Abstract
Photoactivatable aptamer sensing technology is widely used in various detection fields due to its precise spatiotemporal regulation ability, flexible material compatibility, and excellent detection performance. By introducing an optical response mechanism to regulate the efficient recognition of the target by the sensor, this strategy further broadens the regulation means of the aptamer. The application of photoactivated aptamer biosensors in point-of-care testing (POCT) can significantly improve the selectivity, sensitivity, and dynamic response ability of the POCT system. This review systematically explores the design principle and regulation mechanism of photoactivatable aptamers, with a focus on reviewing the application progress of them in the POCT platform. In addition, the existing challenges and future development trends are also discussed. It is expected that this biosensor based on photoactivatable aptamers will continue to drive POCT towards higher sensitivity, intelligence, and scene adaptability, providing innovative tools for precision medicine and environmental health monitoring.
1. Introduction
The advancement of precision medicine has driven an urgent demand for diagnostic technologies that simultaneously achieve rapid analysis, high sensitivity, and portability [1,2,3]. Traditional antibodies have dominated clinical diagnosis as molecular recognition elements for many years, but their deficiencies in production cost, stability, batch consistency, etc., have limited their application in certain scenarios. Since their first report in the early 1990s [4,5], aptamers have rapidly become an ideal alternative to antibodies due to their unique physicochemical properties [6,7,8]. Aptamers are a class of single-stranded sequences composed of RNA, DNA, or artificially synthesized nucleic acid molecules [9,10], which can bind to target molecules (including small molecules [11,12,13], proteins [14,15,16,17,18], viruses [19,20,21], cells [22,23,24], and even tissues [25,26,27]) with high affinity and specificity through specific three-dimensional conformations [28,29,30]. Compared with antibodies, aptamers exhibit advantages in terms of size, chemical modifiability, and stability [31,32]. These characteristics have drawn much attention to the application of aptamers in biosensors [33,34,35,36], especially demonstrating greater flexibility and plasticity in meeting the sensing requirements in harsh environments [37,38] and portable devices [39,40,41,42]. In recent years, researchers have further developed stimulus-responsive aptamer sensors, which have advantages such as high specificity, high sensitivity, and precise dynamic regulation capabilities [43,44,45]. Among them, “photoactivatable aptamers” that regulate aptamers through photosensitive domains or light response mechanisms have become a highly promising research direction [46,47,48,49,50].
Photoactivatable aptamers achieve precise spatiotemporal control of target molecules by introducing photoresponsive chemical groups or constructing photosensitive nucleic acid structures, causing reversible or irreversible changes in their recognition ability or conformation under light of specific wavelengths [51,52,53,54,55,56,57,58]. This strategy not only enhances the dynamic regulation ability of aptamer function, but also endows the sensing system with higher selectivity and background suppression ability [59,60].
In practical applications, the demand for portable point-of-care testing (POCT) technology is increasing day by day [61,62]. POCT emphasizes the miniaturization of equipment, the rapidity of detection, the simplicity of operation, and the feasibility of on-site application [63,64,65]. It is particularly suitable for resource-limited areas, emergency medicine, and home health monitoring [66,67]. Combining the aptamer with POCT can construct a biosensing platform with high sensitivity and high specificity [68,69]. In recent years, aptamer sensors that integrate technologies such as smart phones [70], microfluidic chips [71,72,73], and nanomaterials [74] have been continuously reported, demonstrating excellent performance in detecting heavy metals, bacteria, viruses, and biomarkers [75,76,77,78]. Furthermore, the aptamer sensor empowered by the optical activation mechanism is expected to enhance the controllability and detection accuracy of POCT devices, making them not only suitable for fixed-point detection but also capable of intelligent response and signal amplification.
In summary, aptamers, with their excellent molecular recognition capabilities and engineering modification potential, are gradually becoming key biometric components in POCT platforms [79,80]. This review systematically introduces the design principle qne regulation mechanism of photoactivatable aptamers and their application progress in POCT, and looks forward to their future development potential in precision diagnosis (Figure 1).
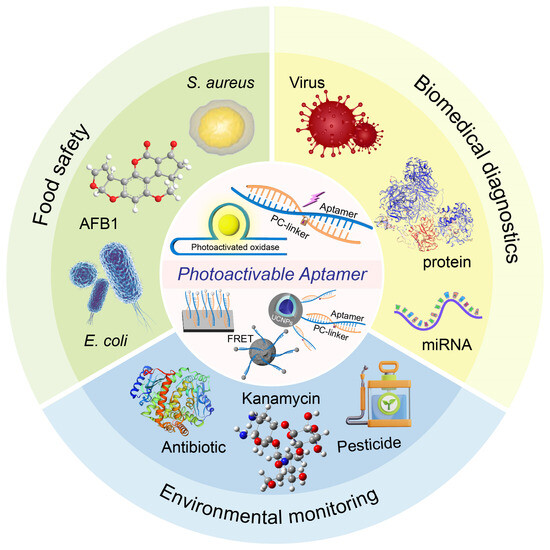
Figure 1.
Overview of aptamer-based photoactivated biosensors for POCT.
2. The Mechanism of Action of Photoactivatable Aptamers
Aptamers can be used to construct various photoactivatable POCT platforms with high sensitivity and specificity by introducing photosensitive linkers and combining them with quantum dots or other nanomaterials.
2.1. Optical Control of Aptamer
A photocleavable linker (PC-linker) is a type of chemical group that can undergo irreversible cleavage under ultraviolet (UV) light (typically 365 nm) irradiation. Its core structure contains o-nitrobenzyl or coumarin derivatives and is covalently linked to the aptamer through ester bonds or amino bonds [81,82]. For instance, Jia et al. [83] exploited the light-cleavable characteristic of a PC-linker by covalently attaching it to a complementary DNA strand capable of hybridizing with the aptamer sequence through Watson–Crick base pairing. This strategic modification creates a sterically hindered conformation that physically blocks aptamer-target recognition in the absence of light irradiation (Figure 2A). Only after the PC-linker is broken by UV light irradiation can the aptamer combine with the target to generate the output signal.
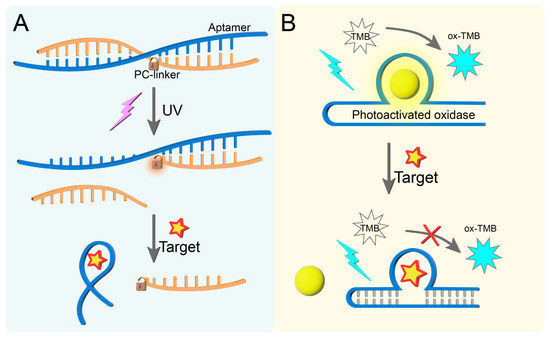
Figure 2.
(A) Photoactivated aptamers based on the action of PC-linker. (B) Photoactivated aptamers based on the action of photoactivated oxidase.
Photoactivated oxidase is an oxidase produced through photoactivation, usually copolymerized from dicyandiamide and barbituric acid [84,85]. This enzyme can catalyze oxidation under visible light to produce a green product. Thioflavin T(ThT) has a unique photoactivated oxidase activity. Therefore, Lu et al. [86] achieved the fluorescence colorimetric detection of estradiol by binding ThT to the cavity site of the estradiol aptamer (Figure 2B). ThT and the aptamer complex can oxidize the colorless TMB substrate to its oxide ox-TMB under 460 nm light exposure, generating a colorimetric signal. In the presence of the target, the specific binding between the aptamer and the target causes ThT to fall off and the signal to weaken. Isoquinoline alkaloids are also a kind of photoactivated oxidase, which show significant photoactivated oxidation capacity after binding to the aptamer sequence. The aptamer–berberine complex can oxidize all substrates under light to show obvious colorimetric changes. In the presence of the target, the aptamer recognizes the target, and the berberine dissociation cannot oxidize the substrate, resulting in a weakened colorimetric signal. Therefore, Lu et al. [87] used the characteristics of isoquinoline alkaloids and combined them with different aptamers to detect different targets under light conditions.
2.2. Optical Control of Materials
Semiconductor materials have unique photoelectric properties. When combined with aptamers, they can be used to construct a POCT detection platform for photoactivatable aptamers [88,89]. For example, an aptamer-based photoelectrochemical sensor can be developed by immobilizing thiol-modified aptamers on an electrode surface via Au-S coordination and hybridizing complementary DNA strands through base pairing (Figure 3A). Upon target binding under UV light irradiation, conformational changes disrupt the DNA duplex, releasing signal reporters and cDNA from the electrode to induce photocurrent reduction. In another case, Wu et al. [90] described a composite material system wherein cDNA is immobilized on the CeO2@MnO2 heterojunction, enabling site-specific molecular interactions through electrostatic anchoring. Due to the unique hairpin structure of cDNA, CeO2@MnO2 on cDNA can approach the ITO surface infinitely, generating a photocurrent response under visible light irradiation. cDNA can complement and pair with the target aptamer to form a double-stranded rigid structure, reducing the photocurrent. In the presence of the target, the aptamer specifically binds to the target and leaves the sensor surface. The cDNA will return to the hairpin structure, resulting in an increase in photocurrent and generating a signal.
