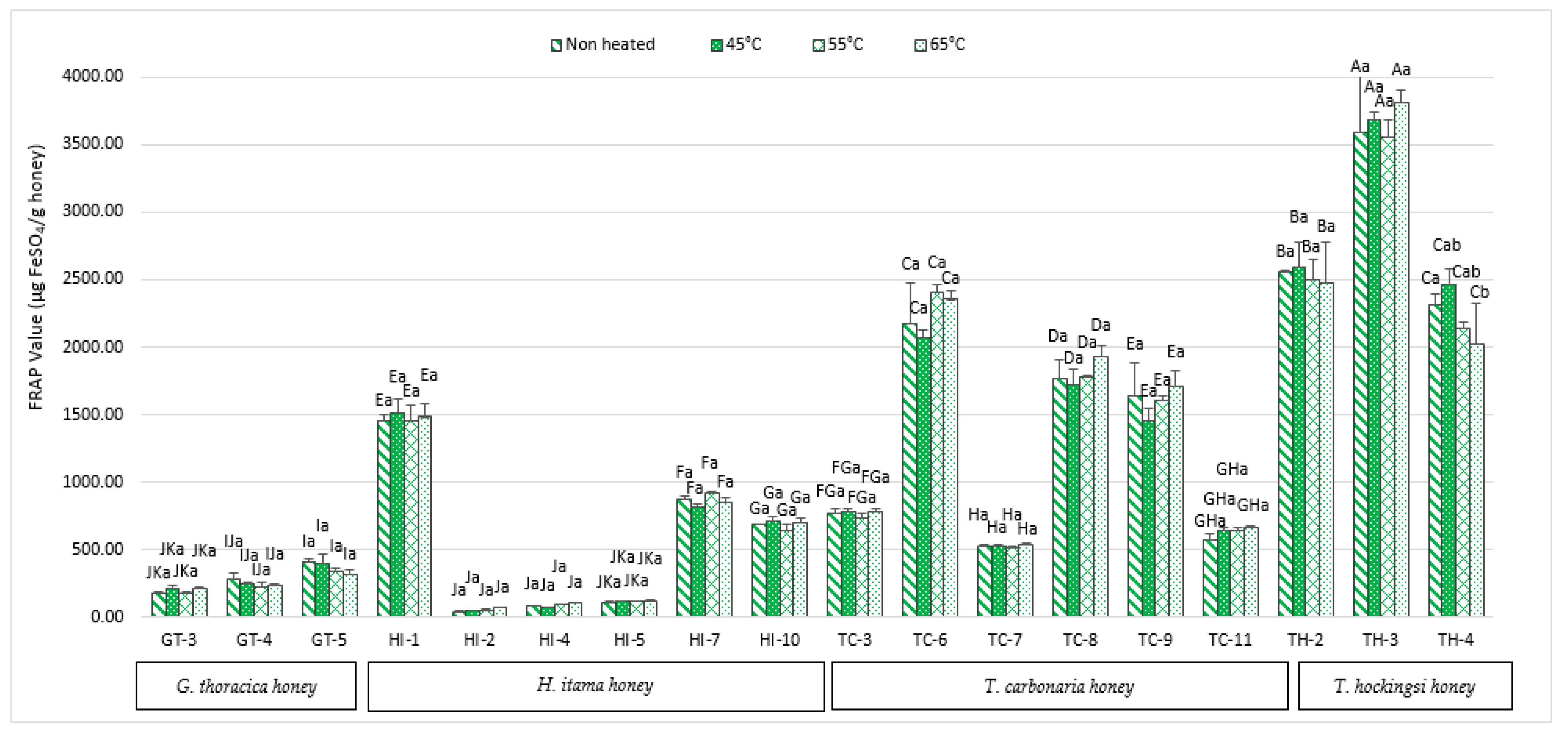
2.4. Ferric Reducing Antioxidant Power (FRAP)
2.5. Correlation between the Total Polyphenol and Total Flavonoid Content with the Antioxidant Activities
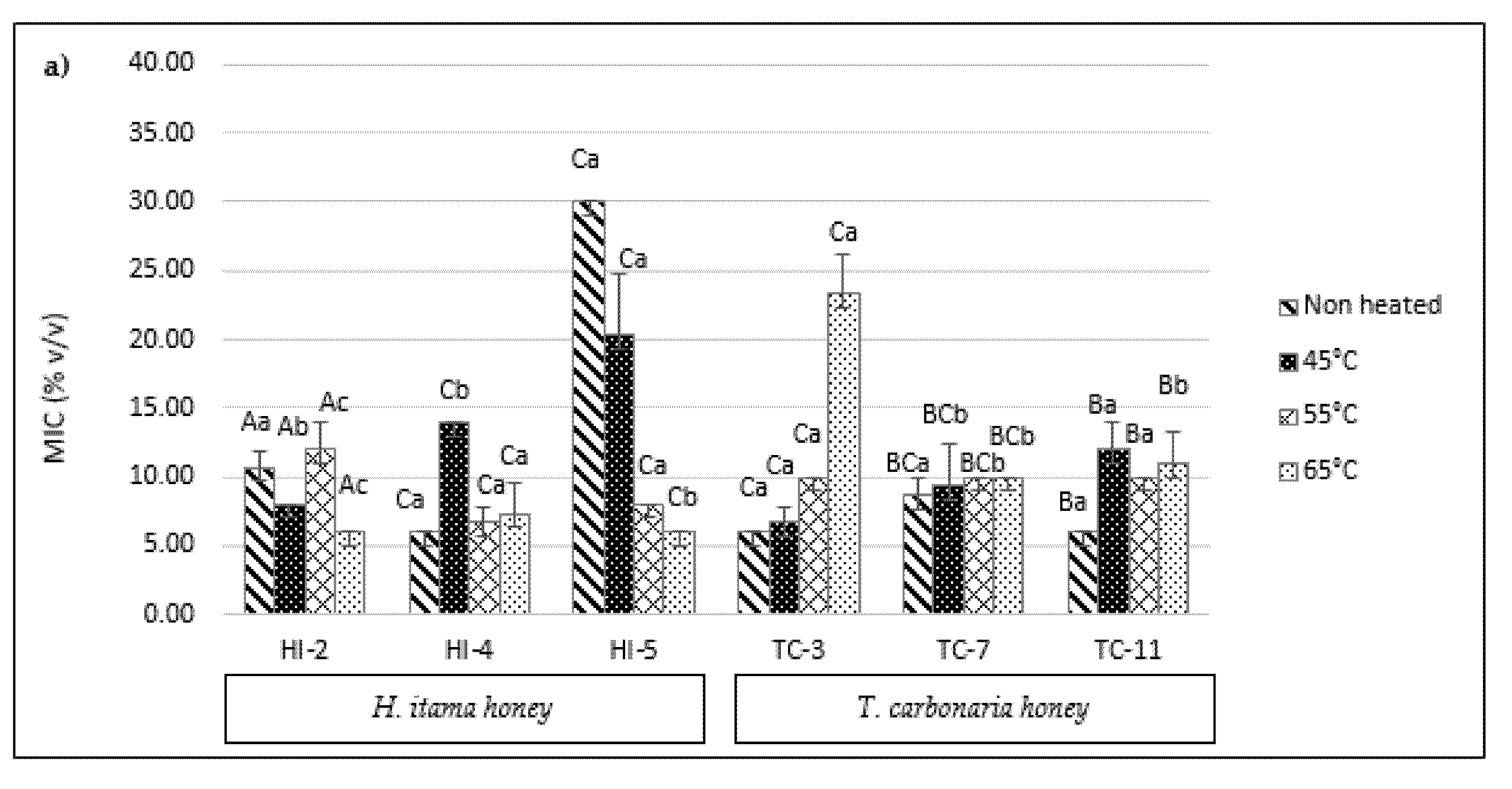
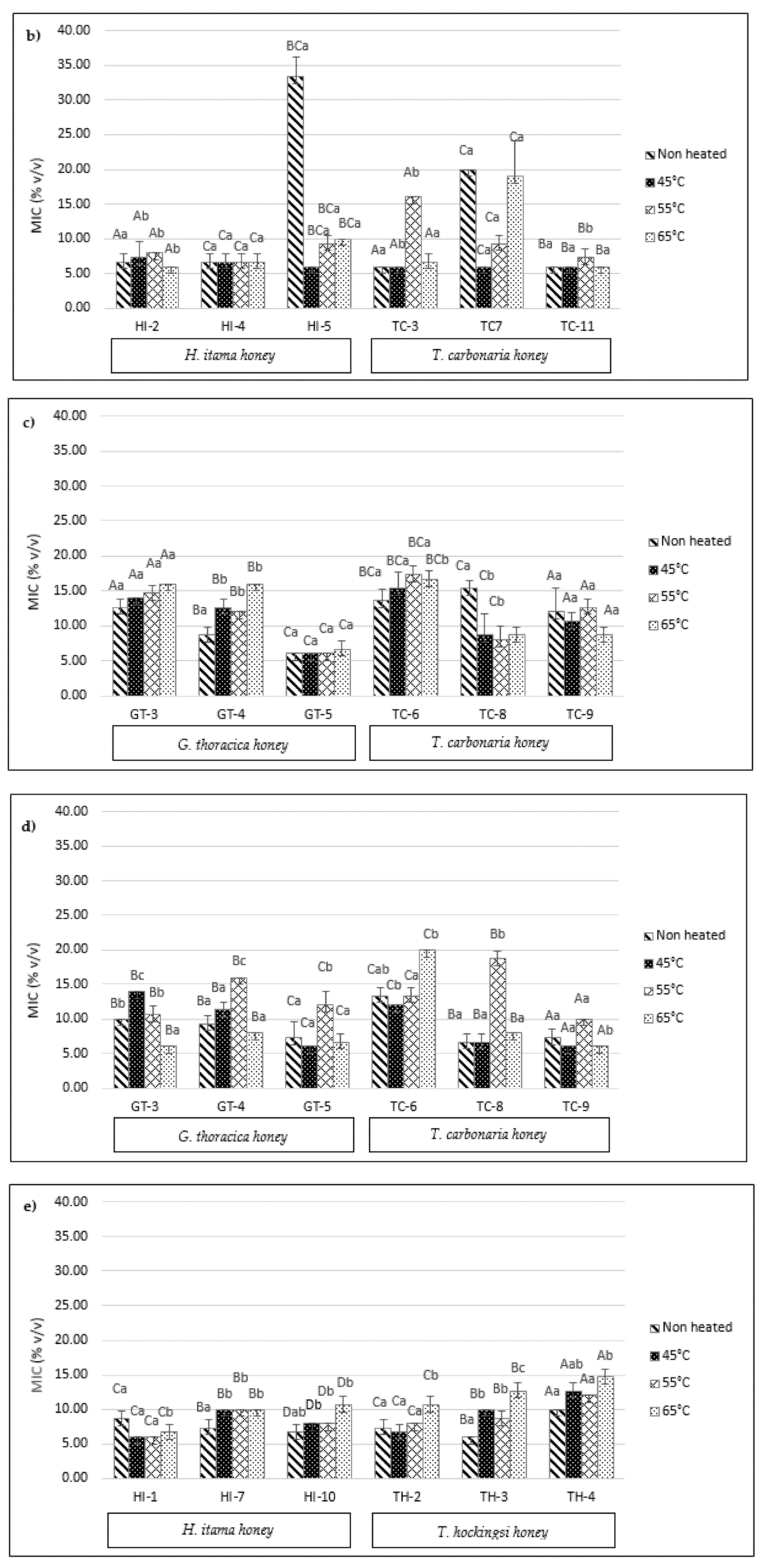
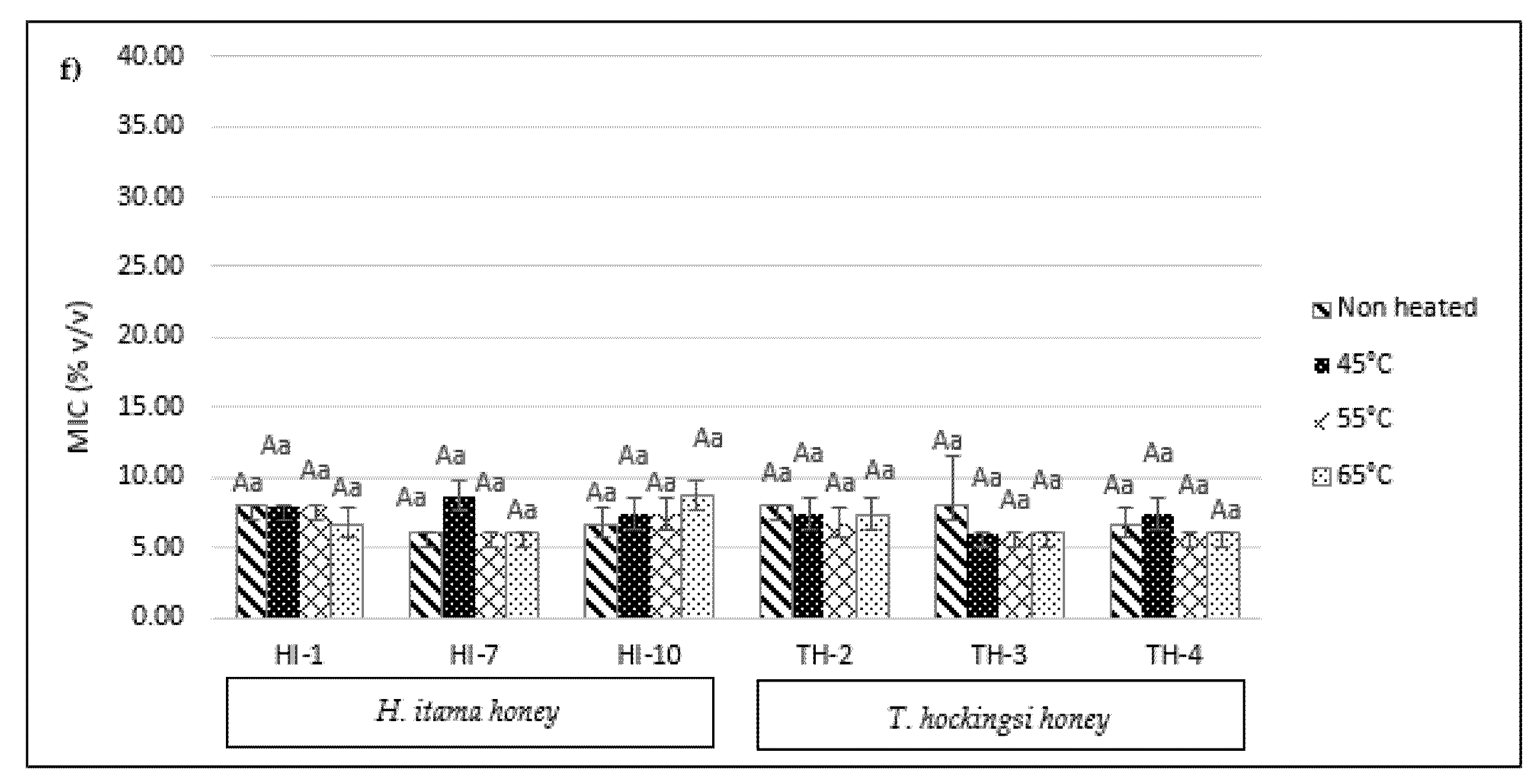
2.6. Minimum Inhibitory Concentration (MIC)
3. Materials and Methods
3.1. Honey Samples
3.2. Bacteria Samples
3.3. Heating Procedure
3.4. Determination of the Total Phenolic Content
3.5. Determination of the Total Flavonoid Content
3.6. Determination of the Radical-Scavenging Effect on DPPH
3.7. Ferric Reducing Antioxidant Power (FRAP) Assay
3.8. Minimum Inhibitory Concentration (MIC) Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afroz, R.; Tanvir, E.; Little, P. Molecular Pharmacology of Honey. Clin. Exp. Pharmacol. 2016, 6, 1000212. [Google Scholar]
- Hagr, T.E.; Mirghani, M.E.S.; Elnour, A.A.H.M.; Bkharsa, B.E. Antioxidant capacity and sugar content of honey from Blue Nile State, Sudan. Int. Food Res. J. 2017, 24, 452–456. [Google Scholar]
- Ávila, S.; Beux, M.R.; Ribani, R.H.; Zambiazi, R.C. Stingless bee honey: Quality parameters, bioactive compounds, health-promotion properties and modification detection strategies. Trends Food Sci. Technol. 2018, 81, 37–50. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; An, C.Y.; Rao, P.V.; Hawlader, M.N.I.; Azlan, S.A.B.M.; Sulaiman, S.A.; Gan, S.H. Identification of Phenolic Acids and Flavonoids in Monofloral Honey from Bangladesh by High Performance Liquid Chromatography: Determination of Antioxidant Capacity. BioMed Res. Int. 2014, 2014, 737490. [Google Scholar] [CrossRef]
