Wearable Sensors for Sleep Monitoring in Free-Living Environments: A Scoping Review on Parkinson’s Disease
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Selection Process
2.4. Data Extraction
2.5. Quality Assessment
3. Results
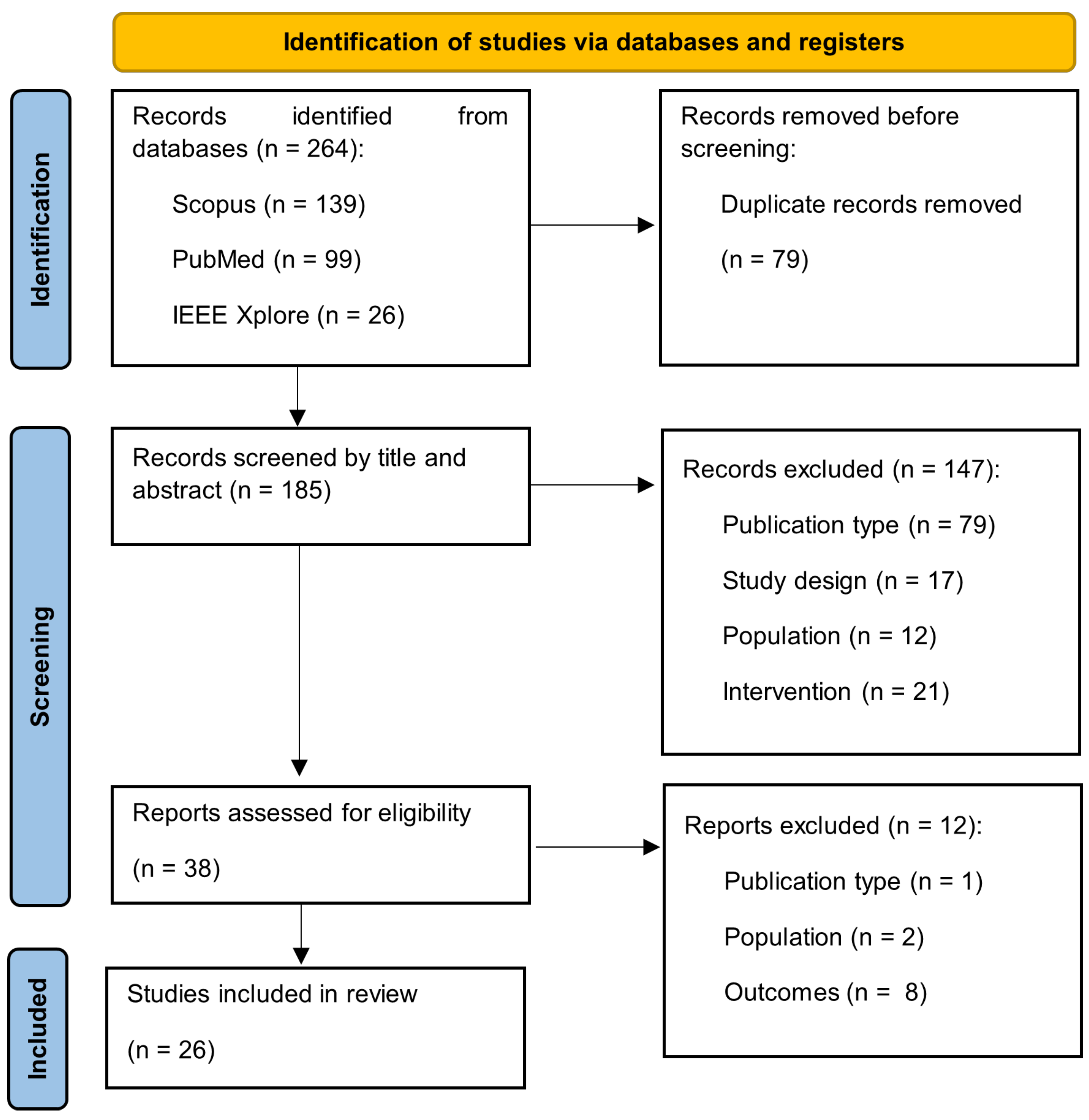
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
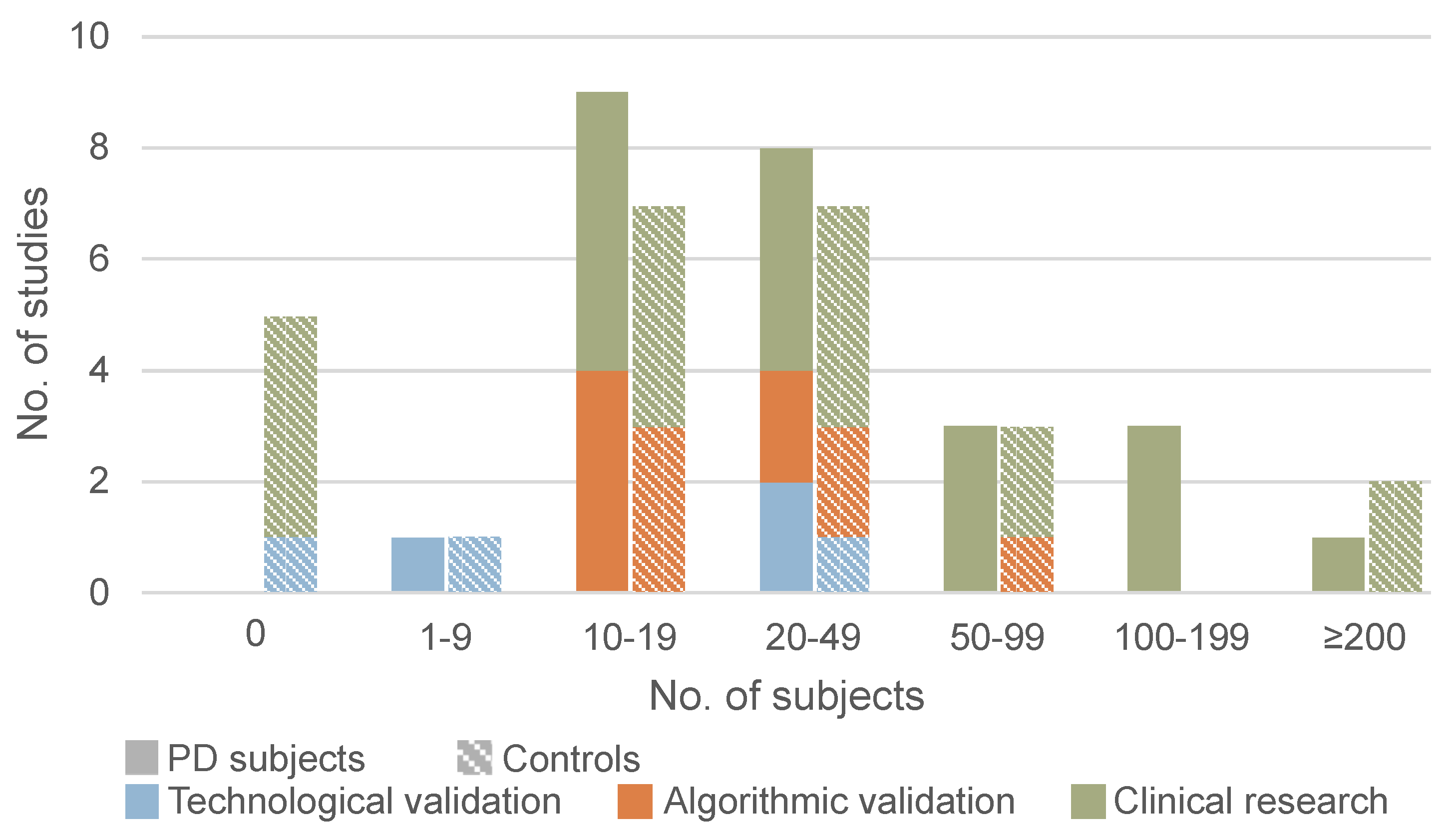
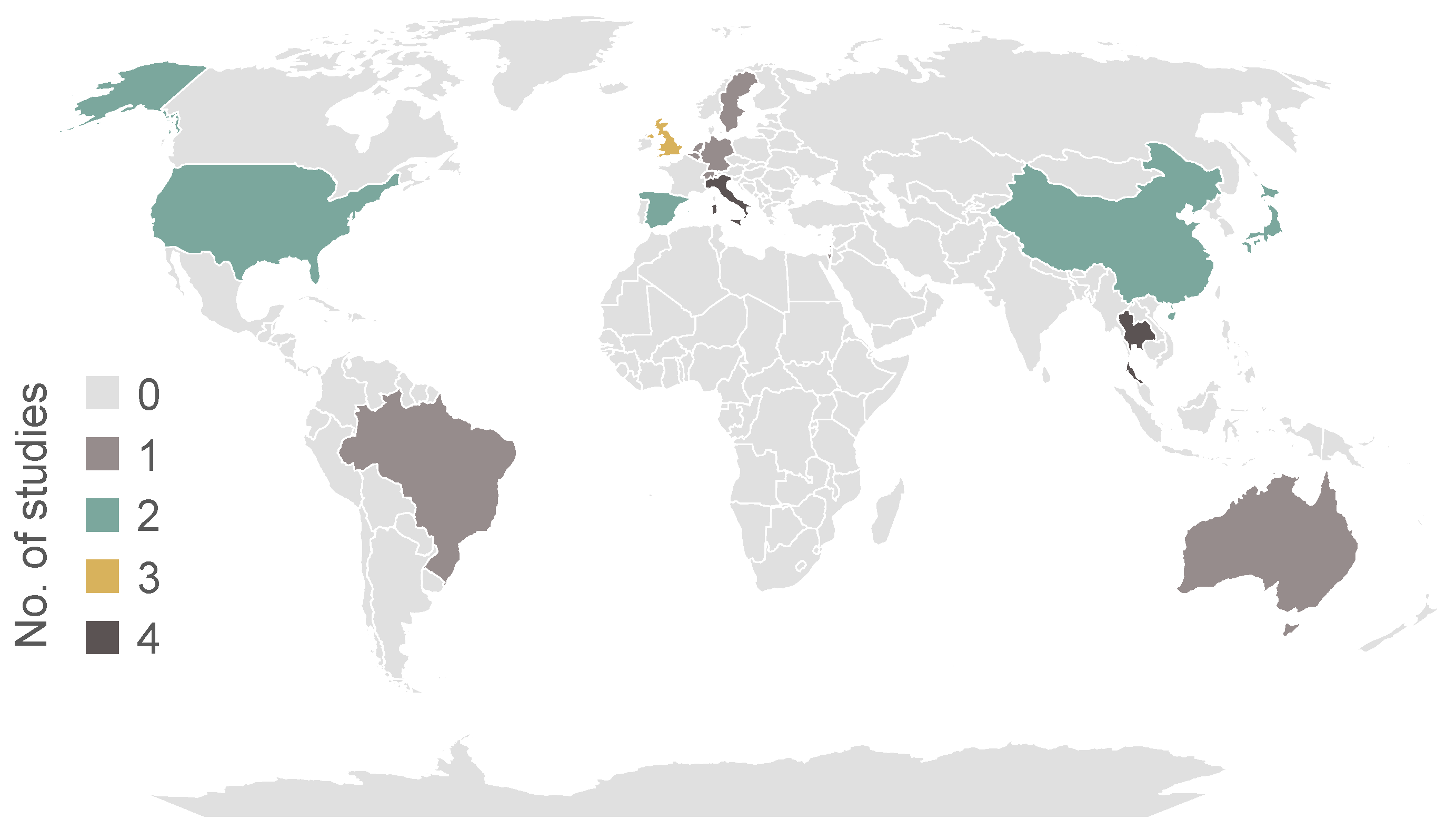
3.4. Participant Characteristics
3.5. Sensor Type and Position
3.6. Correlation with Other Disease Domains
