A Platform for the Glucose Biosensor Based on Dendritic Gold Nanostructures and Polyaniline-Gold Nanoparticles Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
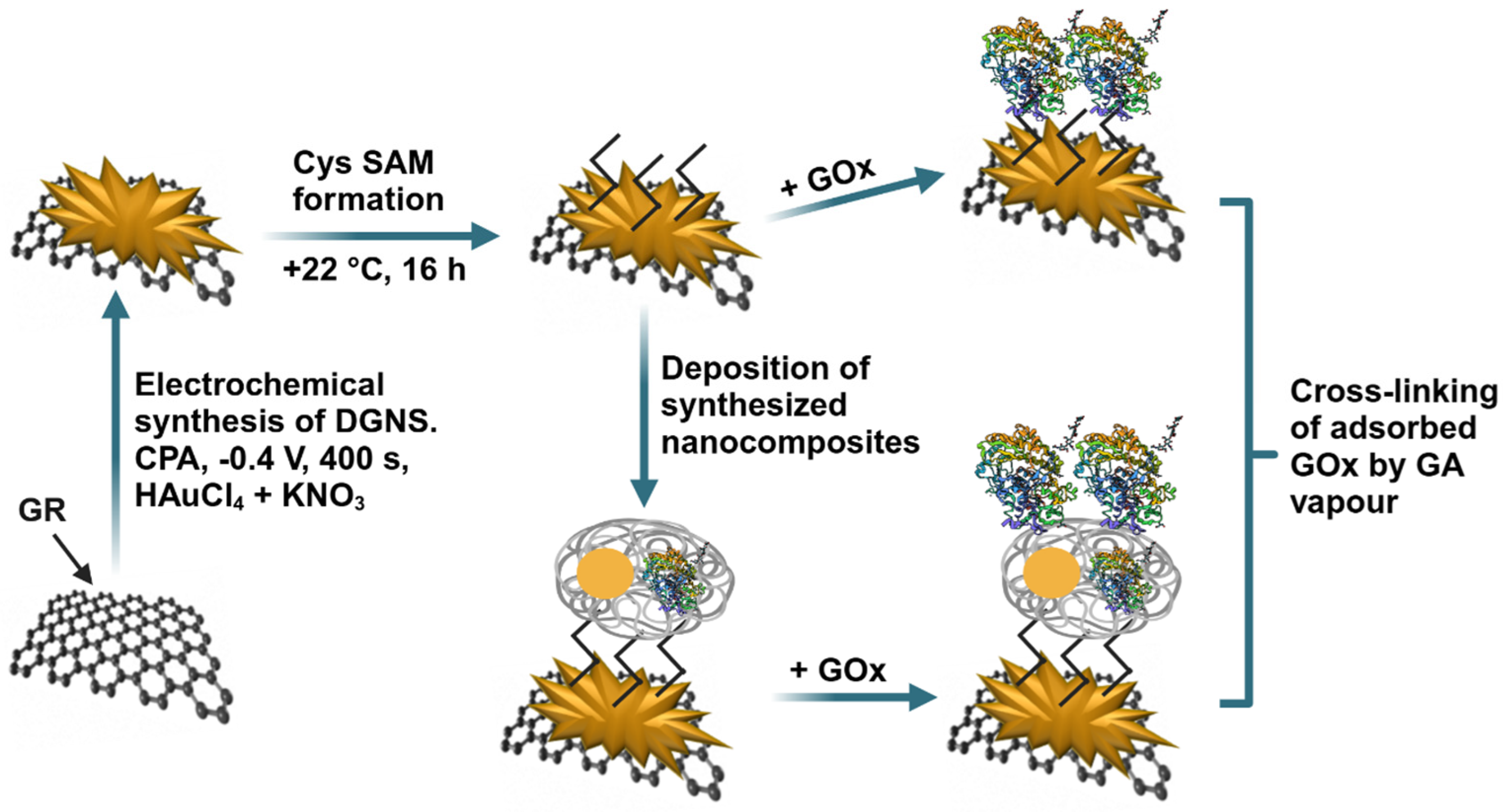
2.2. Synthesis of AuNPs and PANI-AuNPs-GOx Nanocomposites
2.3. The Pre-Treatment and Modification of GR Electrode
2.4. Investigation of Electrochemical Characteristics of Glucose Biosensors
2.5. The Evaluation of the Surface Area of Modified Electrodes
2.6. The Stability and Practical Application of Glucose Biosensors
3. Results and Discussion
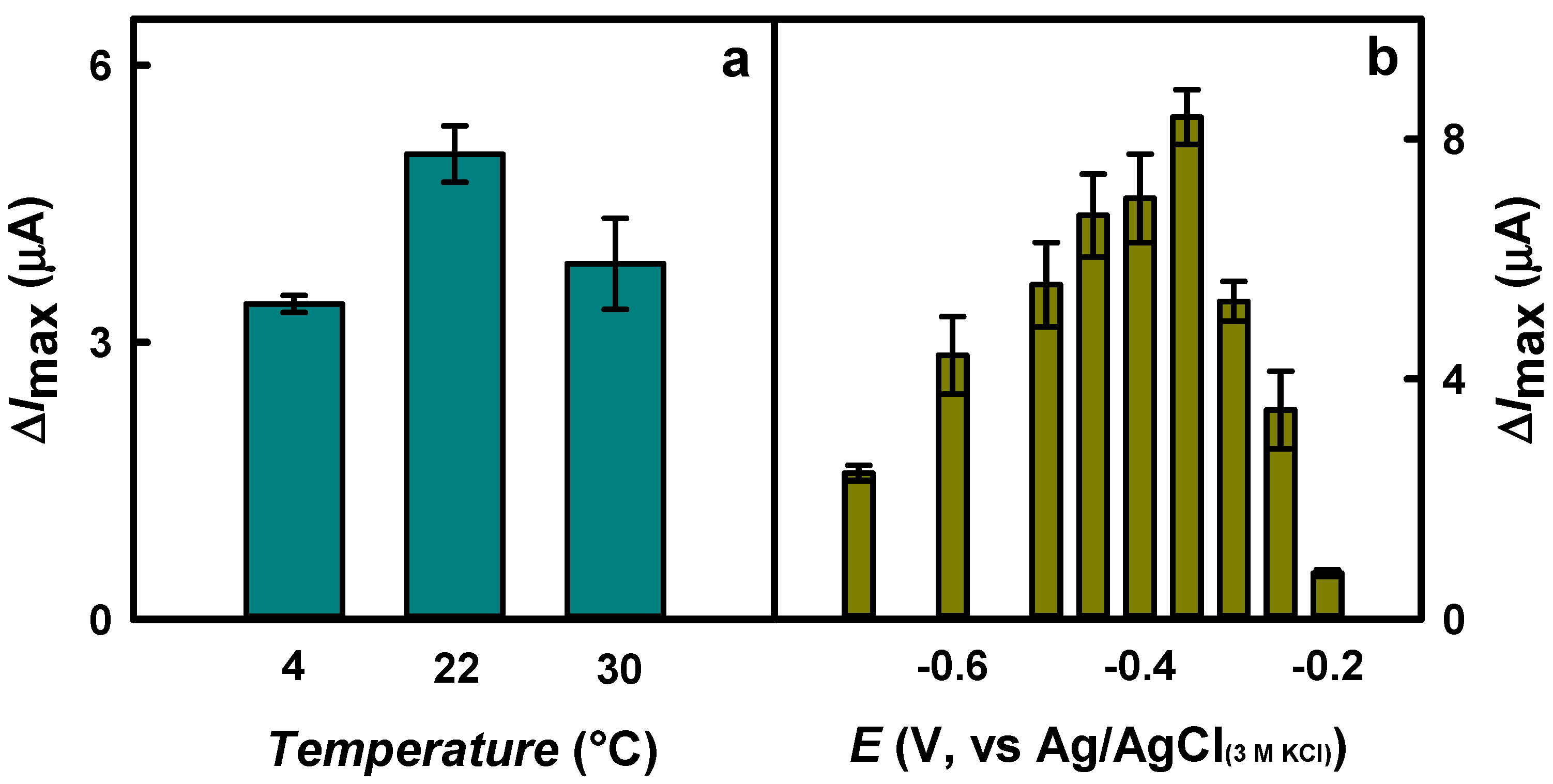
3.1. The Optimization of Biosensor Performance
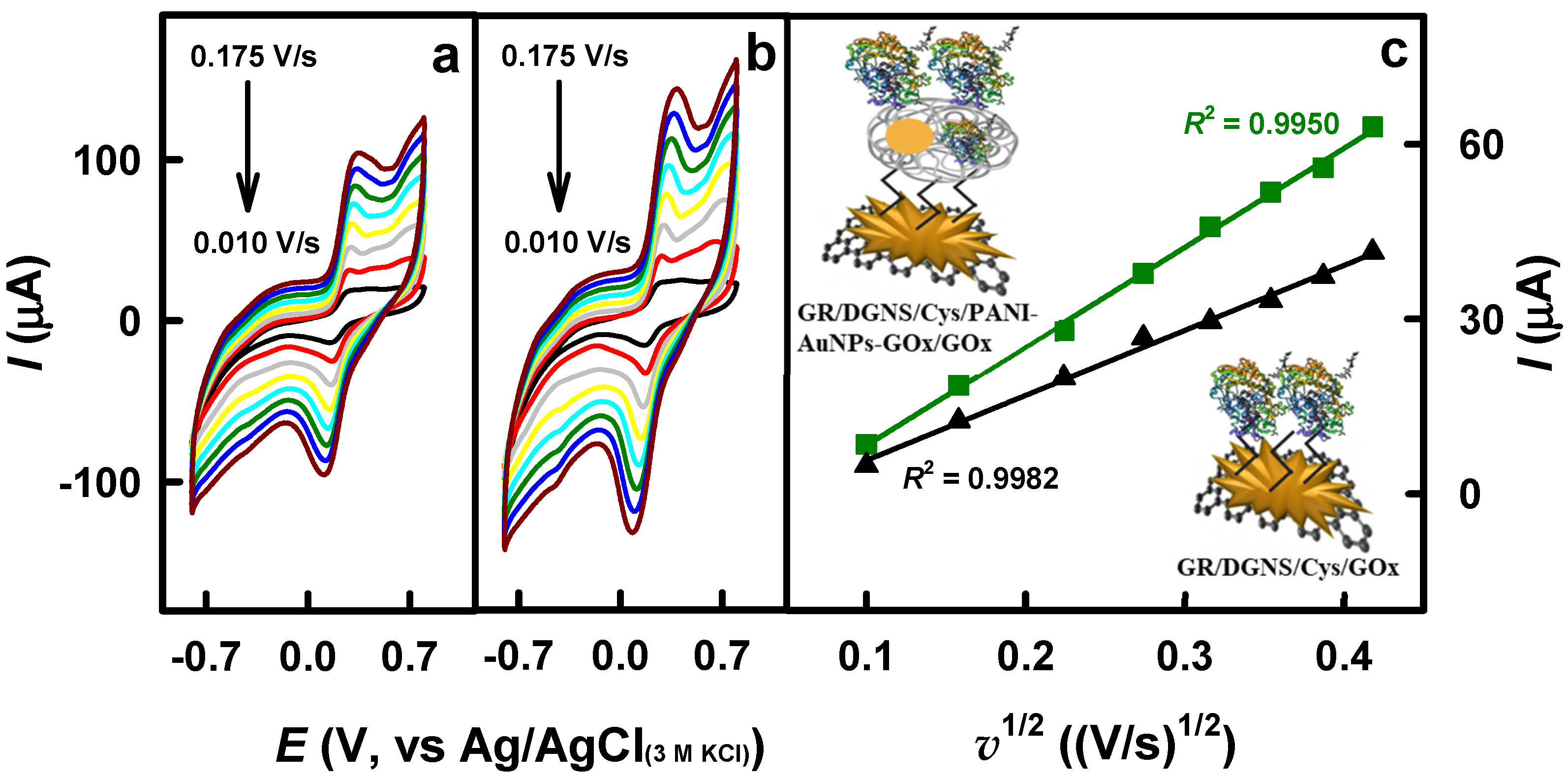
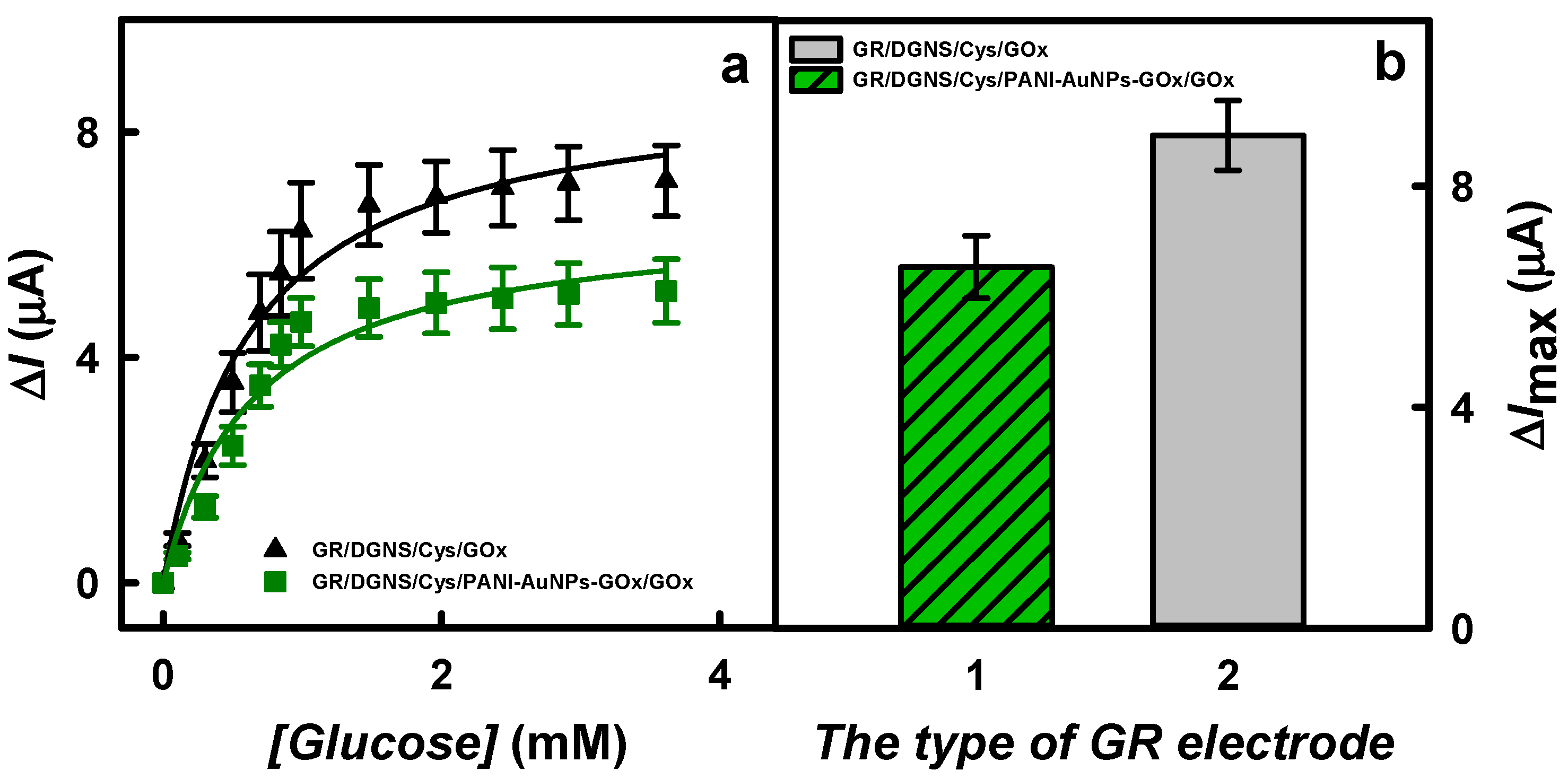
3.2. The Comparison and Characterization of Glucose Biosensors Based on Differently Modified Electrodes
3.3. Determination of Glucose in a Serum Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GR | Graphite rod |
| DGNS | Dendritic gold nanostructures |
| Cys | Cystamine |
| SAM | Self-assembled monolayer |
| GOx | Glucose oxidase |
| PANI | Polyaniline |
| AuNPs | Gold nanoparticles |
| LR | Linear range |
| LOD | Limit of detection |
| H2O2 | Hydrogen peroxide |
| DET | Direct electron transfer |
| O2 | Oxygen |
| GOx(FAD) | Oxidized form of the enzyme |
| GOx(FADH2) | Reduced form of glucose oxidase |
| Fe@Au | Gold-coated magnetic iron oxide nanoparticles |
| AuNC | Gold nanocluster |
| NPAu | Nanoporous gold |
| PtNPs | Platinum nanoparticles |
| RGO-Fe3O4 | Reduced graphene oxide–magnetic nanoparticles |
| Ppy | Polypyrrole |
| GC | Glassy carbon |
| Au@ILs-polysome | Gold nanoparticles and ionic liquids-based polysome |
| Au | Gold |
| K4[Fe(CN)6]·3H2O | Potassium hexacyanoferrate(II) trihydrate |
| HAuCl4·3 H2O | Tetrachloroauric acid trihydrate |
| CH3COONa·3H2O | Sodium acetate trihydrate |
| KCl | Potassium chloride |
