Recent Progress on Rare-Earth-Doped Upconversion Nanomaterials for Bioassay Applications
Abstract
1. Introduction
2. Principles of Fluorescence Probe Construction
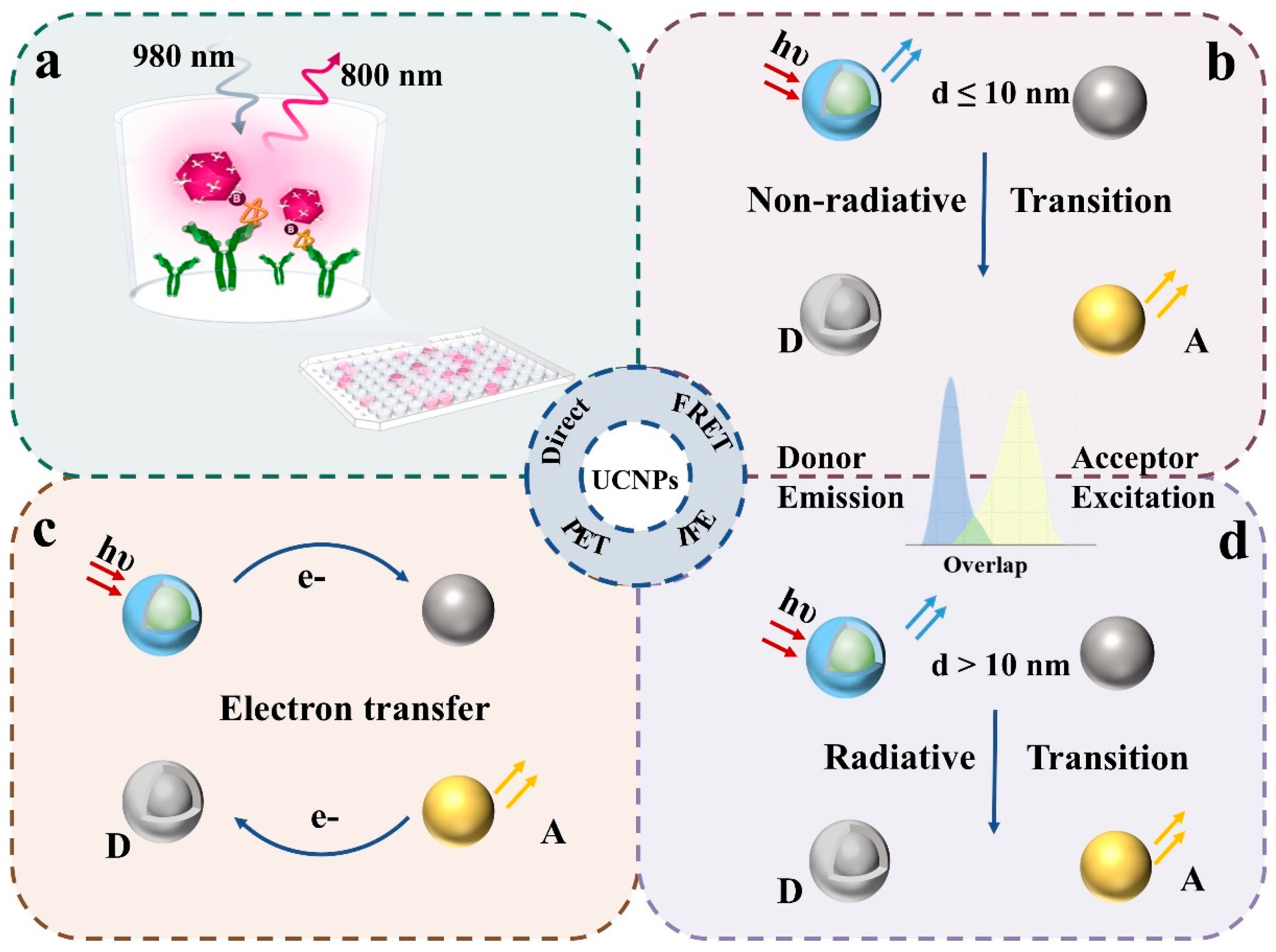
2.1. Direct Detection Method
2.2. Förster Resonance Energy Transfer (FRET)
2.3. Inner Filter Effect (IFE)
2.4. Photo-Induced Electron Transfer (PET)
3. Luminescence Enhancement Strategy
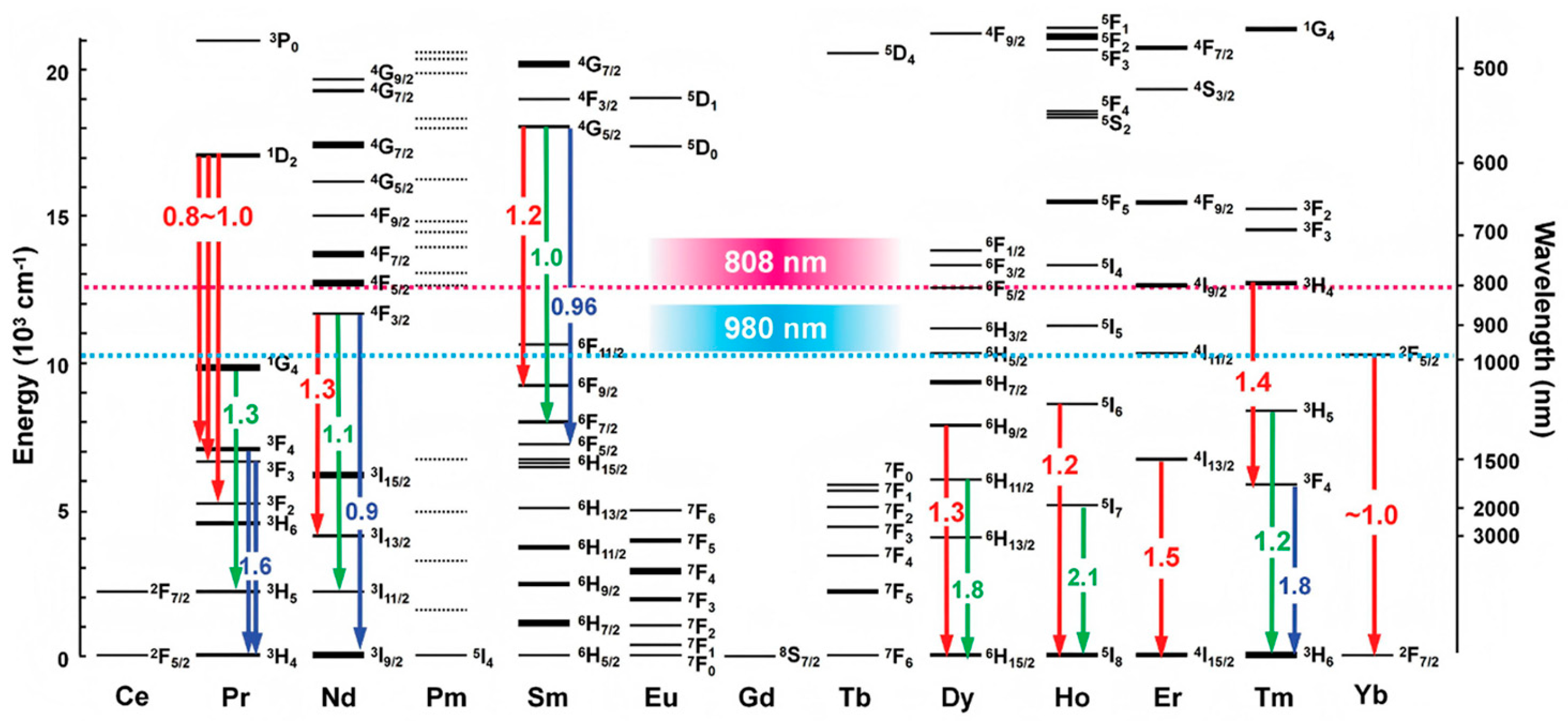
3.1. Structural Composition Regulation

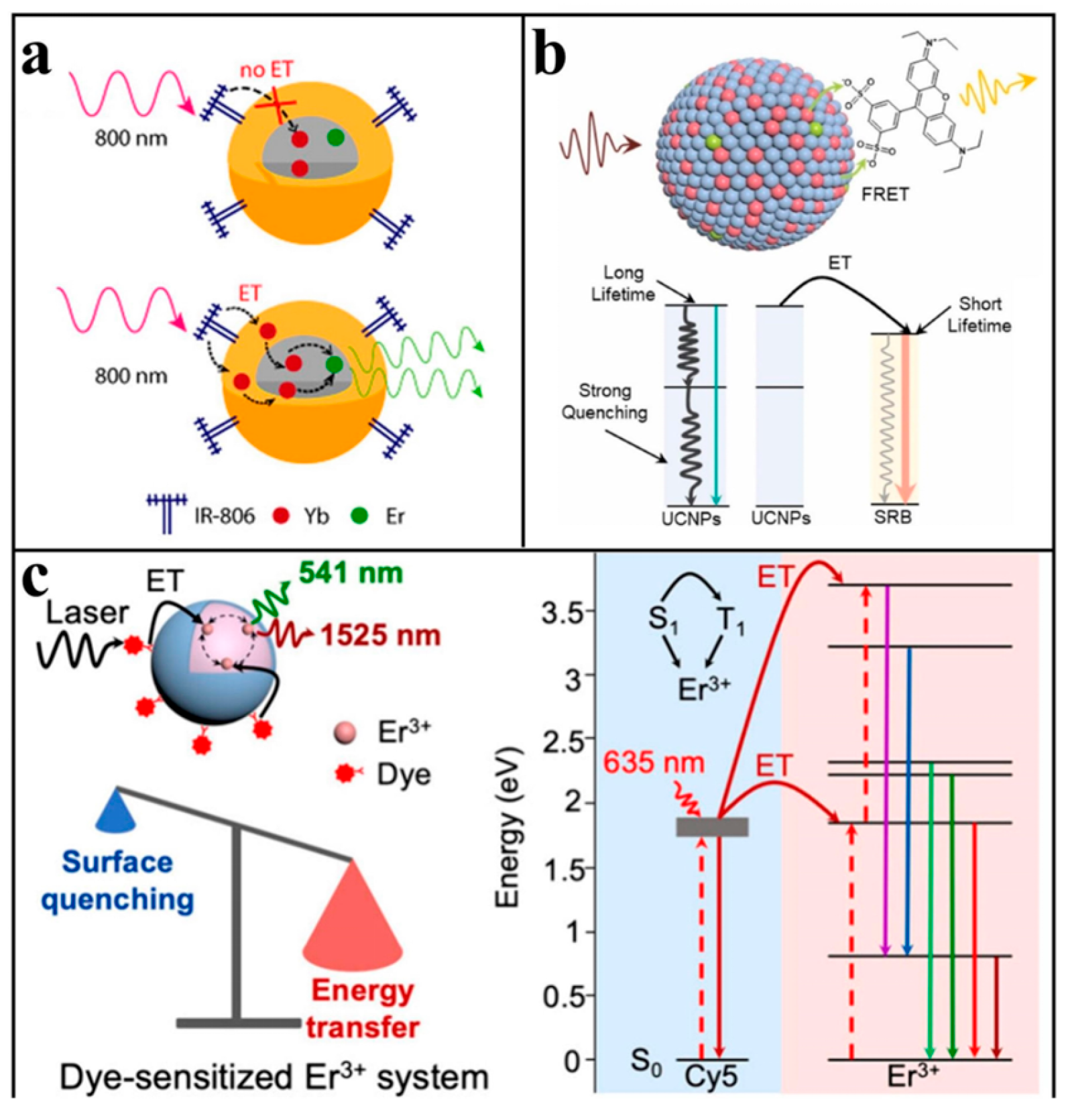
3.2. Dye Sensitization
3.3. Local Field Control

4. Platform for UCNPs in Bioassays
4.1. Solution-Phased Detection
4.2. Lateral Flow Chromatography

4.3. Plate
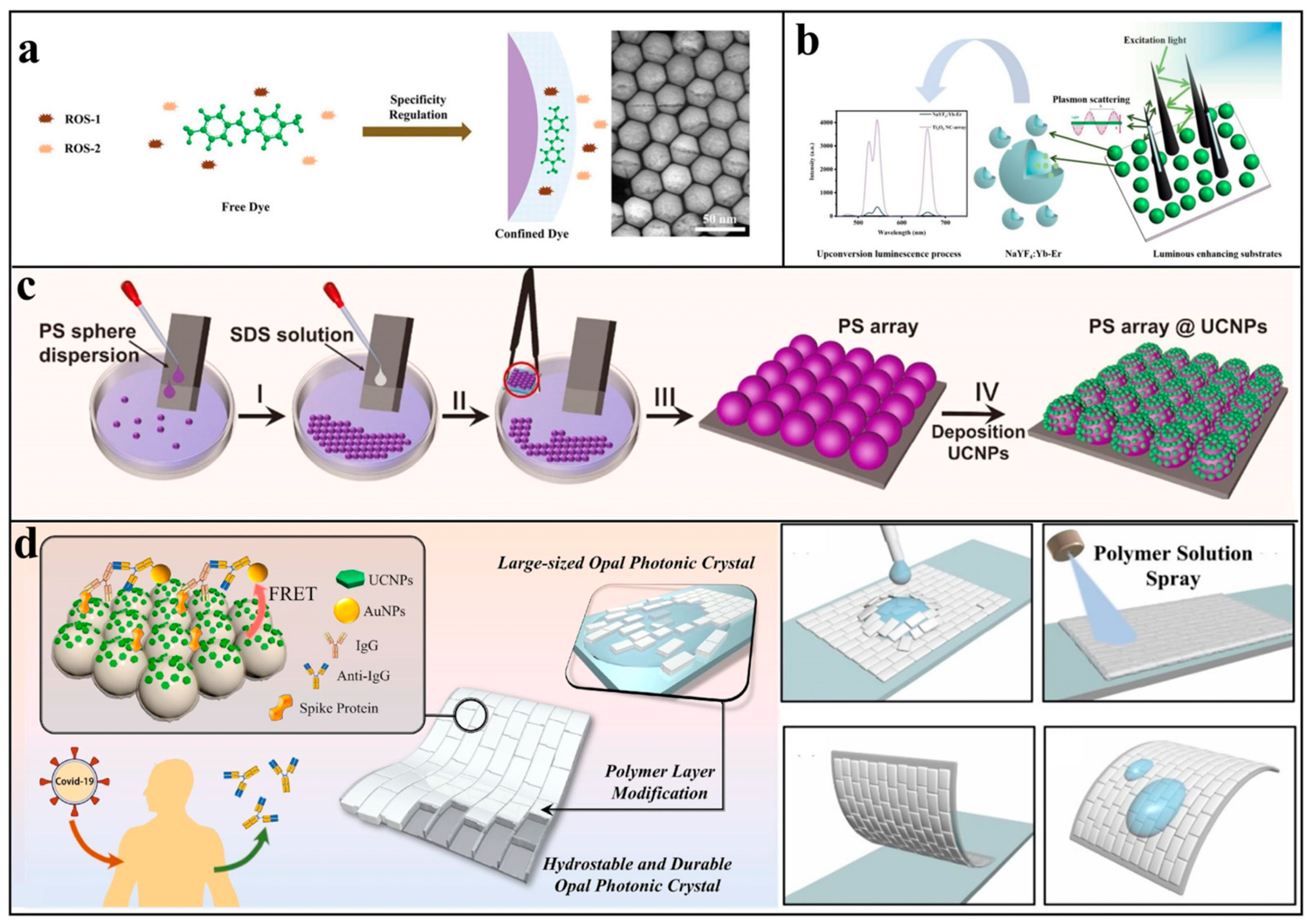
4.4. Linkage with Other Technologies
5. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloembergen, N. Solid State Infrared Quantum Counters. Phys. Rev. Lett. 1959, 2, 84–85. [Google Scholar] [CrossRef]
- Peltomaa, R.; Benito-Pena, E.; Gorris, H.H.; Moreno-Bondi, M.C. Biosensing based on upconversion nanoparticles for food quality and safety applications. Analyst 2021, 146, 13–32. [Google Scholar] [CrossRef]
- Gong, Y.; Yuan, W.; Song, F.; Zhang, Q.; Zhang, P.; Ding, C. A NIR light gated targeting nanoprobe based on DNA-modified upconversion nanoparticles with antifouling properties for ratiometric detection and imaging of microRNA-21. Anal. Chim. Acta 2022, 1235, 340554. [Google Scholar] [CrossRef]
- Han, Q.; Zhao, X.; Zhang, X.; Na, N.; Ouyang, J. A rationally designed triple-qualitative and double-quantitative high precision multi-signal readout sensing platform. Sens. Actuators B-Chem. 2022, 360, 131663. [Google Scholar] [CrossRef]
- Jin, B.; Du, Z.; Ji, J.; Bai, Y.; Tang, D.; Qiao, L.; Lou, J.; Hu, J.; Li, Z. Regulation of probe density on upconversion nanoparticles enabling high-performance lateral flow assays. Talanta 2023, 256, 124327. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Ma, Y.; Sun, X.; Zhang, Y.; Li, H. Simultaneous evolutions in composition, structure, morphology, and upconversion luminescence of BiOxFy:Yb/Er microcrystals and their application for ratiometric temperature sensing. J. Alloys Compd. 2024, 992, 174596. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Liang, R.; Li, P.; Wang, J.; Wang, F. A bright Ce-based downshifting luminescence nanoprobe for NIR-IIb vessel imaging. Chem. Eng. J. 2024, 496, 153917. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, K.; Su, X.; Hu, D.; Luo, Y.; Sun, Y.; Liu, Q.; Chen, L.; Qiao, J.; Xu, M.; et al. A dark-state-dominated photochemical upconversion afterglow via triplet energy transfer relay. Sci. Adv 2025, 11, eadt1225. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, J.; Jiang, D.; Tan, Q.; Li, J.; Yao, C.; Zhu, C.; Zhou, Y. A Hemicyanine-Assembled Upconversion Nanosystem for NIR-Excited Visualization of Carbon Monoxide Bio-Signaling In Vivo. Small 2022, 18, 2202263. [Google Scholar] [CrossRef]
- Li, Y.; Jin, X.; Sun, Y.; Guan, S.; Chang, B.; Li, Y. Dual-mode nanoprobes for reactive oxygen species scavenging and treatment of acute lung injury. Appl. Surf. Sci. 2023, 610, 155458. [Google Scholar] [CrossRef]
- Jiang, Y.; Hong, Y.; Liu, Y.-Y.; Guan, Y.; Zhou, J.; Wang, H.; Sun, L. Switching between upconversion luminescence imaging and therapy in vitro enabled by NIR excitation modulation of nanocomposites. J. Mater. Chem. C 2024, 12, 11938–11947. [Google Scholar] [CrossRef]
- Lv, W.; Wu, Y.; Chen, H. Orthogonal upconversion nanocarriers for combined photodynamic therapy and precisely triggered gene silencing in combating keloids. J. Control. Release 2025, 379, 1–13. [Google Scholar] [CrossRef]
- Molkenova, A.; Choi, H.E.; Lee, G.; Baek, H.; Kwon, M.; Lee, S.B.; Park, J.-M.; Kim, J.-H.; Han, D.-W.; Park, J.; et al. Cold-Responsive Hyaluronated Upconversion Nanoplatform for Transdermal Cryo-Photodynamic Cancer Therapy. Adv. Sci. 2024, 11, 202306684. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Local Structure Engineering in Lanthanide-Doped Nanocrystals for Tunable Upconversion Emissions. J. Am. Chem. Soc. 2021, 143, 20546–20561. [Google Scholar] [CrossRef]
