Recent Advances on Rapid Detection Methods of Steroid Hormones in Animal Origin Foods
Abstract
1. Introduction
2. Rapid Tests for Steroid Hormones
2.1. Electrochemical Methods
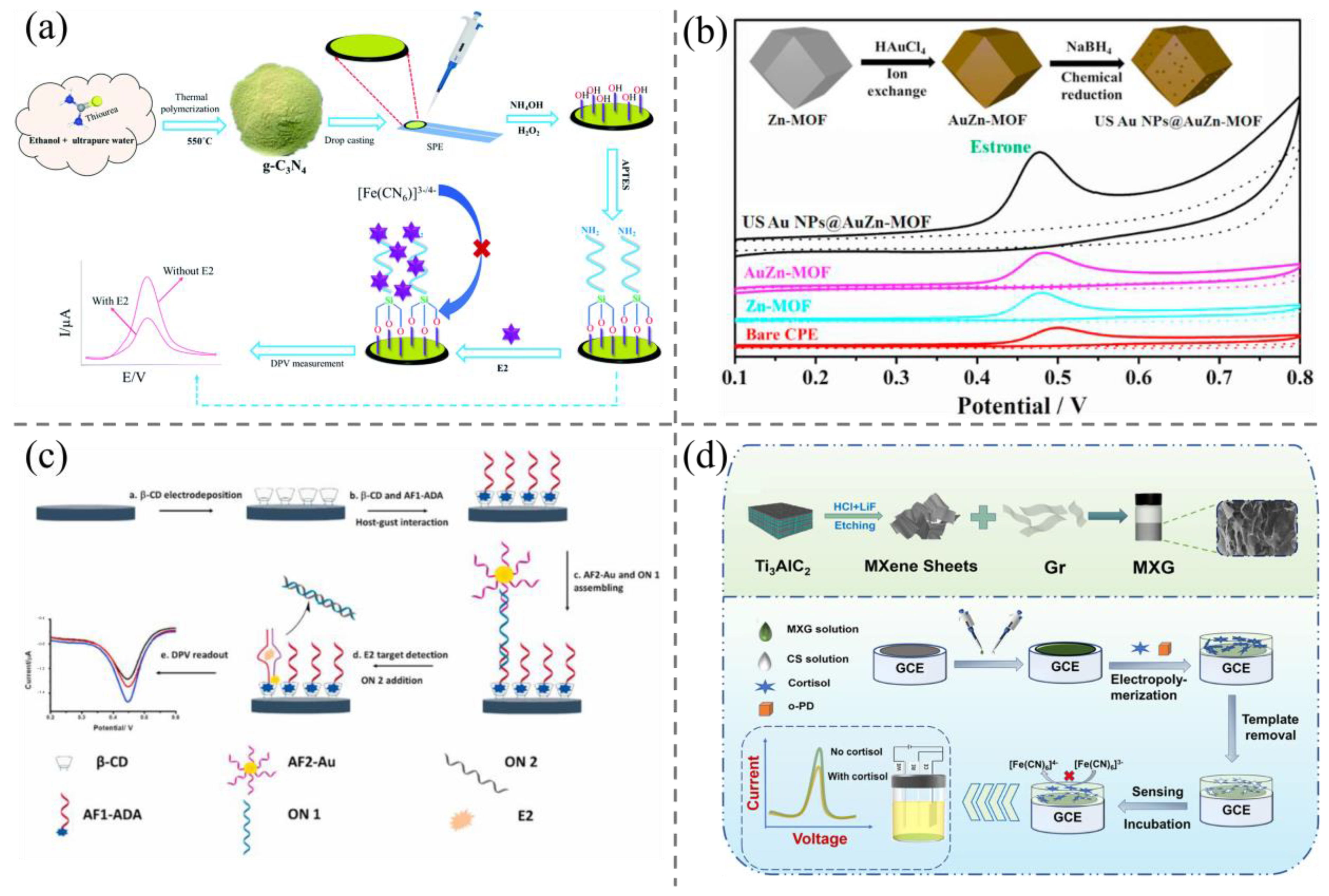
2.2. Colorimetric Methods
2.3. Fluorescence and Phosphorescence Method
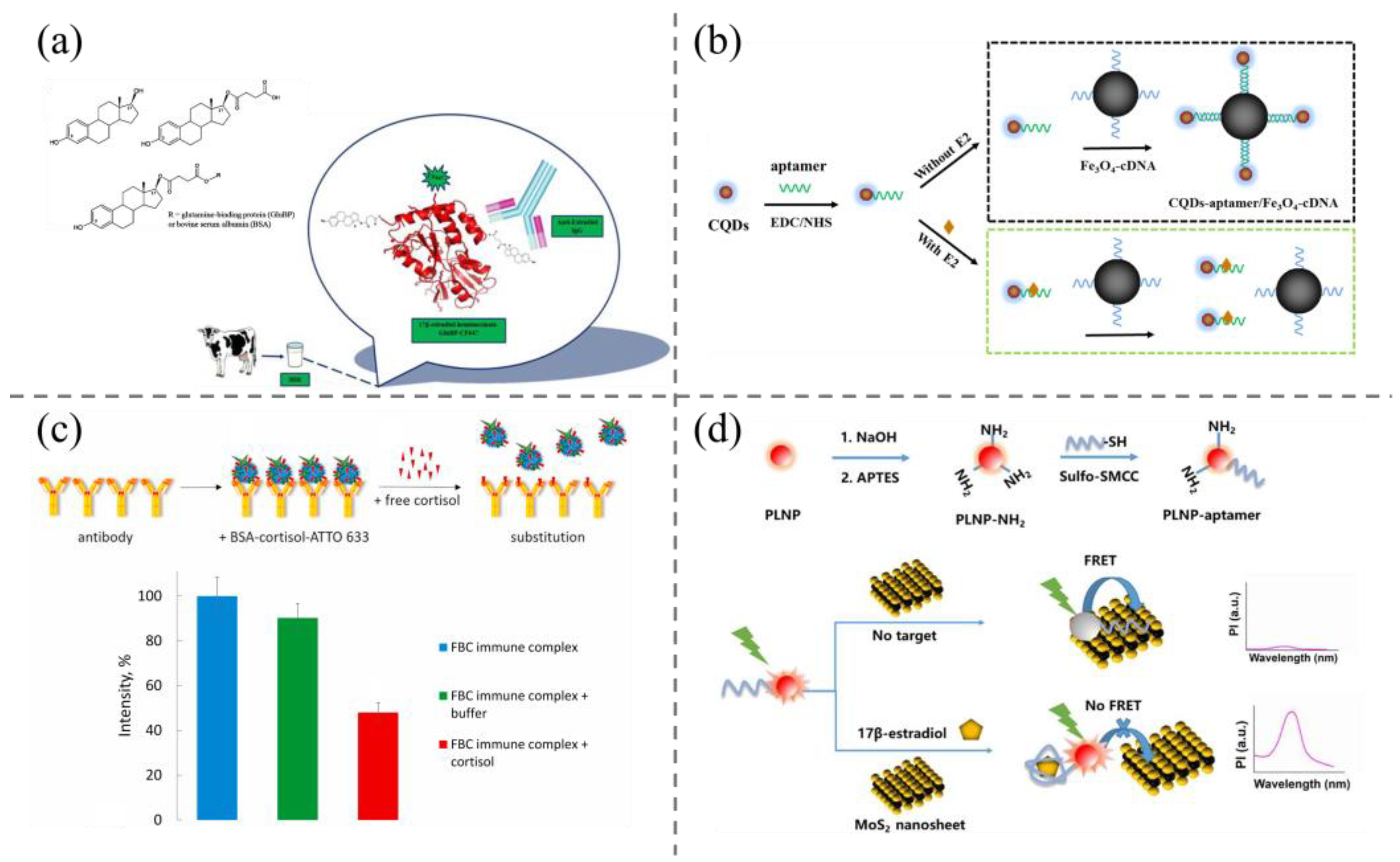
2.4. Surface-Enhanced Raman Spectroscopy Detection Technology
2.5. Lateral Flow Immunochromatography

2.6. Surface Plasmon Resonance Method
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hoga, C.A.; Almeida, F.L.; Reyes, F.G.R. A review on the use of hormones in fish farming: Analytical methods to determine their residues. CyTA-J. Food 2018, 16, 679–691. [Google Scholar] [CrossRef]
- Mlalila, N.; Mahika, C.; Kalombo, L.; Swai, H.; Hilonga, A. Human food safety and environmental hazards associated with the use of methyltestosterone and other steroids in production of all-male tilapia. Environ. Sci. Pollut. Res. 2015, 22, 4922–4931. [Google Scholar] [CrossRef]
- Jiang, X.L.; Hua, X.M.; Zhu, L.L. Production, safety and quality of aquatic products and strategies in China. Rural. Eco-Environ. 2004, 20, 77–80. [Google Scholar]
- Ismail, N.A.H.; Aris, A.Z.; Wee, S.Y.; Nasir, H.M.; Razak, M.R.; Kamarulzaman, N.H.; Omar, T.F.T. Occurrence and distribution of endocrine-disrupting chemicals in mariculture fish and the human health implications. Food Chem. 2021, 345, 128806. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Song, S.; Peng, J.; Liu, L.; Kuang, H.; Xu, C. Sensitive, fast, and specific immunoassays for methyltestosterone detection. Sensors 2015, 15, 10059–10073. [Google Scholar] [CrossRef]
- Gao, H.J.; Cheng, G.Y.; Wang, H.; Chen, T.; Xu, C.; Lv, H.Q.; Zhang, H.Y.; Hou, R.; Wang, Y.L.; Peng, D.P.; et al. Development of a broad-spectrum monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for screening of androgens in animal edible tissues. Microchem. J. 2021, 160, 157–162. [Google Scholar] [CrossRef]
- Huml, L.; Tauchen, J.; Rimpelova, S.; Holubova, B.; Lapcik, O.; Jurasek, M. Advances in the Determination of Anabolic-Androgenic Steroids: From Standard Practices to Tailor-Designed Multidisciplinary Approaches. Sensors 2022, 22, 4. [Google Scholar] [CrossRef]
- Zhu, J.L.; Yang, H.; Xiao, W.; Zou, Z.Y.; Li, D.Y.; Shan, H.Y. Determination of Methyltestosterone Residue in Tilapia Muscle by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Food Sci. 2011, 32, 243–247. [Google Scholar]
- Musee, N.; Kebaabetswe, L.P.; Tichapondwa, S.; Tubatsi, G.; Mahaye, N.; Leareng, S.K.; Nomngongo, P.N. Occurrence, Fate, Effects, and Risks of Dexamethasone: Ecological Implications Post-COVID-19. Int. J. Environ. Res. Public. Health 2021, 18, 11291. [Google Scholar] [CrossRef]
- Zou, D.X.; Gu, Y.J.; Luo, D.; Yang, W.J.; Gao, R.R.; Cao, X.; Dong, W.; Shi, H.H.; Zhao, H.T.; Liu, C. Rapid and ultra-sensitive testosterone detection via aptamer-functional gold nanoparticles. New J. Chem. 2023, 47, 1023–1026. [Google Scholar] [CrossRef]
- Qin, Y.; Qin, Y.; Bubiajiaer, H.; Chen, F.; Yao, J.; Zhang, M. Engineering constructed of high selectivity dexamethasone aptamer based on truncation and mutation technology. Front. Bioeng. Biotechnol. 2022, 10, 994711. [Google Scholar] [CrossRef]
- Fan, Y.B.; Yin, Y.M.; Jiang, W.B.; Chen, Y.P.; Yang, J.W.; Wu, J.; Xie, M.X. Simultaneous determination of ten steroid hormones in animal origin food by matrix solid-phase dispersion and liquid chromatography-electrospray tandem mass spectrometry. Food Chem. 2014, 142, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Conneely, G.; Aherne, M.; Lu, H.; Guilbault, G. Electrochemical immunosensors for the detection of 19-nortestosterone and methyltestosterone in bovine urine. Sens. Actuators B Chem. 2007, 121, 103–112. [Google Scholar] [CrossRef]
- Xia, W.Q.; Hu, Y.J.; Sun, C.; Zhang, M.Z.; Wang, W.P.; Sang, L.Y. Development of a coloidal gold immunochromatographic strip for methyltestosterone residue in aquatic products. Freshw. Fish. 2016, 46, 93–98. [Google Scholar]
- Meng, X.; Xie, S.H.; Xie, S.T.; Xu, J.H.; Liu, W.Z.; Hua, Q. Simultaneous Determination of Six Hormones in Muscle Tissues of Monopterus albus Using Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Sci. 2015, 31, 208–212. [Google Scholar]
- Charithra, M.M.; Manjunatha, J.G.G.; Raril, C. Surfactant Modified Graphite Paste Electrode as an Electrochemical Sensor for the Enhanced Voltammetric Detection of Estriol with Dopamine and Uric acid. Adv. Pharm. Bull. 2020, 10, 247–253. [Google Scholar] [CrossRef]
