Ultrathin Functional Polymer Modified Graphene for Enhanced Enzymatic Electrochemical Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Electropolymerization
2.3. Surface Functionalization
2.4. Enzyme Immobilization
2.5. Characterization Techniques
3. Results and Discussion
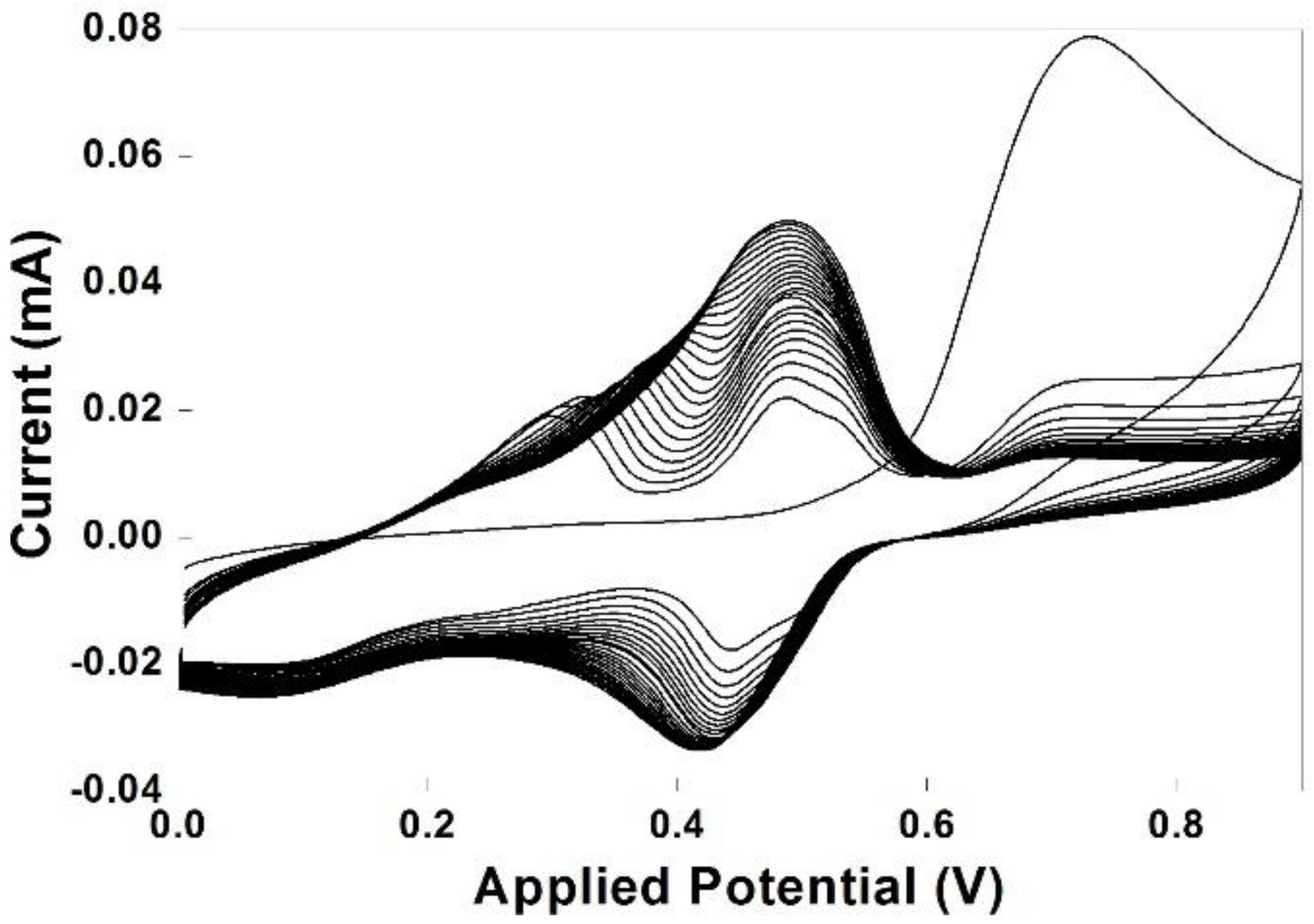
3.1. Electropolymerization
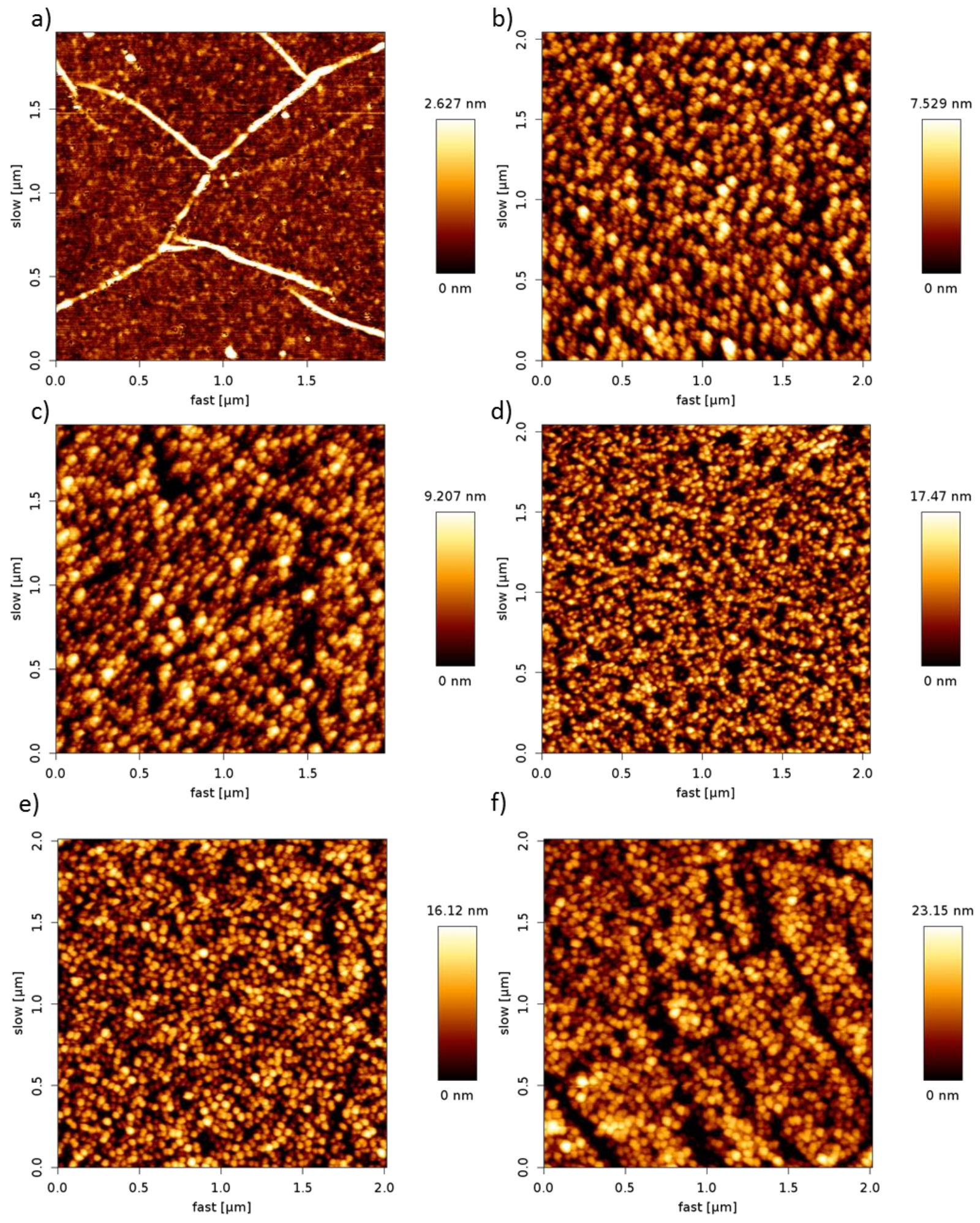
3.2. Surface Analysis
3.3. Chemical Environment Analysis
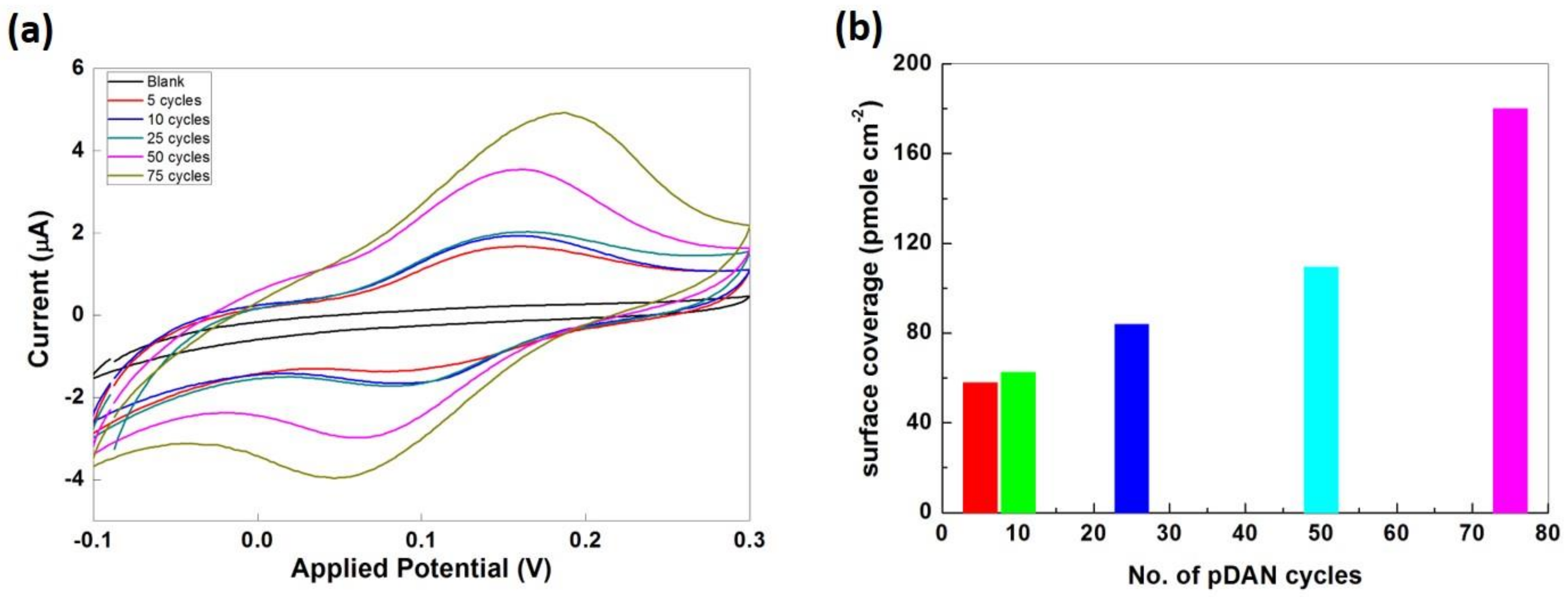
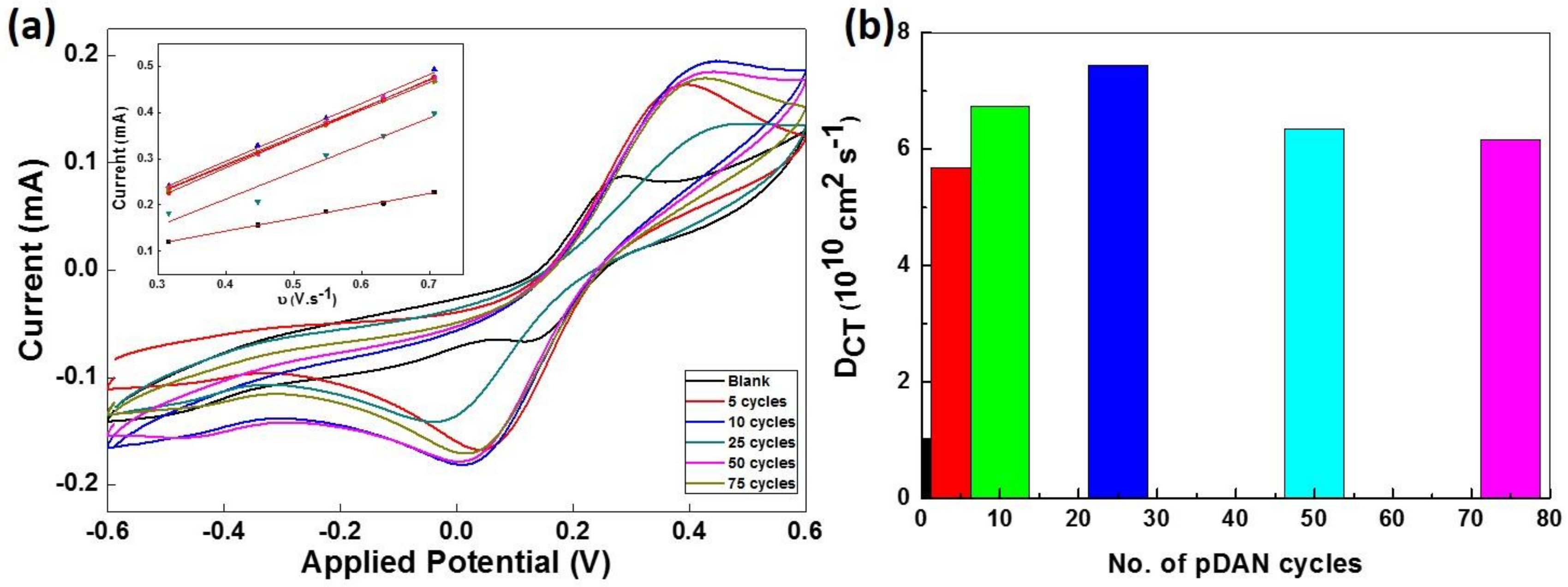
3.4. Electrochemical Analysis
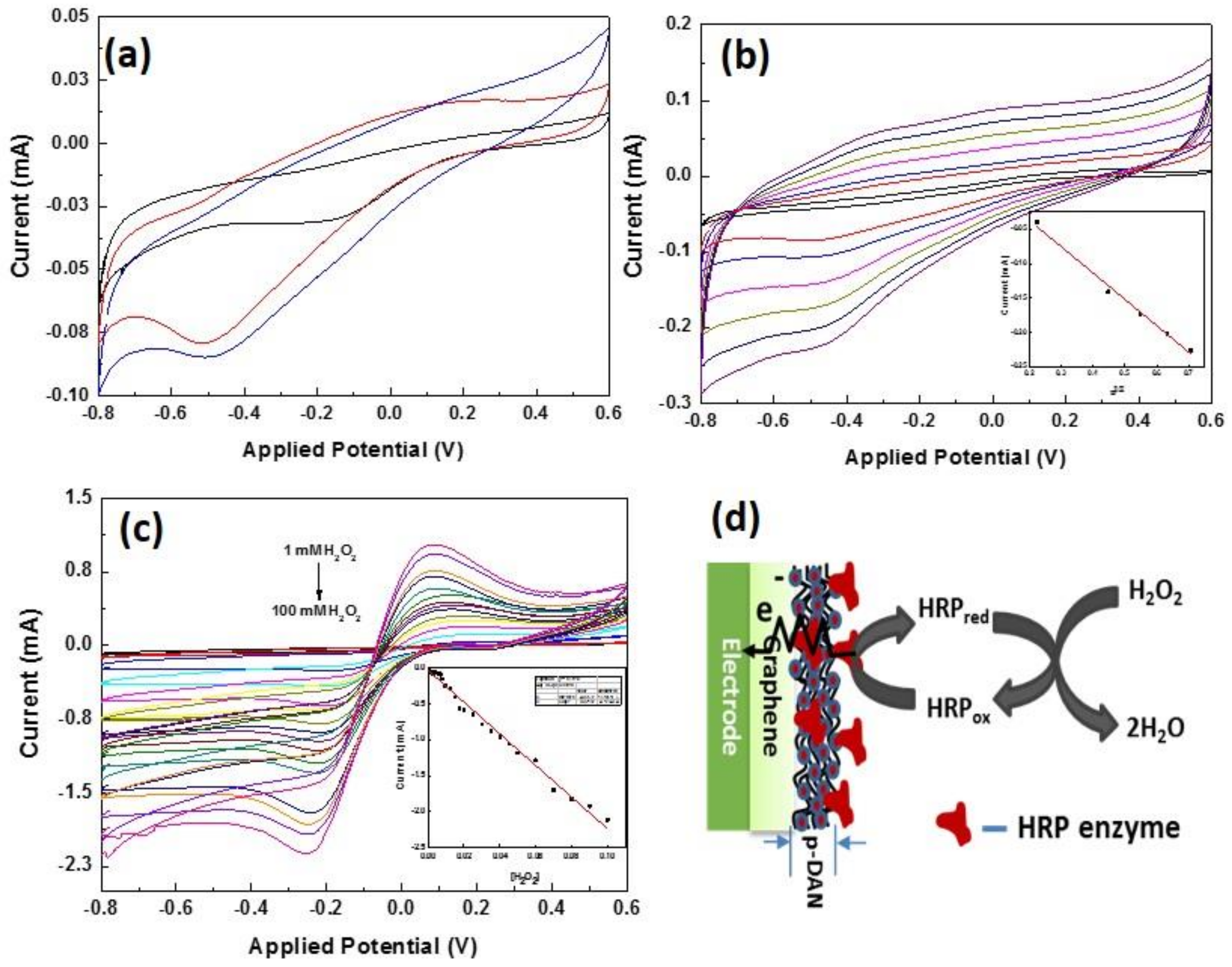
3.5. Enzymatic Electrochemical Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, F.; Li, Y.; Tang, B.; Zhang, C.-Y. Fluorescent Biosensors Based on Single-Molecule Counting. Acc. Chem. Res. 2016, 49, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, A.; Tian, Y. Functional Surface Engineering of C-Dots for Fluorescent Biosensing and in Vivo Bioimaging. Acc. Chem. Res. 2014, 47, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, E. Electrochemical biosensors on platforms of graphene. Chem. Commun. 2013, 49, 9526–9539. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Karapetis, S.; Bratakou, S.; Nikolelis, D.P.; Tzamtzis, N.; Psychoyios, V.N. Graphene-based Electrochemical Biosensors: New Trends and Applications. In Intelligent Nanomaterials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 427–448. [Google Scholar]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76 (Suppl. C), 195–212. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, A.; Sudhagar, P.; Das, S.; Lee, S.Y.; Terashima, C.; Nakata, K.; Fujishima, A.; Choi, W.; Kang, Y.S.; Paik, U. Synergistic Metal–Metal Oxide Nanoparticles Supported Electrocatalytic Graphene for Improved Photoelectrochemical Glucose Oxidation. Acs Appl. Mater. Interfaces 2014, 6, 4864–4871. [Google Scholar] [CrossRef]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Tehrani, Z.; Burwell, G.; Azmi, M.A.M.; Castaing, A.; Rickman, R.; Almarashi, J.; Dunstan, P.; Beigi, A.M.; Doak, S.H.; Guy, O.J. Generic epitaxial graphene biosensors for ultrasensitive detection of cancer risk biomarker. 2d Mater. 2014, 1, 025004. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Vazquez, E.; Prato, M. Organic Functionalization of Graphene in Dispersions. Acc. Chem. Res. 2013, 46, 138–148. [Google Scholar] [CrossRef]
- Englert, J.M.; Dotzer, C.; Yang, G.; Schmid, M.; Papp, C.; Gottfried, J.M.; Steinrück, H.