- Tuksitha, L.; Chen, Y.-L.S.; Wong, K.-Y.; Peng, C.-C. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J. Asia-Pac. Èntomol. 2018, 21, 563–570. [Google Scholar] [CrossRef]
- Khalil, I.; Mahaneem, M.; Jamalullail, S.M.S.; Alam, N.; Sulaiman, S.A. Evaluation of radical scavenging activity and colour intensity of nine Malaysian honeys of different origin. J. ApiProd. ApiMed. Sci. 2011, 3, 4–11. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farm. 2016, 26, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Jalil, M.A.A.; Kasmuri, A.R.; Hadi, H. Stingless Bee Honey, the Natural Wound Healer: A Review. Ski. Pharmacol. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.; Cameron, S.A. Global stingless bee phylogeny supports ancient divergence, vicariance, and long-distance dispersal. Biol. J. Linn. Soc. 2010, 99, 206–232. [Google Scholar] [CrossRef]
- Kelly, N.; Farisya, M.S.N.; Kumara, T.K.; Marcela, P. Species diversity and external nest characteristics of stingless bees in meliponiculture. Pertanika J. Trop. Agric. Sci. 2014, 37, 293–298. [Google Scholar]
- Tosi, E.; Ciappini, M.; Ré, E.; Lucero, H. Honey thermal treatment effects on hydroxymethylfurfural content. Food Chem. 2002, 77, 71–74. [Google Scholar] [CrossRef]
- Escriche, I.; Visquert, M.; Carot, J.M.; Domenech, E.; Fito, P. Effect of Honey Thermal Conditions on Hydroxymethylfurfural Content Prior to Pasteurisation. Food Sci. Technol. Int. 2008, 14, 29–35. [Google Scholar] [CrossRef]
