Improving the Enrichment of Submicron-Sized Particles by Size Decreasing of Cruciform Cross-Sectional Microchannel in Viscoelastic Microfluidics
Abstract
1. Introduction
2. Materials and Methods
2.1. Device Fabrication and Sample Preparation
2.2. Principle of Particle Focusing and Enrichment
3. Results and Discussion
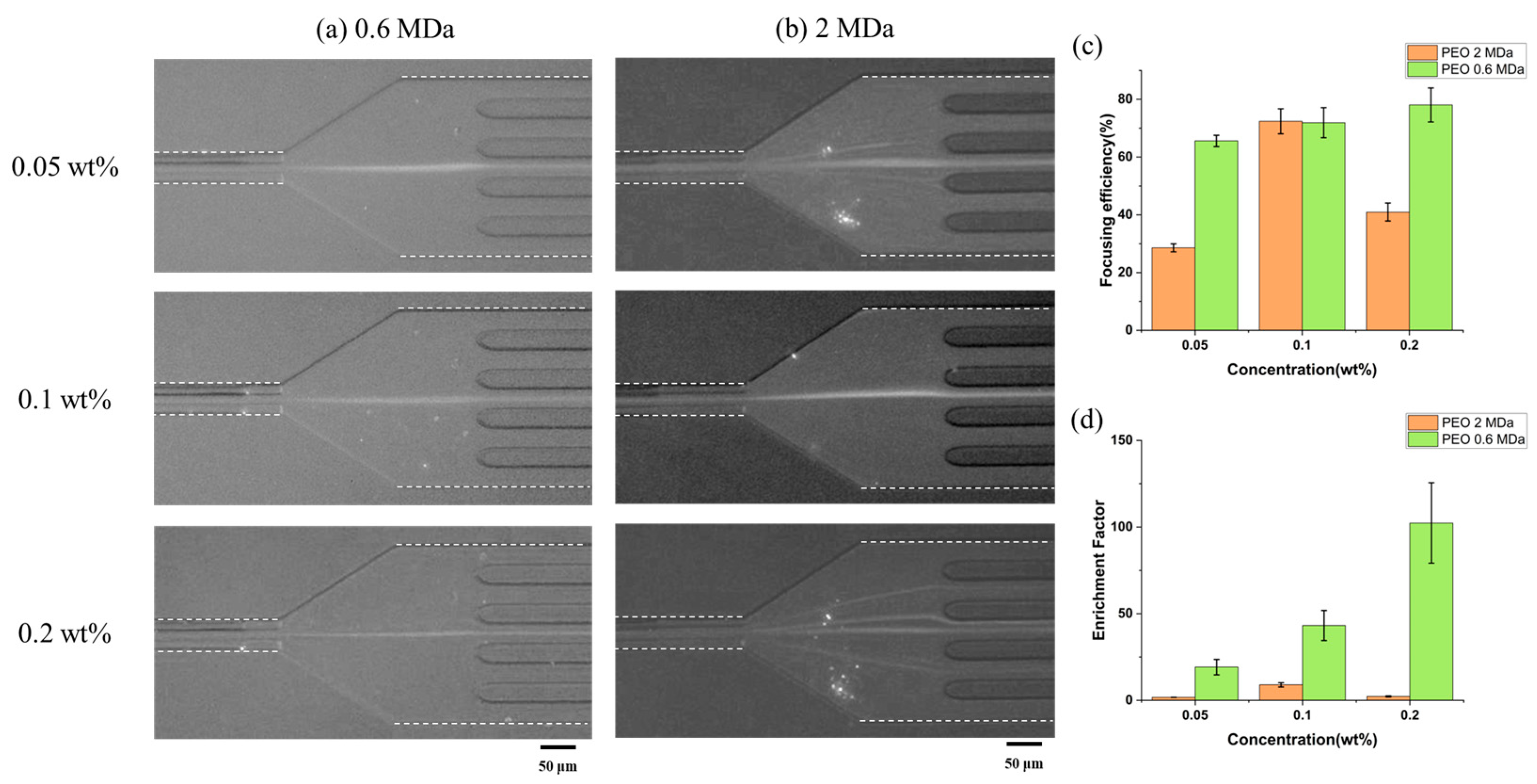
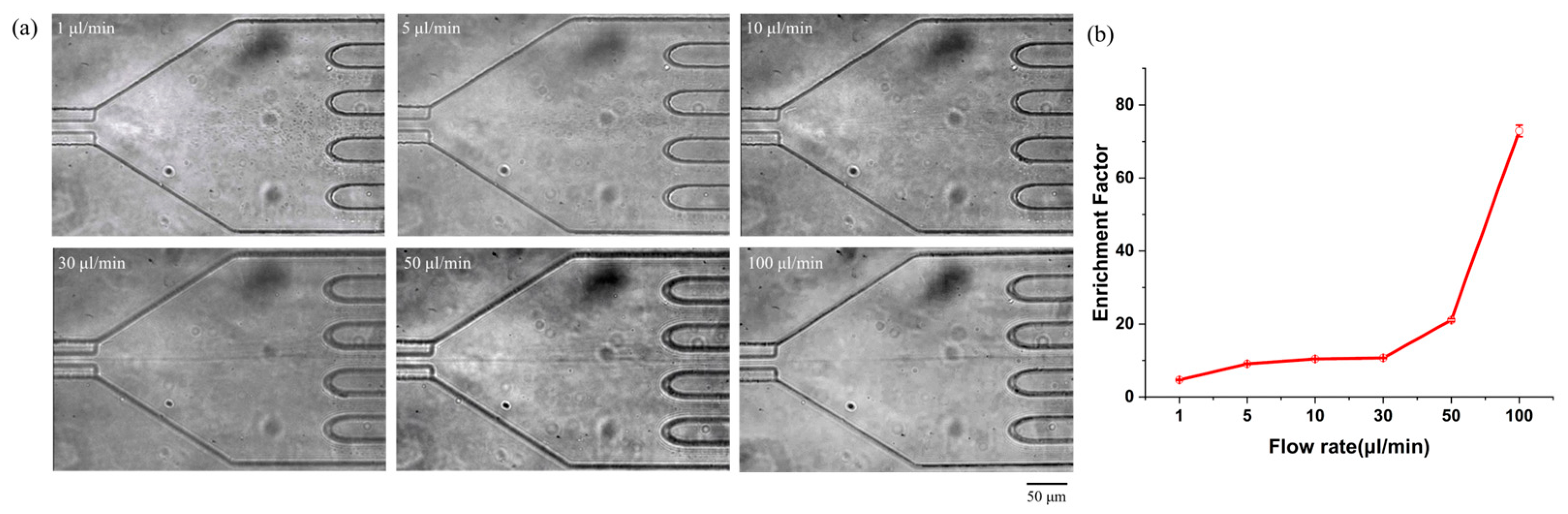
3.1. Submicron-Sized Particle Focusing and Enrichment in a Viscoelastic Fluid
3.2. Bacteria Focusing and Enrichment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, C.; Hu, G.; Xuan, X. Particle manipulations in non-Newtonian microfluidics: A review. J. Colloid Interface Sci. 2017, 500, 182–201. [Google Scholar] [CrossRef] [PubMed]
- D’Avino, G.; Greco, F.; Maffettone, P.L. Particle Migration due to Viscoelasticity of the Suspending Liquid and Its Relevance in Microfluidic Devices. Annu. Rev. Fluid Mech. 2017, 49, 341–360. [Google Scholar] [CrossRef]
- Yuan, D.; Tan, S.H.; Zhao, Q.; Yan, S.; Sluyter, R.; Nguyen, N.T.; Zhang, J.; Li, W. Sheathless Dean-flow-coupled elasto-inertial particle focusing and separation in viscoelastic fluid. RSC Adv. 2017, 7, 3461–3469. [Google Scholar] [CrossRef]
- Cruz, J.; Hjort, K. High-resolution particle separation by inertial focusing in high aspect ratio curved microfluidics. Sci. Rep. 2021, 11, 13959. [Google Scholar] [CrossRef]
- Zhang, T.; Hong, Z.-Y.; Tang, S.-Y.; Li, W.; Inglis, D.W.; Hosokawa, Y.; Yalikun, Y.; Li, M. Focusing of sub-micrometer particles in microfluidic devices. Lab Chip 2020, 20, 35–53. [Google Scholar] [CrossRef]
- Hettiarachchi, S.; Cha, H.; Ouyang, L.; Mudugamuwa, A.; An, H.; Kijanka, G.; Kashaninejad, N.; Nguyen, N.-T.; Zhang, J. Recent microfluidic advances in submicron to nanoparticle manipulation and separation. Lab Chip 2022, 23, 982–1010. [Google Scholar] [CrossRef]
- Xie, Y.; Rufo, J.; Zhong, R.; Rich, J.; Li, P.; Leong, K.W.; Huang, T.J. Microfluidic Isolation and Enrichment of Nanoparticles. ACS Nano 2020, 14, 16220–16240. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Tian, F.; Yang, N.; Yan, F.; Ding, Y.; Wei, J.; Hu, G.; Nie, G.; Sun, J. Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS Nano 2017, 11, 6968–6976. [Google Scholar] [CrossRef]
