Metal Oxide Nanoparticle Based Electrochemical Sensor for Total Antioxidant Capacity (TAC) Detection in Wine Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Electrochemical Measurements
2.3. Electrochemical Method
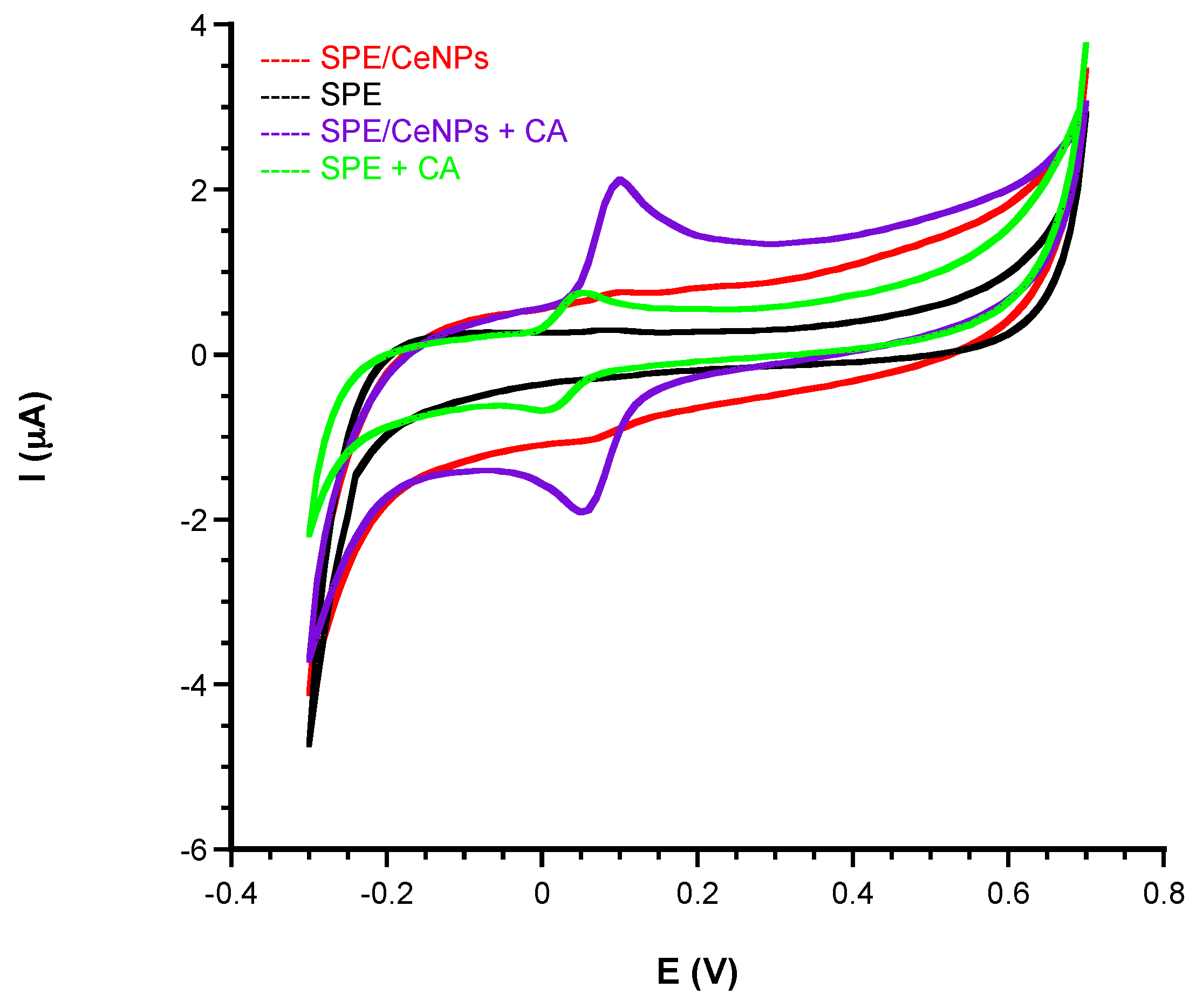
2.4. Sensor Modification by Using CeNPs
2.5. ABTS-Based Method
3. Results and Discussion
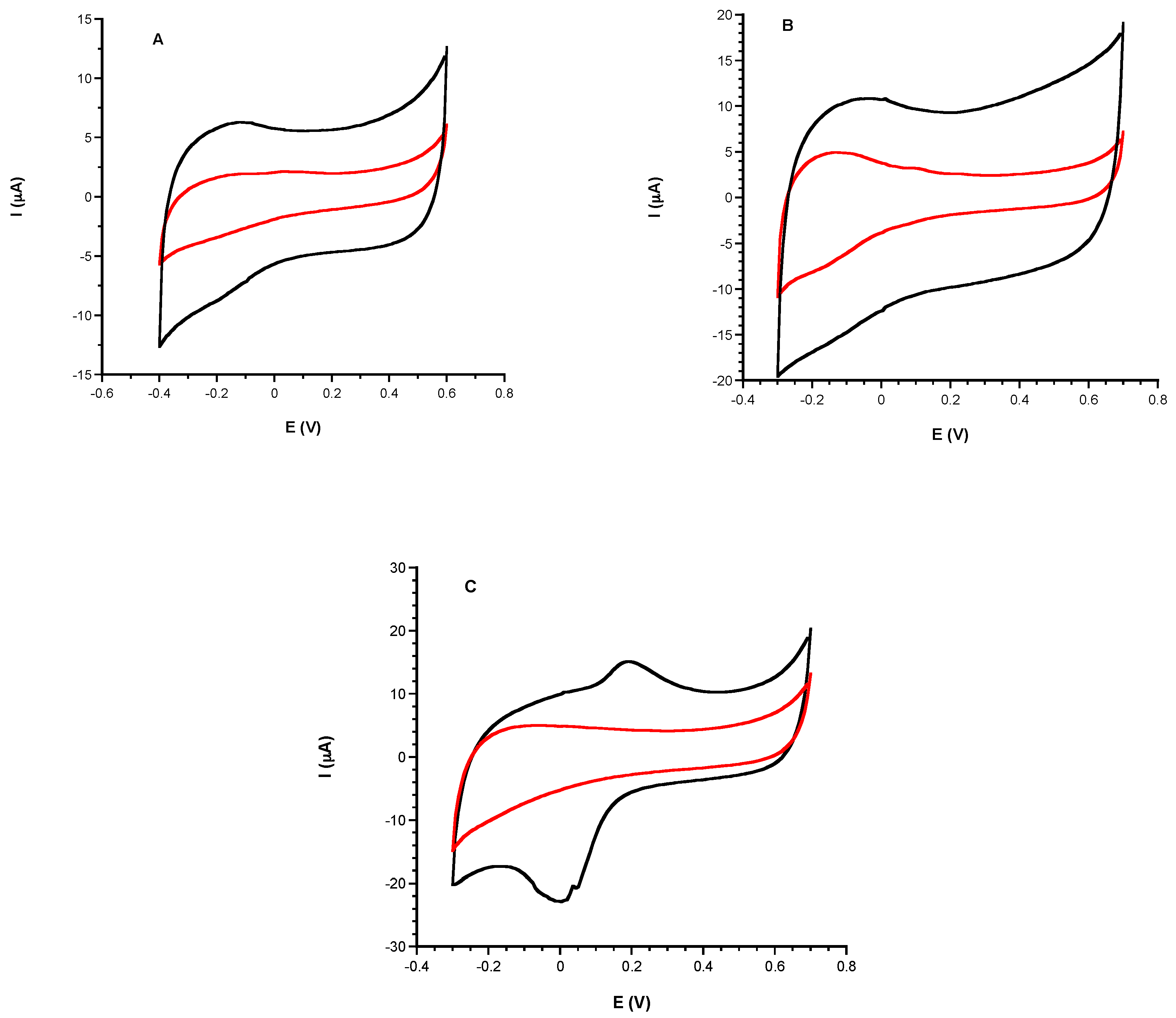
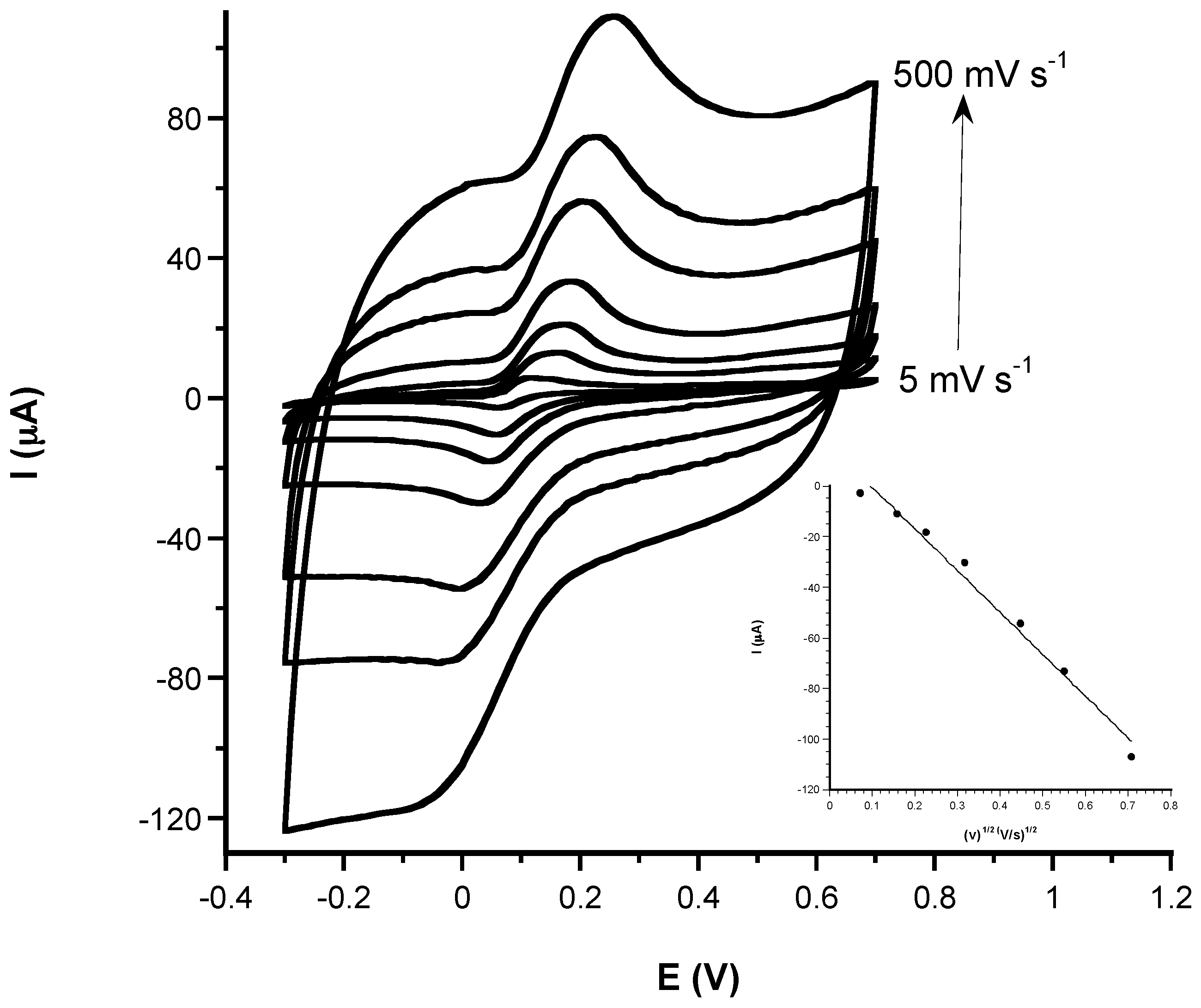
3.1. Electrochemical Characterization
3.1.1. Comparison of Electrochemical Performances before and after CeNPs Sensor Modification
- (1)
- Fe3O4 (s) + 2 e− + 6H+ (aq) → 2Fe2+ (aq) + 3H2O + FeO (s)
- (2)
- Fe2+ (aq) + HPO42− (aq) → FePO4(s) + e− + H+ (aq)
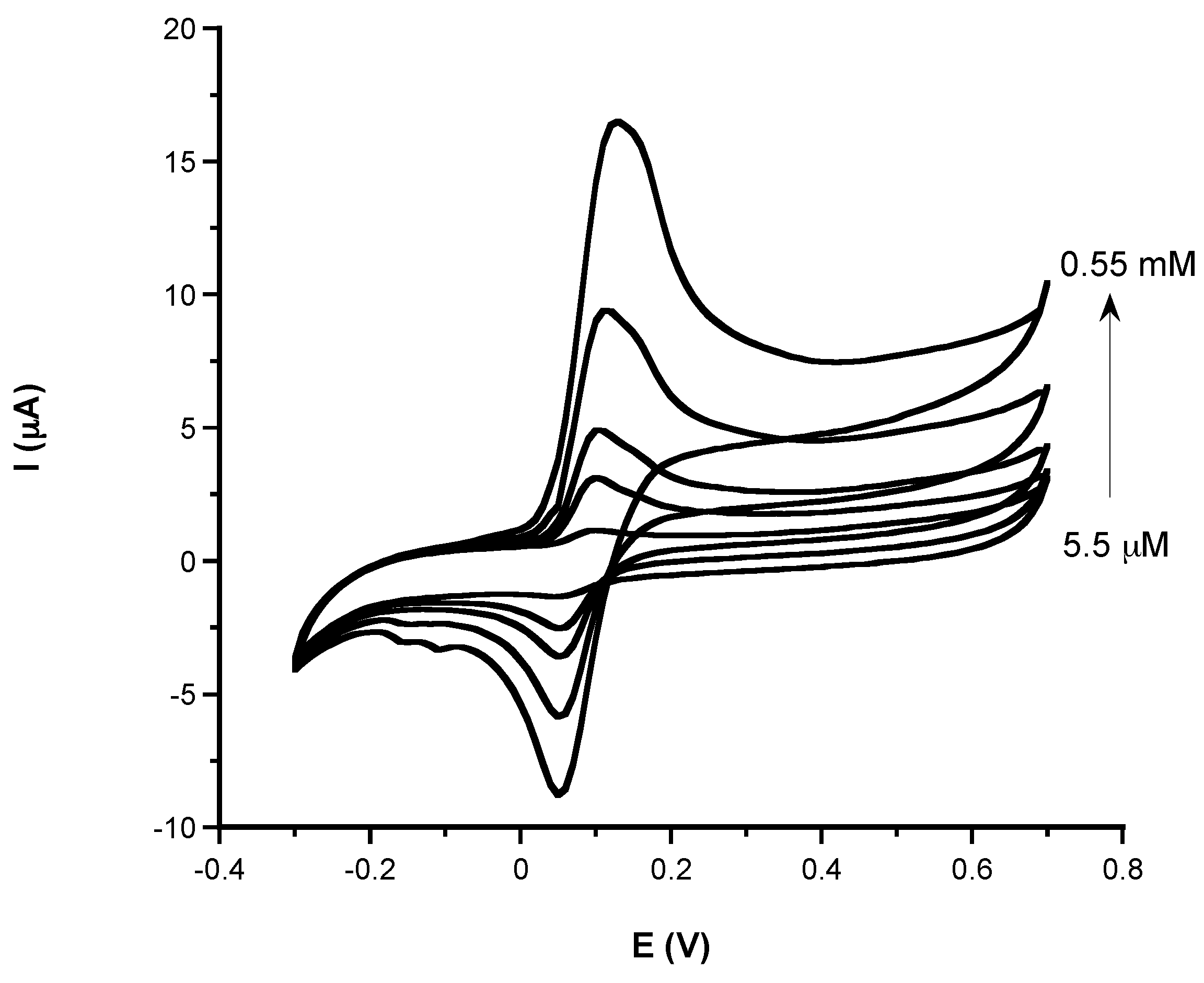
3.1.2. Analytical Characteristic: Sensitivity, LOD, Linear Range, and Response Time of Phenolic and Non-Phenolic Antioxidants
3.2. Spectrophotometric Characterization
3.3. Real Sample Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, B.; Santucci, S.; Benedetti, E.; Di Loreto, S.; Phani, R.A.; Falone, S.; Amicarelli, F.; Ceru, M.P.; Cimini, A. Cerium oxide nanoparticles trigger neuronal survival in a human Alzheimer disease model by modulating BDNF pathway. Curr. Nanosci. 2009, 5, 167–176. [Google Scholar] [CrossRef]
- Tian, Z.; Li, J.; Zhang, Z.; Gao, W.; Zhou, X.; Qu, Y. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials 2015, 59, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bohn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef]
- Lonnrot, K.; Metsa-Ketela, T.; Molnar, G.; Ahonen, J.-P.; Latvala, M.; Peltola, J.; Pietila, T.; Alho, H. The effect of ascorbate and ubiquinone supplementation on plasma and CSF total antioxidant capacity. Free Radic. Biol. Med. 1996, 21, 211–217. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [PubMed]
- Marques, S.S.; Magalhães, L.M.; Tóth, I.V.; Segundo, M.A. Insights on antioxidant assays for biological samples based on the reduction of copper complexes—The importance of analytical conditions. Int. J. Mol. Sci. 2014, 15, 11387–11402. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic. Res. 2010, 44, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Esteve, M.J.; Frigola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Fang, Y.; Zhang, A.; Chen, S.; Xu, T.; Ren, Z.; Han, G.; Liu, J.; Li, H.; Zhang, Z.; et al. Phenolic content and antioxidant capacity of Chinese raisins produced in Xinjiang Province. Food Res. Int. 2011, 44, 2830–2836. [Google Scholar] [CrossRef]