-P.; Spiecker, E.; Hauke, F.; Hirsch, A. Covalent bulk functionalization of graphene. Nat. Chem. 2011, 3, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Vianelli, A.; Candini, A.; Treossi, E.; Palermo, V.; Affronte, M. Observation of different charge transport regimes and large magnetoresistance in graphene oxide layers. Carbon 2015, 89 (Suppl. C), 188–196. [Google Scholar] [CrossRef]
- Hirsch, A.; Englert, J.M.; Hauke, F. Wet Chemical Functionalization of Graphene. Acc. Chem. Res. 2013, 46, 87–96. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Wang, H.; Wang, S. Enhanced sensitivity for biosensors: Functionalized P1,5-diaminonaphthalene-multiwall carbon nanotube composite film-modified electrode. Electrochim. Acta 2012, 85, 467–474. [Google Scholar] [CrossRef]
- Noh, H.-B.; Kumar, P.; Biswas, T.K.; Kim, D.-S.; Shim, Y.-B. Improved Performance of an Amperometric Biosensor with Polydiaminonaphthalene on Electrochemically Deposited Au Nanoparticles. Electroanalysis 2010, 22, 632–638. [Google Scholar] [CrossRef]
- Abdelwahab, A.A.; Lee, H.-M.; Shim, Y.-B. Selective determination of dopamine with a cibacron blue/poly-1,5-diaminonaphthalene composite film. Anal. Chim. Acta 2009, 650, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Won, M.-S.; Kwon, N.-H.; Yoon, J.-H.; Park, D.-S.; Shim, Y.-B. Water Sensor for a Nonaqueous Solvent with Poly(1,5-diaminonapthalene) Nanofibers. Anal. Chem. 2008, 80, 5307–5311. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Tran, L.D.; Le Nguyen, H.; Nguyen, B.H.; Van Hieu, N. Modified interdigitated arrays by novel poly(1,8-diaminonaphthalene)/carbon nanotubes composite for selective detection of mercury(II). Talanta 2011, 85, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.H.; Nguyen, B.T.; Van Vu, H.; Van Nguyen, C.; Nguyen, D.T.; Nguyen, L.T.; Vu, T.T.; Tran, L.D. Development of label-free electrochemical lactose biosensor based on graphene/poly(1,5-diaminonaphthalene) film. Curr. Appl. Phys. 2016, 16, 135–140. [Google Scholar] [CrossRef]
- Noel, S.; Liberelle, B.; Robitaille, L.; De Crescenzo, G. Quantification of Primary Amine Groups Available for Subsequent Biofunctionalization of Polymer Surfaces. Bioconjugate Chem. 2011, 22, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Poli, E.; Chaleix, V.; Damia, C.; Hjezi, Z.; Champion, E.; Sol, V. Efficient quantification of primary amine functions grafted onto apatite ceramics by using two UV-Vis spectrophotometric methods. Anal. Methods 2014, 6, 9622–9627. [Google Scholar] [CrossRef]
- Soto-Cantu, E.; Cueto, R.; Koch, J.; Russo, P.S. Synthesis and Rapid Characterization of Amine-Functionalized Silica. Langmuir 2012, 28, 5562–5569. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Yamamoto, S.; Shimomura, A.; Ojida, A.; Nishino, T.; Maruyama, T. Quantification of Amino Groups on Solid Surfaces Using Cleavable Fluorescent Compounds. Langmuir 2015, 31, 8824–8829. [Google Scholar] [CrossRef]
