Large-Area Nanogap Platforms for Surface-Enhanced Raman Spectroscopy Toward Sensing Applications: Comparison Between Ag and Au
Abstract
1. Introduction
2. Materials and Methods
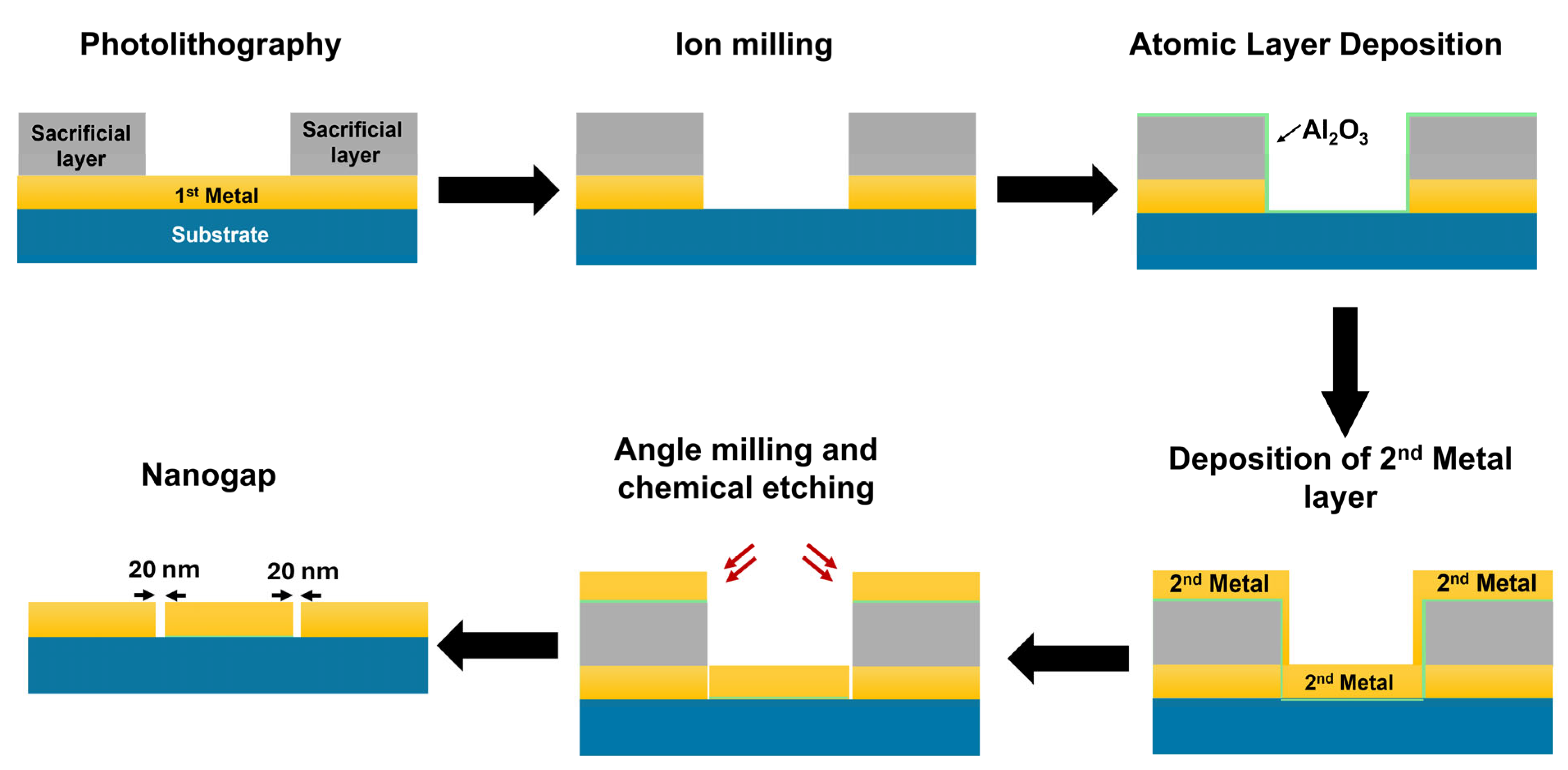
2.1. Fabrication of Nanogap
2.2. Preparation of Molecules
2.3. SERS Measurements
2.4. Numerical Simulation
3. Results and Discussion
3.1. Mass Fabrication of Nanogap Platform
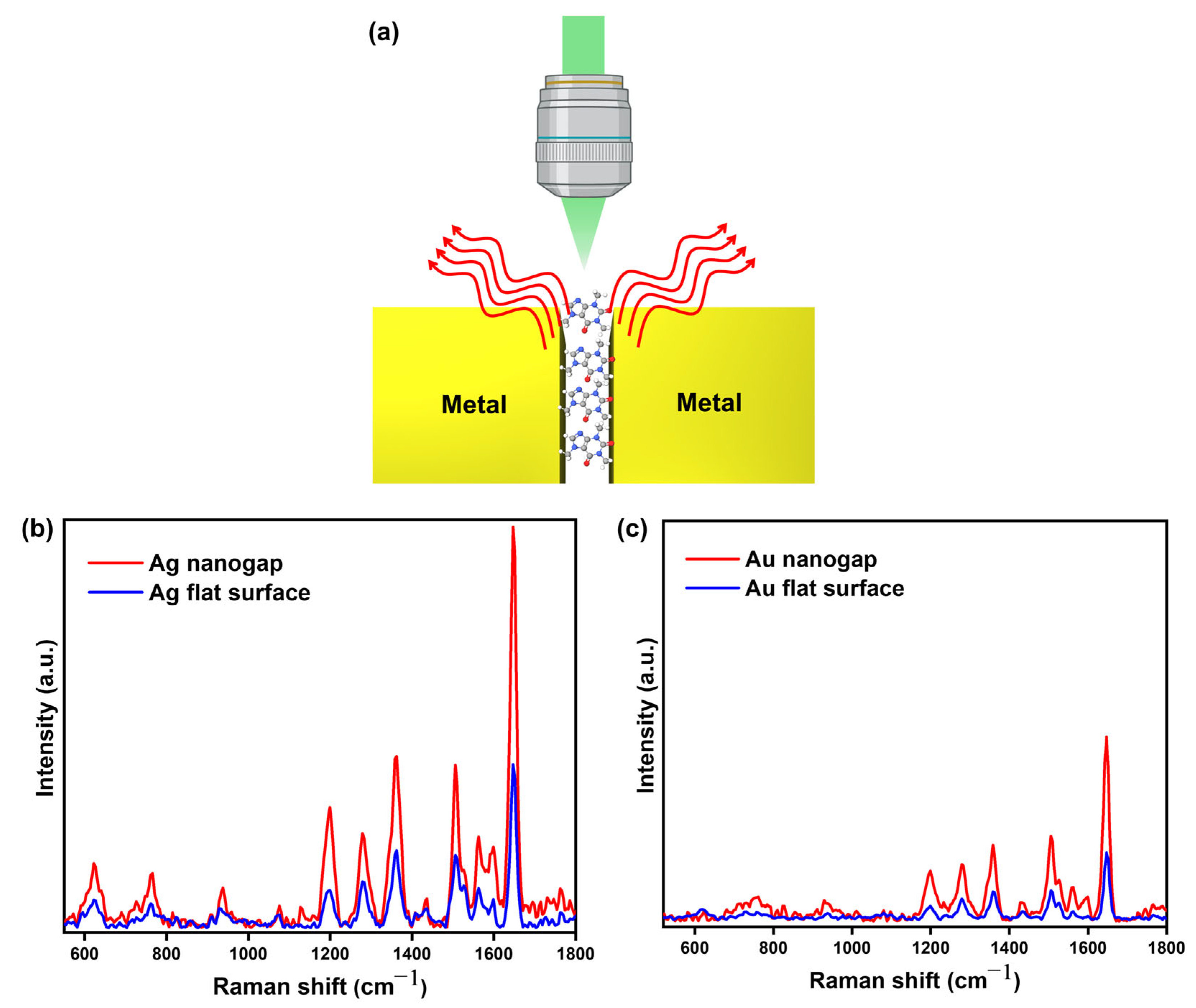
3.2. SERS Measurement
3.3. Numerical Simulation
3.4. Versatile SERS Application of Nanogap Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Sikdar, D.; Fedosyuk, A.; Velleman, L.; Klemme, D.J.; Oh, S.-H.; Kucernak, A.R.J.; Kornyshev, A.A.; Edel, J.B. Electrotunable Nanoplasmonics for Amplified Surface Enhanced Raman Spectroscopy. ACS Nano 2020, 14, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.; Chou, L.-T.; Turchetti, M.; Ritzkowsky, F.; Berggren, K.K.; Keathley, P.D. Lightwave-electronic harmonic frequency mixing. Sci. Adv. 2024, 10, eadq0642. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Rasmita, A.; He, R.; Zhang, Z.; Tan, Q.; Chen, D.; Wang, N.; Mu, Z.; Eng, J.J.H.; She, Y.; et al. Charge-depletion-enhanced WSe2 quantum emitters on gold nanogap arrays with near-unity quantum efficiency. Nat. Photonics 2024, 18, 842–847. [Google Scholar] [CrossRef]
- Choi, H.-K.; Park, S.-M.; Jeong, J.; Lee, H.; Yeon, G.J.; Kim, D.-S.; Kim, Z.H. Spatially Controlled Fabrication of Surface-Enhanced Raman Scattering Hot Spots through Photoinduced Dewetting of Silver Thin Films. J. Phys. Chem. Lett. 2022, 13, 2969–2975. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Yang, R.; Liu, W.; Sun, S. A Sensitive and Stable Surface Plasmon Resonance Sensor Based on Monolayer Protected Silver Film. Sensors 2017, 17, 2777. [Google Scholar] [CrossRef]
- Mason, S.; Meretska, M.L.; Spägele, C.; Ossiander, M.; Capasso, F. Metasurface-Controlled Holographic Microcavities. ACS Photonics 2024, 11, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Decker, M.; Setzpfandt, F.; Gai, X.; Choi, D.-Y.; Kiselev, R.; Chipouline, A.; Staude, I.; Pertsch, T.; Neshev, D.N.; et al. High–bit rate ultra-compact light routing with mode-selective on-chip nanoantennas. Sci. Adv. 2017, 3, e1700007. [Google Scholar] [CrossRef]