- Zhang, T.; Cain, A.K.; Semenec, L.; Liu, L.; Hosokawa, Y.; Inglis, D.W.; Yalikun, Y.; Li, M. Microfluidic Separation and Enrichment of Escherichia coli by Size Using Viscoelastic Flows. Anal. Chem. 2023, 95, 2561–2569. [Google Scholar] [CrossRef]
- Liu, C.; Ding, B.; Xue, C.; Tian, Y.; Hu, G.; Sun, J. Sheathless Focusing and Separation of Diverse Nanoparticles in Viscoelastic Solutions with Minimized Shear Thinning. Anal. Chem. 2016, 88, 12547–12553. [Google Scholar] [CrossRef]
- Asghari, M.; Cao, X.; Mateescu, B.; van Leeuwen, D.; Aslan, M.K.; Stavrakis, S.; Demello, A.J. Oscillatory Viscoelastic Microfluidics for Efficient Focusing and Separation of Nanoscale Species. ACS Nano 2020, 14, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Tian, Z.; Zhe, J.; Zhao, L. Efficient microfluidic enrichment of nano-/submicroparticle in viscoelastic fluid. Electrophoresis 2021, 42, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Ahn, J.; Kim, T.; Cho, Y. Viscoelastic particle focusing and separation in a microfluidic channel with a cruciform section. Biomicrofluidics 2024, 18, 064101. [Google Scholar] [CrossRef]
- Zhang, T.; Di Carlo, D.; Lim, C.T.; Zhou, T.; Tian, G.; Tang, T.; Shen, A.Q.; Li, W.; Li, M.; Yang, Y.; et al. Passive microfluidic devices for cell separation. Biotechnol. Adv. 2024, 71, 108317. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Lin, C.Y.; Huang, C.F.; Tsai, H.H. Liquid flow in a micro-channel. J. Micromech. Microeng. 2004, 14, 436–445. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, M.-H.; Lee, S.; Cho, Y. A long straight square microchannel in viscoelastic fluid for focusing submicron-sized particles and bacteria. Microchim. Acta 2024, 191, 738. [Google Scholar] [CrossRef]
- Song, J.; Jang, J.; Kim, T.; Cho, Y. Particle Separation in a Microchannel with a T-Shaped Cross-Section Using Co-Flow of Newtonian and Viscoelastic Fluids. Micromachines 2023, 14, 1863. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Q.; Yan, S.; Tang, S.-Y.; Alici, G.; Zhang, J.; Li, W. Recent progress of particle migration in viscoelastic fluids. Lab Chip 2018, 18, 551–567. [Google Scholar] [CrossRef]
