Integration of Carbon Nanotubes into Manganese Dioxide Nanorods for Enhanced Enzymeless Electrochemical Glucose Sensing with High Sensitivity and Selectivity
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
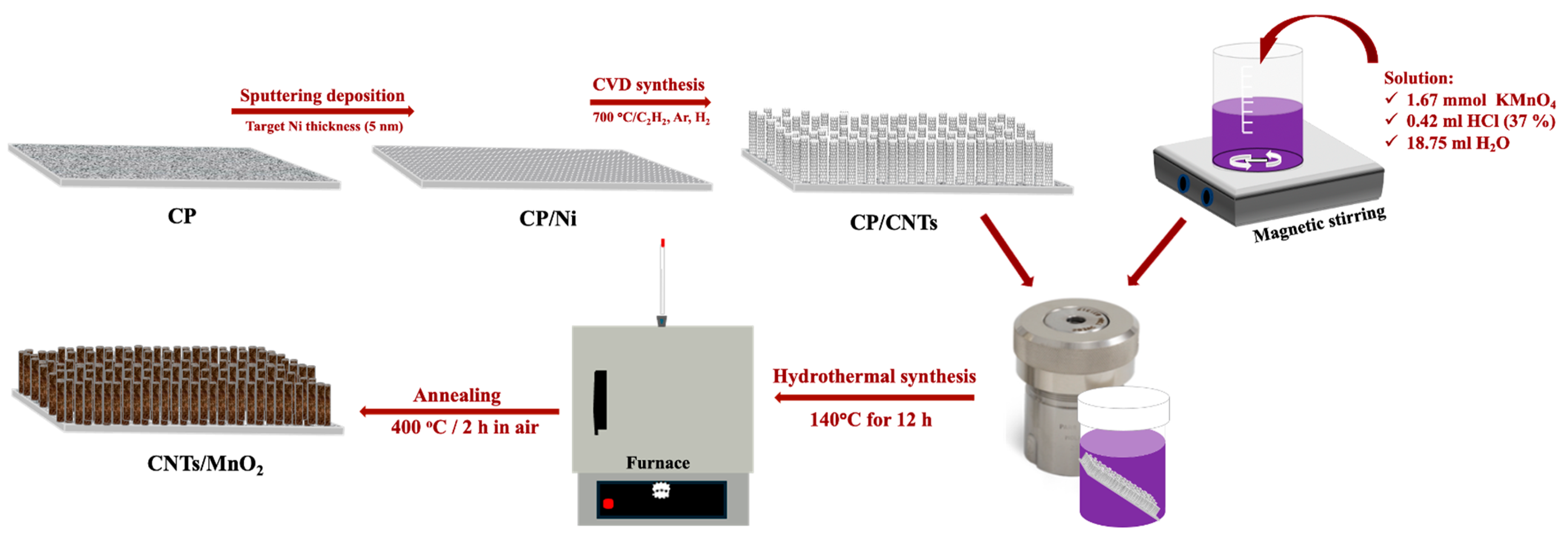
2.2. Synthesis of CNTs/MnO2 Freestanding Nanocomposite
2.3. Materials Structural Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
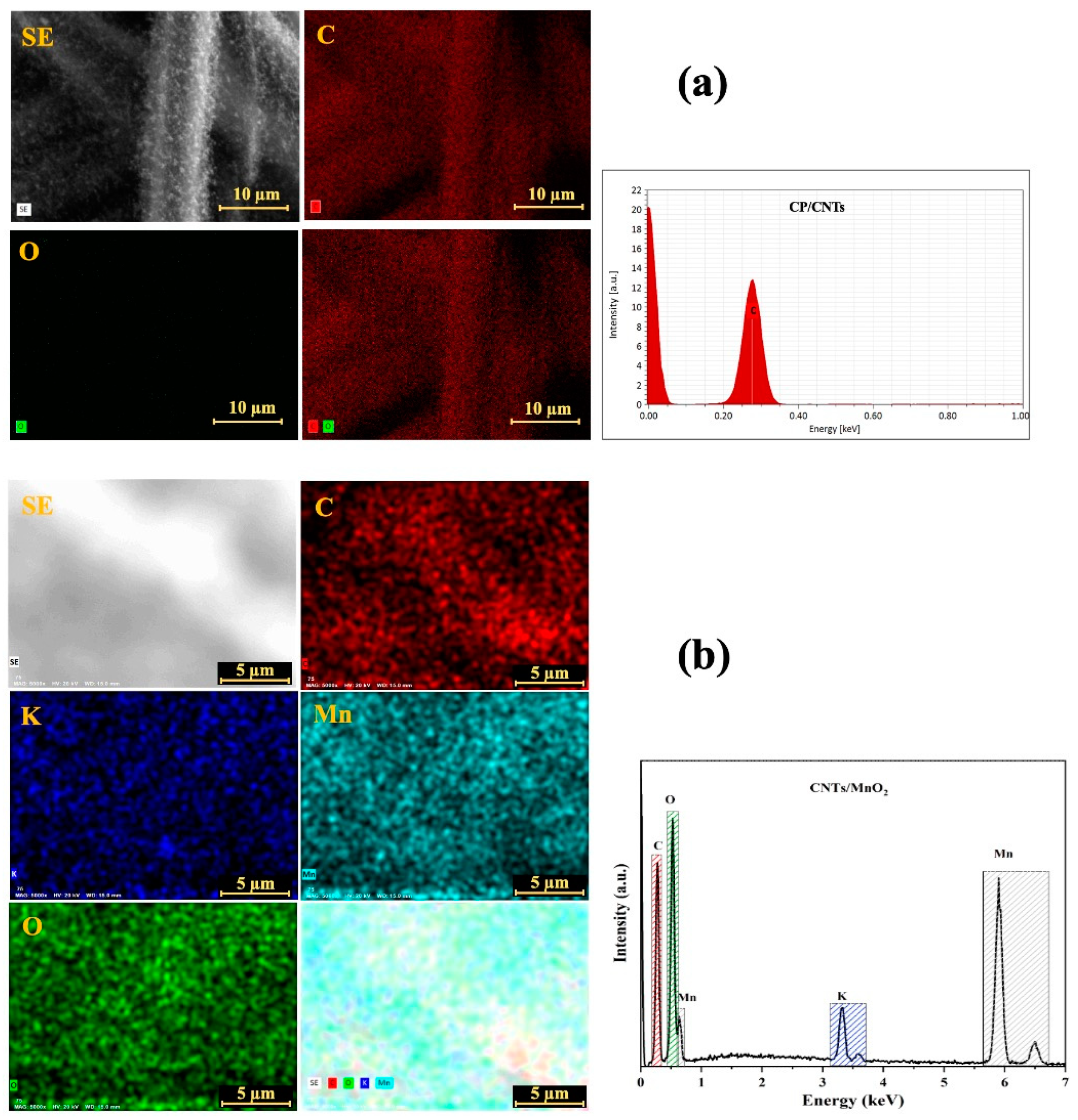
3.1. Structural Characterization
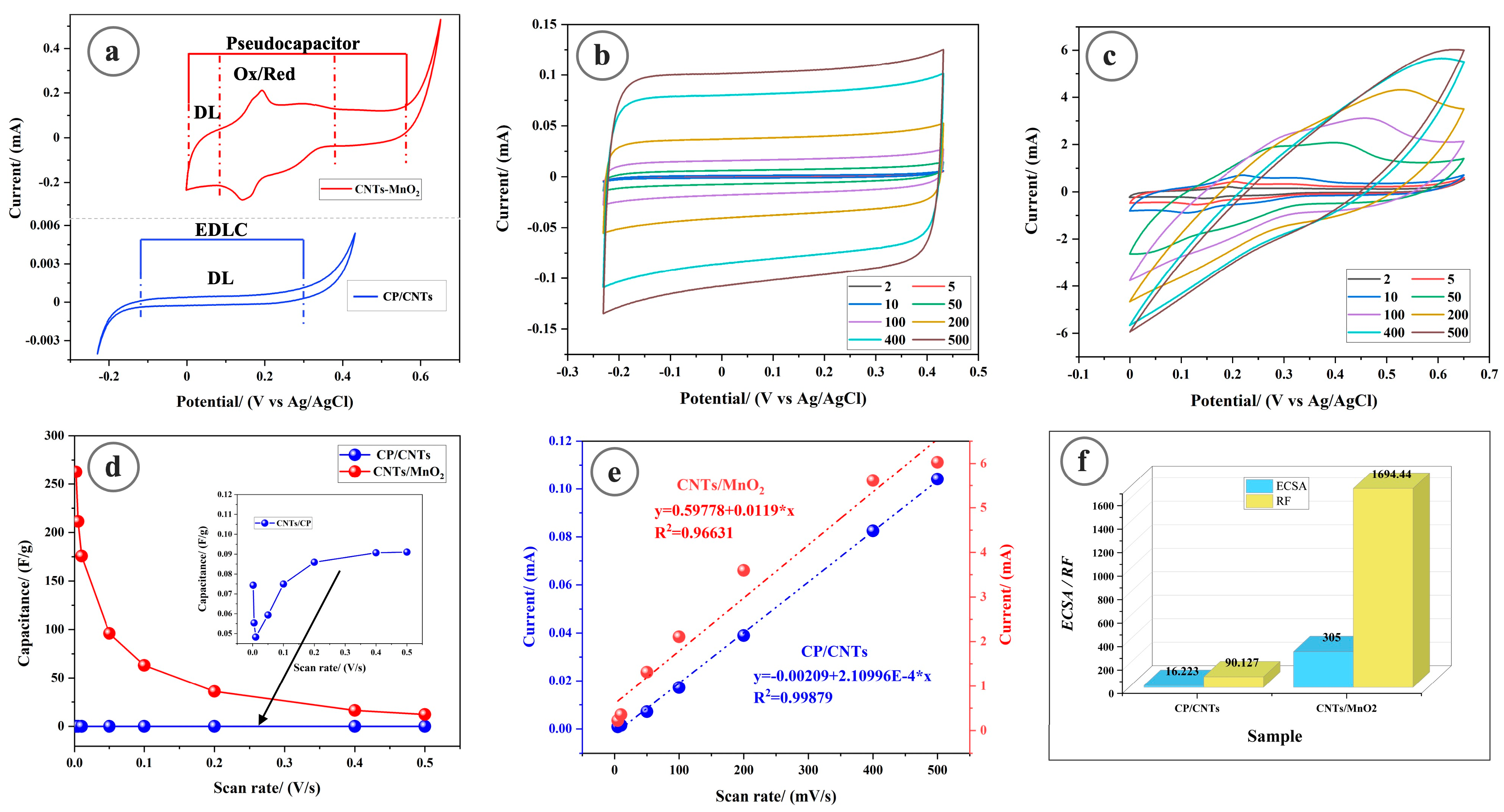
3.2. Electrochemical Surface Area Measurements
3.3. Voltammetric Detection of Glucose
3.4. Chronoamperometric Detection of Glucose
3.5. Chronoamperometry for Detection of Interfering Species in Glucose Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, G.A.; Awan, T.; Salim, H.; Arshad, F.; Hassan, I.U.; Pedram, M.Z.; Ahmed, W.; Faruck, H.L.; Aljabali, A.A.A.; Mishra, V.; et al. Fourth-generation glucose sensors composed of copper nanostructures for diabetes management: A critical review. Bioeng. Transl. Med. 2021, 7, e10248. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.; Tian, X.; Wang, Y.; Cui, B.; Yang, Y.; Dai, S.; Lin, W.; Zhu, J.; Wang, J.; et al. Coin-sized, fully integrated, and minimally invasive continuous glucose monitoring system based on organic electrochemical transistors. Sci. Adv. 2024, 10, eadl1856. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.-H. Carbon nanomaterials and their composites for electrochemical glucose biosensors: A review on fabrication and sensing properties. J. Taiwan Inst. Chem. Eng. 2024, 154, 104957. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Salim, H.; Hassan, I.U.; Awan, T.; Arshad, F.; Pedram, M.Z.; Ahmed, W.; Qurashi, A. Recent Advances in Non-Enzymatic Glucose Sensors Based on Metal and Metal Oxide Nanostructures for Diabetes Management—A Review. Front. Chem. 2021, 9, 748957. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, Y.; Liu, T.; Chu, Z.; Dempsey, E.; Jin, W. Conductive polymer nanocomposites: Recent advances in the construction of electrochemical biosensors. Sens. Diagn. 2024, 3, 165. [Google Scholar] [CrossRef]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Ponnusamy, R.; Gangan, A.; Chakraborty, B.; Late, D.J.; Rout, C.S. Improved Nonenzymatic Glucose Sensing Properties of Pd/MnO2 Nanosheets: Synthesis by Facile Microwave-Assisted Route and Theoretical Insight from Quantum Simulations. J. Phys. Chem. B 2018, 122, 7636–7646. [Google Scholar] [CrossRef]
- Chang, H.W.; Dong, C.L.; Chen, Y.H.; Xu, Y.Z.; Huang, T.C.; Chen, S.C.; Liu, F.J.; Lai, Y.H.; Tsai, Y.C. Extended Graphite Supported Flower-like MnO2 as Bifunctional Materials for Supercapacitors and Glucose Sensing. Nanomaterials 2021, 11, 2881. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Y.; Gao, H.; Ge, S.; Duan, H. Growth of Coral-like PtAu-MnO2 Binary Nanocomposites on Free-Standing Graphene Paper for Flexible Nonenzymatic Glucose Sensors. Biosens. Bioelectron. 2013, 41, 417–423. [Google Scholar] [CrossRef]
- Hao, L.; Li, S.S.; Wang, J.; Tan, Y.; Bai, L.; Liu, A. MnO2/Multi-Walled Carbon Nanotubes Based Nanocomposite with Enhanced Electrocatalytic Activity for Sensitive Amperometric Glucose Biosensing. J. Electroanal. Chem. 2020, 878, 114602. [Google Scholar] [CrossRef]