- Apak, R.; Guculu, K.G.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric iron reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Gil, D.M.A.; Rebelo, M.J.F. Evaluating the antioxidant capacity of wines: A laccase-based biosensor approach. Eur. Food Res. Technol. 2010, 231, 303–308. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, C.; Yu, Z. Electrochemical biosensor for assessment of the total antioxidant capacity of orange juice beverage based on the immobilizing DNA on a poly l-glutamic acid doped silver hybridized membrane. Sens. Actuators B Chem. 2014, 192, 628–633. [Google Scholar] [CrossRef]
- Barroso, M.F.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Delerue-Matos, C.; Oliveira, M.B.P.P.; Tuñón-Blanco, P. DNA-based biosensor for the electrocatalytic determination of antioxidant capacity in beverages. Biosens. Bioelectron. 2011, 26, 2396–2401. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.F.; Delerue-Matos, C.; Oliveira, M.B.P.P. Electrochemical evaluation of total antioxidant capacity of beverages using a purine-biosensor. Food Chem. 2012, 132, 1055–1062. [Google Scholar] [CrossRef]
- Blasco, A.J.; Crevillén, A.G.; González, M.C.; Escarpa, A. Direct electrochemical sensing and detection of natural antioxidants and antioxidant capacity in vitro systems. Electroanalysis 2007, 19, 2275–2286. [Google Scholar] [CrossRef]
- Wang, Y.; Calas-Blanchard, C.; Cortina-Puig, M.; Baohong, L.; Marty, J.L. An electrochemical method for sensitive determination of antioxidant capacity. Electroanalysis 2009, 21, 1395–1400. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem.Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, F.; Favero, G.; Bollella, P.; Tortolini, C.; Mannina, L.; Conti, M.E.; Antiochia, R. Recent trends in electrochemical nanobiosensors for environmental analysis. Int. J. Environ. Health 2015, 7, 267–291. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Taurino, I.; Sanzò, G.; Antiochia, R.; Tortolini, C.; Mazzei, F.; Favero, G.; De Micheli, G.; Carrara, S. Recent advances in third generation biosensors based on Au and Pt nanostructured electrodes. Trends in Anal. Chem. 2016, 79, 151–159. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.; Gao, F.; Ni, J.; Zhang, Y.; Lin, Z. Graphene oxide directed one-step synthesis of flowerlike graphene@HKUST-1 for enzyme-free detection of hydrogen peroxide in biological samples. ACS Appl. Mater. Interfaces 2016, 8, 32477–32487. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Wang, Q.; Gao, F.; Gao, F.; Yang, Y.; Guo, H. Highly dispersible and stable copper terephthalate metal–organic framework–graphene oxide nanocomposite for an electrochemical sensing application. ACS Appl. Mater. Interfaces 2014, 6, 11573–11580. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Q.; Qiu, W.; Guo, H.; Gao, F. Covalent immobilization of Cu3(btc)2 at chitosan–electroreduced graphene oxide hybrid film and its application for simultaneous detection of dihydroxybenzene isomers. J. Phys. Chem. C 2016, 120, 9794–9803. [Google Scholar] [CrossRef]
- Gao, F.; Cai, X.; Wang, X.; Gao, C.; Liu, S.; Gao, F.; Wang, Q. Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode. Sens. Actuators B Chem. 2013, 186, 380–387. [Google Scholar] [CrossRef]
- Pérez-Lópeza, B.; Merkoçi, A. Nanomaterials based biosensors for food analysis applications. Trends Food Sci. Technol. 2011, 22, 625–639. [Google Scholar] [CrossRef]
- Della Pelle, F.; Compagnone, D. Nanomaterial-based sensing and biosensing of phenolic compounds and related antioxidant capacity in food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, M.; Litescu, S.C.; Radu, G.L. Laccase–MWCNT–chitosan biosensor—A new tool for total polyphenolic content evaluation from in vitro cultivated plants. Sens. Actuators B Chem. 2010, 145, 800–806. [Google Scholar] [CrossRef]
- Diaconu, M.; Litescu, S.C.; Radu, G.L. Bienzymatic sensor based on the use of redox enzymes and chitosan–MWCNT nanocomposite. Evaluation of total phenolic content in plant extracts. Microchim. Acta 2010, 172, 177–184. [Google Scholar] [CrossRef]