- Chan, E.M.; Levy, E.S.; Cohen, B.E. Rationally Designed Energy Transfer in Upconverting Nanoparticles. Adv. Mater. 2015, 27, 5753–5761. [Google Scholar] [CrossRef]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.X. Upconversion Nanomaterials: Synthesis, Mechanism, and Applications in Sensing. Sensors 2012, 12, 2414–2435. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Q.; Li, Y. Upconversion Luminescence of Monodisperse CaF2:Yb3+/Er3+ Nanocrystals. J. Am. Chem. Soc. 2009, 131, 14200–14201. [Google Scholar] [CrossRef]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal sodium yttrium fluoride based green and blue emitting upconversion phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Su, Q.; Feng, W.; Yang, D.; Li, F. Resonance Energy Transfer in Upconversion Nanoplatforms for Selective Biodetection. Acc. Chem. Res. 2017, 50, 32–40. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 5642–5643. [Google Scholar] [CrossRef]
- Wang, J.K.; Zhu, Y.L.; Grimes, C.A.; Nie, Z.; Cai, Q.Y. Eu,Sm,Mn-Doped CaS Nanoparticles with 59.3% Upconversion-Luminescence Quantum Yield: Enabling Ultrasensitive and Facile Smartphone-Based Sulfite Detection. Anal. Chem. 2018, 90, 8658–8664. [Google Scholar] [CrossRef]
- Pan, Q.; Cai, Z.; Yang, Y.; Yang, D.; Kang, S.; Chen, Z.; Qiu, J.; Zhan, Q.; Dong, G. Engineering Tunable Broadband Near-Infrared Emission in Transparent Rare-Earth Doped Nanocrystals-in-Glass Composites via a Bottom-Up Strategy. Adv. Opt. Mater 2019, 7, 1801482. [Google Scholar] [CrossRef]
- Gao, C.; Zheng, P.; Liu, Q.; Han, S.; Li, D.; Luo, S.; Temple, H.; Xing, C.; Wang, J.; Wei, Y.; et al. Recent Advances of Upconversion Nanomaterials in the Biological Field. Nanomaterials 2021, 11, 2474. [Google Scholar] [CrossRef]
- Ansari, A.A.; Thakur, V.K.; Chen, G. Functionalized upconversion nanoparticles: New strategy towards FRET-based luminescence bio-sensing. Coord. Chem. Rev. 2021, 436, 213821. [Google Scholar] [CrossRef]
- Al-Salihi, M.; Ghellab, S.E.; Li, Y.; Luo, C.; Kalsoom, U.-e.; Liu, L. Effective Rapid Fluorescence Lifetime Imaging of the Brain: A Novel Approach Using Upconversion Photoluminescence Lifetime Based on Gate-Width Acquisition. Nano Lett. 2024, 24, 14973–14982. [Google Scholar] [CrossRef]
- Mickert, M.J.; Farka, Z.; Kostiv, U.; Hlavacek, A.; Horak, D.; Skladal, P.; Gorris, H.H. Measurement of Sub-femtomolar Concentrations of Prostate-Specific Antigen through Single-Molecule Counting with an Upconversion-Linked Immunosorbent Assay. Anal. Chem. 2019, 91, 9435–9441. [Google Scholar] [CrossRef]
- Song, X.-J.; Ye, F.; Zhang, Y.; Sun, J.; Shentu, X.; Yu, X.; Li, W.; Wu, Y.-F. A clenbuterol detection method based on magnetic separation upconversion fluorescent probe. Food Chem.-X 2024, 24, 101911. [Google Scholar] [CrossRef] [PubMed]
- Lao, X.; Liu, Y.; Li, L.; Song, M.; Ma, Y.; Yang, M.; Chen, G.; Hao, J. Plasmon-enhanced FRET biosensor based on Tm3+/Er3+ co-doped core-shell upconversion nanoparticles for ultrasensitive virus detection. Aggregate 2024, 5, e448. [Google Scholar] [CrossRef]
- Chen, H.; Tian, P.; Guo, J.; Sun, M.; Zhu, W.; Li, Z.; Liu, Z. Synergistic synthesis of gold nanoflowers as upconversion near-infrared nanoprobe energy acceptor and recognition unit for improved hydrogen sulfide sensing. Talanta 2024, 273, 125908. [Google Scholar] [CrossRef]
- Che, D.; Cao, X.; Chen, C.; Yan, H. A point-of-care aptasensor based on the upconversion nanoparticles/MoS2 FRET system for the detection of Pseudomonas aeruginosa infection. Microchim. Acta 2024, 191, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, J.; Zhang, Y.; Xiao, D.; Zhou, C. Blue emission-dominated NaYbF4@NaYF4:2%Ho@NaYF4 upconversion nanoparticles for detecting ascorbic acid. Nanoscale 2024, 16, 18910–18917. [Google Scholar] [CrossRef]