- Ding, N.; Zhao, H.; Peng, W.B.; He, Y.J.; Zhou, Y.; Yuan, L.F.; Zhang, Y.X. A simple colorimetric sensor based on anti-aggregation of gold nanoparticles for Hg2+ detection. Colloids Surf. A-Physicochem. Eng. Asp. 2012, 395, 161–167. [Google Scholar] [CrossRef]
- Liu, J.C.; Bai, W.H.; Niu, S.C.; Zhu, C.; Yang, S.M.; Chen, A.L. Highly sensitive colorimetric detection of 17β-estradiol using split DNA aptamers immobilized on unmodified gold nanoparticles. Sci. Rep. 2014, 4, 7571. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, X.; Yang, J.; Tang, C.; Jiang, H.; Cai, T.; Chen, M.; Wei, Z.; Yu, P. A label-free fluorescent aptamer sensor for testosterone based on SYBR Green I. Anal. Methods 2023, 15, 1546–1552. [Google Scholar] [CrossRef]
- Wang, R.; Chon, H.; Lee, S.; Cheng, Z.Y.; Hong, S.H.; Yoon, Y.H.; Choo, J. Highly Sensitive Detection of Hormone Estradiol E2 Using Surface-Enhanced Raman Scattering Based Immunoassays for the Clinical Diagnosis of Precocious Puberty. ACS Appl. Mater. Interfaces 2016, 8, 10665–10672. [Google Scholar] [CrossRef]
- Choi, J.M.; Hahm, E.; Park, K.; Jeong, D.; Rho, W.-Y.; Kim, J.; Jeong, D.H.; Lee, Y.-S.; Jhang, S.H.; Chung, H.J.; et al. SERS-Based Flavonoid Detection Using Ethylenediamine-β-Cyclodextrin as a Capturing Ligand. Nanomaterials 2017, 7, 8. [Google Scholar] [CrossRef]
- Wang, Z.X.; Sun, L.; Liu, L.Q.; Kuang, H.; Xu, L.G.; Xu, C.L. Ultrasensitive detection of seventeen chemicals simultaneously using paper-based sensors. Mater. Chem. Front. 2018, 2, 1900–1910. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, W.L.; Yang, C.; Xu, Z.H.; Zhang, Y.; Shi, X.L. Detection of 17beta-Estradiol in Water by Time-resolved Fluorescence Lateral Flow Assay. J. Instrum. Anal. 2021, 40, 1409–1416. [Google Scholar]
- Ren, J.; Yin, X.C.; Hu, H.L.; Wang, S.C.; Tian, Y.L.; Chen, Y.Q.; Li, Y.C.; Wang, J.L.; Zhang, D.H. A multi-scenario dip-stick immunoassay of 17β-estradiol based on multifunctional and non-composite nanoparticles with colorimetric-nanozyme-magnetic properties. Sens. Actuators B-Chem. 2022, 367, 132150. [Google Scholar] [CrossRef]
- Qin, Y.; Bubiajiaer, H.; Yao, J.; Zhang, M. Based on Unmodified Aptamer-Gold Nanoparticles Colorimetric Detection of Dexamethasone in Food. Biosensors 2022, 12, 242. [Google Scholar] [CrossRef]
- Barreto, F.C.; Leme Silva, M.K.; Cesarino, I. An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation. Chemosensors 2022, 10, 395. [Google Scholar] [CrossRef]
- Bacchu, M.S.; Ali, M.R.; Hasan, M.N.; Mamun, M.R.A.; Hossain, M.I.; Khan, M.Z.H. Graphitic carbon nitride and APTES modified advanced electrochemical biosensor for detection of 17beta-estradiol in spiked food samples. RSC Adv. 2022, 12, 16581–16588. [Google Scholar] [CrossRef]
- Chang, Z.; Zhu, B.C.; Liu, J.J.; Zhu, X.; Xu, M.T.; Travas-Sejdic, J. Electrochemical aptasensor for 17β-estradiol using disposable laser scribed graphene electrodes. Biosens. Bioelectron. 2021, 185, 113247. [Google Scholar] [CrossRef]
- Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. Graphene-Based Electrodes for Monitoring of Estradiol. Chemosensors 2023, 11, 337. [Google Scholar] [CrossRef]
- Zhao, Q.A.; Faraj, Y.; Liu, L.Y.; Wang, W.; Xie, R.; Liu, Z.; Ju, X.J.; Wei, J.; Chu, L.Y. Simultaneous determination of dopamine, uric acid and estriol in maternal urine samples based on the synergetic effect of reduced graphene oxide, silver nanowires and silver nanoparticles in their ternary 3D nanocomposite. Microchem. J. 2020, 158, 105185. [Google Scholar] [CrossRef]
- Cao, Q.A.; Zhao, H.; Zeng, L.X.; Wang, J.; Wang, R.; Qiu, X.H.; He, Y.J. Electrochemical determination of melamine using oligonucleotides modified gold electrodes. Talanta 2009, 80, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Samie, H.A.; Arvand, M. Label-free electrochemical aptasensor for progesterone detection in biological fluids. Bioelectrochemistry 2020, 133, 107489. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ye, D.X.; Yang, J.; Zhu, W.Y.; Li, L.; Ding, Y.P. Dual recognition elements for selective determination of progesterone based on molecularly imprinted electrochemical aptasensor. Anal. Chim. Acta 2023, 1264, 341288. [Google Scholar] [CrossRef]
- Singh, A.C.; Asif, M.H.; Bacher, G.; Danielsson, B.; Willander, M.; Bhand, S. Nanoimmunosensor based on ZnO nanorods for ultrasensitive detection of 17β-Estradiol. Biosens. Bioelectron. 2019, 126, 15–22. [Google Scholar] [CrossRef]