- Pimentel-González, D.J.; Basilio-Cortes, U.A.; Hernández-Fuentes, A.D.; Figueira, A.C.; Quintero-Lira, A.; Campos-Montiel, R.G. Effect of Thermal Processing on Antibacterial Activity of Multifloral Honeys. J. Food Process Eng. 2015, 40, e12279. [Google Scholar] [CrossRef] [Green Version]
- Jahan, N.; Alam, F.; Gan, S.H.; Centre, H.G. Prolonged heating of honey increases its antioxidant potential but decreases its antimicrobial activity. Afr. J. Tradit. Complement Altern. Med. 2015, 12, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Turkmen, N.; Sari, F.; Poyrazoglu, E.S.; Velioglu, Y.S. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2006, 95, 653–657. [Google Scholar] [CrossRef]
- Majid, M.; Ellulu, M.S.; Abu Bakar, M.F. Melissopalynological Study, Phenolic Compounds, and Antioxidant Properties of Heterotrigona itama Honey from Johor, Malaysia. Scientifica 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Da Silva, I.A.; da Silva, T.M.; Camara, C.A.; Queiroz, N.; Magnani, M.; de Novais, J.S.; Soledade, L.E.; de Oliveira Lima, E.; de Souza, A.L.; de Souza, A.G. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013, 141, 3552–3558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braghini, F.; Biluca, F.C.; Gonzaga, L.V.; Kracik, A.S.; Vieira, C.R.; Vitali, L.; Micke, G.A.; Costa, A.C.; Fett, R. Impact of short-term thermal treatment on stingless bee honey (Meliponinae): Quality, phenolic compounds and antioxidant capacity. J. Food Process. Preserv. 2019, 43, e13954. [Google Scholar] [CrossRef]
- Braghini, F.; Biluca, F.C.; Gonzaga, L.V.; Vitali, L.; Costa, A.C.O.; Fett, R. Effect thermal processing in the honey of Tetragonisca angustula: Profile physicochemical, individual phenolic compounds and antioxidant capacity. J. Apic. Res. 2020, 60, 290–296. [Google Scholar] [CrossRef]