- Wu, M.; Yi, J.; Yin, C.; Sun, Q.; Gao, L.; Niu, N.; Chen, L. An upconversion nanosensor with phenolic-like functionality for accurate identification of chlorpyrifos in grapes. Food Chem. 2023, 416, 135859. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, X.; Yang, F.; Jia, W.; Yang, L.; Jiang, C. Zinc Doping-Induced Lattice Growth Regulation for Enhanced Upconversion Emission in Serum Bilirubin Detection. Anal. Chem. 2025, 97, 3515–3524. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, L.; Yang, L.; Jiang, C. Ratiometric upconversion luminescence sensing platform for visual monitoring of antiarrhythmic drug level in serum. Chem. Eng. J. 2023, 476, 146923. [Google Scholar] [CrossRef]
- Shao, X.; Cao, L.; Lu, L. Ultrasensitive detection of glucose oxidase and alkaline phosphatase in milk based on valence regulated upconversion nanoprobes. Food Chem. 2024, 432, 137212. [Google Scholar] [CrossRef]
- Wu, W.; Ahmad, W.; Hassan, M.M.; Wu, J.; Ouyang, Q.; Chen, Q. An upconversion biosensor based on inner filter effect for dual-role recognition of sulfadimethoxine in aquatic samples. Food Chem. 2024, 437, 137832. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, S.; Wang, J.; Li, J.; Tang, J.; Aaron, A.A.; Cai, Q.; Wang, N.; Li, Z. Highly sensitive and ratiometric detection of nitrite in food based on upconversion-carbon dots nanosensor. Anal. Chim. Acta 2023, 1263, 341245. [Google Scholar] [CrossRef]
- David, M.; Gutkin, S.; Nithun, R.V.; Jbara, M.; Shabat, D. Unprecedented Photoinduced-Electron-Transfer Probe with a Turn-ON Chemiluminescence Mode-of-Action. Angew. Chem.-Int. Ed. 2025, 64, 202417924. [Google Scholar] [CrossRef]
- Li, Y.; Jia, D.; Ren, W.; Shi, F.; Liu, C. A Versatile Photoinduced Electron Transfer-Based Upconversion Fluorescent Biosensing Platform for the Detection of Disease Biomarkers and Nerve Agent. Adv. Funct. Mater. 2019, 29, 1903191. [Google Scholar] [CrossRef]
- Li, L.; Ding, Y.; Zhang, C.; Xian, H.; Chen, S.; Dai, G.; Wang, X.; Ye, C. Ratiometric Fluorescence Detection of Mg2+ Based on Regulating Crown-Ether Modified Annihilators for Triplet-Triplet Annihilation Upconversion. J. Phys. Chem. B 2022, 126, 3276–3282. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wen, S.; Kong, M.; Liu, Y.; Hu, W.; Shi, B.; Shi, X.; Jin, D. Highly Doped Upconversion Nanoparticles for In Vivo Applications Under Mild Excitation Power. Anal. Chem. 2020, 92, 10913–10919. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, X.; Wang, Y.; Liu, E.; Hu, X.; Fan, J. Enhancement of the red upconversion luminescence in NaYF4:Yb3+, Er3+nanoparticles by the transition metal ions doping. Ceram. Int. 2015, 41, 14545–14553. [Google Scholar] [CrossRef]
- Du, K.; Xu, X.; Yao, S.; Lei, P.; Dong, L.; Zhang, M.; Feng, J.; Zhang, H. Enhanced upconversion luminescence and controllable phase/shape of NaYF4:Yb/Er crystals through Cu2+ ion doping. Crystengcomm 2018, 20, 1945–1953. [Google Scholar] [CrossRef]
- Yi, M.; Liu, Y.; Gao, H.; Huang, Z.; Liang, J.; Mao, Y. Upconversion effective enhancement of NaYF4:Yb3+/Er3+ nanoparticles by Ni2+ doping. J. Mater. Sci. 2018, 53, 1395–1403. [Google Scholar] [CrossRef]
- Tang, H.; Xu, Y.; Cheng, X. Growth and enhanced upconversion luminescence intensity of Mg2+ and Cr3+ co-doped β-NaYF4:Yb3+/Er3+ microcrystals. J. Solid State Chem. 2020, 285, 121229. [Google Scholar] [CrossRef]
- Bi, S.; Deng, Z.; Huang, J.; Wen, X.; Zeng, S. NIR-II Responsive Upconversion Nanoprobe with Simultaneously Enhanced Single-Band Red Luminescence and Phase/Size Control for Bioimaging and Photodynamic Therapy. Adv. Mater. 2023, 35, 2207038. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, B.; Zhou, L.; Wu, Y.; Wang, F.; Ding, C.; Hu, J. Large enhancement of red upconversion luminescence in beta Ba2Sc0.67Yb0.3Er0.03AlO5 phosphor via Mn2+ ions doping for thermometry. Sci. Rep. 2024, 14, 8893. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. Combating Concentration Quenching in Upconversion Nanoparticles. Acc. Chem. Res. 2020, 53, 358–367. [Google Scholar] [CrossRef]
- Drees, C.; Raj, A.N.; Kurre, R.; Busch, K.B.; Haase, M.; Piehler, J. Engineered Upconversion Nanoparticles for Resolving Protein Interactions inside Living Cells. Angew. Chem.-Int. Ed. 2016, 55, 11668–11672. [Google Scholar] [CrossRef]