- Disha; Kumari, P.; Nayak, M.K.; Kumar, P. A bio-sensing platform based on graphene quantum dots for label free electrochemical detection of progesterone. In Proceedings of the International Conference on Advances in Nanomaterials and Devices for Energy and Environment (ICAN), Gwalior, India, 27–29 January 2019; pp. 583–586. [Google Scholar] [CrossRef]
- Masikini, M.; Ghica, M.E.; Baker, P.G.L.; Iwuoha, E.I.; Brett, C.M.A. Electrochemical Sensor Based on Multi-walled Carbon Nanotube/Gold Nanoparticle Modified Glassy Carbon Electrode for Detection of Estradiol in Environmental Samples. Electroanalysis 2019, 31, 1925–1933. [Google Scholar] [CrossRef]
- Chai, C.X.; Gao, J.; Zhao, G.Q.; Li, L.L.; Tang, Y.; Wu, C.; Wan, C.D. In-situ synthesis of ultrasmall Au nanoparticles on bimetallic metal-organic framework with enhanced electrochemical activity for estrone sensing. Anal. Chim. Acta 2021, 1152, 157–162. [Google Scholar] [CrossRef]
- Wu, L.; Yang, W.; Xia, C.; Xu, C.; Zhang, H.Y. An electrochemical immunosensor for detecting progesterone in milk from dairy cows. Vet. Arh. 2018, 88, 49–57. [Google Scholar] [CrossRef]
- Liu, H.C.; Qin, W.J.; Li, X.X.; Feng, L.; Gu, C.S.; Chen, J.J.; Tian, Z.H.; Chen, J.X.; Yang, M.; Qiao, H.Y.; et al. Molecularly Imprinted Electrochemical Sensors Based on Ti3C2Tx-MXene and Graphene Composite Modifications for Ultrasensitive Cortisol Detection. Anal. Chem. 2023, 95, 16079–16088. [Google Scholar] [CrossRef]
- Yeasmin, S.J.; Wu, B.; Liu, Y.; Ullah, A.; Cheng, L.J. Nano gold-doped molecularly imprinted electrochemical sensor for rapid and ultrasensitive cortisol detection. Biosens. Bioelectron. 2022, 206, 114142. [Google Scholar] [CrossRef]
- Lee, M.H.; Thomas, J.L.; Liu, W.C.; Zhang, Z.X.; Liu, B.D.; Yang, C.H.; Lin, H.Y. A multichannel system integrating molecularly imprinted conductive polymers for ultrasensitive voltammetric determination of four steroid hormones in urine. Microchim. Acta 2019, 186, 695. [Google Scholar] [CrossRef] [PubMed]
- Manickam, P.; Arizaleta, F.; Gurusamy, M.; Bhansali, S. Theoretical Studies of Cortisol-Imprinted Prepolymerization Mixtures: Structural Insights into Improving the Selectivity of Affinity Sensors. J. Electrochem. Soc. 2017, 164, B3077–B3080. [Google Scholar] [CrossRef]
- Tlili, C.; Myung, N.V.; Shetty, V.; Mulchandani, A. Label-free, chemiresistor immunosensor for stress biomarker cortisol in saliva. Biosens. Bioelectron. 2011, 26, 4382–4386. [Google Scholar] [CrossRef]
- Na, W.; Park, J.W.; An, J.H.; Jang, J. Size-controllable ultrathin carboxylated polypyrrole nanotube transducer for extremely sensitive 17β-estradiol FET-type biosensors. J. Mater. Chem. B 2016, 4, 5025–5034. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.Q.; Zhi, H.; Keefe, D.W.; Gao, B.T.; LeFevre, G.H.; Toor, F. Sensitive and Specific Detection of Estrogens Featuring Doped Silicon Nanowire Arrays. ACS Omega 2022, 7, 47341–47348. [Google Scholar] [CrossRef]
- Lee, S.; Kang, C.; Song, J.; Kwon, Y.; Kim, J. Development of HRP-assisted rGO-FET biosensors for high-precision measurement of serological steroid hormones. Anal. Chim. Acta 2025, 1336, 9. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.Y.; Jia, M.M.; Yang, Z.K.; Liang, Z.W.; Guo, J.J.; Luo, Y.L.; Shen, F.; Sun, C.Y. Colorimetric determination of 17β-estradiol based on the specific recognition of aptamer and the salt-induced aggregation of gold nanoparticles. Mater. Lett. 2015, 159, 221–224. [Google Scholar] [CrossRef]
- Tu, E.; Pearlmutter, P.; Tiangco, M.; Derose, G.; Begdache, L.; Koh, A. Comparison of Colorimetric Analyses to Determine Cortisol in Human Sweat. ACS Omega 2020, 5, 8211–8218. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Z.J.; Su, W.H.; Wu, H.Y.; Gopinath, S.C.B.; Chen, R.X. Gold nanoparticle assembly and disassembly in colorimetric immunoassay to detect 17β-estradiol and determine gynecological disorder. Process Biochem. 2020, 99, 21–26. [Google Scholar] [CrossRef]
- Chang, C.C.; Li, C.F.; Yang, Z.H.; Lin, P.Y.; Chang, H.C.; Yang, C.W. Target-induced recycling assembly of split aptamer fragments by DNA toehold-mediated displacement for the amplified colorimetric detection of estradiol. Sens. Actuators B-Chem. 2022, 364, 131823. [Google Scholar] [CrossRef]
- Pule, B.O.; Degni, S.; Torto, N. Electrospun fibre colorimetric probe based on gold nanoparticles for on-site detection of 17β-estradiol associated with dairy farming effluents. Water SA 2015, 41, 27–32. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zhang, S.Q.; Du, S.Y.; Pang, S.X.; Liu, X.Y.; Zhang, H.Y. A high throughput screening method for endocrine disrupting chemicals in tap water and milk samples based on estrogen receptor α and gold nanoparticles. Anal. Methods 2020, 12, 200–204. [Google Scholar] [CrossRef]