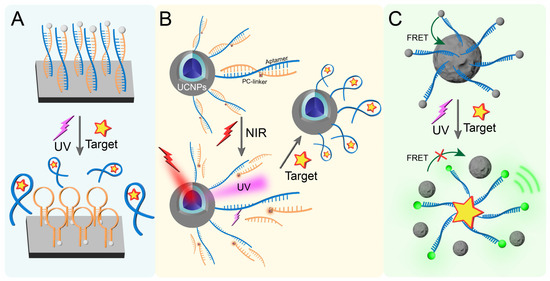
Figure 3.
(A) Schematic diagram of photoactivated aptamers based on semiconductor materials. (B) Illustration of NIR light-activated aptamer sensing based on UCNPs and PC-linker combination. (C) Photoactivated sensing based on the FRET effect of PDANS.
Because upconversion nanoparticles (UCNPs) can absorb near-infrared (NIR) light and convert it into UV light and visible light, UCNPs have been widely used in the light sensors of near-infrared light-activated corresponding systems [91,92,93]. Combining the aptamer with UCNPs can construct an aptamer sensor activated by NIR light. As shown in Figure 3B, Zhang et al. [94] covalently coupled the aptamer hybrid chain modified with a PC-linker to the surface of UCNPs. Under NIR light irradiation, UCNPs can absorb photons and convert them into ultraviolet light, thereby breaking the PC-linker of the aptamer. In the presence of the target, the aptamer binds and recognizes the target.
Another strategy involves intelligent nanomaterials utilizing fluorescence resonance energy transfer (FRET) [95]. For instance, Ye et al. [96] designed and synthesized an aptamer-based nanoprobe, which is composed of polydopamine nanospheres (PDANSs) as energy receptors and aptamers (FAM-Apt) modified with the energy donor FAM through π-π stacking interactions (Figure 3C). In the presence of the target, the specific binding of the target to the aptamer will reduce the π-π stacking interaction, thereby releasing free FAM-Apt, which can produce significant green fluorescence under UV light excitation. Moreover, under the irradiation of 808 nm laser, this nanoprobe can produce photothermal effects.
3. The Application of POCT Based on Photoactivatable Aptamers in Different Fields
In the field of modern analytical testing, POCT has attracted much attention due to its advantages of rapidity, convenience, and on-site presence. As an emerging recognition molecule, photoactivatable aptamers have shown great potential in POCT applications in fields such as food safety, environmental monitoring, and biomedical diagnostics, thanks to their unique photoresponse characteristics and high specificity. We have summarized the detection targets, detection methods, optical control strategies and detection limits of photoactivatable aptamer POCT in different fields, as shown in Table 1.

Table 1.
Summary of the optical activation aptamer sensing strategy.
3.1. Food Safety
In food safety testing, expeditious and accurate identification and quantification of hazardous substances is the key to protect public health. There are various types of contaminants in food, such as Escherichia coli (E. coli) [98], Staphylococcus aureus (S. aureus) [88,119,120], Salmonella typhimurium (S. typhimurium), AFB1, other bacteria [121] and biological toxins [101,102]. Although traditional detection methods (high-performance liquid chromatography, gas chromatography, mass spectrometry, etc.) can accurately detect them, these methods have disadvantages such as complex pretreatment, long detection time, and expensive equipment. Therefore, it is difficult to meet the demand for on-site rapid detection. Based on this, researchers have developed a series of POCT based on photoactivatable aptamers for the field of food safety.
For example, Zhang et al. [98] constructed a dynamic and reversible bacterial detection system by integrating photoresponsive magnetic beads, rolling ring amplification (RCA), and photothermal sensing technology (Figure 4A). In this system, aptamers specifically recognizing E. coli are fixed on the surface of magnetic beads. The binding of the target bacteria triggers the RCA reaction, generating a large number of DNA products with repetitive sequences. Subsequently, ultraviolet light irradiation causes the photoresponsive DNA to break and release RCA products. These products hybridize with near-infrared excited CuxS-modified DNA probes to enhance the photothermal signal. Through thermal imaging analysis on smart phones, the visual detection of E. coli was achieved, with a detection limit as low as 1.8 CFU/mL.
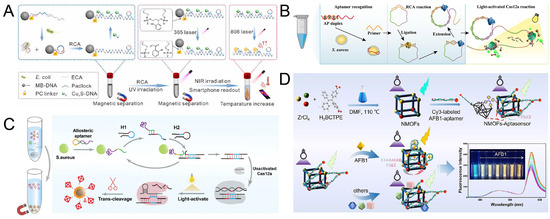
Figure 4.
(A) Schematic of RCA-amplified photothermal biosensor for detection of E. coli. Reproduced with permission from ref. [98]. Copyright 2022, American Chemical Society. (B) The scheme of light-activated RCA-Cas12a method for S. aureus detection. Reproduced with permission from ref. [97]. Copyright 2023, Elsevier. (C) The scheme of the photoactivated CHA/Cas12a-based SERS platform for detecting S. aureus. Reproduced with permission from ref. [122]. Copyright 2023, Elsevier. (D) The scheme of the NMOFs-aptasensor for sensitive detection of AFB1. Reproduced with permission from ref. [123]. Copyright 2025, Springer Nature.
Based on this light-controlled dynamic interface, the optimization of the signal amplification strategy has become the key to improving the detection performance. As shown in Figure 4B, Wu et al. [97] designed a CRISPR/Cas12a system triggered by light-controlled crRNA and achieved dual-signal amplification in combination with RCA. The DNA of the target bacteria triggers RCA to generate repetitive sequences, which serve as the activation template for CRISPR/Cas12a. The crRNA modified by the photosensitive protective group releases active crRNA under UV light, activates the cleavage activity of Cas12a, and generates a fluorescence signal. This method can achieve rapid detection of S. aureus within 30 min. This light-controlled precise activation mechanism effectively reduces the background signal and improves the detection efficiency.
To further enhance the detection sensitivity, Fan et al. [122] designed a SERS detection platform for S. aureus detection, which combines a photoactivatable catalytic hairpin self-assembly (CHA)/Cas12a cascade system with a multifunctional core-shell DNA tetrahedron (Figure 4C). The CHA reaction is triggered in the presence of S. aureus by using allosteric aptamers that can specifically bind to S. aureus. After the CHA reaction is completed, under UV light, it can activate the trans-cleavage activity of CRISPR/Cas12a bound to the photocleavage group. After S. aureus triggers cleavage, the released DNA tetrahedral probe carries Raman molecules embedded in the core-shell structure (AuNPs@Fe3O4), enhancing the SERS signal through magnetic enrichment. The detection limit of this platform for S. aureus is 3.16 CFU/mL, which is 20 times more sensitive than traditional PCR. Moreover, the detection can be completed within 40 min, providing an efficient solution for the rapid detection of low-abundance pathogenic bacteria in clinical samples.
In the scenario of complex matrix detection, the anti-interference ability and signal self-calibration will become a difficult problem to be solved. As shown in Figure 4D, Dou et al. [123] constructed a ratio fluorescence aptamer sensor based on Zr-MOF (UiO-66-NH2). The MOF used in this sensor is prepared with H2BCTPE as the organic binder, enabling the MOF to be excited at 460 nm to produce blue-green luminescence. The AFB1 aptamer modified by Cy3 binds to NMOF through the Zr-OP bond. In the presence of AFB1, the specific binding of AFB1 and the aptamer induces conformational changes, enhancing the FRET effect and generating fluorescence signals. The detection limit of this sensor for actual corn samples can reach 0.08 ng/mL, and the detection recovery rate for actual samples can reach 89.11–102.4%. With the high stability of MOF materials and the specific recognition of aptamers, the anti-interference detection of toxins in complex food matrices was achieved.
3.2. Environmental Monitoring
The problem of environmental pollution is becoming increasingly serious. Conducting real-time and rapid detection of pollutants in the environment is an important link in environmental governance. Environmental pollutants include organic pollutants, biological toxins, etc. Their existence poses a serious threat to the ecological environment and human health. Traditional environmental detection methods require complex laboratory equipment and professional technicians, making it difficult to realize the on-site rapid detection of pollutants. Photoactivatable aptamers can specifically recognize target pollutants in the environment. Aptamers can be combined with the technology of POCT, which establishes rapid and sensitive detection of pollutants.
Di (2-ethylhexyl) phthalate (DEHP) is often used as a plasticizer for plastic products. DEHP can easily leak from the plastic substrate into the environmental medium, thus causing great pollution to the environment. As shown in Figure 5A, Yang et al. [104] developed a NIR light-responsive aptamer sensor based on the Z-type heterojunction poly(pyrrole-co-thiophene)/ZnIn2S4 for the detection of DEHP. This heterojunction matches the energy band of ZnIn2S4 through the copolymer, forming a direct Z-type charge transfer path. The aptamer is fixed on the surface of the heterojunction through π-π stacking. After the aptamer specifically recognizes DEHP, the conformational change of the aptamer leads to an increase in the steric hindrance on the surface of the heterojunction, hindering electron transfer and causing the photocurrent signal to decrease. Under the excitation of NIR light, the efficient charge separation of Z-type heterojunction enables the detection limit of the target to be as low as 0.45 pM, and the recovery rate in lake water samples can reach 91.85–103.41%. This sensor utilizes the strong penetration characteristic of NIR light to achieve the direct detection of DEHP in turbid water bodies.