| SA | Sodium acetate |
| Al2O3 | α-Aluminium oxide |
| KNO3 | Potassium nitrate |
| K3[Fe(CN)6] | Potassium hexacyanoferrate(III) |
| GA | Glutaraldehyde |
| AA | L-ascorbic acid |
| UA | Uric acid |
| CPA | Constant potential amperometry |
| CV | Cyclic voltammetry |
| Pt | Platinum |
| Ag/AgCl(3 M KCl) | Reference electrode |
| R2 | Determination coefficient |
| ΔImax | Maximal current |
| KM(app) | Michaelis constant |
| EASA | Electroactive surface area |
| Ip | Maximal peak current |
| n | Number of electrons appearing in the half-reaction |
| D | Diffusion coefficient |
| C | Concentration of electroactive species |
| v | Potential scan rate |
| Cu-nanoflower | Copper nanoflower |
| PVA | Polyvinyl alcohol |
| GO NFs | Graphene oxide nanofibers |
| HRP | Horseradish peroxidase |
| AuNNs | Gold nanopine needles |
| BSA | Bull serum albumin |
| PEGDE | Poly(ethylene glycol) diglycidylether |
| DENPs | Dualenzyme nanoparticles |
| CP | Carbon paste |
| OOPpy | Overoxidized polypyrrole |
| RSD | Relative standard deviation |
| PMS | Phenazine methosulfate |
| PD | 1,10-Phentroline-5,6-dione |
References
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Sense and Sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Chambers, J.P.; Arulanandam, B.P.; Matta, L.L.; Weis, A.; Valdes, J.J. Biosensor Recognition Elements. Curr. Issues Mol. Biol. 2008, 10, 1–12. [Google Scholar] [CrossRef]
- D’Orazio, P. Biosensors in Clinical Chemistry. Clin. Chim. Acta 2003, 334, 41–69. [Google Scholar] [CrossRef]
- Ratautas, D.; Dagys, M. Nanocatalysts Containing Direct Electron Transfer-Capable Oxidoreductases: Recent Advances and Applications. Catalysts 2019, 10, 9. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, Applications and Electrochemical Techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and Analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, T.; Chu, Z.; Jin, W. Recent Advances in Electrochemical Enzymatic Biosensors Based on Regular Nanostructured Materials. J. Electroanal. Chem. 2021, 893, 115328. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Poo-Arporn, R.P. Self-Assembly of Glucose Oxidase on Reduced Graphene Oxide-Magnetic Nanoparticles Nanocomposite-Based Direct Electrochemistry for Reagentless Glucose Biosensor. Mater. Sci. Eng. C 2017, 76, 398–405. [Google Scholar] [CrossRef]
- Pereira, S.O.; Santos, N.F.; Carvalho, A.F.; Fernandes, A.J.S.; Costa, F.M. Electrochemical Response of Glucose Oxidase Adsorbed on Laser-Induced Graphene. Nanomaterials 2021, 11, 1893. [Google Scholar] [CrossRef]
- Wu, C.; Sun, H.; Li, Y.; Liu, X.; Du, X.; Wang, X.; Xu, P. Biosensor Based on Glucose Oxidase-Nanoporous Gold Co-Catalysis for Glucose Detection. Biosens. Bioelectron. 2015, 66, 350–355. [Google Scholar] [CrossRef]
- Baek, S.H.; Roh, J.; Park, C.Y.; Kim, M.W.; Shi, R.; Kailasa, S.K.; Park, T.J. Cu-Nanoflower Decorated Gold Nanoparticles-Graphene Oxide Nanofiber as Electrochemical Biosensor for Glucose Detection. Mater. Sci. Eng. C 2020, 107, 110273. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold Nanoparticle-Based Electrochemical Biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Semashko, T.; Mikhailova, R.; Ramanaviciene, A. The Use of Different Glucose Oxidases for the Development of an Amperometric Reagentless Glucose Biosensor Based on Gold Nanoparticles Covered by Polypyrrole. Electrochim. Acta 2015, 169, 326–333. [Google Scholar] [CrossRef]
- Sakalauskiene, L.; Brasiunas, B.; Popov, A.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. The Development of Reagentless Amperometric Glucose Biosensor Based on Gold Nanostructures, Prussian Blue and Glucose Oxidase. Biosensors 2023, 13, 942. [Google Scholar] [CrossRef]