- Ahmed, K.H.; Mohamedi, M. Microfibrous carbon paper decorated with high-density manganese dioxide nanorods: An electrochemical nonenzymatic platform of glucose sensing. Sensors 2024, 18, 5864. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Mohammadi, N.; Moreno Zuria, A.; Mohamedi, M. Advanced Zinc–Air Batteries with Free-Standing Hierarchical Nanostructures of the Air Cathode for Portable Applications. ACS Appl. Mater. Interfaces 2021, 13, 61374–61385. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Liao, C.; Liang, W.; Lou, X.; Liu, Z.; Zhang, J.; Tang, Y.; Mai, L.; Zhou, L.; et al. High mass loading potassium ion stabilized manganese dioxide nanowire forests for rechargeable Zn batteries. Nano Energy 2024, 126, 109607. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, S.; Hu, Y.H. 3D Graphene Materials: From Understanding to Design and Synthesis Control. Chem. Rev. 2020, 120, 10336–10453. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhan, C.; He, K.; Chen, H.; Yao, W.; Sharifi-Asl, S.; Song, B.; Yang, Z.; Nie, A.; Luo, X.; et al. The influence of large cations on the electrochemical properties of tunnel-structured metal oxides. Nat. Commun. 2016, 7, 13374. [Google Scholar] [CrossRef]
- Ingole, S.M.; Navale, S.T.; Navale, Y.H.; Dhole, I.A.; Mane, R.S.; Stadler, F.J.; Patil, V.B. Galvanostatically electroplated MnO2 nanoplate-type electrode for potential electrochemical pseudocapacitor application. J. Solid State Electrochem. 2017, 21, 1817–1826. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, X.; Tynan, B.; Sha, Z.; Huang, F.; Islam, M.S.; Zhang, J.; Rider, A.N.; Dai, L.; Chu, D.; et al. High-performance hierarchical MnO2/CNT electrode for multifunctional supercapacitors. Carbon 2021, 184, 504. [Google Scholar] [CrossRef]