- Chawla, S.; Rawal, R.; Sharma, S.; Pundir, C.S. An amperometric biosensor based on laccase immobilized onto nickel nanoparticles/carboxylated multiwalled carbon nanotubes/polyaniline modified gold electrode for determination of phenolic content in fruit juices. Biochem. Eng. J. 2012, 68, 76–84. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Li, M.; Pan, Z.; Li, D. A disposable biosensor based on immobilization of laccase with silica spheres on the MWCNTs-doped screen-printed electrode. Chem. Cent. J. 2012, 6, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.; Chawla, S.; Devender Pundir, C.S. An amperometric biosensor based on laccase immobilized onto Fe3O4NPs/cMWCNT/PANI/Au electrode for determination of phenolic content in tea leaves extract. Enzyme Microb. Technol. 2012, 51, 179–185. [Google Scholar] [CrossRef]
- Lanzellotto, C.; Favero, G.; Antonelli, M.L.; Tortolini, C.; Cannistraro, S.; Coppari, E.; Mazzei, F. Nanostructured enzymatic biosensor based on fullerene and gold nanoparticles: Preparation, characterization and analytical applications. Biosens. Bioelectron. 2014, 55, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Zappi, D.; Nasci, G.; Sadun, C.; Tortolini, C.; Antonelli, M.L.; Bollella, P. Evaluation of new cholinium-amino acids based room temperature ionic liquids (RTILs) as immobilization matrix for electrochemical biosensor development: Proof-of-concept with Trametes Versicolor laccase. Microchem. J. 2018, 141, 346–352. [Google Scholar] [CrossRef]
- Nasir, M.; Nawaz, M.H.; Latif, U.; Yaqub, M.; Hayat, A.; Rahim, A. An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim. Acta 2017, 184, 323–342. [Google Scholar] [CrossRef]
- Andrei, V.; Sharpe, E.; Vasilescu, A.; Andreescu, S. A single use electrochemical sensor based on biomimetic nanoceria for the detection of wine antioxidants. Talanta 2016, 156–157, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Solanki, P.R.; Malhotra, B.D. Hydrogen peroxide sensor based on horseradish peroxidase immobilized nanostructured cerium oxide film. J. Biotechnol. 2009, 142, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.L.; Luan, Q.F.; Yao, X.; Zhou, K.B. Single-crystal CeO2 nanocubes used for the direct electron transfer and electrocatalysis of horseradish peroxidase. Biosens. Bioelectron. 2010, 24, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Özel, R.E.; Ispas, C.; Ganesana, M.; Leiter, J.C.; Andreescu, S. Glutamate oxidase biosensor based on mixed ceria and titania nanoparticles for the detection of glutamate in hypoxic environments. Biosens. Bioelectron. 