- Alsager, O.A.; Kumar, S.; Zhu, B.; Travas-Sejdic, J.; McNatty, K.P.; Hodgkiss, J.M. Ultrasensitive Colorimetric Detection of 17β-Estradiol: The Effect of Shortening DNA Aptamer Sequences. Anal. Chem. 2015, 87, 4201–4209. [Google Scholar] [CrossRef]
- Soh, J.H.; Lin, Y.Y.; Rana, S.; Ying, J.Y.; Stevens, M.M. Colorimetric Detection of Small Molecules in Complex Matrixes via Target-Mediated Growth of Aptamer-Functionalized Gold Nanoparticles. Anal. Chem. 2015, 87, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.L.; Li, J.W.; Liu, Y. Colourimetric assay for β-estradiol based on the peroxidase-like activity of Fe3O4@mSiO2@HP-β-CD nanoparticles. RSC Adv. 2015, 5, 107670–107679. [Google Scholar] [CrossRef]
- Kongpreecha, P.; Chumpol, J.; Siri, S. Highly sensitive colorimetric aptasensor for 17 beta-estradiol detection in milk based on the repetitive-loop aptamer. Biotechnol. Appl. Biochem. 2023, 70, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Yang, B.; Zhu, J.; Lou, S.Y.; Hao, H.M.; Lu, W.Y. Light-activated, dual-mode fluorescence and colorimetric detection of estradiol with high fidelity based on aptamer’s special recognition. Food Chem. 2024, 436, 137702. [Google Scholar] [CrossRef]
- Tan, C.X.; Schenk, J.A.; Gajovic-Eichelmann, N.; Sellrie, F.; Bier, F.F. A new one-step antigen heterologous homogeneous fluorescence immunoassay for progesterone detection in serum. Talanta 2015, 134, 508–513. [Google Scholar] [CrossRef]
- Sarkar, I.; Mishra, A.K. Fluorophore tagged bio-molecules and their applications: A brief review. Appl. Spectrosc. Rev. 2018, 53, 552–601. [Google Scholar] [CrossRef]
- Lee, W.-I.; Shrivastava, S.; Le-Thai, D.; Kim, B.Y.; Son, Y.-M.; Lee, N.-E. A smartphone imaging-based label-free and dual-wavelength fluorescent biosensor with high sensitivity and accuracy. Biosens. Bioelectron. 2017, 94, 643–650. [Google Scholar] [CrossRef]
- Varriale, A.; Pennacchio, A.; Pinto, G.; Oliviero, G.; D’Errico, S.; Majoli, A.; Scala, A.; Capo, A.; Pennacchio, A.; Di Giovanni, S.; et al. A Fluorescence Polarization Assay to Detect Steroid Hormone Traces in Milk. J. Agric. Food Chem. 2015, 63, 9159–9164. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Li, J.; Liang, A.H.; Wen, G.Q.; Jiang, Z.L. Aptamer Turn-On SERS/RRS/Fluorescence Tri-mode Platform for Ultra-trace Urea Determination Using Fe/N-Doped Carbon Dots. Front. Chem. 2021, 9, 613083. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Dong, L.Y.; Chen, L.; Fan, Y.B.; Wu, J.; Wang, X.F.; Xie, M.X. A novel water-soluble quantum dot-neutral red fluorescence resonance energy transfer probe for the selective detection of megestrol acetate. New J. Chem. 2015, 39, 555–565. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Zhang, P.Y.; Pu, H.B.; Sun, D.W. A fluorescence aptasensor based on carbon quantum dots and magnetic Fe3O4 nanoparticles for highly sensitive detection of 17β-estradiol. Food Chem. 2022, 373, 131591. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Tanyi, E.K.; Yeasmin, S.; Cheng, L.J. Label-Free Sensitive Detection of Steroid Hormone Cortisol Based on Target-Induced Fluorescence Quenching of Quantum Dots. Langmuir 2020, 36, 7781–7788. [Google Scholar] [CrossRef]
- Ni, X.; Xia, B.; Wang, L.M.; Ye, J.; Du, G.S.; Feng, H.W.; Zhou, X.T.; Zhang, T.; Wang, W.H. Fluorescent aptasensor for 17β-estradiol determination based on gold nanoparticles quenching the fluorescence of Rhodamine B. Anal. Biochem. 2017, 523, 17–23. [Google Scholar] [CrossRef]
- Hong, S.C.; Murale, D.P.; Lee, M.; Lee, S.M.; Park, J.S.; Lee, J.-S. Bulk Aggregation Based Fluorescence Turn-On Sensors for Selective Detection of Progesterone in Aqueous Solution. Angew. Chem. 2017, 56, 14642–14647. [Google Scholar] [CrossRef]
- Arafa, R.M.; Yehia, A.M.; Abbas, S.S.; Amer, S.M. Exploiting steroid-cyclodextrin complexes for selective determination of Estradiol Valerate and Norethisterone Acetate by synchronous fluorescence spectrofluorimetry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117237. [Google Scholar] [CrossRef]
- Haynes, A.Z.; Levine, M. Detection of anabolic steroids via cyclodextrin-promoted fluorescence modulation. RSC Adv. 2020, 10, 25108–25115. [Google Scholar] [CrossRef]