- Misiak, M.; Prorok, K.; Cichy, B.; Bednarkiewicz, A.; Strek, W. Thulium concentration quenching in the up-converting α-Tm3+/Yb3+ NaYF4 colloidal nanocrystals. Opt. Mater. 2013, 35, 1124–1128. [Google Scholar] [CrossRef]
- Tu, L.; Wu, K.; Luo, Y.; Wang, E.; Yuan, J.; Zuo, J.; Zhou, D.; Li, B.; Zhou, J.; Jin, D.; et al. Significant Enhancement of the Upconversion Emission in Highly Er3+-Doped Nanoparticles at Cryogenic Temperatures. Angew. Chem.-Int. Ed. 2023, 62, 202217100. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Tao, L.; Zhang, Q.; Huang, H.; Zhang, Q.; Zhou, B. Amplifying Photon Upconversion in Alloyed Nanoparticles for a Near-Infrared Photodetector. Nano Lett. 2024, 24, 4580–4587. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, S.; Deng, X.; Zhou, J.; Zhang, R.; Shao, Q. Opposite luminescence thermal behavior of upconversion core/shell nanocrystals for anticounterfeiting. Nanoscale 2023, 15, 15552–15557. [Google Scholar] [CrossRef]
- Grauel, B.; Wuerth, C.; Homann, C.; Krukewitt, L.; Andresen, E.; Roik, J.; Recknagel, S.; Haase, M.; Resch-Genger, U. Volume and surface effects on two-photonic and three-photonic processes in dry co-doped upconversion nanocrystals. Nano Res. 2022, 15, 2362–2373. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Bai, Y.; Wang, R. Activating ultralow upconversion nanothermometry in neodymium sublattice for heart tissue imaging rapid-responsive. Talanta 2023, 264, 124764. [Google Scholar] [CrossRef]
- Alzahrani, Y.A.; Alessa, A.M.; Almosaind, M.K.; Alarifi, R.S.; Alromaeh, A.; Alkahtani, M. 17Preparation and Characterization of Uniform and Controlled Silica Encapsulating on Lithium Yttrium Fluoride-Based Upconversion Nanoparticles. Nanomaterials 2024, 14, 685. [Google Scholar] [CrossRef]
- Li, F.; Tu, L.; Zhang, Y.; Huang, D.; Liu, X.; Zhang, X.; Du, J.; Fan, R.; Yang, C.; Kraemer, K.W.; et al. Size-dependent lanthanide energy transfer amplifies upconversion luminescence quantum yields. Nat. Photonics 2024, 18, 440–449. [Google Scholar] [CrossRef]
- Wang, Y.; Rui, J.; Song, H.; Yuan, Z.; Huang, X.; Liu, J.; Zhou, J.; Li, C.; Wang, H.; Wu, S.; et al. Antithermal Quenching Upconversion Luminescence via Suppressed Multiphonon Relaxation in Positive/Negative Thermal Expansion Core/Shell NaYF4:Yb/Ho@ScF3 Nanoparticles. J. Am. Chem. Soc. 2024, 146, 6530–6535. [Google Scholar] [CrossRef]
- Garfield, D.J.; Borys, N.J.; Hamed, S.M.; Torquato, N.A.; Tajon, C.A.; Tian, B.; Shevitski, B.; Barnard, E.S.; Suh, Y.D.; Aloni, S.; et al. Enrichment of molecular antenna triplets amplifies upconverting nanoparticle emission. Nat. Photonics 2018, 12, 402–407. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Takle, K.; Bilsel, O.; Li, Z.; Lee, H.; Zhang, Z.; Li, D.; Fan, W.; Duan, C.; et al. Dye-Sensitized Core/Active Shell Upconversion Nanoparticles for Optogenetics and Bioimaging Applications. ACS Nano 2016, 10, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shen, T.; Zhang, W.; Luo, R.; Zhou, J.; Liao, R.; Zhao, R.; Cao, C. Energy Aggregation for Illuminating Upconversion Multicolor Emission Based on Ho3+ Ions. Acs Appl. Mater. Interfaces 2025, 17, 8191–8197. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Luo, Y.; Liu, Y.; Huang, Y.; Yu, P.; Ma, H.; Li, X.; Zhang, Z.; Zhang, C.; Chen, C.; et al. A hybrid strategy to enhance small-sized upconversion nanocrystals. Biosens. Bioelectron. 2025, 271, 117003. [Google Scholar] [CrossRef]
- Zhao, F.; Ling, H.; Zhang, W.; Zhang, Y.; Liu, Q. Dye-to-Er3+ Direct Energy Transfer for Enhancing Up- and Down-conversion Luminescence in Sub-10 nm NaErF4. Nano Lett. 2024, 24, 14838–14846. [Google Scholar] [CrossRef]
- Chikkaraddy, R.; de Nijs, B.; Benz, F.; Barrow, S.J.; Scherman, O.A.; Rosta, E.; Demetriadou, A.; Fox, P.; Hess, O.; Baumberg, J.J. Single-molecule strong coupling at room temperature in plasmonic nanocavities. Nature 2016, 535, 127–130. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Poh, E.T.; Liang, L.; Liu, H.; Yang, J.K.W.; Qiu, C.-W.; Vallee, R.A.L.; Liu, X. Upconversion superburst with sub-2 μs lifetime. Nat. Nanotechnol. 2019, 14, 1110–1115. [Google Scholar] [CrossRef]