- Men, D.; Feng, S.; Liu, G.; Hang, L.; Zhang, T. A Sensitive “Optical Nose” for Detection of Volatile Organic Molecules Based on Au@MOFs Nanoparticle Arrays through Surface-Enhanced Raman Scattering. Part. Part. Syst. Charact. 2020, 37, 1900452. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Yu, E.-S.; Lee, S.; Woo, H.; Kwak, J.; Chung, S.; Choi, I.; Ryu, Y.-S. Fabry–Perot Cavity Control for Tunable Raman Scattering. Small 2023, 19, 2207003. [Google Scholar] [CrossRef]
- Rao, V.K.; Radhakrishnan, T.P. Tuning the SERS Response with Ag-Au Nanoparticle-Embedded Polymer Thin Film Substrates. ACS Appl. Mater. Interfaces 2015, 7, 12767–12773. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, S.Z.; Yang, C.; Li, C.H.; Huo, Y.Y.; Liu, X.Y.; Liu, A.H.; Wei, Q.; Gao, S.S.; Gao, X.G.; et al. Gold@silver bimetal nanoparticles/pyramidal silicon 3D substrate with high reproducibility for high-performance SERS. Sci. Rep. 2016, 6, 25243. [Google Scholar] [CrossRef]
- Gu, Z.; Zhao, D.; He, H.; Wang, Z. SERS-Based Microneedle Biosensor for In Situ and Sensitive Detection of Tyrosinase. Biosensors 2024, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-M.; Oh, J.-W.; Lee, H.; Suh, Y.D. Plasmonic Nanogap-Enhanced Raman Scattering with Nanoparticles. Acc. Chem. Res. 2016, 49, 2746–2755. [Google Scholar] [CrossRef]
- Lin, G.; Yang, B.; Zhang, C.; Zhu, J.; Wang, Y.; Li, L.; Xiong, S.; Zhang, J.; Qi, Z.; Wu, W. A Surface Enhanced Raman Scattering (SERS) Sensing Method Enhanced by All-metal Metasurface. Phys. Scr. 2023, 98, 125964. [Google Scholar] [CrossRef]
- Ayas, S.; Güner, H.; Türker, B.; Ekiz, O.Ö.; Dirisaglik, F.; Okyay, A.K.; Dâna, A. Raman Enhancement on a Broadband Meta-Surface. ACS Nano 2012, 6, 6852–6861. [Google Scholar] [CrossRef]