- Lee, M.J.; Choi, J.H.; Shin, J.H.; Yun, J.; Kim, T.; Kim, Y.J.; Oh, B.K. Gold Nanoclusters with Two Sets of Embedded Enzyme Nanoparticles for Applications as Electrochemical Sensors for Glucose. ACS Appl. Nano Mater. 2023, 6, 12567–12577. [Google Scholar] [CrossRef]
- Liu, S.; Ju, H. Reagentless Glucose Biosensor Based on Direct Electron Transfer of Glucose Oxidase Immobilized on Colloidal Gold Modified Carbon Paste Electrode. Biosens. Bioelectron. 2003, 19, 177–183. [Google Scholar] [CrossRef]
- Dimcheva, N. Nanostructures of Noble Metals as Functional Materials in Biosensors. Curr. Opin. Electrochem. 2020, 19, 35–41. [Google Scholar] [CrossRef]
- Haghighi, B.; Tabrizi, M.A. Direct Electron Transfer from Glucose Oxidase Immobilized on an Overoxidized Polypyrrole Film Decorated with Au Nanoparticles. Colloids Surf. B Biointerfaces 2013, 103, 566–571. [Google Scholar] [CrossRef]
- Ratautas, D.; Ramonas, E.; Marcinkevičienė, L.; Meškys, R.; Kulys, J. Wiring Gold Nanoparticles and Redox Enzymes: A Self-Sufficient Nanocatalyst for the Direct Oxidation of Carbohydrates with Molecular Oxygen. ChemCatChem 2018, 10, 971–974. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Graphene/AuNPs/Chitosan Nanocomposites Film for Glucose Biosensing. Biosens. Bioelectron. 2010, 25, 1070–1074. [Google Scholar] [CrossRef]
- Gineitytė, J.; Meškys, R.; Dagys, M.; Ratautas, D. Highly Efficient Direct Electron Transfer Bioanode Containing Glucose Dehydrogenase Operating in Human Blood. J. Power Sources 2019, 441, 227163. [Google Scholar] [CrossRef]
- Chen, H.; Fan, J.; Chen, X.; Ma, Z.; Zhang, L.; Chen, X. Gold Nanoparticle (Au NP)-Decorated Ionic Liquid (IL) Based Liposome: A Stable, Biocompatible, and Conductive Biomimetic Platform for the Fabrication of an Enzymatic Electrochemical Glucose Biosensor. Anal. Lett. 2023, 56, 2021–2039. [Google Scholar] [CrossRef]