- Xu, F.; Li, Z.; Gu, Y.; Luo, W.; Sun, Z. 26Dual-resonance enhancement and polarization control of upconversion luminescence of NaYF4:Yb,Er nanoparticles on 2D metal gratings. J. Alloys Compd. 2025, 1010, 177919. [Google Scholar] [CrossRef]
- Ju, Z.; Wang, M.; Chen, Y.; Wang, Z.; Yang, M.; Meng, F.; Lv, R. An Optoelectronic Sensing Real-Time Glucose Detection Film Using Photonic Crystal Enhanced Rare Earth Fluorescence and Additive Manufacturing. Small 2025, 21, e2409725. [Google Scholar] [CrossRef]
- Hu, S.; Xu, H.; Zhou, B.; Xu, S.; Shen, B.; Dong, B.; Yin, Z.; Xu, S.; Sun, L.; Lv, J.; et al. Double Stopband Bilayer Photonic Crystal Based Upconversion Fluorescence PSA Sensor. Sens. Actuators B-Chem. 2021, 326, 128816. [Google Scholar] [CrossRef]
- Meng, Z.; Wu, Y.; Ren, J.; Li, X.; Zhang, S.; Wu, S. Upconversion Nanoparticle-Integrated Bilayer Inverse Opal Photonic Crystal Film for the Triple Anticounterfeiting. Acs Appl. Mater. Interfaces 2022, 14, 12562–12570. [Google Scholar] [CrossRef]
- Hou, M.; Ma, L.; Yang, H.; Si, F.; Liu, Y. Background-free and signal-amplified upconversion fluorescent biosensing platform for sensitive detection of CYFRA21-1. Talanta 2023, 262, 124659. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Ma, Y.; Li, L.; Wong, M.-C.; Wang, P.; Chen, J.; Chen, H.; Wang, F.; Hao, J. Multiplexed detection of SARS-CoV-2 based on upconversion luminescence nanoprobe/MXene biosensing platform for COVID-19 point-of-care diagnostics. Mater. Des. 2022, 223, 111249. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, M.; Li, L.; Lao, X.; Liu, Y.; Wong, M.-c.; Yang, M.; Chen, H.; Hao, J. Attomolar-level detection of respiratory virus long-chain oligonucleotides based on FRET biosensor with upconversion nanoparticles and Au-Au dimer. Biosens. Bioelectron. 2024, 243, 115778. [Google Scholar] [CrossRef]
- Huang, X.; Aguilar, Z.P.; Xu, H.; Lai, W.; Xiong, Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef]
- Lee, L.G.; Nordman, E.S.; Johnson, M.D.; Oldham, M.F. A low-cost, high-performance system for fluorescence lateral flow assays. Biosensors 2013, 3, 360–373. [Google Scholar] [CrossRef]
- Gong, X.; Cai, J.; Zhang, B.; Zhao, Q.; Piao, J.; Peng, W.; Gao, W.; Zhou, D.; Zhao, M.; Chang, J. A review of fluorescent signal-based lateral flow immunochromatographic strips. J. Mater. Chem. B 2017, 5, 5079–5091. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, C.; Ma, C.; Yin, H.; Li, S.; Du, Z.; Zhao, G.; Huang, H.; Li, Z. Innovative strategies and approaches for enhancing performance in optical probe-based biosensors for point-of-care testing. Trac-Trends Anal. Chem. 2024, 176, 117775. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Zhang, C.; Ma, C.; Hu, J.; Wang, J.; Li, Z. Systematic optimization of UCNPs-LFA for Helicobacter pylori nucleic acid detection at point-of-care. Microchim. Acta 2024, 191, 650. [Google Scholar] [CrossRef]
- Ji, T.; Xu, X.; Wang, X.; Cao, N.; Han, X.; Wang, M.; Chen, B.; Lin, Z.; Jia, H.; Deng, M.; et al. Background-Free Chromatographic Detection of Sepsis Biomarker in Clinical Human Serum through Near-Infrared to Near-Infrared Upconversion Immunolabeling. ACS Nano 2020, 14, 16864–16874. [Google Scholar] [CrossRef]
- Wang, W.; Ye, Z.; Ma, X.; Guo, J. Smartphone enabled upconversion nanoparticle-based lateral flow strip for ultra-low concentration of methamphetamine detection. Sens. Actuators B-Chem. 2022, 370, 132421. [Google Scholar] [CrossRef]
- Chen, C.; Hu, S.; Tian, L.; Qi, M.; Chang, Z.; Li, L.; Wang, L.; Dong, B. A versatile upconversion-based multimode lateral flow platform for rapid and ultrasensitive detection of microRNA towards health monitoring. Biosens. Bioelectron. 2024, 252, 116135. [Google Scholar] [CrossRef] [PubMed]
- Masoumeh Ghorbanpour, S.; Wen, S.; Kaitu’u-Lino, T.u.J.; Hannan, N.J.; Jin, D.; McClements, L. 9 Quantitative Point of Care Tests for Timely Diagnosis of Early-Onset Preeclampsia with High Sensitivity and Specificity. Angew. Chem.-Int. Ed. 2023, 62, 202301193. [Google Scholar] [CrossRef]