- Abu Bakar, M.F.; Sanusi, S.B.; Abu Bakar, F.I.; Cong, O.J.; Mian, Z. Physicochemical and Antioxidant Potential of Raw Unprocessed Honey From Malaysian Stingless Bees. Pak. J. Nutr. 2017, 16, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J. Food Compos. Anal. 2016, 50, 61–69. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2017, 20, S2723–S2738. [Google Scholar] [CrossRef]
- Sousa, J.M.; de Souza, E.L.; Marques, G.; Meireles, B.; de Magalhães Cordeiro, Â.T.; Gullón, B.; Pintado, M.M.; Magnani, M. Polyphenolic profile and antioxidant and antibacterial activities of monofloral honeys produced by Meliponini in the Brazilian semiarid region. Food Res. Int. 2016, 84, 61–68. [Google Scholar] [CrossRef]
- De Oliveira, R.G.; Jain, S.; Luna, A.C.; Freitas, L.D.S.; De Araújo, E.D. Screening for quality indicators and phenolic compounds of biotechnological interest in honey samples from six species of stingless bees (Hymenoptera: Apidae). Food Sci. Technol. 2017, 37, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Shaimaa, G.; Mahmoud, M.; Mohamed, M.; Emam, A. Effect of Heat Treatment on Phenolic and Flavonoid Compounds and Antioxidant Activities of Some Egyptian Sweet and Chilli Pepper. Nat. Prod. Chem. Res. 2016, 4, 1–6. [Google Scholar]