- Tamer, U.; Gündoǧdu, Y.; Boyaci, I.H.; Pekmez, K. Synthesis of Magnetic Core-Shell Fe3O4-Au Nanoparticle for Biomolecule Immobilization and Detection. J. Nanoparticle Res. 2010, 12, 1187–1196. [Google Scholar] [CrossRef]
- Baniukevic, J.; Hakki Boyaci, I.; Goktug Bozkurt, A.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic Gold Nanoparticles in SERS-Based Sandwich Immunoassay for Antigen Detection by Well Oriented Antibodies. Biosens. Bioelectron. 2013, 43, 281–288. [Google Scholar] [CrossRef]
- Eskandari, K.; Zarei, H.; Ghourchian, H.; Amoozadeh, S.M. The Electrochemical Study of Glucose Oxidase on Gold-Coated Magnetic Iron Oxide Nanoparticles. J. Anal. Chem. 2015, 70, 1254–1260. [Google Scholar] [CrossRef]
- Gružauskaitė, J.; Jasinskaitė, J.; Meškys, R.; Gaidamavičienė, G.; Žalga, A.; Laurynėnas, A.; Tetianec, L.; Dagys, M. Gold-Coated Magnetic Nanocatalyst Containing Wired Oxidoreductases for Mediatorless Catalysis of Carbohydrate Oxidation by Oxygen. Catal. Commun. 2020, 135, 105848. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A. Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods. Chemosensors 2021, 9, 188. [Google Scholar] [CrossRef]
- Yu, M.; Li, Y.T.; Hu, Y.; Tang, L.; Yang, F.; Lv, W.L.; Zhang, Z.Y.; Zhang, G.J. Gold Nanostructure-Programmed Flexible Electrochemical Biosensor for Detection of Glucose and Lactate in Sweat. J. Electroanal. Chem. 2021, 882, 115029. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Development and Practical Application of Glucose Biosensor Based on Dendritic Gold Nanostructures Modified by Conducting Polymers. Biosensors 2022, 12, 641. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A. The Development and Evaluation of Reagentless Glucose Biosensors Using Dendritic Gold Nanostructures as a Promising Sensing Platform. Biosensors 2023, 13, 727. [Google Scholar] [CrossRef]