- Stilwell, D.E.; Park, S.M. Electrochemistry of Conductive Polymers: II. Electrochemical Studies on Growth Properties of Polyaniline. J. Electrochem. Soc. 1988, 135, 2254–2262. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, D.S.; Shim, Y.B.; Park, S.M. Electrochemical Characterization of Poly(1,8-diaminonaphthalene): A Functionalized Polymer. J. Electrochem. Soc. 1992, 139, 3507–3514. [Google Scholar] [CrossRef]
- Zorn, G.; Liu, L.-H.; Árnadóttir, L.; Wang, H.; Gamble, L.J.; Castner, D.G.; Yan, M. X-ray Photoelectron Spectroscopy Investigation of the Nitrogen Species in Photoactive Perfluorophenylazide-Modified Surfaces. J. Phys. Chem. C 2014, 118, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Eby, D.M.; Artyushkova, K.; Paravastu, A.K.; Johnson, G.R. Probing the molecular structure of antimicrobial peptide-mediated silica condensation using X-ray photoelectron spectroscopy. J. Mater. Chem. 2012, 22, 9875–9883. [Google Scholar] [CrossRef]
- Devadoss, A.; Spehar-Délèze, A.-M.; Tanner, D.A.; Bertoncello, P.; Marthi, R.; Keyes, T.E.; Forster, R.J. Enhanced Electrochemiluminescence and Charge Transport Through Films of Metallopolymer-Gold Nanoparticle Composites. Langmuir 2010, 26, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, A.; Han, H.; Song, T.; Kim, Y.-P.; Paik, U. Gold nanoparticle-composite nanofibers for enzymatic electrochemical sensing of hydrogen peroxide. Analyst 2013, 138, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, Q.; Zhang, L.; Li, J. Direct electron transfer of horseradish peroxidase and its biosensor based on chitosan and room temperature ionic liquid. Electrochem. Commun. 2006, 8, 874–878. [Google Scholar] [CrossRef]
- Piccinini, E.; Bliem, C.; Reiner-Rozman, C.; Battaglini, F.; Azzaroni, O.; Knoll, W. Enzyme-polyelectrolyte multilayer assemblies on reduced graphene oxide field-effect transistors for biosensing applications. Biosens. Bioelectron. 2017, 92, 661–667. [Google Scholar] [CrossRef]
- Deng, C.; Chen, J.; Chen, X.; Xiao, C.; Nie, L.; Yao, S. Direct electrochemistry of glucose oxidase and biosensing for glucose based on boron-doped carbon nanotubes modified electrode. Biosens. Bioelectron. 2008, 23, 1272–1277. [Google Scholar] [CrossRef]
- Bharath, G.; Madhu, R.; Chen, S.-M.; Veeramani, V.; Balamurugan, A.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Enzymatic electrochemical glucose biosensors by mesoporous 1D hydroxyapatite-on-2D reduced graphene oxide. J. Mater. Chem. B 2015, 3, 1360–1370. [Google Scholar] [CrossRef]