- Amarowicz, R. Antioxidant activity of Maillard reaction products. Eur. J. Lipid Sci. Technol. 2009, 111, 109–111. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzie, I.F.F.; Choi, S.W. Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Šarić, G.; Marković, K.; Vukičević, D.; Lež, E.; Hruškar, M.; Vahčić, N. Changes of antioxidant activity in honey after heat treatment. Czech J. Food Sci. 2013, 31, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Shamsudin, S.; Selamat, J.; Sanny, M.; Shamsul Bahari, A.R.; Jambari, N.N.; Khatib, A. A comparative characterisation of physicochemical and antioxidants properties of processed Heterotrigona itama honey from different origins and classification by chemometrics analysis. Molecules 2020, 25, 3874. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaiman, N.H.I.; Sarbon, N.M. Physicochemical, antioxidant and antimicrobial properties of selected Malaysian honey as treated at different temperatures: A comparative study. J. Apic. Res. 2020, 60, 1–9. [Google Scholar] [CrossRef]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase—Its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Campbell, L.T.; Blair, S.E.; Carter, D.A. The effect of standard heat and filtration processing procedures on antimicrobial activity and hydrogen peroxide levels in honey. Front. Microbiol. 2012, 3, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucekova, M.; Juricova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Canini, A.; Majtan, J. Effect of thermal liquefying of crystallised honeys on their antibacterial activities. Food Chem. 2018, 269, 335–341. [Google Scholar] [CrossRef] [PubMed]
| Antioxidant Analyses | TPC | TFC | DPPH | FRAP | TPC | TFC | DPPH | FRAP | TPC | TFC | DPPH | FRAP | TPC | TFC | DPPH | FRAP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | RT | RT | RT | 45 °C | 45 °C | 45 °C | 45 °C | 55 °C | 55 °C | 55 °C | 55 °C | 65 °C | 65 °C | 65 °C | 65 °C | |