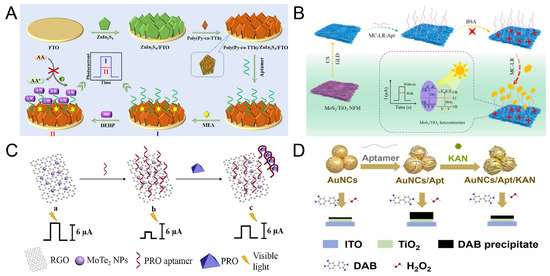
Figure 5.
(A) Schematic of the near-infrared light-responsive aptamer sensor for DEHP detection. Reproduced with permission from ref. [104]. Copyright 2025, Elsevier. (B) The Scheme of the PEC aptasensor for MC-LR detection. Reproduced with permission from ref. [108]. Copyright 2025, Elsevier. (C) The Scheme of PRO aptamer sensor activated by visible light. Reproduced with permission from ref. [106]. Copyright 2021, Elsevier. (D) Schematic of PEGFET sensor constructed with TiO2 photoresponse gate and aptamer probe for detecting kanamycin. Reproduced with permission from ref. [107]. Copyright 2025, Elsevier.
For the trace detection of microcystin toxin-LR (MC-LR), Liao et al. [108] established an aptamer sensor using MoS2/TiO2 nanofiber membranes (NFM) type II heterostructures (Figure 5B). The band offset of MoS2 and TiO2 forms Type II heterojunctions, effectively promoting the separation of photogenerated electron-hole pairs. The aptamer is fixed on the electrode surface and is used to specifically recognize MC-LR molecules. Under visible light irradiation, the combination of MC-LR and the aptamer causes the sensor to generate a photocurrent signal, and the sensor detection limit reaches 0.34 pM. This sensor realizes the portable and sensitive detection of MC-LR, and also has a good recovery rate for the detection of actual samples.
With the development of detection technology, the rapid and accurate detection of residual pesticides has become a hot topic worldwide. The increasing application of pesticides may lead to the consequence of environmental deterioration, thereby causing damage to human health. Profenofos (PRO) is a common moderately toxic organophosphate insecticide and has been classified as a Class II toxicity by the World Health Organization. As shown in Figure 5C, Wang’s team [106] developed a label-free PRO aptamer sensor using a one-step hydrothermal synthesis of molybdenum telluride/reduced graphene oxide (MoTe2/rGO) Schottky junction. The Schottky barrier formed by MoTe2 and rGO effectively inhibits the recombination of photogenerated electron-hole pairs and significantly improves the photoelectric conversion efficiency. The aptamer is fixed on the surface of MoTe2 through physical adsorption. In the presence of PRO, the stronger binding force between the aptamer and PRO causes the aptamer /PRO compound to be released from the electrode, reducing the resistance value. Under visible light, the detection limit of this sensor is 3.3 × 10−10 g/L.
Antibiotic residues in the environment may pose a serious threat to human health, so it is necessary to develop effective monitoring and control analysis strategies. As shown in Figure 5D, Ye et al. [107] proposed a PEGFET sensor based on TiO2 light-response gates and aptamer probes for the detection of kanamycin. This sensor uses TiO2 as the photosensitive layer for adsorbing UV light. The aptamer is attached to AuNCs for the recognition of kanamycin. When kanamycin is present, kanamycin molecules will bind to the surface of AuNCs through interaction with the aptamer to weaken the catalytic ability of AuNCs and increase the photocurrent. The high-sensitivity detection of kanamycin was achieved through the offset of the transmission curve and the current change after adding kanamycin. Under the condition of light exposure, the detection limit can reach 1.13 nM. Meanwhile, the detection of actual samples also proved the potential of this sensor in practical applications.
3.3. Biomedical Diagnostics
In the field of biomedical diagnostics, light-controlled aptamer technology is opening up new paths for the precise detection of biomarkers with its unique spatiotemporal regulation ability. Different research teams have developed a series of highly sensitive detection systems based on aptamers and light regulation, achieving certain progress in cancer diagnosis [124,125,126,127,128], organelle metabolism analysis [129,130,131,132,133,134], and intracellular molecular monitoring [135,136].
As shown in Figure 6A, Liu et al. [117] constructed a PEC aptamer sensor based on UCNPs and Y-type DNA, achieving highly sensitive detection of carcinoembryonic antigen (CEA). This sensor can regulate the dynamic switching of the signal pathway through near-infrared (NIR) light. Upconversion nanomaterials (ITO/SnS2/ZnIn2S4/UCNPs) were synthesized by electrostatic adsorption, and CDSe-modified DNA1 was connected to their surfaces through gold–sulfur bonds to further enhance the photoelectric conversion efficiency. The UCNPs in this nanomaterial can convert NIR light into ultraviolet-visible light, excite CdSe, and generate enhanced photocurrent. The Y-type DNA in the sensor is composed of DNA1, SiO2 NPS-labeled auxiliary DNA2, and CEA aptamer DNA. In the absence of a target, due to the relatively long distance between CdSe NPs and the electrode surface and the shielding effect of SiO2, the photocurrent decreases, resulting in the signal being turned off. When CEA is present, the aptamer specifically binds to CEA, causing conformational changes, resulting in the destruction of the Y-type structure. CdSe approaches the electrode, and the photocurrent recovers to generate a signal. By monitoring the photocurrent signal, highly sensitive detection of CEA can be achieved, with a detection limit of up to 0.3 pg/mL, and it has been successfully applied to the clinical detection of CEA in human serum samples. This light-controlled signal switching strategy provides a reliable technical means for the early diagnosis of cancer.
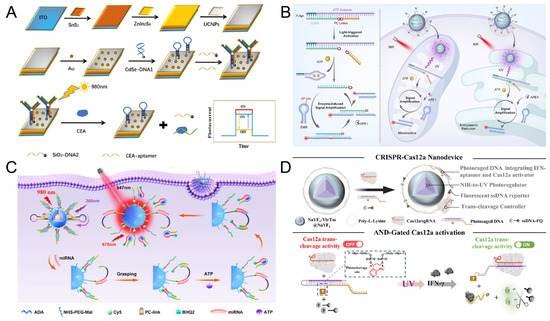
Figure 6.
(A) The scheme of PEC aptamer sensor based on UCNPs and Y-type DNA. Reproduced with permission from ref. [117]. Copyright 2023, Elsevier. (B) Schematic of the design principle of the light and enzyme dual-regulated aptamer sensor. Reproduced with permission from ref. [114]. Copyright 2025, Wiley-VCH. (C) Schematic diagram of ATP and NIR photocontrolled sensors used to detect intracellular miRNAs. Reproduced with permission from ref. [110]. Copyright 2023, American Chemical Society. (D) Schematic of the NIR photoactivatable aptamer-CRISPR Nanodevice for monitoring IFN-γ release. Reproduced with permission from ref. [116]. Copyright 2024, American Chemical Society.
ATP is the main energy currency within cells, and subcellular ATP dysregulation is associated with various disease conditions. As shown in Figure 6B, Feng et al. [114] developed a DNA nanodevice based on photoactivatable aptamers. This device combines exogenous light control and endogenous enzymatic reactions, achieving precise regulation of DNA nanostructures and specific signal amplification of ATP in organelles. The ATP aptamer was hybridized with the block chain (bDNA) modified by PC-linker to form the aptamer probe. Under ultraviolet light irradiation, the PC-linker breaks, releasing the hybrid chain of the aptamer and the bDNA fragment. In the presence of ATP, the aptamer binds to ATP and releases a bDNA fragment. This fragment can hybridize with EMB modified with AP site. In the presence of APE1, AP site is cleaved, fluorescent groups and quenching groups are separated, and a fluorescence signal is generated. Meanwhile, the released bDNA fragment can initiate the next hybridization cycle. The specificity and sensitivity of ATP detection have been further enhanced, and the detection limit of ATP can reach 0.44 μM. Subsequently, UCNPs were combined with the aptamer probe and EMB and functionalized with organelle targeting ligands to form DNA nanodevices. Notably, UCNPs enable the conversion of NIR light into UV emission, thereby activating the sensing function of the aptamer probe. This strategy achieves highly selective and sensitive imaging of ATP within subcellular organelles by coupling photonic energy transfer with aptamer-target recognition dynamics.
As shown in Figure 6C, Ren et al. [110] further advanced this paradigm by integrating endogenous ATP signaling with exogenous light-controlled regulation. They engineered a bioresponsive nanomaterial that responds to intracellular ATP levels to modulate NIR-switchable fluorescence output, enabling spatiotemporally resolved detection of miRNA within living cells. This DNA nanomachine consists of three parts: double-stranded DNA (A/B), miRNA recognition hairpins modified with PC-linker (DNA-C), and UCNPs. In double-stranded DNA, fluorescent groups are modified on DNA-A for signal output, and the DNA-b sequence contains ATP aptamer sequences for ATP recognition. Under 808 nm laser irradiation, UCNPs convert NIR light into UV light to induce the cleavage of the PC-linker. Subsequently, in the presence of miR-21, DNA-C hybridizes with miR-21 and captured DNA-B. The captured DNA-B combines with ATP to release DNA-C again to form a cycle. The generation of fluorescence signals was further enhanced. The detection limit of miR-21 triggered by ATP was 5.5 pM, achieving sensitive detection of miR-21. This strategy of combining intracellular metabolites (ATP) with the detection of target miR-21 provides a new idea for the monitoring of complex intracellular molecular networks.