- Jin, B.; Yang, Y.; He, R.; Park, Y.I.; Lee, A.; Bai, D.; Li, F.; Lu, T.J.; Xu, F.; Lin, M. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens. Actuators B-Chem. 2018, 276, 48–56. [Google Scholar] [CrossRef]
- Ekman, M.; Salminen, T.; Raiko, K.; Soukka, T.; Gidwani, K.; Martiskainen, I. Spectrally separated dual-label upconversion luminescence lateral flow assay for cancer-specific STn-glycosylation in CA125 and CA15-3. Anal. Bioanal. Chem. 2024, 416, 3251–3260. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, Y.; Liu, J.; Fu, S.; Zhang, J.; Mei, Q.; Zhang, Y. Single-Line Flow Assay Platform Based on Orthogonal Emissive Upconversion Nanoparticles. Anal. Chem. 2021, 93, 3010–3017. [Google Scholar] [CrossRef]
- Ai, Z.; Cai, H.; Liu, C.; Zhao, Y.; Fu, Q.; Fan, N.; Li, Y.; Li, S.; Zhou, S.; Li, C.; et al. Ultrasensitive Bi-Mode Lateral-Flow Assay via UCNPs-Based Host-Guest Assembly of Fluorescent-Colorimetric Nanoparticles. Small 2025, 21, 2410947. [Google Scholar] [CrossRef]
- Mao, M.; Chen, X.; Cai, Y.; Yang, H.; Zhang, C.; Zhang, Y.; Wang, Z.; Peng, C. Accelerated and signal amplified nanozyme-based lateral flow assay of acetamiprid based on bivalent triple helix aptamer. Sens. Actuators B-Chem. 2023, 378, 133148. [Google Scholar] [CrossRef]
- Song, Z.; Guo, H.; Suo, Y.; Zhang, Y.; Zhang, S.; Qiu, P.; Liu, L.; Chen, B.; Cheng, Z. Enhanced NIR-II Fluorescent Lateral Flow Biosensing Platform Based on Supramolecular Host-Guest Self-Assembly for Point-of-Care Testing of Tumor Biomarkers. ACS Appl. Mater. Interfaces 2023, 15, 52038–52050. [Google Scholar] [CrossRef]
- Peng, S.; Fan, M.; Xiao, C.; Chen, Y.; You, R.; Xu, Y.; Chen, Y.; Liu, Y.; Xiao, X.; Feng, S.; et al. Portable SERS-based lateral flow immunoassay strips with self-calibration for detection of a prostate cancer biomarker. Sens. Actuators B-Chem. 2024, 401, 135012. [Google Scholar] [CrossRef]
- Sun, Y.; Mao, M.; Tai, S.; Chao, M.; Xu, H.; Cai, Y.; Peng, C.; Ma, W.; Wang, Z. Lateral flow assay with automatic signal amplification based on delayed substrate release. Talanta 2025, 286, 127557. [Google Scholar] [CrossRef]
- Nandhakumar, P.; Martin, C.M.S.; Arevalo, B.; Ding, S.; Lunker, M.; Vargas, E.; Djassemi, O.; Campuzano, S.; Wang, J. Redox Cycling Amplified Electrochemical Lateral-Flow Immunoassay: Toward Decentralized Sensitive Insulin Detection. ACS Sens. 2023, 8, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, S.; Kang, D.; Lee, S.-H.; Cho, A.Y.; Lee, H.; Kwon, J.-H.; Shin, Y.; Kim, Y.-P.; Lee, J. Near-Infrared Long-Lived Luminescent Nanoparticle-Based Time-Gated Imaging for Background-Free Detection of Avian Influenza Virus. ACS Sens. 2025, 10, 1312–1320. [Google Scholar] [CrossRef]
- Tu, Z.; Yang, X.; Dong, H.; Yu, Q.; Zheng, S.; Cheng, X.; Wang, C.; Rong, Z.; Wang, S. Ultrasensitive Fluorescence Lateral Flow Assay for Simultaneous Detection of Pseudomonas aeruginosa and Salmonella typhimurium via Wheat Germ Agglutinin-Functionalized Magnetic Quantum Dot Nanoprobe. Biosensors 2022, 12, 942. [Google Scholar] [CrossRef]
- Ramin, B.B.S.; Santos, W.G.; Messaddeq, Y.; Deffune, E.; Moraes, M.L.; Ribeiro, S.J.L. Layer-by-layer assembly of NaYF4:Yb3+/Er3+UCNPs@Cystein and UCNPs@Cys-Ab as hybrid bio-optical sensors for E. coli bacteria. J. Lumin. 2024, 271, 120590. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Jiang, C.; Liao, X.; Chen, Y.; Jia, T.; Chen, G.; Feng, X. Specific and Long-Term Luminescent Monitoring of Hydrogen Peroxide in Tumor Metastasis. Adv. Mater. 2023, 35, 2210948. [Google Scholar] [CrossRef]
- Ouyang, Q.; Liu, S.; Sun, R.; Zhang, M.; Riaz, T.; Chen, Q. Au@Ag NPs-based probes with enhanced quenching effect immobilized on polymer platforms as solid-phase fluorescent sensors for malachite green detection. Sens. Actuators B-Chem. 2024, 405, 135351. [Google Scholar] [CrossRef]
- Rong, Y.; Hassan, M.M.; Wu, J.; Chen, S.; Yang, W.; Li, Y.; Zhu, J.; Huang, J.; Chen, Q. Enhanced detection of acrylamide using a versatile solid-state upconversion sensor through spectral and visual analysis. J. Hazard. Mater. 2024, 466, 133369. [Google Scholar] [CrossRef]