- Tan, Y.N.; Hu, X.X.; Liu, M.; Liu, X.W.; Lv, X.B.; Li, Z.H.; Wang, J.; Yuan, Q. Simultaneous Visualization and Quantitation of Multiple Steroid Hormones Based on Signal-Amplified Biosensing with Duplex Molecular Recognition. Chem.-A Eur. J. 2017, 23, 10683–10689. [Google Scholar] [CrossRef]
- Wu, J.Z.; Ahmad, W.; Zhang, J.G.; Wei, W.Y.; Yu, J.H.; Zhang, W.Q.; Chen, Q.S.; Ouyang, Q. Ratiometric upconversion-luminescence in-situ sampling aptasensing platform integrated with smartphone-based device for visual detection of 17β-estradiol. Sens. Actuators B-Chem. 2023, 390, 133999. [Google Scholar] [CrossRef]
- Safarian, S.M.; Kusov, P.A.; Kosolobov, S.S.; Borzenkova, O.V.; Khakimov, A.V.; Kotelevtsev, Y.V.; Drachev, V.P. Surface-specific washing-free immunosensor for time-resolved cortisol monitoring. Talanta 2021, 225, 122070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Zhang, X.X.; Wang, W.L.; Yang, C.; Shi, X.L.; Feng, Y.W.; Abdurahman, R. NIR persistent luminescence nanoparticles based turn-on aptasensor for autofluorescence-free determination of 17β-estradiol in milk. Food Chem. 2022, 373, 131432. [Google Scholar] [CrossRef]
- Pomelova, V.G.; Osin, N.S.; Bychenkova, T.A.; Paramonov, D.V.; Kostryukova, T.S. Application of Eu(III) Nanoparticle Labels in Time-Resolved Phosphorescence Analysis for Detection of Thyroid Stimulating Hormone. Russ. J. Bioorganic Chemistry 2017, 43, 377–385. [Google Scholar] [CrossRef]
- Chen, G.L.; Zhou, J.; Feng, H.; Feng, F.F.; Xu, P.F.; Pan, S.F.; Xu, J.; Qian, Z.S. A simple and efficient phosphorescent probe for iodide-specific detection based on crystallization-induced phosphorescence of organic ionic crystals. J. Mater. Chem. C. 2019, 7, 43–47. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Qin, W.; Du, C.; Gao, H.Y.; Zhu, F.M.; Liang, G.D. Long-Lived Room-Temperature Phosphorescence for Visual and Quantitative Detection of Oxygen. Angew. Chem. 2019, 58, 12102–12106. [Google Scholar] [CrossRef]
- Feng, C.; Dong, K.; Wu, Y.J.; Zhang, P.X. Study of Raman Technique Applied to Estrogen. Spectrosc. Spectr. Anal. 2011, 31, 2127–2130. [Google Scholar] [CrossRef]
- Xue, J.Q.; Li, D.W.; Qu, L.L.; Long, Y.T. Surface-imprinted core-shell Au nanoparticles for selective detection of bisphenol A based on surface-enhanced Raman scattering. Anal. Chim. Acta 2013, 777, 57–62. [Google Scholar] [CrossRef]
- Vedad, J.; Mojica, E.-R.E.; Desamero, R.Z.B. Raman spectroscopic discrimination of estrogens. Vib. Spectrosc. 2018, 96, 93–100. [Google Scholar] [CrossRef]
- Albuquerque, C.D.L.; Nogueira, R.B.; Poppi, R.J. Determination of 17β-estradiol and noradrenaline in dog serum using surface-enhanced Raman spectroscopy and random Forest. Microchem. J. 2016, 128, 95–101. [Google Scholar] [CrossRef]
- Mugo, S.M.; Lu, W. Determination of β-Estradiol by Surface-Enhance Raman Spectroscopy (SERS) Using a Surface Imprinted Methacrylate Polymer on Nanoporous Biogenic Silica. Anal. Lett. 2022, 55, 378–387. [Google Scholar] [CrossRef]
- Liu, X.H.; Deng, H.; Chang, L.; Zhang, W.; Jiang, S. Recent Progress of SERS for Environmental Estrogen Detection. Spectrosc. Spectr. Anal. 2020, 40, 3038–3047. [Google Scholar] [CrossRef]
- Liu, J.X.; Pan, M.D.; Jin, J.Z.; Lu, Y.S.; Feng, Z.M.; Sun, T. Prepared Three-Dimensional Flowerlike MoS2/Ag@rGO Nanocomposite as a Self-Cleaning SERS Substrate for the Trace Detection of 17β-Estradiol in Environmental Water. ACS Sustain. Chem. Eng. 2024, 12, 893–903. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Wang, J.J.; Lai, Y.T.; Wei, Z.; Li, J.B. Rapid detection of estrogen compounds using surface-enhanced Raman spectroscopy with a Zn/Au-Ag/Ag sandwich-structured substrate. Opt. Mater. 2021, 112, 110759. [Google Scholar] [CrossRef]
- Liu, S.Y.; Cheng, R.J.; Chen, Y.Q.; Shi, H.J.; Zhao, G.H. A simple one-step pretreatment, highly sensitive and selective sensing of 17β-estradiol in environmental water samples using surface-enhanced Raman spectroscopy. Sens. Actuators B-Chem. 2018, 254, 1157–1164. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wu, Z.Y.; Yang, Y.H.; Ye, J.; Han, S.; Li, Y.T. Fabrication of molecularly-imprinted gold nanoparticle-embedded Fe-MOFs for highly selective SERS detection of 17β-estradiol in milk. Analyst 2023, 148, 2472–2481. [Google Scholar] [CrossRef]
- Liu, J.X.; Zheng, J.; Lu, Y.S.; Feng, Z.M.; Zhang, S.Q.; Sun, T. Prepared Sandwich structure WS2/ag@MIP composite for ultrasensitive SERS detection of trace 17beta-estradiol in food. Food Chem. 2024, 460, 140731. [Google Scholar] [CrossRef]