As shown in Figure 6D, Liu et al. [116] constructed a photoactivatable aptamer-CRISPR nanodevice for the demand of in vivo detection, which was used to precisely analyze interferon -γ (IFN-γ) released by T cells in humanized mice. This device consists of UV-cleavable PC-DNA probes embedded with aptamers, UCNPs, and a CRISPR-Cas12a-enhanced fluorescence system. Under the irradiation of 980 nm NIR light, UCNPs converts NIR light into UV light, activates the PC-DNA probe, and releases the aptamer sequence. After the aptamer specifically binds to IFN-γ, it triggers the fluorescence signal amplification mediated by CRISPR-Cas12a, achieving real-time dynamic imaging of IFN-γ. The detection limit of IFN-γ can reach 0.28 pg/mg, and it has been successfully applied to real-time dynamic imaging in a humanized mouse inflammation model. This detection method based on photoactivatable aptamers shows high sensitivity and specificity both in vivo and in vitro, and has great potential in precise in vivo analysis.
4. Summary and Outlook
Aptamers, with their high affinity, high specificity, and versatile modification capabilities, have become important candidates in biosensing applications. The integration of light-responsive mechanisms not only expands the modulation strategies for aptamer functionality but also significantly enhances the selectivity, sensitivity, and dynamic response capabilities of POCT systems. This article reviews the applications of photoactivatable aptamers-based POCT platforms across diverse fields. The combination of the optical response element and the aptamer realizes the precise spatiotemporal regulation and highly sensitive detection of the target molecule. The photoactivatable aptamer can significantly improve the selectivity and anti-interference ability of detection through light-induced conformational changes, charge separation or energy transfer, combined with signal amplification strategies such as CRISPR/Cas systems and RCA. At present, photoactivatable aptamers have demonstrated broad utility in multiple fields such as food safety, environmental pollution, and biomedicine. Their advantages of rapid response, low cost, and high sensitivity provide a new direction for the development of POCT technology.
Despite these advancements, practical implementation of photoactivatable aptamers faces several challenges. For instance, the dependence of some photosensitive structures on UV or visible light limits their applicability for in vivo or deep tissue detection [137,138], while their high-energy photons may also cause DNA damage and cellular phototoxicity [139]. Although the NIR response system has strong penetration, it is limited by the Stokes shift effect and the non-radiative transition mechanism [140]. Its energy conversion efficiency is generally low, and it is easily interfered by the absorption bands of hemoglobin and water molecules in deep tissues, resulting in a significant attenuation of the effective photon flux actually reaching the target [141,142,143]. Furthermore, the inconsistency of lighting conditions not only leads to signal fluctuations but also faces more complex biological constraints in live applications. Especially for clinical transformation, the existing light control devices lack unified norms in terms of irradiation area, angle and dose standardization [144]. In terms of sensor construction, although the traditional covalent coupling strategy can achieve the stable combination of photoresponsive materials and aptamers, the immobilization orientation may lead to the distortion of the three-dimensional conformation of the aptamer, significantly reducing the molecular recognition sensitivity [145]. Therefore, the coupling mode of photoresponsive materials and adaptors still needs to be further optimized to improve the response efficiency and signal-to-noise ratio.
Looking ahead, the future directions can be expanded from the following aspects to promote photoactivatable aptamer POCT. Efforts should be made to develop more efficient photoresponsive materials to address the issues of insufficient UV light penetration and light scattering interference in biological samples, while enhancing the stability and biological safety of the materials in physiological environments. An attempt has been made to build a multi-functional integrated platform, combining the light control aptamer with microfluidic chips [146], smartphone imaging, and artificial intelligence algorithms [147] to construct a fully integrated POCT platform integrating “sample processing-light control response-multi-signal output-data analysis [148]”, achieving simultaneous detection of multiple targets and real-time feedback. In addition, the standardization and clinical transformation of photoactivatable aptamer POCT are also of vital importance. It is necessary to establish a unified evaluation system for photoactivatable aptamer detection, solve the problem of non-specific adsorption in complex samples, promote the transformation of related technologies from the laboratory to commercial POCT kits, and enable them to play a role in rapid diagnosis and personalized medicine.
In conclusion, as the intersection frontier of molecular recognition and light control technology, the light-activated aptamer will continue to drive POCT to evolve towards higher sensitivity, intelligence, and scene adaptability, providing innovative tools for precision medicine and environmental health monitoring.
Author Contributions
S.W.: Conceptualization, Investigation, Visualization, and Writing—original draft and review and editing. X.C.: Investigation and Writing—review and editing. Z.Z.: Writing—review and editing. J.Z.: Conceptualization, Supervision, Project administration, Writing—original draft preparation, Writing—review and editing, and Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (no. BK20242022), the Fundamental Research Funds for the Central Universities (no. 2024300408), and the State Key Laboratory of Analytical Chemistry for Life Science (no. 5431ZZXM2505).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mu, Y.; Chen, Z.; Zhan, J.; Zhang, J. Recent Advances in Aptamer-Based sensors for in vitro detection of small molecules. Anal. Sens. 2024, 4, e202400027. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Z.; Chen, M.; Xing, H.; Zhang, P.; Zhang, J. Cooperatively designed aptamer-PROTACs for spatioselective degradation of nucleocytoplasmic shuttling protein for enhanced combinational therapy. Chem. Sci. 2024, 15, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, T.; Lu, Y. Overcoming major barriers to developing successful sensors for practical applications using functional nucleic acids. Annu. Rev. Anal. Chem. 2022, 15, 151–171. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Shi, Y.; Yun, Y.; Wang, R.; Liu, Z.; Wu, Z.; Xiang, Y.; Zhang, J. Engineering covalent aptamer chimeras for enhanced autophagic degradation of membrane proteins. Angew. Chem. Int. Ed. 2025, 64, e202425123. [Google Scholar] [CrossRef]
- Wong, K.-Y.; Liu, Y.; Wong, M.-S.; Liu, J. Cornea-SELEX for aptamers targeting the surface of eyes and liposomal drug delivery. Exploration 2024, 4, 20230008. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Huang, W.; Yang, N.; Yuan, Q.; Yang, Y. Aptamer-functionalized field-effect transistor biosensors for disease diagnosis and environmental monitoring. Exploration 2023, 3, 20210027. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, Z.; An, Y.; Zhang, W.; Zhang, H.; Liu, D.; Yu, C.; Duan, W.; Yang, C.J. Selection of DNA aptamers against epithelial cell adhesion molecule for cancer cell imaging and circulating tumor cell capture. Anal. Chem. 2013, 85, 4141–4149. [Google Scholar] [CrossRef]
- Zheng, L.; Ye, Q.; Wang, M.; Sun, F.; Chen, Q.; Yu, X.; Wang, Y.; Liang, P. Research progress in small-molecule detection using aptamer-based SERS techniques. Biosensors 2025, 15, 29. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Cui, G.; Wang, X.; Xiang, S.; Huang, W.; Liu, C. RNA aptamer-based CRISPR-Cas12a system for enhanced small molecule detection and point-of-care testing. Int. J. Biol. Macromol. 2025, 303, 140675. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.H.; Fraser, A.G. Quantifying metabolites using structure-switching aptamers coupled to DNA sequencing. Nat. Biotechnol. 2025, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, J.-M.; Zha, C.-J.; Su, M.; Ying, Z.-M. Split T7 switch-based orthogonal logic operation of fluorogenic RNA aptamer for small molecules detection. Spectrochim. Acta Part A 2025, 336, 126044. [Google Scholar] [CrossRef]