2014, 52, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ouyang, Y.; Wu, F.; Hu, Y.; Zhang, H.; Wu, Z. In situ & controlled preparation of platinum nanoparticles dopping into graphene sheets @cerium oxide nanocomposites sensitized screen printed electrode for nonenzymatic electrochemical sensing of hydrogen peroxide. J. Electroanal. Chem. 2016, 777, 85–91. [Google Scholar] [CrossRef]
- Ispas, C.; Njagi, J.; Cates, M.; Andreescu, S. Electrochemical Studies of Ceria as Electrode Material for Sensing and Biosensing Applications. J. Electrochem. Soc. 2008, 155, F169–F176. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.L.; Wang, X.D.; Li, W.C. Complex impedance study on nano-CeO2 coating TiO2. Mater. Design 2006, 27, 489–493. [Google Scholar] [CrossRef]
- Preda, G.; Migani, A.; Neyman, K.M.; Bromley, S.T.; Illas, F.; Pacchioni, G. Formation of superoxide anions on ceria nanoparticles by interaction of molecular oxygen with Ce3+ sites. J. Phys. Chem. C 2011, 115, 5817–5822. [Google Scholar] [CrossRef]
- Dutta, P.; Pal, S.; Seehra, M.S.; Shi, Y.; Eyring, E.M.; Ernst, R.D. Concentration of Ce3+ and oxygen vacancies in cerium oxide nanoparticles. Chem. Mater. 2006, 18, 5144–5146. [Google Scholar] [CrossRef]
- Wang, D.; Kang, Y.; Doan-Nguyen, V.; Chen, J.; Küngas, R.; Wieder, N.L.; Bakhmutsky, K.; Gorte, R.J.; Murray, C.B. Synthesis and oxygen storage capacity of two-dimensional ceria nanocrystals. Angew. Chem. Int. Ed. Engl. 2011, 50, 4378–4381. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Tsang, S.C. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef] [PubMed]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.G.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Kaittanis, C.; Santra, S.; Perez, J.M. The pH-tunable oxidase-like activity of cerium oxide nanoparticles achieves sensitive fluorigenic detection of cancer biomarkers at neutral pH. Anal. Chem. 2011, 83, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, L.; Ge, A.; Guo, Y. Enhanced chemiluminescence detection of thrombin based on cerium oxide nanoparticles. Chem. Commun. 