- Tamer, U.; Seçkin, A.İ.; Temur, E.; Torul, H. Fabrication of Biosensor Based on Polyaniline/Gold Nanorod Composite. Int. J. Electrochem. 2011, 2011, 869742. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, M.; Yan, Z.; Chen, J. Highly Selective and Stable Glucose Biosensor Based on Incorporation of Platinum Nanoparticles into Polyaniline-Montmorillonite Hybrid Composites. Microchem. J. 2020, 152, 104266. [Google Scholar] [CrossRef]
- Rong, L.Q.; Yang, C.; Qian, Q.Y.; Xia, X.H. Study of the Nonenzymatic Glucose Sensor Based on Highly Dispersed Pt Nanoparticles Supported on Carbon Nanotubes. Talanta 2007, 72, 819–824. [Google Scholar] [CrossRef]
- Liu, L.M.; Wen, J.; Liu, L.; He, D.; Kuang, R.Y.; Shi, T. A Mediator-Free Glucose Biosensor Based on Glucose Oxidase/Chitosan/ α-Zirconium Phosphate Ternary Biocomposite. Anal. Biochem. 2014, 445, 24–29. [Google Scholar] [CrossRef]
- Gao, Y.; Kyratzis, I. Covalent Immobilization of Proteins on Carbon Nanotubes Using the Cross-Linker 1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide—A Critical Assessment. Bioconjug. Chem. 2008, 19, 1945–1950. [Google Scholar] [CrossRef]
- Singh, K.; Singh, B.P.; Chauhan, R.; Basu, T. Fabrication of Amperometric Bienzymatic Glucose Biosensor Based on MWCNT Tube and Polypyrrole Multilayered Nanocomposite. J. Appl. Polym. Sci. 2012, 125, E235–E246. [Google Scholar] [CrossRef]
- Freund, M.S.; Deore, B.A. Self-Doped Conducting Polymers; John Wiley & Sons: Hoboken, NK, USA, 2007. [Google Scholar]
- Cai, Y.; Liang, B.; Chen, S.; Zhu, Q.; Tu, T.; Wu, K.; Cao, Q.; Fang, L.; Liang, X.; Ye, X. One-Step Modification of Nano-Polyaniline/Glucose Oxidase on Double-Side Printed Flexible Electrode for Continuous Glucose Monitoring: Characterization, Cytotoxicity Evaluation and in Vivo Experiment. Biosens. Bioelectron. 2020, 165, 112408. [Google Scholar] [CrossRef]