- Hayat, M.; Bukhari, S.A.R.; Raza, M.; Aslam, A.; Liu, Z. Nanostructured aptasensors for ricin detection and tumor therapy: Exploring aptamer-protein interactions and conformational stability in biological complexities. Int. J. Biol. Macromol. 2025, 310, 143282. [Google Scholar] [CrossRef]
- Filius, M.; Fasching, L.; van Wee, R.; Rwei, A.Y.; Joo, C. Decoding aptamer-protein binding kinetics for continuous biosensing using single-molecule techniques. Sci. Adv. 2025, 11, eads9687. [Google Scholar] [CrossRef]
- Cui, S.; Wang, F.; Yang, W. Highly sensitive electrochemical PDGF-BB sensor based on protein-templated split aptamer ligation reaction. Sens. Actuators B 2025, 425, 136996. [Google Scholar] [CrossRef]
- Li, D.; Chen, Q.; Li, Y.; Yuan, R.; Xiang, Y. Programming catalytic nucleic acid amplification cascade for highly sensitive electrochemical aptamer-based angiopoietin like protein 4 biosensor. Sens. Actuators B 2025, 427, 137207. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Liu, R.; Li, X.; Zhang, J. Harnessing the CRISPR-Cas13d system for protein detection by dual-aptamer-based transcription amplification. Chem.—Eur. J. 2023, 29, e202202693. [Google Scholar] [CrossRef]
- Kalsoom, I.; Shehzadi, K.; Irfan, M.; Qiu, L.; Wang, Y.; Xu, Z.; Meng, Z. Structure switching aptamer enhance sensitivity and specificity of photonic crystal-based sensors for RSV-G protein detection. Biosens. Bioelectron. 2025, 273, 117091. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Li, X.; Gao, Y.; Yong, W.; Jin, Y.; Dong, Y. Development of aptamer-based lateral flow devices for rapid detection of SARS-CoV-2 S protein and uncertainty assessment. Talanta 2025, 281, 126825. [Google Scholar] [CrossRef]
- Goto, K.; Amano, R.; Ichinose, A.; Michishita, A.; Hamada, M.; Nakamura, Y.; Takahashi, M. Generation of RNA aptamers against chikungunya virus E2 envelope protein. J. Virol. 2025, 29, e02095-24. [Google Scholar] [CrossRef] [PubMed]
- Nourry, J.; Chevalier, P.; Laurenceau, E.; Cattoen, X.; Bertrand, X.; Peres, B.; Oukacine, F.; Peyrin, E.; Choisnard, L. Whole-cell aptamer-based techniques for rapid bacterial detection: Alternatives to traditional methods. J. Pharm. Biomed. Anal. 2025, 255, 116661. [Google Scholar] [CrossRef] [PubMed]
- Fatah, S.A.; Omer, K.M. Aptamer-modified MOFs (Aptamer@ MOF) for efficient detection of bacterial pathogens: A Review. ACS Appl. Mater. Interfaces 2025, 17, 11578–11594. [Google Scholar] [CrossRef]
- Liu, R.; Yun, Y.; Feng, Z.-Y.; Chen, M.; Zhang, J. Rational design of trident aptamer scaffold for rapid and accurate monitoring of 25-Hydroxyvitamin D3 metabolism in living cells. Anal. Chem. 2023, 95, 10322–10329. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Chen, P.; Zhong, S.; Yang, Y. Nucleic acid aptamer-based sensors for bacteria detection: A Review. BioEssays 2025, 47, e202400111. [Google Scholar] [CrossRef]
- Li, L.; Wan, J.; Wen, X.; Guo, Q.; Jiang, H.; Wang, J.; Ren, Y.; Wang, K. Identification of a new DNA aptamer by tissue-SELEX for cancer recognition and imaging. Anal. Chem. 2021, 93, 7369–7377. [Google Scholar] [CrossRef]
- Hsu, Y.-W.; Ma, L.; Tang, Y.; Li, M.; Zhou, C.; Geng, Y.; Zhang, C.; Wang, T.; Guo, W.; Li, M. The application of aptamers in the repair of bone, nerve, and vascular tissues. J. Mater. Chem. B 2025, 13, 1872–1889. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Zhang, S.; Liu, F.; Mao, C.; Li, M.; Jiang, J.; Zhang, Y.; Fan, C.; Zuo, X. DNA Framework-ensembled aptamers enhance fluid stability in circulating tumor cells capture for tumor treatment evaluation. Angew. Chem. Int. Ed. 2025, e202425252. [Google Scholar] [CrossRef]
- Qin, X.; Li, D.; Qin, X.; Chen, F.; Guo, H.; Gui, Y.; Zhao, J.; Jiang, L.; Luo, D. Electrochemical detection of the cardiac biomarker cardiac troponin I. View 2024, 5, 20240025. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, D.; Yang, M.; Liu, S.; Fang, Y.; Huang, S. A Highly sensitive electrochemical aptasensor for kanamycin: Leveraging recJf exonuclease-assisted target recycling and hybridization chain reaction signal amplification. J. Anal. Test. 2025, 9, 96–108. [Google Scholar] [CrossRef]
- Adachi, T.; Nakamura, Y. Aptamers: A review of their chemical properties and modifications for therapeutic application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl. Mater. Interfaces 2020, 13, 9500–9519. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T.; Sharma, T.K. Aptasensors for full body health checkup. Biosens. Bioelectron. 2022, 11, 100199. [Google Scholar] [CrossRef]
- Farid, S.; Ghosh, S.; Dutta, M.; Stroscio, M.A. Aptamer-based optical and electrochemical sensors: A review. Chemosensors 2023, 11, 569. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; He, S.; Deng, L.; Wang, H.; Cao, Z.; Feng, Z.; Xiong, B.; Yin, Y. A brief review of aptamer-based biosensors in recent years. Biosensors 2025, 15, 120. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, M.; Liu, R.; Fan, H.; Zhang, J. A cocktail therapeutic strategy based on clofarabine-containing aptamer-PROTAC for enhanced cancer therapy. Chem. Comm. 2023, 59, 11560–11563. [Google Scholar] [CrossRef]
- Yu, H.; Alkhamis, O.; Canoura, J.; Liu, Y.; Xiao, Y. Advances and challenges in small-molecule DNA aptamer isolation, characterization, and sensor development. Angew. Chem. Int. Ed. 2021, 60, 16800–16823. [Google Scholar] [CrossRef]
- Ali, G.K.; Omer, K.M. Molecular imprinted polymer combined with aptamer (MIP-aptamer) as a hybrid dual recognition element for bio (chemical) sensing applications. Review. Talanta 2022, 236, 122878. [Google Scholar] [CrossRef]
- Wang, K.; Wang, M.; Ma, T.; Li, W.; Zhang, H. Review on the selection of aptamers and application in paper-based sensors. Biosensors 2022, 13, 39. [Google Scholar] [CrossRef]
- Chung, Y.-D.; Tsai, Y.-C.; Wang, C.-H.; Lee, G.-B. Aptamer selection via versatile microfluidic platforms and their diverse applications. Lab Chip 2025, 25, 1047–1080. [Google Scholar] [CrossRef]
- Yuan, M.; Li, C.; Wang, M.; Cao, H.; Ye, T.; Hao, L.; Wu, X.; Yin, F.; Yu, J.; Xu, F. Low-cost, portable, on-site fluorescent detection of As (III) by a paper-based microfluidic device based on aptamer and smartphone imaging. Microchim. Acta 2023, 190, 109. [Google Scholar] [CrossRef] [PubMed]
- Inês, A.; Cosme, F. Biosensors for Detecting Food Contaminants—An Overview. Processes 2025, 13, 380. [Google Scholar] [CrossRef]
- Shaban, S.M.; Kim, D.-H. Recent advances in aptamer sensors. Sensors 2021, 21, 979. [Google Scholar] [CrossRef]
- Chen, Y.; Morihiro, K.; Nemoto, Y.; Ichimura, A.; Ueki, R.; Sando, S.; Okamoto, A. Selective inhibition of cancer cell migration using a pH-responsive nucleobase-modified DNA aptamer. Chem. Sci. 2024, 15, 17097–17102. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, Y.; Zeng, J.; Han, S.; Wang, Y.; Su, X.; Li, X.; Liang, T.; Liu, J.; Qu, P. Temporal responsive vesicle-INFγ aptamer-PEI/HA system targeted activation of B-CD4T-Tfh-CD8T cells cascade inhibits TNBC progression. Nano Today 2025, 62, 102708. [Google Scholar] [CrossRef]
- Wang, C.; O’Hagan, M.P.; Li, Z.; Zhang, J.; Ma, X.; Tian, H.; Willner, I. Photoresponsive DNA materials and their applications. Chem. Soc. Rev. 2022, 51, 720–760. [Google Scholar] [CrossRef]
- Aqib, R.M.; Umer, A.; Li, J.; Liu, J.; Ding, B. Light responsive DNA nanomaterials and their biomedical applications. Chem.-Asian J. 2024, 19, e202400226. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, C.; Liu, Q.; Zhong, Y.; Zhu, L.; Zhao, Y. Combination of adenosine blockade and ferroptosis for photo-immunotherapy of triple negative breast cancer with aptamer-modified copper sulfide. J. Mater. Chem. B 2025, 13, 2504–2519. [Google Scholar] [CrossRef]