- Connor, P.; Schuch, J.; Kaiser, B.; Wolfram Jaegermann, W. The Determination of Electrochemical Active Surface Area and Specific Capacity Revisited for the System MnOx as an Oxygen Evolution Catalyst. Z. Phys. Chem. 2020, 234, 979–994. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, T.; Zhang, J.; Cui, E.; Yue, L.; Jiang, R.; Hou, G. The potassium hydroxide-urea synergy in improving the capacitive energy-storage performance of agar-derived carbon aerogels. Carbon 2019, 147, 451–459. [Google Scholar] [CrossRef]
- Ji, H.X.; Zhao, X.; Qiao, Z.H.; Jung, J.; Zhu, Y.W.; Lu, Y.L.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 3317. [Google Scholar] [CrossRef]
- Larew, L.; Johnson, D. Concentration dependence of the mechanism of glucose oxidation at gold electrodes in alkaline media. J. Electroanal. Chem. 1989, 262, 167–182. [Google Scholar] [CrossRef]
- Adzic, R.; Hsiao, M.; Yeager, E. Electrochemical oxidation of glucose on single crystal gold surfaces. J. Electroanal. Chem. 1989, 260, 475–485. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Conen, K.; Scanni, R.; Gombert, M.-T.; Hulter, H.N.; Krapf, R. Effects of potassium citrate or potassium chloride in patients with combined glucose intolerance: A placebo-controlled pilot study. J. Diabetes Its Complicat. 2016, 30, 1158–1161. [Google Scholar] [CrossRef]
- Kettritz, R.; Loffing, J. Potassium homeostasis—Physiology and pharmacology in a clinical context. Pharmacol. Ther. 2023, 249, 108489. [Google Scholar] [CrossRef]
- Thatikayala, D.; Ponnamma, D.; Sadasivuni, K.K.; Cabibihan, J.-J.; Al-Ali, A.K.; Malik, R.A.; Min, B. Progress of Advanced Nanomaterials in the Non-Enzymatic Electrochemical Sensing of Glucose and H2O2. Biosensors 2020, 10, 151. [Google Scholar] [CrossRef]