- Gou, Y.; Jia, Y.; Wang, P.; Sun, C. Progress of Inertial Microfluidics in Principle and Application. Sensors 2018, 18, 1762. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.-T.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-Y.; Kim, T.; Kim, J.; Cho, Y. Particle Focusing under Newtonian and Viscoelastic Flow in a Straight Rhombic Microchannel. Micromachines 2020, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Kang, Y.J.; Lee, S.J. Lateral migration and focusing of microspheres in a microchannel flow of viscoelastic fluids. Phys. Fluids 2014, 26, 063301. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.M. Elasto-inertial particle focusing under the viscoelastic flow of DNA solution in a square channel. Biomicrofluidics 2016, 10, 024111. [Google Scholar] [CrossRef]
- Zhao, T.; Zeng, P.; Zhang, Y.; Li, J.; Sun, H.; Gablech, I.; Chang, H.; Yuan, X.; Neužil, P.; Feng, J. Inertial co-focusing of heterogeneous particles in hybrid microfluidic channels with constantly variable cross-sections. Lab Chip 2024, 24, 5032–5042. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, J.Y.; Lee, S.J.; Lee, S.S.; Kim, J.M. Sheathless elasto-inertial particle focusing and continuous separation in a straight rectangular microchannel. Lab Chip 2010, 11, 266–273. [Google Scholar] [CrossRef]
- Liu, C.; Xue, C.; Chen, X.; Shan, L.; Tian, Y.; Hu, G. Size-Based Separation of Particles and Cells Utilizing Viscoelastic Effects in Straight Microchannels. Anal. Chem. 2015, 87, 6041–6048. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.; Kim, E.; Kim, S.; Jeong, O.-C.; Lee, S.; Cho, Y. Improving the Enrichment of Submicron-Sized Particles by Size Decreasing of Cruciform Cross-Sectional Microchannel in Viscoelastic Microfluidics. Biosensors 2025, 15, 370. https://doi.org/10.3390/bios15060370
Jang J, Kim E, Kim S, Jeong O-C, Lee S, Cho Y. Improving the Enrichment of Submicron-Sized Particles by Size Decreasing of Cruciform Cross-Sectional Microchannel in Viscoelastic Microfluidics. Biosensors. 2025; 15(6):370. https://doi.org/10.3390/bios15060370
Chicago/Turabian StyleJang, Jaekyeong, Eunjin Kim, Sungdong Kim, Ok-Chan Jeong, Sangwook Lee, and Younghak Cho. 2025. "Improving the Enrichment of Submicron-Sized Particles by Size Decreasing of Cruciform Cross-Sectional Microchannel in Viscoelastic Microfluidics" Biosensors 15, no. 6: 370. https://doi.org/10.3390/bios15060370
APA StyleJang, J., Kim, E., Kim, S., Jeong, O.-C., Lee, S., & Cho, Y. (2025). Improving the Enrichment of Submicron-Sized Particles by Size Decreasing of Cruciform Cross-Sectional Microchannel in Viscoelastic Microfluidics. Biosensors, 15(6), 370. https://doi.org/10.3390/bios15060370