- Cesarini, V.; Appleton, S.L.; de Franciscis, V.; Catalucci, D. The recent blooming of therapeutic aptamers. Mol. Asp. Med. 2025, 102, 101350. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, T.; Wang, S.; Wang, W. A biomimetic 3D DNA nanoplatform for enhanced capture and high-purity isolation of stem cell exosomes. Anal. Methods 2025, 17, 388–394. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Liang, H.; Lin, X.H.; Li, J.; Yang, H.H. Light-induced activation of c-Met signalling by photocontrolled DNA assembly. Chem.-Eur. J. 2018, 24, 15988–15992. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zhang, X.; Lake, R.J.; Pawel, G.T.; Guo, Z.; Pei, R.; Lu, Y. A photo-regulated aptamer sensor for spatiotemporally controlled monitoring of ATP in the mitochondria of living cells. Chem. Sci. 2020, 11, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Z.; Lu, Y. Photocaged functional nucleic acids for spatiotemporal imaging in biology. Curr. Opin. Chem. Biol. 2020, 57, 95–104. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, G.; Liu, Q.; Liu, W.; Weng, Y.; Zhao, Y.; Qu, F.; Li, L.; Huang, Y. A photo-triggerable aptamer nanoswitch for spatiotemporal controllable siRNA delivery. Nanoscale 2020, 12, 10939–10943. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, D.; Yun, Y.; Feng, Z.; Zheng, F.; Xiang, Y.; Fan, H.; Zhang, J. Photoactivatable engineering of CRISPR/Cas9-inducible DNAzyme probe for in situ imaging of nuclear zinc ions. Angew. Chem. Int. Ed. 2024, 63, e202315536. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Li, B.; Zhang, J. Photoactivable CRISPR for biosensing and cancer therapy. ChemBioChem 2024, 25, e202400685. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, Y.; Yuan, E.; Li, W.; Jiang, Z.; Wei, L.; Su, H.; Zeng, W.; Gan, Y.; Wang, Z. Obtaining more accurate signals: Spatiotemporal imaging of cancer sites enabled by a photoactivatable aptamer-based strategy. ACS Appl. Mater. Interfaces 2016, 8, 23542–23548. [Google Scholar] [CrossRef]
- Ranzani, A.T.; Buchholz, K.; Blackholm, M.; Kopkin, H.; Möglich, A. Induction of bacterial expression at the mRNA level by light. Nucleic Acids Res. 2024, 52, 10017–10028. [Google Scholar] [CrossRef]
- Motohashi, Y.; Nishihara, T.; Tanabe, K. Preparation of a multifunctional photoactivated prodrug on a streptavidin scaffold bearing a DNA aptamer. Bioorg. Med. Chem. Lett. 2022, 71, 128819. [Google Scholar] [CrossRef]
- Ye, M.; Hu, S.; Zhou, L.; Tang, X.; Zhao, S.; Zhao, J. Fluidic membrane accelerating the kinetics of photoactivatable hybridization chain reaction for accurate imaging of tumor-derived exosomes. Anal. Chem. 2022, 94, 17645–17652. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, S.; Li, X.; Zhang, J. Molecular engineering of functional DNA molecules toward point-of-care diagnostic devices. Chem. Commun. 2025, 61, 4316–4338. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Yu, C.; Han, F.; Mao, Q.; Jing, W.; Yang, Z.; Pires, N.M.M.; Correia, J.H.; Lin, Q.; Hu, F. Cutting-edge RNA quantitative detection methods for rapid and on-site analysis. TrAC Trends Anal. Chem. 2025, 190, 118274. [Google Scholar] [CrossRef]
- Khan, A.R.; Hussain, W.L.; Shum, H.C.; Hassan, S.U. Point-of-care testing: A critical analysis of the market and future trends. Front. Lab Chip Technol. 2024, 3, 1394752. [Google Scholar] [CrossRef]
- Han, G.-R.; Goncharov, A.; Eryilmaz, M.; Ye, S.; Palanisamy, B.; Ghosh, R.; Lisi, F.; Rogers, E.; Guzman, D.; Yigci, D. Machine learning in point-of-care testing: Innovations, challenges, and opportunities. Nat. Commun. 2025, 16, 3165. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, C.; Lv, Q.; Lei, L. Emerging point-of-care testing technology for the detection of animal pathogenic microorganisms. Chem. Eng. J. 2025, 512, 162548. [Google Scholar] [CrossRef]
- Raja, M.; Musi, R.; Fattorini, M.; Piva, E.; Putoto, G. Point of care testing and transfusion safety in resource limited settings: A review. J. Blood Disord. Transfus. 2015, 6, 269. [Google Scholar]
- Fonseca, W.T.; Parra Vello, T.; Lelis, G.C.; Ferreira Deleigo, A.V.r.; Takahira, R.K.; Martinez, D.S.T.; de Oliveira, R.F. Chemical sensors and biosensors for point-of-care testing of pets: Opportunities for individualized diagnostics of companion animals. ACS Sens. 2025, 10, 3222–3238. [Google Scholar] [CrossRef]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-based rapid diagnosis for point-of-care application. Microfluid. Nanofluid. 2023, 27, 15. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Zhao, Y.; Zhu, C.; Yang, R.; Fang, M.; Luan, Y. Aptamer-based point-of-care-testing for small molecule targets: From aptamers to aptasensors, devices and applications. TrAC Trends Anal. Chem. 2023, 169, 117408. [Google Scholar] [CrossRef]
- Lan, Y.; He, B.; Tan, C.S.; Ming, D. Applications of smartphone-based aptasensor for diverse targets detection. Biosensors 2022, 12, 477. [Google Scholar] [CrossRef]
- Yin, B.; Wan, X.; Sohan, A.M.F.; Lin, X. Microfluidics-based POCT for SARS-CoV-2 diagnostics. Micromachines 2022, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, L.; Yang, S. Advancements in the research of finger-actuated POCT chips. Microchim. Acta 2024, 191, 65. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Amin, K.; Yu, H.; Yang, H.; Guo, Z.; Liu, J. Advances in aptamers, and application of mycotoxins detection: A review. Food Res. Int. 2023, 170, 113022. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Su, X.; Ji, S.; Ma, Z.; Gao, Y.; Song, X. The development potential of AuNPs-based lateral flow technology combined with other advanced technologies in POCT. Appl. Biochem. Biotechnol. 2025, 197, 2867–2886. [Google Scholar] [CrossRef]
- Song, W.; Hu, J.-J.; Song, S.-J.; Xu, Y.; Yang, H.; Yang, F.; Zhou, Y.; Yu, T.; Qiu, W.-X. Aptamer-gold nanocage composite for photoactivated immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 42931–42939. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, J.; Dong, C.; Li, Y.; Yu, Q.; Wang, X.; Chen, Z.; Li, J.; Yang, Y.; Wang, H. Polyvalent aptamer-functionalized NIR-II quantum dots for targeted theranostics in high PD-L1-expressing tumors. ACS Appl. Mater. Interfaces 2024, 16, 21571–21581. [Google Scholar] [CrossRef]
- Prante, M.; Segal, E.; Scheper, T.; Bahnemann, J.; Walter, J. Aptasensors for point-of-care detection of small molecules. Biosensors 2020, 10, 108. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Y.; Jiang, X.; Tan, H.; Ying, B. Translation of aptamers toward clinical diagnosis and commercialization. Biosens. Bioelectron. 2022, 208, 114168. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, T.; Lu, Y. Translating in vitro diagnostics from centralized laboratories to point-of-care locations using commercially-available handheld meters. TrAC Trends Anal. Chem. 2020, 124, 115782. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, J.; Yang, Z.; Mou, Q.; Ma, Y.; Xiong, Y.; Lu, Y. Functional DNA regulated CRISPR-Cas12a sensors for point-of-care diagnostics of non-nucleic-acid targets. J. Am. Chem. Soc. 2019, 142, 207–213. [Google Scholar] [CrossRef]
- Duan, Z.; Tan, L.; Duan, R.; Chen, M.; Xia, F.; Huang, F. Photoactivated biosensing process for dictated ATP detection in single living cells. Anal. Chem. 2021, 93, 11547–11556. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Lin, M.; Duan, R.; Lou, X.; Xia, F.; Willner, I. Photoactivated specific mRNA detection in single living cells by coupling “signal-on” fluorescence and “signal-off” electrochemical signals. Nano Lett. 2018, 18, 5116–5123. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Han, B.; Zhou, G.; Li, Z.; Xue, M.; Liang, B.; Liu, P.; Cheng, Y. Light-activated aptasensor for plug-and-play detection of Aflatoxin B1 in food samples. Anal. Chim. Acta 2025, 1346, 343786. [Google Scholar] [CrossRef]
- Quillin, A.L.; Karloff, D.B.; Ayele, T.M.; Flores, T.F.; Chen, G.; McEachin, Z.T.; Valdez-Sinon, A.N.; Heemstra, J.M. Imaging and tracking RNA in live mammalian cells via fluorogenic photoaffinity labeling. ACS Chem. Biol. 2025, 20, 707–720. [Google Scholar] [CrossRef]