2011, 47, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Kaittanis, C.; Santra, S.; Asati, A.; Perez, J.M. A cerium oxide nanoparticle based device for the detection of chronic inflammation via optical and magnetic resonance imaging. Nanoscale 2012, 4, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Ornatska, M.; Sharpe, E.; Andreescu, D.; Andreescu, S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal. Chem. 2011, 83, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, C.; Ren, J.; Qu, X. Using thermally regenerable cerium oxide nanoparticles in biocomputing to perform label-free, resettable, and colorimetric logic operations. Angew. Chem. Int. Ed. Engl. 2012, 51, 12579–12583. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, P.; Xu, C.; Ren, J.; Qu, X. Cerium oxide caged metal chelator: Anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer’s disease treatment. Chem. Sci. 2013, 4, 2536–2542. [Google Scholar] [CrossRef]
- Xu, C.; Lin, Y.; Wang, J.; Wu, L.; Wei, W.; Ren, J.; Qu, X. Nanoceria-triggered synergetic drug release based on CeO(2)-capped mesoporous silica host–guest interactions and switchable enzymatic activity and cellular effects of CeO(2). Adv. Healthc. Mater. 2013, 2, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Cerium oxide based nanozymes: Redox phenomenon at biointerfaces. Biointerphases 2016, 11, 04B202. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Cunningham, J.; Bulbul, G.; Andreescu, S. Evaluation of the oxidase like activity of nanoceria and its application in colorimetric assays. Anal. Chim. Acta 2015, 885, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,20-azinobis (3-ethylenebenzothiazoline-6-sulfonic acid) radical cation decolourisation assay. Methods Enzymol. 1999, 299, 379–389. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Njagi, J.; Chernov, M.M.; Leiter, J.C.; Andreescu, S. Amperometric detection of dopamine in vivo with an enzyme based carbon fiber microbiosensor. Anal. Chem. 2010, 82, 989–996. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.J.; Marken, F. Direct electrochemistry of nanoparticulate Fe2O3 in aqueous solution and adsorbed onto tin-doped indium oxide. Pure Appl. Chem. 2001, 73, 1885–1894. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.A.S.S.; Nogueira, J.M.F.; Rebelo, M.J.F. A New Laccase biosensor for polyphenols determination. Sensors 2003, 3, 166–175. [Google Scholar] [CrossRef]