- Jakob, L.A.; Deacon, W.M.; Zhang, Y.; De Nijs, B.; Pavlenko, E.; Hu, S.; Carnegie, C.; Neuman, T.; Esteban, R.; Aizpurua, J.; et al. Giant optomechanical spring effect in plasmonic nano- and picocavities probed by surface-enhanced Raman scattering. Nat. Commun. 2023, 14, 3291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yi, Y.; Zhou, C.; Ying, G.; Zhou, X.; Fu, C.; Zhu, Y.; Shen, Y. SERS detection of microRNA biomarkers for cancer diagnosis using gold-coated paramagnetic nanoparticles to capture SERS-active gold nanoparticles. RSC Adv. 2017, 7, 52782–52793. [Google Scholar] [CrossRef]
- Kim, Y.B.; Behera, S.; Lee, D.; Namgung, S.; Park, K.-D.; Kim, D.-S.; Das, B. Active Surface-Enhanced Raman Scattering Platform Based on a 2D Material–Flexible Nanotip Array. Biosensors 2024, 14, 619. [Google Scholar] [CrossRef]
- Cha, J.; Lee, G.; Lee, D.; Kim, D.S.; Kim, S. A progressive wafer scale approach for Sub-10 nm nanogap structures. Sci. Rep. 2025, 15, 11323. [Google Scholar] [CrossRef]
- He, S.; Tian, R.; Wu, W.; Li, W.-D.; Wang, D. Helium-ion-beam nanofabrication: Extreme processes and applications. Int. J. Extrem. Manuf. 2021, 3, 012001. [Google Scholar] [CrossRef]
- Duan, H.; Fernández-Domínguez, A.I.; Bosman, M.; Maier, S.A.; Yang, J.K.W. Nanoplasmonics: Classical down to the Nanometer Scale. Nano Lett. 2012, 12, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Ahn, H.; Kim, S.; Song, H.; Choi, J.-R.; Kim, K. Advanced optical nanolithography by enhanced transmission through bull’s eye nanostructured meta-mask. Nanophotonics 2023, 12, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Y.; Sun, B.; Wang, D.; Li, G. Nanogap Electrode-Enabled Versatile Electrokinetic Manipulation of Nanometric Species in Fluids. Biosensors 2022, 12, 451. [Google Scholar] [CrossRef]
- Chen, X.; Park, H.-R.; Pelton, M.; Piao, X.; Lindquist, N.C.; Im, H.; Kim, Y.J.; Ahn, J.S.; Ahn, K.J.; Park, N.; et al. Atomic layer lithography of wafer-scale nanogap arrays for extreme confinement of electromagnetic waves. Nat. Commun. 2013, 4, 2361. [Google Scholar] [CrossRef] [PubMed]