| TPC | ||||||||||||||||
| r | - | 0.937 | 0.233 | 0.952 | - | 0.942 | 0.359 | 0.962 | - | 0.953 | 0.313 | 0.962 | - | 0.956 | 0.321 | 0.941 |
| p | - | 0.000 * | 0.091 | 0.000 * | - | 0.000 * | 0.008 * | 0.000 * | - | 0.000 * | 0.021 * | 0.000 * | - | 0.000 * | 0.018 | 0.000 * |
| TFC | ||||||||||||||||
| r | - | - | 0.201 | 0.899 | - | - | 0.325 | 0.907 | - | - | 0.290 | 0.895 | - | - | 0.265 | 0.896 |
| p | - | - | 0.146 | 0.000 * | - | - | 0.017 * | 0.000 * | - | - | 0.034 | 0.000 * | - | - | 0.053 | 0.000 * |
| DPPH | ||||||||||||||||
| r | - | - | - | 0.193 | - | - | - | 0.310 | - | - | - | 0.315 | - | - | - | 0.281 |
| p | - | - | - | 0.161 | - | - | - | 0.023 * | - | - | - | 0.020 | - | - | - | 0.040 * |
| No | Sample Code | Bee Species | Location |
|---|---|---|---|
| 1 | GT-3 | Geniotrigona thoracica | Selangor, Malaysia |
| 2 | GT-4 | Geniotrigona thoracica | Selangor, Malaysia |
| 3 | GT-5 | Geniotrigona thoracica | Selangor, Malaysia |
| 4 | HI-1 | Heterotrigona itama | Sarawak, Malaysia |
| 5 | HI-2 | Heterotrigona itama | Selangor, Malaysia |
| 6 | HI-4 | Heterotrigona itama | Selangor, Malaysia |
| 7 | HI-5 | Heterotrigona itama | Johor, Malaysia |
| 8 | HI-7 | Heterotrigona itama | Selangor, Malaysia |
| 9 | HI-10 | Heterotrigona itama | Selangor, Malaysia |
| 10 | TC-3 | Tetragonula carbonaria | Brisbane, Queensland, Australia |
| 11 | TC-6 | Tetragonula carbonaria | Brisbane, Queensland, Australia |
| 12 | TC-7 | Tetragonula carbonaria | Brisbane, Queensland, Australia |