- Yang, Y.J.; Hu, S. Electrodeposited MnO2/Au composite film with improved electrocatalytic activity for oxidation of glucose and hydrogen peroxide. Electrochim. Acta 2010, 55, 3471–3476. [Google Scholar] [CrossRef]
- Hosseini, H.; Rezaei, S.J.T.; Rahmani, P.; Sharifi, R.; Nabid, M.R.; Bagheri, A. Nonenzymatic glucose and hydrogen peroxide sensors based on catalytic properties of palladium nanoparticles/poly(3,4-ethylenedioxythiophene) nanofibers. Sens. Actuators B Chem. 2014, 195, 85–91. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, K.H.; Zuria, A.M.; Mohamedi, M. Integration of Carbon Nanotubes into Manganese Dioxide Nanorods for Enhanced Enzymeless Electrochemical Glucose Sensing with High Sensitivity and Selectivity. Biosensors 2025, 15, 215. https://doi.org/10.3390/bios15040215
Ahmed KH, Zuria AM, Mohamedi M. Integration of Carbon Nanotubes into Manganese Dioxide Nanorods for Enhanced Enzymeless Electrochemical Glucose Sensing with High Sensitivity and Selectivity. Biosensors. 2025; 15(4):215. https://doi.org/10.3390/bios15040215
Chicago/Turabian StyleAhmed, Khawtar Hasan, Alonso Moreno Zuria, and Mohamed Mohamedi. 2025. "Integration of Carbon Nanotubes into Manganese Dioxide Nanorods for Enhanced Enzymeless Electrochemical Glucose Sensing with High Sensitivity and Selectivity" Biosensors 15, no. 4: 215. https://doi.org/10.3390/bios15040215
APA StyleAhmed, K. H., Zuria, A. M., & Mohamedi, M. (2025). Integration of Carbon Nanotubes into Manganese Dioxide Nanorods for Enhanced Enzymeless Electrochemical Glucose Sensing with High Sensitivity and Selectivity. Biosensors, 15(4), 215. https://doi.org/10.3390/bios15040215