- Praturi, A.S.; Kim, D.-S.; Singh, B.P.; Vasa, P. Relaxation and Excitation Rate Modifications by Metal Nanostructures for Solar Energy Conversion Applications. J. Phys. Chem. C 2021, 125, 8090–8097. [Google Scholar] [CrossRef]
- Rhie, J.; Hong, S.J.; Lee, D.; Lee, D.; Yun, H.S.; Bahk, Y.-M.; Kim, D.-S. Twofold Plasmonic Resonator Based on Polyethylene Terephthalate Thin Films for Terahertz Sensing Applications. ACS Appl. Nano Mater. 2021, 4, 8753–8760. [Google Scholar] [CrossRef]
- Semple, J.; Georgiadou, D.G.; Wyatt-Moon, G.; Yoon, M.; Seitkhan, A.; Yengel, E.; Rossbauer, S.; Bottacchi, F.; McLachlan, M.A.; Bradley, D.D.C.; et al. Large-area plastic nanogap electronics enabled by adhesion lithography. npj Flex. Electron. 2018, 2, 18. [Google Scholar] [CrossRef]
- Mandal, S.; Mantilla, H.M.; Loganathan, K.; Faber, H.; Sharma, A.; Gedda, M.; Yengel, E.; Goswami, D.K.; Heeney, M.; Anthopoulos, T.D. Ultra-Fast Moisture Sensor for Respiratory Cycle Monitoring and Non-Contact Sensing Applications. Adv. Mater. 2025, 37, 2414005. [Google Scholar] [CrossRef]
- Moghaddam, M.H.; Dalayoan, D.J.C.; Park, D.; Wang, Z.; Kim, H.; Im, S.; Ji, K.; Kang, D.; Das, B.; Kim, D.S. Lithographically Defined Zerogap Strain Sensors. ACS Photonics 2024, 11, 3239–3249. [Google Scholar] [CrossRef]
- Moon, T.; Joo, H.; Das, B.; Koo, Y.; Kang, M.; Lee, H.; Kim, S.; Chen, C.; Suh, Y.D.; Kim, D.-S.; et al. Adaptive Gap-Tunable Surface-Enhanced Raman Spectroscopy. Nano Lett. 2024, 24, 3777–3784. [Google Scholar] [CrossRef]
- Moghaddam, M.H.; Sharma, S.; Park, D.; Kim, D.S. Tuning 1D Plasmonic Gap at Nanometer Scale for Advanced SERS Detection. Adv. Opt. Mater. 2025, 13, 2403021. [Google Scholar] [CrossRef]
- Ertsgaard, C.T.; Wittenberg, N.J.; Klemme, D.J.; Barik, A.; Shih, W.-C.; Oh, S.-H. Integrated Nanogap Platform for Sub-Volt Dielectrophoretic Trapping and Real-Time Raman Imaging of Biological Nanoparticles. Nano Lett. 2018, 18, 5946–5953. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Todeschini, M.; Bastos da Silva Fanta, A.; Jensen, F.; Wagner, J.B.; Han, A. Influence of Ti and Cr Adhesion Layers on Ultrathin Au Films. ACS Appl. Mater. Interfaces 2017, 9, 37374–37385. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, L.N.; Kang, T.; Bahk, Y.-M.; Han, S.; Choi, G.; Rhie, J.; Jeong, J.; Kim, D.-S. Quantum dots-nanogap metamaterials fabrication by self-assembly lithography and photoluminescence studies. Opt. Express 2015, 23, 14937–14945. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Mun, C.; Linh, V.T.N.; Kwon, J.-D.; Jung, H.S.; Kim, D.-H.; Park, S.-G. Highly Sensitive and Selective Nanogap-Enhanced SERS Sensing Platform. Nanomaterials 2019, 9, 619. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, A.Y.; Wheeler, D.A.; Bond, T.C.; Gu, C.; Li, Y. Direct molecule-specific glucose detection by Raman spectroscopy based on photonic crystal fiber. Anal. Bioanal. Chem. 2012, 402, 687–691. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alagurasu, A.; Behera, S.; Yang, J.-M.; Kim, D.-S.; Namgung, S. Large-Area Nanogap Platforms for Surface-Enhanced Raman Spectroscopy Toward Sensing Applications: Comparison Between Ag and Au. Biosensors 2025, 15, 369. https://doi.org/10.3390/bios15060369
Alagurasu A, Behera S, Yang J-M, Kim D-S, Namgung S. Large-Area Nanogap Platforms for Surface-Enhanced Raman Spectroscopy Toward Sensing Applications: Comparison Between Ag and Au. Biosensors. 2025; 15(6):369. https://doi.org/10.3390/bios15060369
Chicago/Turabian StyleAlagurasu, Arunkumar, Satyabrat Behera, Joon-Mo Yang, Dai-Sik Kim, and Seon Namgung. 2025. "Large-Area Nanogap Platforms for Surface-Enhanced Raman Spectroscopy Toward Sensing Applications: Comparison Between Ag and Au" Biosensors 15, no. 6: 369. https://doi.org/10.3390/bios15060369
APA StyleAlagurasu, A., Behera, S., Yang, J.-M., Kim, D.-S., & Namgung, S. (2025). Large-Area Nanogap Platforms for Surface-Enhanced Raman Spectroscopy Toward Sensing Applications: Comparison Between Ag and Au. Biosensors, 15(6), 369. https://doi.org/10.3390/bios15060369