- Wakano, M.; Tsunoda, M.; Murayama, K.; Morimoto, J.; Ueki, R.; Aoyama-Ishiwatari, S.; Hirabayashi, Y.; Asanuma, H.; Sando, S. Reversible optical control of receptor tyrosine kinase activity and ERK dynamics using azobenzene-carrying DNA aptamer agonist. J. Am. Chem. Soc. 2025, 147, 11477–11484. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, B.; Zhu, J.; Lou, S.; Hao, H.; Lu, W. Light-activated, dual-mode fluorescence and colorimetric detection of estradiol with high fidelity based on aptamer’s special recognition. Food Chem. 2024, 436, 137702. [Google Scholar] [CrossRef]
- Lu, W.; Lou, S.; Yang, B.; Guo, Z.; Tian, Z. Light-activated oxidative capacity of isoquinoline alkaloids for universal, homogeneous, reliable, colorimetric assays with DNA aptamers. Talanta 2024, 279, 126667. [Google Scholar] [CrossRef]
- Zhu, A.; Jiao, T.; Ali, S.; Xu, Y.; Ouyang, Q.; Chen, Q. SERS sensors based on aptamer-gated mesoporous silica nanoparticles for quantitative detection of staphylococcus aureus with signal molecular release. Anal. Chem. 2021, 93, 9788–9796. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Etemad-Moghadam, S.; Alaeddini, M.; Miri Mousavi, R.s.; Bahador, A. DNA-aptamer-nanographene oxide as a targeted bio-theragnostic system in antimicrobial photodynamic therapy against Porphyromonas gingivalis. Sci. Rep. 2022, 12, 12161. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, J.; Wen, R.; Tian, J.; Lu, J. A photoelectrochemical aptasensor for omethoate determination based on a photocatalysis of CeO2@ MnO2 heterojunction for glucose oxidation. Anal. Chim. Acta 2024, 1293, 342284. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, K.; Liu, Y.; Qin, Y.; Wang, X.; Qi, L.; Shi, D. Photo-controlled aptamers delivery by dual surface gold-magnetic nanoparticles for targeted cancer therapy. Mater. Sci. Eng. 2017, 80, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Chen, M.; Zhang, J.; Sun, X.; Zhou, H.; Gao, Z. Application of near-infrared-activated and ATP-responsive trifunctional upconversion nano-jelly for in vivo tumor imaging and synergistic therapy. Biosens. Bioelectron. 2024, 250, 116094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, J.; Xue, W.; Di, Z.; Xing, H.; Lu, Y.; Li, L. Upconversion luminescence-activated DNA nanodevice for ATP sensing in living cells. J. Am. Chem. Soc. 2018, 140, 578–581. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, P.; Li, W.; Ye, L.; Li, L.; Li, Z.; Li, M. Near-infrared light-activatable spherical nucleic acids for conditional control of protein activity. Angew. Chem. Int. Ed. 2022, 61, e202117562. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, T.; Zhang, Y.; Ling, N.; Zheng, L.; Zhang, S.-L.; Sun, Y.; Wang, X.; Ye, Y. Engineering of a dual-recognition ratiometric fluorescent nanosensor with a remarkably large stokes shift for accurate tracking of pathogenic bacteria at the single-cell level. Anal. Chem. 2020, 92, 13396–13404. [Google Scholar] [CrossRef]
- Ye, Y.; Zheng, L.; Wu, T.; Ding, X.; Chen, F.; Yuan, Y.; Fan, G.-C.; Shen, Y. Size-dependent modulation of polydopamine nanospheres on smart nanoprobes for detection of pathogenic bacteria at single-cell level and imaging-guided photothermal bactericidal activity. ACS Appl. Mater. Interfaces 2020, 12, 35626–35637. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, S.; Huang, Y.; Zhang, L.; Li, Z.; Hou, Y.; Zhang, J.; Wang, Y.; Zhu, C.; Zhou, D. A one-pot method based on rolling circle amplification and light-activated CRISPR/Cas12a reaction for simple and highly sensitive detection of Staphylococcus aureus. Chem. Eng. J. 2023, 477, 146814. [Google Scholar] [CrossRef]
- Zhang, J.J.; Nie, C.; Fu, W.L.; Cheng, F.L.; Chen, P.; Gao, Z.F.; Wu, Y.; Shen, Y. Photoresponsive DNA-modified magnetic bead-assisted rolling circle amplification-driven visual photothermal sensing of Escherichia coli. Anal. Chem. 2022, 94, 16796–16802. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, B.; Hamed, E.M.; Qin, M.; Ji, L.; Qi, S.; Li, S.F.Y.; Wang, Z. Aptamer-driven multifunctional nanoplatform for near-infrared fluorescence imaging and rapid in situ inactivation of salmonella typhimurium. Anal. Chem. 2025, 97, 1889–1899. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Zong, F.; Li, J.; Xu, Y.; Zhang, Z. Visible light-activated signal amplification PEC aptasensor for ultrasensitive zearalenone detection based on double Z-type BiOI/g-C3N4@ Au/Bi2S3 heterojunctions. Microchem. J. 2024, 207, 112159. [Google Scholar] [CrossRef]
- He, Z.; Zhang, J.; Liu, M.; Meng, Y. Polyvalent aptamer scaffold coordinating light-responsive oxidase-like nanozyme for sensitive detection of zearalenone. Food Chem. 2024, 431, 136908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, Z.; Lin, X.; Han, F.; Liang, Z.; Huang, L.; Dai, M.; Han, D.; Han, L.; Niu, L. A label-free PEC aptasensor platform based on g-C3N4/BiVO4 heterojunction for tetracycline detection in food analysis. Food Chem. 2023, 402, 134258. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Du, X.; Sun, J.; Zhang, B.; Lian, Y.; Zheng, M.; Zhu, R.; Geng, L. Persulfate mediated single-channel bimodal self-checking light-assisted self-powered aptasensor for highly accurate assay. Sens. Actuators B 2023, 397, 134647. [Google Scholar] [CrossRef]
- Yang, H.; Tu, C.; Zhuang, Y.; Li, Y.; Hao, Y.; Yang, J.; Zhang, L.; Zhang, Y.; Yu, J. Near-infrared light-driven photoelectrochemical aptasensor based on direct Z-scheme poly (pyrrole-co-thiophene)/ZnIn2S4 heterostructure for sensitive detection of di (2-ethylhexyl) phthalate. Sens. Actuators B 2025, 427, 137183. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Pu, L.; Wang, T.; Liu, Y.; Zhou, X.; Wang, K. Visible light-responsive enrofloxacin PEC aptasensor based on CN QDs sensitized Bi4O5Br2 nanosheets. Anal. Chim. Acta 2025, 1337, 343545. [Google Scholar] [CrossRef]
- Ding, L.; Wei, J.; Qiu, Y.; Wang, Y.; Wen, Z.; Qian, J.; Hao, N.; Ding, C.; Li, Y.; Wang, K. One-step hydrothermal synthesis of telluride molybdenum/reduced graphene oxide with Schottky barrier for fabricating label-free photoelectrochemical profenofos aptasensor. Chem. Eng. J. 2021, 407, 127213. [Google Scholar] [CrossRef]
- Ye, Z.; Qin, H.; Wei, X.; Tao, T.; Li, Q.; Mao, S. Antibiotic residue detection by novel photoelectrochemical extended-gate field-effect transistor sensor. J. Hazard. Mater. 2025, 485, 136897. [Google Scholar] [CrossRef]
- Liao, X.; Liu, S.; Li, L.; Li, S.; Zhang, Y.; Zhao, F.; Tang, Y.; Liu, R. Visible light-activated photoelectrochemical aptasensor for microcystin-LR detection based on DFT-proved Type-Ⅱ heterojunction MoS2/TiO2 NFM. Microchem. J. 2025, 212, 113497. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, Y.; Zhao, W.; Zhang, S. Light-activated nanodevice for on-demand imaging of miRNA in living cells via logic assembly. ACS Appl. Mater. Interfaces 2022, 14, 13070–13078. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, C.; Zhang, Y.; Li, M.; Zhang, Y.; Shi, X.; Wang, Q.; Zhang, S.; Song, X. Construction of a near-infrared photoswitched nanomachine powered by an endogenous trigger for activatable imaging of intracellular microRNA and amplified photodynamic therapy for cancer cells. ACS Appl. Mater. Interfaces 2023, 15, 56881–56894. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, L.; Chen, Y.; Lin, Z.; Ruan, X.; Lin, Q.; Xing, C.; Lu, C. Construction of an exogenously and endogenously Co-activated DNA logic amplifier for highly reliable intracellular MicroRNA imaging. Biosens. Bioelectron. 2024, 259, 116409. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Yun, D.; Jung, C. One-pot RPA/CRISPR-Cas12a assay with photomodulated aptamer-based inhibitors. Sens. Actuators B 2024, 412, 135790. [Google Scholar] [CrossRef]
- Zhang, X.; Song, C.; Yang, K.; Hong, W.; Lu, Y.; Yu, P.; Mao, L. Photoinduced regeneration of an aptamer-based electrochemical sensor for sensitively detecting adenosine triphosphate. Anal. Chem. 2018, 90, 4968–4971. [Google Scholar] [CrossRef]
- Feng, X.; Yi, D.; Li, L.; Li, M. Exogenously and endogenously sequential regulation of DNA nanodevices enables organelle-specific signal amplification in subcellular ATP profiling. Angew. Chem. Int. Ed. 2025, 64, e202422651. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, X.; Li, Y.; Ye, Y.; Yang, L.; Liao, L.; Deng, Y.; Liu, J.; Zhang, J.; Zheng, C. Scandium-mediated photosensitization oxidation: A new strategy for fast and neutral pH colorimetric detection of cocaine by coupling split aptamer. Sens. Actuators B 2023, 380, 133349. [Google Scholar] [CrossRef]