- Di Fusco, M.; Tortolini, C.; Deriu, D.; Mazzei, F. Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 2010, 81, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Odaci, D.; Timur, S.; Pazarlioglu, N.; Montereali, M.R.; Vastarella, W.; Pilloton, R.; Telefoncu, A. Determination of phenolic acids using Trametes versicolor laccase. Talanta 2007, 71, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Montereali, M.R.; Vastarella, W.; Della Seta, L.; Pilloton, R. Tyrosinase biosensor based on modified screen printed electrodes: Measurements of total phenol content. Int. J. Environ. Anal. Chem. 2005, 85, 795–806. [Google Scholar] [CrossRef]
- Granero, A.M.; Fernández, H.; Agostini, E.; Zón, M.A. An amperometric biosensor based on peroxidases from Brassica napus for the determination of the total polyphenolic content in wine and tea samples. Talanta 2012, 83, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Amatatongchai, M.; Laosing, S.; Chailapakul, O.; Nacapricha, D. Simple flow injection for screening of total antioxidant capacity by amperometric detection of DPHH radical on carbon nanotube modified-glassy carbon electrode. Talanta 2012, 97, 267–272. [Google Scholar] [CrossRef] [PubMed]

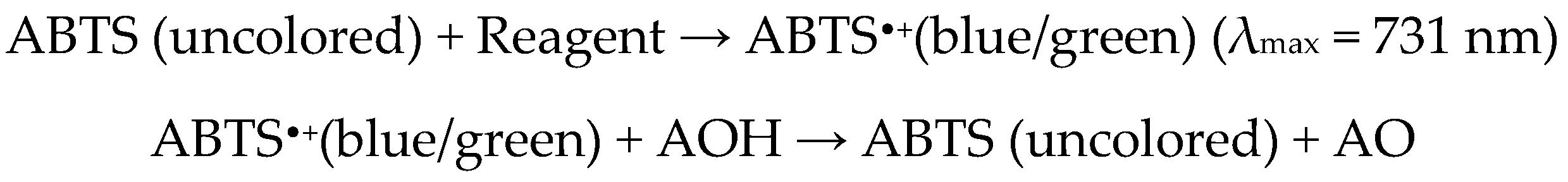
| Sensor | Ae a (mm2) | Ae b (mm2) | ρ a | ρ b |
|---|---|---|---|---|
| OHT-000 | 2.42 ± 0.02 | 16.82 ± 0.04 | 0.19 | 1.34 |
| OHT-102 | 6.26 ± 0.02 | 18.74 ± 0.02 | 0.50 | 1.49 |
| OHT-069 | 8.50 ± 0.03 | 21.93 ± 0.01 | 0.68 | 1.74 |
| Antioxidant Compound | Linear Range (mM) | Slope (µA mM−1) | R | LOD (mM) | |
|---|---|---|---|---|---|
| GA | LLR | 0.025–0.05 | 6.55 | 0.990 | 0.007 |
| HLR | 0.5–5.0 | 0.57 | 0.987 | 0.151 | |
| CA | LLR | 0.033–0.1 | 0.793 | 0.999 | 0.010 |
| HLR | 0.25–5.0 | 0.180 | 0.998 | 0.083 | |
| Q | LLR | 0.025–0.1 | 3.978 | 0.978 | 0.009 |
| HLR | 1.0–5.0 | 0.120 | 0.994 | 0.303 | |
| t-R | LLR | 0.025–0.05 | 4.791 | 0.982 | 0.008 |
| HLR | 0.1–1.0 | 0.262 | 0.999 | 0.033 | |
| AA | LLR | 0.025–1 | 1.667 | 0.999 | 0.007 |
| HLR | 1.0–5.0 | 0.180 | 0.996 | 0.334 | |
| Sensor | Antioxidant Compound | LR (µM) | LOD (µM) | Storage/Response Time | Ref. |
|---|---|---|---|---|---|
| Lac/pESm/Pt electrode | CA | 10–80 | - | - | [87] |
| Lac/PAP/SWCNTs/SPE | GA | 0.53–96 | - | 10 d at 4 °C/- | [88] |