- Kotanen, C.N.; Karunwi, O.; Guiseppi-Elie, A. Biofabrication Using Pyrrole Electropolymerization for the Immobilization of Glucose Oxidase and Lactate Oxidase on Implanted Microfabricated Biotransducers. Bioengineering 2014, 1, 85–110. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Dispersed Conducting Polymer Nanocomposites with Glucose Oxidase and Gold Nanoparticles for the Design of Enzymatic Glucose Biosensors. Polymer 2021, 13, 2173. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Evaluation of Polyaniline and Polypyrrole Nanocomposites Based on Glucose Oxidase and Gold Nanostructures. Polymers 2020, 12, 3026. [Google Scholar] [CrossRef]
- Zavada, S.R.; Battsengel, T.; Scott, T.F. Radical-Mediated Enzymatic Polymerizations. Int. J. Mol. Sci. 2016, 17, 195. [Google Scholar] [CrossRef]
- Banks, C.E.; Compton, R.G.; Fisher, A.C.; Henley, I.E. The Transport Limited Currents at Insonated Electrodes. Phys. Chem. Chem. Phys. 2004, 6, 3147–3152. [Google Scholar] [CrossRef]
- Hu, I.F.; Karweik, D.H.; Kuwana, T. Activation and Deactivation of Glassy Carbon Electrodes. J. Electroanal. Chem. 1985, 188, 59–72. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef]
- Koch, P.; Sidloi, M.; Tonks, D.B. Estimation of Serum Ascorbic Acid in Patients and the Effect of Ascorbic Acid and Its Oxidation Products on SMA 12/60 Parameters. Clin. Biochem. 1980, 13, 73–77. [Google Scholar] [CrossRef]
- Gonzales, W.V.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]

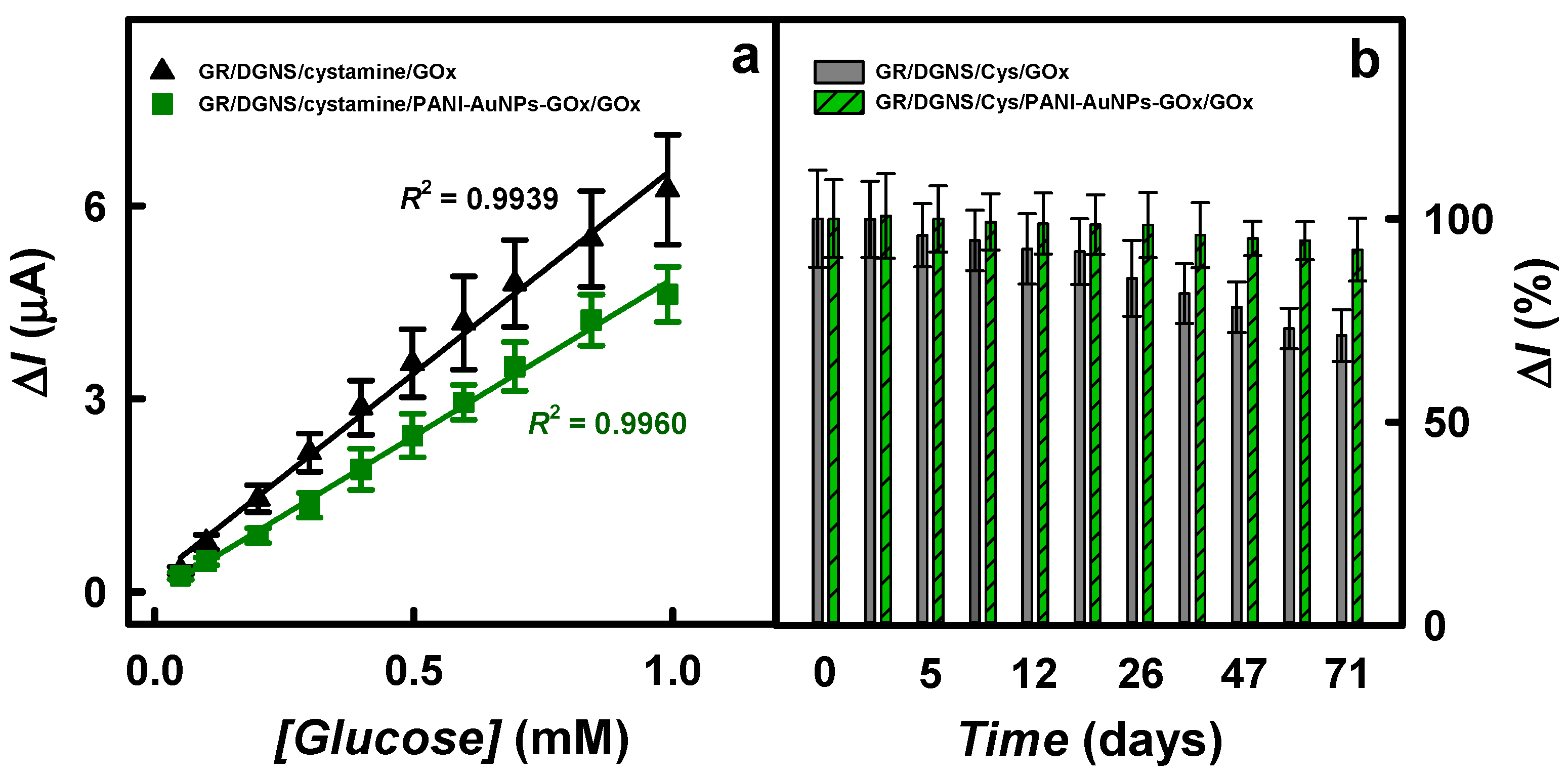
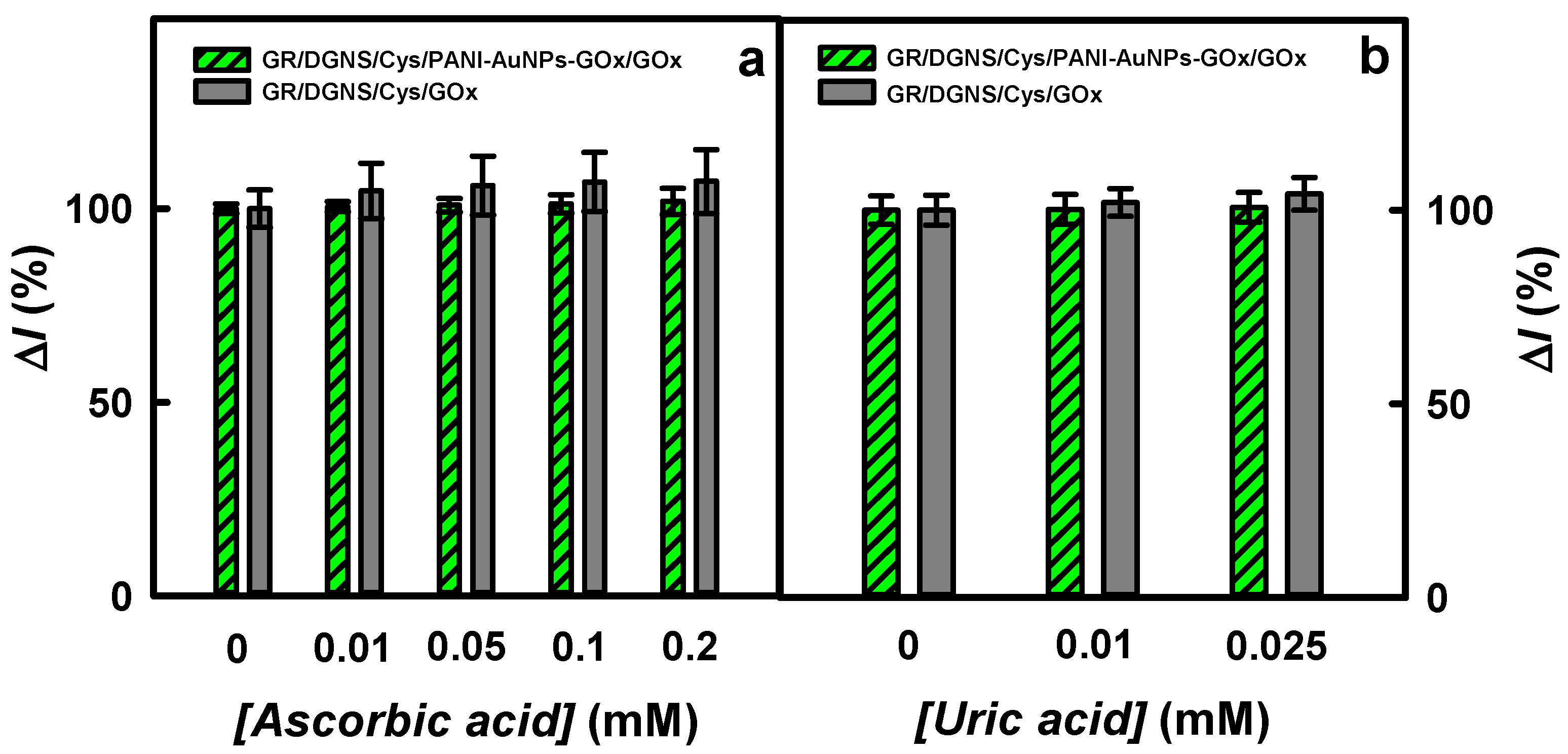
| Working Electrode | LOD (mM)/ Sensitivity (μA/(mM cm2)) | LR (mM) | Reference |
|---|---|---|---|
| Au/PVA-GO NFs/cysteamine-AuNPs/Cu-nanoflower/GOx-HRP | 0.018 × 10−3/332.68 | 0.001–0.10 | [14] |
| GC/NPAu/GOx | 0.00102/12.1 | 0.05–10 | [13] |