- Kissell, L.N.; Sheokand, M.; Strobbia, P. Rational design enabling aptamer-based sensors for surface-enhanced Raman detection of small molecules. J. Raman Spectrosc. 2023, 54, 966–975. [Google Scholar] [CrossRef]
- Wang, Z.W.; Yan, R.X.; Liao, S.W.; Miao, Y.R.; Zhang, B.; Wang, F.; Yang, H.F. In situ reduced silver nanoparticles embedded molecularly imprinted reusable sensor for selective and sensitive SERS detection of Bisphenol A. Appl. Surf. Sci. 2018, 457, 323–331. [Google Scholar] [CrossRef]
- Xing, C.R.; Liu, L.Q.; Song, S.S.; Feng, M.; Kuang, H.; Xu, C.L. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens. Bioelectron. 2015, 66, 445–453. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.Q.; Yu, M.; Zhao, H. The application of a lateral flow immunographic assay to rapidly test for dexamethasone in commercial facial masks. Anal. Bioanal. Chem. 2019, 411, 5703–5710. [Google Scholar] [CrossRef]
- Xu, Z.H.; Sun, T.Q.; He, H.Q.; Liu, W.T.; Fan, L.X.; Zhao, L.D.; Wu, X.L.; Han, Z.Y.; Zhang, Y.C.; Wang, Q.Q.; et al. Simultaneous detection of diethylstilbestrol and estradiol residues with a single immunochromatographic assay strip. Food Sci. Nutr. 2021, 9, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Guo, L.L.; Liu, L.Q.; Kuang, H.; Xu, C.L. Colloidal gold-based immunochromatographic strip assay for the rapid detection of three natural estrogens in milk. Food Chem. 2018, 259, 122–129. [Google Scholar] [CrossRef]
- Yao, X.L.; Wang, Z.H.; Zhao, M.; Liu, S.J.; Su, L.H.; Dou, L.; Li, T.; Wang, J.L.; Zhang, D.H. Graphite-like carbon nitride-laden gold nanoparticles as signal amplification label for highly sensitive lateral flow immunoassay of 17β-estradiol. Food Chem. 2021, 347, 129001. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Wang, L.L.; Yao, X.L.; Yin, X.C.; Zhang, H.; Zhao, M.; Liu, S.J.; Wang, Z.H.; Wang, J.L.; Zhang, D.H. Small size nanoparticles-Co3O4 based lateral flow immunoassay biosensor for highly sensitive and rapid detection of furazolidone. Talanta 2020, 211, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Sun, C.Y.; Zhang, X.Y.; Shu, R.; Zhang, J.Y.; Wang, B.; Wang, K.X.; Dou, L.N.; Huang, L.J.; Yang, Q.Y.; et al. Advances in enhancement-type signal tracers and analysis strategies driven Lateral flow immunoassay for guaranteeing the agri-food safety. Biosens. Bioelectron. 2025, 268, 13. [Google Scholar] [CrossRef]
- Hao, L.W.; Chen, J.; Chen, X.R.; Ma, T.T.; Cai, X.X.; Duan, H.; Leng, Y.K.; Huang, X.L.; Xiong, Y.H. A novel magneto-gold nanohybrid-enhanced lateral flow immunoassay for ultrasensitive and rapid detection of ochratoxin A in grape juice. Food Chem. 2021, 336, 9. [Google Scholar] [CrossRef]
- Geballa-Koukoula, A.; Gerssen, A.; Nielen, M.W.F. Direct analysis of lateral flow immunoassays for deoxynivalenol using electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 7547–7558. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, H.; Deng, S.L.; Xiao, X.Y.; Xiong, Y.H.; Peng, J.; Lai, W.H. An integrated colorimetric and photothermal lateral flow immunoassay based on bimetallic Ag-Au urchin-like hollow structures for the sensitive detection of E. coli O157:H7. Biosens. Bioelectron. 2023, 225, 10. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.Y.; Guo, L.L.; Li, J.X.; He, F.; Zhang, H.T.; Chi, H. The Developments on Lateral Flow Immunochromatographic Assay for Food Safety in Recent 10 Years: A Review. Chemosensors 2024, 12, 18. [Google Scholar] [CrossRef]
- Sun, B.Y.; Wu, H.Y.; Jia, P.; Cao, Y.Y.; Xuan, C.Y.; Feng, Q.L.; Yan, H.Q.; Wang, L. Dual-modal lateral flow immunoassay based on cauliflower-like ReS2@Pt core-shell nanospheres mediated ultra-sensitive detection of deoxynivalenol in food samples. Chem. Eng. J. 2024, 497, 11. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.J.; Xu, Z.H.; Liu, X.; Zhou, J.; Qu, X.F.; Wang, W.L.; Feng, Y.W.; Peng, C.F. An ultra-sensitive photothermal lateral flow immunoassay for 17?-estradiol in food samples. Food Chem. 2023, 404, 134482. [Google Scholar] [CrossRef]
- Yao, X.L.; Wang, Z.H.; Dou, L.; Zhao, B.X.; He, Y.X.; Wang, J.L.; Sun, J.; Li, T.; Zhang, D.H. An innovative immunochromatography assay for highly sensitive detection of 17β-estradiol based on an indirect probe strategy. Sens. Actuators B-Chem. 2019, 289, 48–55. [Google Scholar] [CrossRef]