- Liu, Z.; Duan, X.; Yun, Y.; Li, S.; Feng, Z.; Zhan, J.; Liu, R.; Li, Y.; Zhang, J. Photoactivatable aptamer-CRISPR nanodevice enables precise profiling of interferon-gamma release in humanized mice. ACS Nano 2024, 18, 3826–3838. [Google Scholar] [CrossRef]
- Liu, B.; Ge, Y.; Lu, Y.; Huang, Y.; Zhang, X.; Yuan, X. An NIR light-responsive “on-off-on” photoelectrochemical aptasensor for carcinoembryonic antigen assay based on Y-shaped DNA. Biosens. Bioelectron. 2023, 229, 115241. [Google Scholar] [CrossRef]
- Zhang, J.; Smaga, L.P.; Satyavolu, N.S.R.; Chan, J.; Lu, Y. DNA aptamer-based activatable probes for photoacoustic imaging in living mice. J. Am. Chem. Soc. 2017, 139, 17225–17228. [Google Scholar] [CrossRef]
- Kang, Q.; Xing, X.-y.; Zhang, S.-q.; He, L.; Li, J.-z.; Jiao, J.-b.; Du, X.-j.; Wang, S. A novel aptamer-induced CHA amplification strategy for ultrasensitive detection of Staphylococcus aureus and NIR-triggered photothermal bactericidal activity based on aptamer-modified magnetic Fe3O4@ AuNRs. Sens. Actuators B 2023, 382, 133554. [Google Scholar] [CrossRef]
- Yu, T.; Xu, H.; Zhao, Y.; Han, Y.; Zhang, Y.; Zhang, J.; Xu, C.; Wang, W.; Guo, Q.; Ge, J. Aptamer based high throughput colorimetric biosensor for detection of staphylococcus aureus. Sci. Rep. 2020, 10, 9190. [Google Scholar] [CrossRef]
- Taguchi, Y.; Toma, K.; Iitani, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. In vitro performance of a long-range surface plasmon hydrogel aptasensor for continuous and real-time vancomycin measurement in human serum. ACS Appl. Mater. Interfaces 2024, 16, 28162–28171. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Luo, S.; He, Y.; Xiao, Y.; Liang, Y.; Zhang, L.; Li, W.; Zhang, Y.; Li, L. Simple and sensitive SERS platform for Staphylococcus aureus one-pot determination by photoactivated CRISPR/Cas12a cascade system and core–shell DNA tetrahedron@ AuNP@ Fe3O4 reporter. Microchim. Acta 2025, 192, 240. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Wu, G.; Ding, Z.; Xie, J. Construction of a nanoscale metal-organic framework aptasensor for fluorescence ratiometric sensing of AFB1 in real samples. Food Chem. 2023, 416, 135805. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Zhang, J.; Cao, D.-X.; Tang, A.-N.; Kong, D.-M. Telomerase-activated Au@ DNA nanomachine for targeted chemo-photodynamic synergistic therapy. RSC Med. Chem. 2023, 14, 2268–2276. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Qi, Y.; Wang, G.; Li, L.; Jin, Z.; Tian, J.; Du, Y. PD-L1 aptamer-functionalized metal-organic framework nanoparticles for robust photo-immunotherapy against cancer with enhanced safety. Angew. Chem. Int. Ed. 2023, 62, e202214750. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Duan, J.; Ma, Y.; Gao, H.; Zhang, Z.; Liu, J.; Shi, J.; Zhang, K. Photocontrolled spatiotemporal delivery of DNA immunomodulators for enhancing membrane-targeted tumor photodynamic immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 44183–44198. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, L.; Jehan, S.; Wang, H.; Chen, X.; Zhao, S.; Zhou, W. Activable photodynamic DNA probe with an “AND” logic gate for precision skin cancer therapy. Research 2024, 7, 0295. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Shu, Y.; Wang, J. DNA hairpin self-assembly on cell membrane triggered by Dual-aptamer logic circuit for cancer cell recognition and photodynamic therapy. Sens. Actuators B 2023, 391, 134063. [Google Scholar] [CrossRef]
- Zheng, L.-e.; Huang, M.; Liu, Y.; Bao, Q.; Huang, Y.; Ye, Y.; Liu, M.; Sun, P. Colorimetric aptasensor based on temporally controllable light-stimulated oxidase-mimicking fluorescein for the sensitive detection of exosomes in mild conditions. Anal. Methods 2024, 16, 3577–3586. [Google Scholar] [CrossRef]
- Liu, B.; Ma, R.; Zhao, J.; Zhao, Y.; Li, L. A smart DNA nanodevice for ATP-activatable bioimaging and photodynamic therapy. Sci. China Chem. 2020, 63, 1490–1497. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Ruan, Y.-F.; Liu, Y.-L.; Han, D.-M.; Zhou, H.; Zhao, W.-W.; Jiang, D.; Xu, J.-J.; Chen, H.-Y. Photocontrolled nanopipette biosensor for ATP gradient electroanalysis of single living cells. ACS Sens. 2021, 6, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xie, G.; Liu, P.; Kong, X.-Y.; Song, Y.; Wen, L.; Jiang, L. Light-driven ATP transmembrane transport controlled by DNA nanomachines. J. Am. Chem. Soc. 2018, 140, 16048–16052. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Sun, X.-M.; Xin, M.-K.; Chen, Y.-L.; He, J.-W.; Liu, D.; Li, C.-Y. Light-gated cascade amplification DNA circuit under the encapsulation of a natural protein-derived facilitate nanocarrier for spatiotemporal sensing in living cells. Sens. Actuators B 2022, 373, 132715. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Liu, D.; Zhang, L.; Zheng, L.; Nie, G. Highly efficient photoelectrochemical aptasensor based on CdS/CdTe QDs co-sensitized TiO2 nanoparticles designed for thrombin detection. Microchim. Acta 2024, 191, 216. [Google Scholar] [CrossRef]
- Duan, Z.; Ouyang, Y.; Fu, Y.; Huang, F.; Xia, F.; Willner, I. Optical and electrochemical probes for monitoring cytochrome c in subcellular compartments during apoptosis. Angew. Chem. Int. Ed. 2023, 62, e202301476. [Google Scholar] [CrossRef]
- Newman, S.S.; Wilson, B.; Zheng, L.; Eisenstein, M.; Soh, T. Multiplexed assay for small-molecule quantification via photo-cross-linking of structure switching aptamers. ACS Omega 2024, 9, 43785–43792. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Li, C.; Zuo, J.; Zhang, L.; Chang, Y.; Zhang, Y.; Tu, L.; Liu, X.; Xue, B.; Li, Q.; Zhao, H. Accurate quantitative sensing of intracellular pH based on self-ratiometric upconversion luminescent nanoprobe. Sci. Rep. 2016, 6, 38617. [Google Scholar] [CrossRef]
- de Gruijl, F.R.; van Kranen, H.J.; Mullenders, L.H. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J. Photochem. Photobiol. B 2001, 63, 19–27. [Google Scholar] [CrossRef]
- MacKenzie, L.E.; Harvey, A.R. Oximetry using multispectral imaging: Theory and application. J. Opt. 2018, 20, 063501. [Google Scholar] [CrossRef]
- Mironova, K.; Khochenkov, D.; Generalova, A.; Rocheva, V.; Sholina, N.; Nechaev, A.; Semchishen, V.; Deyev, S.; Zvyagin, A.; Khaydukov, E. Ultraviolet phototoxicity of upconversion nanoparticles illuminated with near-infrared light. Nanoscale 2017, 9, 14921–14928. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Mooney, R.; Cornejo, Y.R.; Schena, E.; Berlin, J.M.; Aboody, K.S.; Saccomandi, P. Thermal analysis of laser irradiation-gold nanorod combinations at 808 nm, 940 nm, 975 nm and 1064 nm wavelengths in breast cancer model. Int. J. Hyperth. 2021, 38, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E.U. Activatable photosensitizers: Agents for selective photodynamic therapy. Adv. Funct. Mater. 2017, 27, 1604053. [Google Scholar] [CrossRef]
- Allison, R.R. Photodynamic therapy: Oncologic horizons. Future Oncol. 2014, 10, 123–124. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, X.; Zhao, Z.; Zhu, G.; Chen, T.; Fu, T.; Tan, W. DNA nanoflowers for multiplexed cellular imaging and traceable targeted drug delivery. Angew. Chem. Int. Ed. 2014, 126, 5931–5936. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, J.; Zhao, K. A finger actuated POCT microfluidic chip for on-site multiple detection. Microchem. J. 2025, 212, 113390. [Google Scholar] [CrossRef]
- de Plaza, M.A.P.; Lambrakis, K.; Marmolejo-Ramos, F.; Beleigoli, A.; Archibald, M.; Yadav, L.; McMillan, P.; Clark, R.; Lawless, M.; Morton, E. Human-centred AI for emergency cardiac care: Evaluating RAPIDx AI with PROLIFERATE_AI. Int. J. Med. Inf. 2025, 196, 105810. [Google Scholar] [CrossRef]
- Rajput, Y.; Tarif, T.; Wolfe, A.; Dawson, E.; Fox, K. AI in Point-of-Care—A sustainable healthcare revolution at the edge. Pac. Symp. Biocomput. 2025, 30, 734–747. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).