| Au/AuNC-DENPs | 2.58 a/18.944 | 5.5–320 a | [18] |
| Pt/PANI/gold nanorod/GOx | 0.0058/13.8 | 0.0176–1 | [34] |
| Au/AuNNs/cysteamine/GOx-BSA-PEGDE | 0.007/– | 0.025–0.25 | [31] |
| CP/AuNPs(24 nm)/GOx | 0.01/8.4 b | 0.04–0.28 | [19] |
| GC/Au@ILs-polysome/GOx | 0.02/32.52 | 0.05–0.5 | [25] |
| Au/graphene/AuNPs/chitosan/GOx | 0.18/0.55 b | 2–10 | [23] |
| GC/OOPpy-AuNPs/GOx | 0.5/0.217 b | 1.0–8.0 | [21] |
| GR/DGNS/Cys/GOx | 0.027/93.7 | 0.050–1.0 | This work |
| GR/DGNS/Cys/PANI-AuNPs-GOx/GOx | 0.034/72.0 | 0.050–1.0 | This work |
| Total Concentration (mM) | Detected * Concentration (mM) | Recovery Ratio (%) |
|---|---|---|
| 0.466 | 0.448 ± 0.025 | 96.1 |
| 0.520 | 0.501 ± 0.027 | 96.3 |
| 0.790 | 0.763 ± 0.053 | 96.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
German, N.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. A Platform for the Glucose Biosensor Based on Dendritic Gold Nanostructures and Polyaniline-Gold Nanoparticles Nanocomposite. Biosensors 2025, 15, 196. https://doi.org/10.3390/bios15030196
German N, Popov A, Ramanavicius A, Ramanaviciene A. A Platform for the Glucose Biosensor Based on Dendritic Gold Nanostructures and Polyaniline-Gold Nanoparticles Nanocomposite. Biosensors. 2025; 15(3):196. https://doi.org/10.3390/bios15030196
Chicago/Turabian StyleGerman, Natalija, Anton Popov, Arunas Ramanavicius, and Almira Ramanaviciene. 2025. "A Platform for the Glucose Biosensor Based on Dendritic Gold Nanostructures and Polyaniline-Gold Nanoparticles Nanocomposite" Biosensors 15, no. 3: 196. https://doi.org/10.3390/bios15030196
APA StyleGerman, N., Popov, A., Ramanavicius, A., & Ramanaviciene, A. (2025). A Platform for the Glucose Biosensor Based on Dendritic Gold Nanostructures and Polyaniline-Gold Nanoparticles Nanocomposite. Biosensors, 15(3), 196. https://doi.org/10.3390/bios15030196