- Brandmeier, J.C.; Jurga, N.; Grzyb, T.; Hlavácek, A.; Oborilová, R.; Skládal, P.; Farka, Z.; Gorris, H.H. Digital and Analog Detection of SARS-CoV-2 Nucleocapsid Protein via an Upconversion-Linked Immunosorbent Assay. Anal. Chem. 2023, 95, 4753–4759. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Yan, J.; Wu, Y.; Zhang, Y.; Yang, Y.; Zhou, D.; Long, Z.; Wang, Q.; Qiu, J. Enhancement of Upconversion Luminescence through Three-Dimensional Design of Plasma Ti3O5-Coupled Structural Domain-Limiting Effects. ACS Appl. Mater. Interfaces 2024, 16, 24879–24888. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, S.; Huang, G.; Zhan, S.; Nie, G.; Su, X.; Cheng, D.; Liu, Y. Flexible composite film with enhanced upconversion emission for ultrasensitive gas detection. Ceram. Int. 2024, 50, 3843–3851. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Dong, B.; Tang, Z.; Zhou, B.; Wang, Y.; Sun, L.; Xu, L.; Wang, L.; Zhang, X.; et al. Highly hydrostable and flexible opal photonic crystal film for enhanced up-conversion fluorescence sensor of COVID-19 antibody. Biosens. Bioelectron. 2023, 237, 115484. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Li, H.; Xu, W.; Cui, S.; Zhou, D.; Chen, X.; Zhu, Y.; Qin, G.; Song, H. Local Field Modulation Induced Three-Order Upconversion Enhancement: Combining Surface Plasmon Effect and Photonic Crystal Effect. Adv. Mater. 2016, 28, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Y.; Wu, P.-W.; Chen, J.-C.; Tsou, N.-T.; Lin, Y.-Y.; Lo, Y.-C.; Li, S.-J.; Chang, C.-W.; Chen, B.-W.; Tsai, C.-L.; et al. Flexible Optogenetic Transducer Device for Remote Neuron Modulation Using Highly Upconversion-Efficient Dendrite-Like Gold Inverse Opaline Structure. Adv. Healthc. Mater. 2022, 11, 2101310. [Google Scholar] [CrossRef]
- Wang, G.; Li, L.; Zheng, H.; Li, Q.; Huang, J.; Zhang, L.; Yang, H.; Cui, K.; Yu, J. Bifunctional Strategy toward Constructing Perovskite/Upconversion Lab-on-Paper Photoelectrochemical Device for Sensitive Detection of Malathion. Acs Nano 2023, 17, 13418–13429. [Google Scholar] [CrossRef]
- He, Y.; Rao, H.; Wang, J.; Wu, Y.; Han, C.; Yan, C.; Temple, H.; Zhang, L.; Chen, W.; Liu, Y. Perovskite quantum dots modulating upconversion nanomaterials for cancer early detections. Cancer Nanotechnol. 2023, 14, 52. [Google Scholar] [CrossRef]
- Yan, J.; Yin, B.; Zhang, Q.; Li, C.; Chen, J.; Huang, Y.; Hao, J.; Yi, C.; Zhang, Y.; Wong, S.H.D.; et al. A CRISPR-Cas12a-mediated dual-mode luminescence and colorimetric nucleic acid biosensing platform based on upconversion nanozyme. Biosens. Bioelectron. 2025, 270, 116963. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Y.; Fan, X.; Ma, L.; Feng, T.; Zeng, J.; Xue, C.; Xu, J. A smartphone-assisted portable sensing hydrogel modules based on UCNPs and Co3O4 NPs for fluorescence quantitation of hypoxanthine in aquatic products. Talanta 2024, 276, 12625. [Google Scholar] [CrossRef]
- Ding, X.; Ahmad, W.; Rong, Y.; Wu, J.; Ouyang, Q.; Chen, Q. A dual-mode fluorescence and colorimetric sensing platform for efficient detection of ofloxacin in aquatic products using iron alkoxide nanozyme. Food Chem. 2024, 442, 13841. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, S.; Xie, X.; Li, Q.; Ding, L.; Chen, M.; Tu, J.; Xing, K.; Cheng, Y. Target-triggered Fe3O4@NPC-UCNPs assembly for photoactivatable biosensing of Aflatoxin B1. Chem. Eng. J. 2023, 470, 144028. [Google Scholar] [CrossRef]
- Chen, J.; Ho, W.K.H.; Yin, B.; Zhang, Q.; Li, C.; Yan, J.; Huang, Y.; Hao, J.; Yi, C.; Zhang, Y.; et al. Magnetic-responsive upconversion luminescence resonance energy transfer (LRET) biosensor for ultrasensitive detection of SARS-CoV-2 spike protein. Biosens. Bioelectron. 2024, 248, 115969. [Google Scholar] [CrossRef]
- Liao, H.Z.; Ye, S.; Zhang, H.R.; Huang, R.H.; Zheng, T.N.; Wang, D.P. Core-satellite upconversion nanocrystals@metal organic frameworks composite for the detection of hydrogen peroxide. J. Alloys Compd. 2025, 1014, 17870. [Google Scholar] [CrossRef]