| Lac/oxygen electrode | CA | 0.10–1.00 | 0.06 | - | [89] |
| Lac/Fc/SPE | 2.0–30.0 | 1.6 | - | ||
| Tyr/BSA/GA/Fc/SPE | GA | 30.0–300 | 57 | 30 d at 4 °C/- | [90] |
| CA | 10.6–266 | 10.5 | |||
| ITO/Lac/Tyr/CS/MWCNTs electrode | GA | 1.6–8.1 | 1.5 | 8 d at 4 °C dry atmosphere/- | [50] |
| CA | 0.4–7.4 | 0.3 | |||
| POx/Fc/MWCNTs/ MO electrode | CA | 0.3–383 | 0.1 | - | [91] |
| t-R | 0.2–228 | 0.1 | |||
| GC/MWCNTs/PEI/electrode | GA | 0.6–12 | 0.04 | ||
| CA | 0.6–12 | 0.08 | - | [92] | |
| Q | 0.3–6 | 0.03 | |||
| CeNPs/C/SPE | GA | 2–20 | 1.5 | RT/40 s | [57] |
| CA | 50–100 | 15.3 | |||
| Q | 20–200 | 8.6 | |||
| AA | 0.5–20 | 0.4 | |||
| CeNPs/MWCNTs-COOH/SPE | GA | 25–50 | 7 | Our work | |
| CA | 33–100 | 10 | |||
| Q | 25–100 | 8 | RT/30 s | ||
| t-R | 25–50 | 8 | |||
| AA | 25–100 | 7 |
| Wine Sample | CeNPs-SPE a | ABTS-Based Method a |
|---|---|---|
| white 1 | 204 ± 10 | 208 ± 17 |
| white 2 | 183 ± 11 | 187 ± 9 |
| white 3 | 212 ± 18 | 209 ± 15 |
| red 1 | 2067 ± 207 | 2078 ± 187 |
| red 2 | 2340 ± 210 | 2538 ± 228 |
| red 3 | 3938 ± 236 | 4172 ± 292 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortolini, C.; Bollella, P.; Zumpano, R.; Favero, G.; Mazzei, F.; Antiochia, R. Metal Oxide Nanoparticle Based Electrochemical Sensor for Total Antioxidant Capacity (TAC) Detection in Wine Samples. Biosensors 2018, 8, 108. https://doi.org/10.3390/bios8040108
Tortolini C, Bollella P, Zumpano R, Favero G, Mazzei F, Antiochia R. Metal Oxide Nanoparticle Based Electrochemical Sensor for Total Antioxidant Capacity (TAC) Detection in Wine Samples. Biosensors. 2018; 8(4):108. https://doi.org/10.3390/bios8040108
Chicago/Turabian StyleTortolini, Cristina, Paolo Bollella, Rosaceleste Zumpano, Gabriele Favero, Franco Mazzei, and Riccarda Antiochia. 2018. "Metal Oxide Nanoparticle Based Electrochemical Sensor for Total Antioxidant Capacity (TAC) Detection in Wine Samples" Biosensors 8, no. 4: 108. https://doi.org/10.3390/bios8040108
APA StyleTortolini, C., Bollella, P., Zumpano, R., Favero, G., Mazzei, F., & Antiochia, R. (2018). Metal Oxide Nanoparticle Based Electrochemical Sensor for Total Antioxidant Capacity (TAC) Detection in Wine Samples. Biosensors, 8(4), 108. https://doi.org/10.3390/bios8040108