- Kim, H.T.; Jin, E.; Lee, M.-H. Portable Chemiluminescence-Based Lateral Flow Assay Platform for the Detection of Cortisol in Human Serum. Biosensors 2021, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.W.; Wang, Y.; Lin, M.F.; Chen, Y.H.; Qian, Z.J.; Liu, J.W.; Li, X.M. High-Density Au Anchored to Ti3C2-Based Colorimetric-Fluorescence Dual-Mode Lateral Flow Immunoassay for All-Domain-Enhanced Performance and Signal Intercalibration. Anal. Chem. 2024, 96, 5106–5114. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, X.; Li, M.; Ma, W.; Du, S.; Wang, Z.; Qiu, N.; Zhao, X. Dual-plasmonic eccentric nanostructure with prominent colorimetric and photothermal performance to detect zearalenone by dual signal immunochromatography assay. Talanta 2024, 286, 127487. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yin, X.; Li, J.; Dou, L.; Wang, S.; Jia, C.; Li, Y.; Chen, Y.; Yan, S.; Wang, J.; et al. A dual-mode lateral flow immunoassay by ultrahigh signal-to background ratio SERS probes for nitrofurazone metabolites ultrasensitive detection. Food Chem. 2024, 441, 138374. [Google Scholar] [CrossRef]
- Wu, Q.; Yin, X.; Cheng, Y.; Wang, C.; Ma, J.; Zhang, Q.; Liu, H.; Youssef, A.; Wang, J.; Zhang, D. Layer-By-Layer Designed Spark-Type AuCuPt Alloy with Robust Broadband Absorption to Enhance Sensitivity in Flexible Detection of Estriol by a Lateral Flow Immunoassay. Anal. Chem. 2024, 96, 10714–10723. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, Y.; Zhang, C.; Peng, C. Fabrication of Immunochromatography Strip for Tebuconazole Based on Colorimetric and Fluorescent Dual-Signal Detection. J. Food Sci. Biotechnol. 2022, 41, 36–43. [Google Scholar]
- Gutiérrez-Gallego, R.; Llop, E.; Bosch, J.; Segura, J. Surface plasmon resonance in doping analysis. Anal. Bioanal. Chem. 2011, 401, 389–403. [Google Scholar] [CrossRef]
- Mitchell, J.S.; Wu, Y.Q.; Cook, C.J.; Main, L. Sensitivity enhancement of surface plasmon resonance biosensing of small molecules. Anal. Biochem. 2005, 343, 125–135. [Google Scholar] [CrossRef]
- Sari, E.; Üzek, R.; Duman, M.; Alagöz, H.Y.; Denizli, A. Prism coupler-based sensor system for simultaneous screening of synthetic glucocorticosteroid as doping control agent. Sens. Actuators B-Chem. 2018, 260, 432–444. [Google Scholar] [CrossRef]
- Kausaite, A.; Ramanaviciene, A.; Mostovojus, V.; Ramanavicius, A. Surface plasmon resonance and its application to biomedical research. Med. Lith. 2007, 43, 355–365. [Google Scholar] [CrossRef]
- Mitchell, J. Small Molecule Immunosensing Using Surface Plasmon Resonance. Sensors 2010, 10, 7323–7346. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wei, T.X. Detection of 17β-estradiol in water samples by a novel double-layer molecularly imprinted film-based biosensor. Talanta 2015, 141, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; McDermott, M.T. A surface plasmon resonance based inhibition immunoassay for measurement of steroid hormones. Anal. Biochem. 2018, 557, 7–12. [Google Scholar] [CrossRef]
- Blackburn, C.; Sullivan, M.V.; Wild, M.I.; O’Connor, A.J.; Turner, N.W. Utilisation of molecularly imprinting technology for the detection of glucocorticoids for a point of care surface plasmon resonance (SPR) device. Anal. Chim. Acta 2024, 1285, 11. [Google Scholar] [CrossRef]
- Jia, Y.T.; Peng, Y.; Bai, J.L.; Zhang, X.H.; Cui, Y.G.; Ning, B.A.; Cui, J.S.; Gao, Z.X. Magnetic nanoparticle enhanced surface plasmon resonance sensor for estradiol analysis. Sens. Actuators B-Chem. 2018, 254, 629–635. [Google Scholar] [CrossRef]
- Qu, L.J.; Bai, J.L.; Peng, Y.; Han, D.P.; Ning, B.A.; Zhou, H.Y.; Li, S.; Gao, Z.X. Detection of Three Different Estrogens in Milk Employing SPR Sensors Based on Double Signal Amplification Using Graphene. Food Anal. Meth. 2021, 14, 54–65. [Google Scholar] [CrossRef]
- Tan, J.S.; Dai, Z.R.; Zhou, K.M.; Zhang, L.; Zhou, X.H.; Tan, Y.D. Ultrasensitive Screening of Endocrine-Disrupting Chemicals Using a Surface Plasmon Resonance Biosensor with Polarization-Compensated Laser Heterodyne Feedback. Anal. Chem. 2023, 95, 8687–8695. [Google Scholar] [CrossRef]
- Juma, M.W.; Birech, Z.; Mwenze, N.M.; Ondieki, A.M.; Maaza, M.; Mokhotjwa, S.D. Localized surface plasmon resonance sensing of Trenbolone acetate dopant using silver nanoparticles. Sci. Rep. 2024, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, J.P.; Li, D.J.; Li, Y.B. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Sha, J.Y.; Li, Z.H.; Wang, W.J.; Zhang, H.Y. High affinity truncated aptamers for ultra-sensitive colorimetric detection of bisphenol A with label-free aptasensor. Food Chem. 2020, 317, 126459. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.; Godjevargova, T.; Dimova, N. Magnetic nanoparticle-based fluorescent immunoassay for determination of progesterone in milk. Int. J. Dairy. Technol. 2018, 71, 309–320. [Google Scholar] [CrossRef]