- Sharma, P.; Duhan, U.; Dubey, R.; Kumar, S.; Goswami, T. Upconverting Luminescent MOF for Highly Sensitive Dual-Mode Recognition of Synthetic Dyes. Inorg. Chem. 2024, 63, 23651–23661. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, X.; Yan, D.; Mi, Y.; Sun, P.; Yan, X.; Liu, X.; Lu, G. Upconversion-based chiral nanoprobe for highly selective dual-mode sensing and bioimaging of hydrogen sulfide in vitro and in vivo. Light-Sci. Appl. 2024, 13, 180. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Li, S.; Han, D.; Ren, S.; Qin, K.; Zhou, H.; Han, T.; Gao, Z. The development of a fluorescence/colorimetric biosensor based on the cleavage activity of CRISPR-Cas12a for the detection of non-nucleic acid targets. J. Hazard. Mater. 2023, 449, 131044. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, D.; Zhang, Y.; Zhang, Q.-H.; Ji, Q.-Y.; Zhou, S.-K.; Huang, S.; Li, L.-l.; Lu, F.; Yao, W.-F.; et al. DNAzymes-conjugated upconversion nanoamplicon for in-situ ultrasensitive detection and imaging of microRNA in vivo. Chem. Eng. J. 2023, 454, 140489. [Google Scholar] [CrossRef]
| Biomass Category | Sensing Method | LOD | Linear Range | Ref | |
|---|---|---|---|---|---|
| Organic small molecules | Methamphetamine | UCNP-based | 47.25 pg/mL | 5.012 × 10–5.012 × 105 pg/mL | [86] |
| Acetamiprid | nanoenzymatic colorimetry | 25 cells/mL | 102–104 cells/mL | [87] | |
| Antigen | PSA | UCNP-based | 0.65 ng/mL | 1.5–80 ng/mL | [88] |
| PSA | SERS | 0.08 ng/mL | 0–400 ng/mL | [89] | |
| Nucleic acid | miRNA-21 | UCNP-based | 1 fM | 1 fM–2 nM | [81] |
| H1N1 | nanoenzymatic colorimetry | 0.02 nM | 0.02–50 nM | [90] | |
| Protein | FKBPL | UCNP-based | 10 pg/mL | 0.025–100 ng/mL | [82] |
| Insulin | electrochemical | 690 pM | 0–5 nM | [91] | |
| Microorganism | Avian influenza virus | UCNP-based | 103.5 EID50/mL | 103.5–105.5 EID50/mL | [92] |
| Salmonella typhimurium | QDs | 25 cells/mL | 102–104 cells/mL | [93] | |
| Type | Materials | Target | LOD | Mechanism | Ref. |
|---|---|---|---|---|---|
| Perovskite | Donor: CsPbBr2I@UCNP Acceptor: NiMn-LDH/CdS | Malathion | 4.8 fg/L | FRET PET | [104] |
| Donor: UCNP@SiO2 Acceptor: CsPbX3 | miRNA-155 | 73.5 pM | FRET | [105] | |
| Nano-enzyme | Donor: UCNP@SiO2/CeO2 Acceptor: MGO | S-cDNA | UCL: 320 fM Colorimetric: 28.4 pM | FRET | [106] |
| Donor: UCNPs Acceptor: reaction product | Hypoxanthine | 0.69 mg/L | IFE | [107] | |
| Donor: UCNPs Acceptor: oxTMB | Ofloxacin | UCL: 0.048 μg/kg Colorimetric: 0.165 μg/kg | IFE | [108] | |
| Donor: UCNPs Acceptor: Fe3O4@NPC | Aflatoxin B1 | 0.56 pg/mL | FRET | [109] | |
| Magnetic material | Donor: UCNPs Acceptor: Fe3O4@Au | SARS-CoV-2 | 2.1 pg/mL | FRET | [110] |
| MOF | Donor: UCNP@UiO-66-NH2 Acceptor: NiSx | H2O2 | 0.15 μM | IFE | [111] |
| Donor: 30%Yb3+-EuMOF Acceptor: MG | Malachite green | 36.33 nM | FRET | [112] | |
| Chiral material | Donor: UCNPs Acceptor: CuxOS | H2S | UCL: 43 μM CD: 22 μM | FRET | [113] |
| CHA | UCNPs-ssDNA-BHQ1 | PSA | 0.648 pg/mL | FRET | [114] |
| UCNPs@DNAzyme-BHQ1 | miRNA-21 | 31 fM | FRET | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Cao, H.; Wu, C.; Wang, T.; Sun, L.; Dong, B. Recent Progress on Rare-Earth-Doped Upconversion Nanomaterials for Bioassay Applications. Biosensors 2025, 15, 335. https://doi.org/10.3390/bios15060335
Xu J, Cao H, Wu C, Wang T, Sun L, Dong B. Recent Progress on Rare-Earth-Doped Upconversion Nanomaterials for Bioassay Applications. Biosensors. 2025; 15(6):335. https://doi.org/10.3390/bios15060335
Chicago/Turabian StyleXu, Jiling, Hengyuan Cao, Chenwei Wu, Ting Wang, Liheng Sun, and Biao Dong. 2025. "Recent Progress on Rare-Earth-Doped Upconversion Nanomaterials for Bioassay Applications" Biosensors 15, no. 6: 335. https://doi.org/10.3390/bios15060335
APA StyleXu, J., Cao, H., Wu, C., Wang, T., Sun, L., & Dong, B. (2025). Recent Progress on Rare-Earth-Doped Upconversion Nanomaterials for Bioassay Applications. Biosensors, 15(6), 335. https://doi.org/10.3390/bios15060335