- Zhou, K.; Zhu, Y.; Yang, X.; Luo, J.; Li, C.; Luan, S. A novel hydrogen peroxide biosensor based on Au–graphene–HRP–chitosan biocomposites. Electrochim. Acta 2010, 55, 3055–3060. [Google Scholar] [CrossRef]
- Li, M.; Xu, S.; Tang, M.; Liu, L.; Gao, F.; Wang, Y. Direct electrochemistry of horseradish peroxidase on graphene-modified electrode for electrocatalytic reduction towards H2O2. Electrochim. Acta 2011, 56, 1144–1149. [Google Scholar] [CrossRef]
- Nandini, S.; Nalini, S.; Manjunatha, R.; Shanmugam, S.; Melo, J.S.; Suresh, G.S. Electrochemical biosensor for the selective determination of hydrogen peroxide based on the co-deposition of palladium, horseradish peroxidase on functionalized-graphene modified graphite electrode as composite. J. Electroanal. Chem. 2013, 689, 233–242. [Google Scholar] [CrossRef]
- Hossain, M.F.; Park, J.Y. Fabrication of sensitive enzymatic biosensor based on multi-layered reduced graphene oxide added PtAu nanoparticles-modified hybrid electrode. PLoS ONE 2017, 12, e0173553. [Google Scholar] [CrossRef] [PubMed]
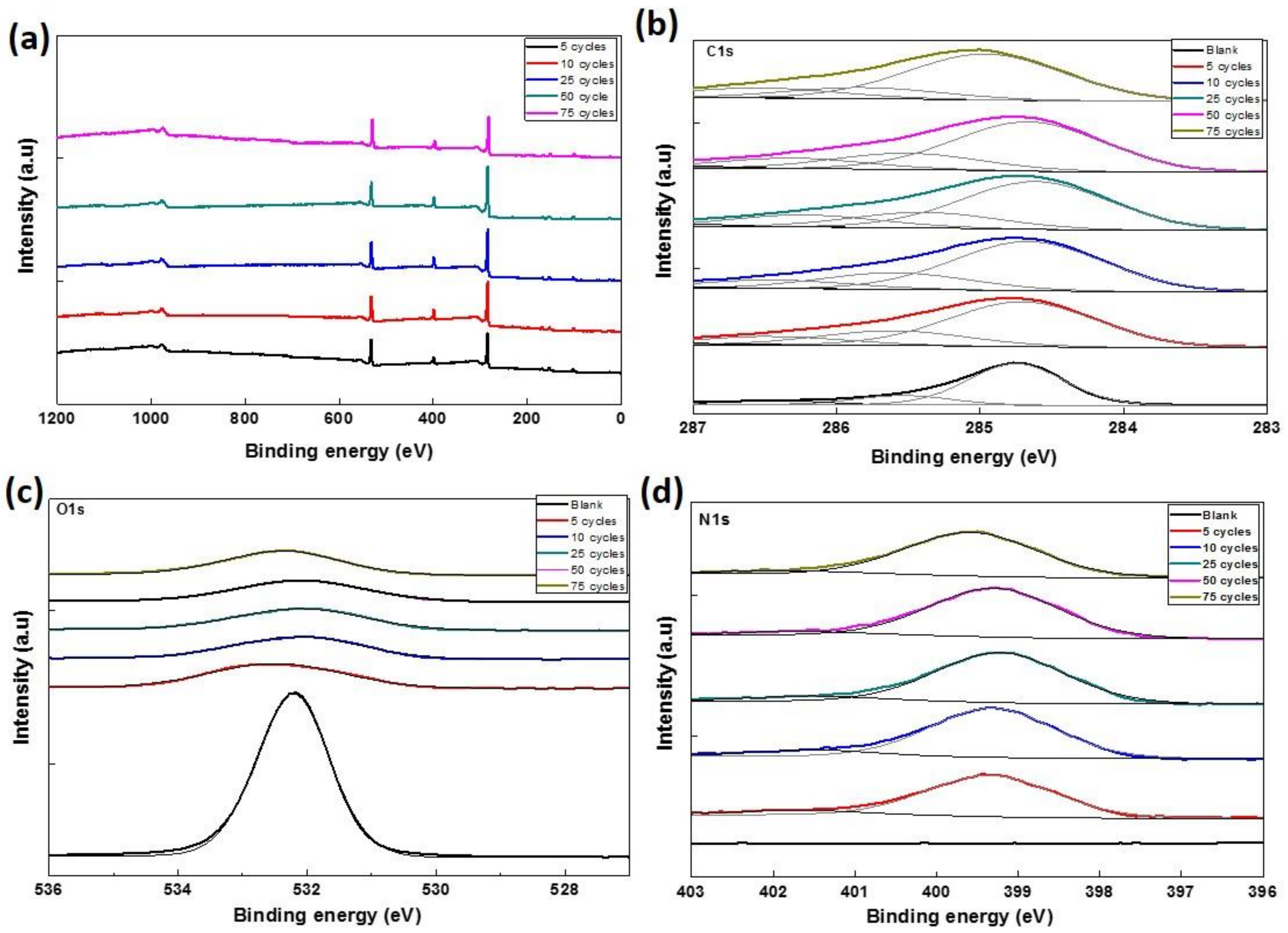
| Samples. | Atomic Percentage (%) | ||
|---|---|---|---|
| C | O | N | |
| Graphene | 25.64 | 49.47 | 0.00 |