- Li, Q.Y.; Li, G.L.; Fan, L.H.; Yu, Y.X.; Liu, J. Click reaction triggered turn-on fluorescence strategy for highly sensitive and selective determination of steroid hormones in food samples. Food Chem. 2022, 374, 131565. [Google Scholar] [CrossRef]
- Xu, X.X.; Guo, L.L.; Xu, L.G.; Kuang, H.; Xu, C.L.; Liu, L.Q. Multiplex lateral flow immunochromatographic assay for the qualitative and quantitative detection of six steroid hormone residues in chicken and pork. Food Biosci. 2023, 53, 102498. [Google Scholar] [CrossRef]

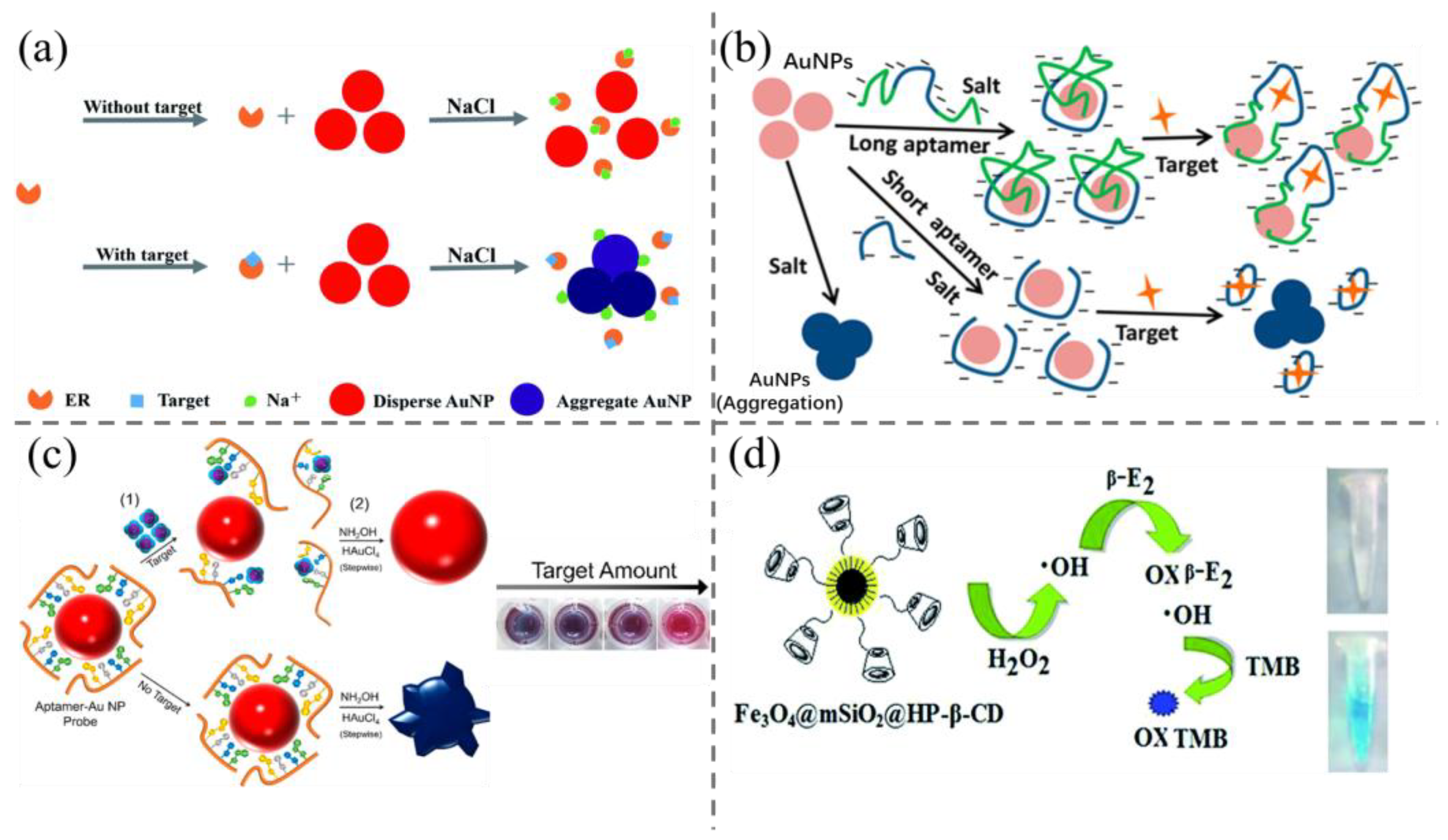


| Methodologies | Advantages | Disadvantages |
| Electrochemical method | Fast response, low cost, and high sensitivity | Relatively low stability, complex electrode modification, and poor reproducibility |
| Colorimetric method | Visualization, simple, and ready for on-site analysis | Low sensitivity and poor selectivity (non-specific aggregation of probe causes false positive) |
| Fluorescence method | High sensitivity and high-throughput | Serious optical interference, complicated biomodification |
| SERS | Rapid, sensitive, non-destructive, in situ analysis | Complicated design and fabrication of SERS substrate, relatively low stability, and poor repeatability |
| LFIAs | Good specificity, relatively high sensitivity, low cost, easy to operate, rapid and on-site detection | Poor reproducibility, instability of the antibody, difficult to quantify |
| SPR | Sensitive, fast, real-time monitoring | Sensitive to substrate and temperature, high equipment cost, requires specialized personnel to operate |
| Methodologies | Samples | Targets | Detection Limit | Linear Range | Recovery | Analysis Time | Refs. |
|---|---|---|---|---|---|---|---|
| Electrochemical method | Milk | Bisphenol A | 5 nM | 0.01–10 μM | 90–116% | 30 min | [122] |
| Milk, Meat | Estradiol | 9.9 × 10−19 M | 10−18–10−6 M | 95.1–104.8% | 20 min | [27] | |
| Colorimetric method | Milk | Estradiol | 13.1 pM | 0.05–0.8 nM | 100.1–113.0% | 1.5 h | [56] |
| Bisphenol A | 7.60 pM | 10–100 pM | 96.10–106.5% | 55 min | [123] | ||
| Meats | Estradiol | 0.15 μM | 0–10 μM | 95.70–113.8% | 40 min | [57] | |
| Fluorescence method | Milk | Progesterone | 0.065 ng/mL | 0.25–25 ng/mL | 90–105% | 15 min | [124] |
| Estradiol | 3.48 × 10−12 M | 10−11–10−6 M | 82–107% | 1.5 h | [125] | ||
| 0.29 ng/mL | 0.5 ng/mL–1.2 μg/mL | 93.6–102.4% | 30 min | [73] | |||
| SERS | Chicken, Milk | Estradiol | 9.58 × 10−14 M | 10−12–10−4 M | 95.00–98.72% | 1 h | [87] |
| Milk | 1.95 × 10−16 M | 10−14–10−6 M | 90.56–109.40% | 35 min | [86] | ||
| LFIAs | Aquatic products, milk | Estradiol | 65 ng/g | 75 ng/g | - | 7–10 min | [92] |
| Chicken, Pork | Testosterone Propionate | 0.32 μg/kg | 0.05–0.94 ng/mL | 90–110% | 40 min | [126] | |
| Milk | Dexamethasone | 0.0013 μg/kg | 0.05–0.08 μg/kg | 81.1–113.7% | 20 min | [105] | |
| Beef | 0.08 μg/kg | 0.35–1.50 μg/kg | 89.2–115.4% | ||||
| Pork | 0.07 μg/kg | 0.25–1.00 μg/kg | |||||
| SPR | Water | Estradiol | 2.50 × 10−13 mol/L | 2.50 × 10−13–2.50 × 10−9 mol/L | 96.1–101.4% | 10 min | [120] |
| Milk | Estradiol | 0.81 ng/mL | 1.95–2000 ng/mL | 99.63–114.91% | 30 min | [118] | |
| Milk | Estradiol | 3.605 × 10−6 ng/mL | 2.105 × 10−5–1.403 × 10−2 ng/mL | 87.26–105.30% | 20 min | [119] | |
| Diethylstilboestrol | 2.901 × 10−6 ng/mL | 1.687 × 10−5–1.141 × 10−2 ng/mL | |||||
| Bisphenol A | 5.169 × 10−5 ng/mL | 2.288 × 10−4–9.211 × 10−2 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Li, J.; Ma, M.; Fu, P.; Qian, S.; Han, C.; Wang, Y. Recent Advances on Rapid Detection Methods of Steroid Hormones in Animal Origin Foods. Biosensors 2025, 15, 216. https://doi.org/10.3390/bios15040216
Xue Y, Li J, Ma M, Fu P, Qian S, Han C, Wang Y. Recent Advances on Rapid Detection Methods of Steroid Hormones in Animal Origin Foods. Biosensors. 2025; 15(4):216. https://doi.org/10.3390/bios15040216
Chicago/Turabian StyleXue, Yaohui, Jinhua Li, Ming Ma, Pan Fu, Sihua Qian, Chao Han, and Yuhui Wang. 2025. "Recent Advances on Rapid Detection Methods of Steroid Hormones in Animal Origin Foods" Biosensors 15, no. 4: 216. https://doi.org/10.3390/bios15040216
APA StyleXue, Y., Li, J., Ma, M., Fu, P., Qian, S., Han, C., & Wang, Y. (2025). Recent Advances on Rapid Detection Methods of Steroid Hormones in Animal Origin Foods. Biosensors, 15(4), 216. https://doi.org/10.3390/bios15040216