| 13 | TC-8 | Tetragonula carbonaria | Brisbane, Queensland, Australia |
| 14 | TC-9 | Tetragonula carbonaria | Brisbane, Queensland, Australia |
| 15 | TC-11 | Tetragonula carbonaria | Brisbane, Queensland, Australia |
| 16 | TH-2 | Tetragonula hockingsi | Bargara, Queensland, Australia |
| 17 | TH-3 | Tetragonula hockingsi | Bargara, Queensland, Australia |
| 18 | TH-4 | Tetragonula hockingsi | Bargara, Queensland, Australia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Ramlan, N.A.F.; Md Zin, A.S.; Safari, N.F.; Chan, K.W.; Zawawi, N. Application of Heating on the Antioxidant and Antibacterial Properties of Malaysian and Australian Stingless Bee Honey. Antibiotics 2021, 10, 1365. https://doi.org/10.3390/antibiotics10111365
Mat Ramlan NAF, Md Zin AS, Safari NF, Chan KW, Zawawi N. Application of Heating on the Antioxidant and Antibacterial Properties of Malaysian and Australian Stingless Bee Honey. Antibiotics. 2021; 10(11):1365. https://doi.org/10.3390/antibiotics10111365
Chicago/Turabian StyleMat Ramlan, Nurul Ainaa Farhanah, Aina Syahirah Md Zin, Nur Fatihah Safari, Kim Wei Chan, and Norhasnida Zawawi. 2021. "Application of Heating on the Antioxidant and Antibacterial Properties of Malaysian and Australian Stingless Bee Honey" Antibiotics 10, no. 11: 1365. https://doi.org/10.3390/antibiotics10111365
APA StyleMat Ramlan, N. A. F., Md Zin, A. S., Safari, N. F., Chan, K. W., & Zawawi, N. (2021). Application of Heating on the Antioxidant and Antibacterial Properties of Malaysian and Australian Stingless Bee Honey. Antibiotics, 10(11), 1365. https://doi.org/10.3390/antibiotics10111365