| 5 cycles pDAN | 66.86 | 17.67 | 8.61 |
| 10 cycles pDAN | 71.14 | 14.65 | 9.64 |
| 25 cycles pDAN | 71.77 | 14.39 | 9.89 |
| 50 cycles pDAN | 72.90 | 14.04 | 9.55 |
| 75 cycles pDAN | 71.76 | 14.90 | 9.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devadoss, A.; Forsyth, R.; Bigham, R.; Abbasi, H.; Ali, M.; Tehrani, Z.; Liu, Y.; Guy, O.J. Ultrathin Functional Polymer Modified Graphene for Enhanced Enzymatic Electrochemical Sensing. Biosensors 2019, 9, 16. https://doi.org/10.3390/bios9010016
Devadoss A, Forsyth R, Bigham R, Abbasi H, Ali M, Tehrani Z, Liu Y, Guy OJ. Ultrathin Functional Polymer Modified Graphene for Enhanced Enzymatic Electrochemical Sensing. Biosensors. 2019; 9(1):16. https://doi.org/10.3390/bios9010016
Chicago/Turabian StyleDevadoss, Anitha, Rhiannan Forsyth, Ryan Bigham, Hina Abbasi, Muhammad Ali, Zari Tehrani, Yufei Liu, and Owen. J. Guy. 2019. "Ultrathin Functional Polymer Modified Graphene for Enhanced Enzymatic Electrochemical Sensing" Biosensors 9, no. 1: 16. https://doi.org/10.3390/bios9010016
APA StyleDevadoss, A., Forsyth, R., Bigham, R., Abbasi, H., Ali, M., Tehrani, Z., Liu, Y., & Guy, O. J. (2019). Ultrathin Functional Polymer Modified Graphene for Enhanced Enzymatic Electrochemical Sensing. Biosensors, 9(1), 16. https://doi.org/10.3390/bios9010016