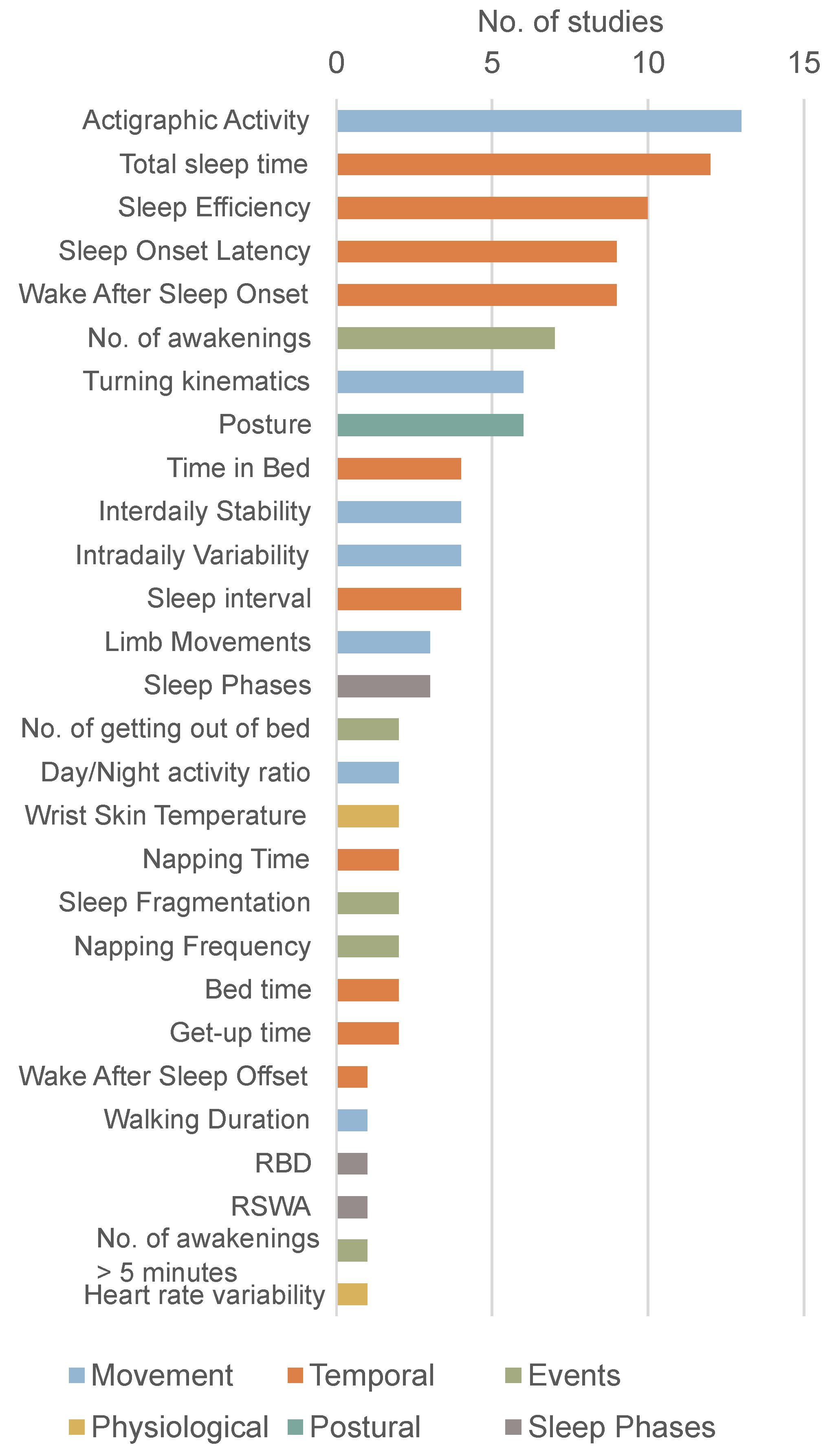
3.7. Sleep Parameters
3.7.1. Temporal Parameters
3.7.2. Movement Parameters
3.7.3. Nighttime Events
3.7.4. Postural Parameters
3.7.5. Sleep Architecture and Physiological Parameters
3.8. Sleep Disorders
3.9. Machine Learning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACM | Ambulatory circadian monitoring |
| DBS | Deep brain stimulation |
| ECG | Electrocardiography |
| EDS | Excessive daytime sleepiness |
| EEG | Electroencephalography |
| EMG | Electromyography |
| EOG | Electrooculography |
| ESS | Epworth Sleepiness Scale |
| H&Y | Hoehn and Yahr Scale |
| HCs | Healthy controls |
| HRV | Heart rate variability |
| IS | Interdaily stability |
| IV | Intradaily variability |
| MeSH | Medical Subject Headings |
| ML | Machine learning |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| PD | Parkinson’s disease |
| PDQ39 | The Parkinson’s Disease Questionnaire |
| PLMI | Periodic limb movements |
| PPG | Photoplethysmography |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSG | Polysomnography |
| PSQI | Pittsburgh Sleep Quality Index |
| PTI | Proportion of time immobile |
| RBD | REM sleep behaviour disorder |
| RBDSQ | RBD screening questionnaire |
| REM | Rapid eye movement |
| RLS | Restless legs syndrome |
| RSWA | REM sleep without atonia |
| SE | Sleep efficiency |
| SOL | Sleep onset latency |
| SVM | Support vector machine |
| SWA | Slow wave activity |
| TST | Total sleep time |
| UPDRS | Unified Parkinson’s disease rating scale |
| vPSG | Video polysomnography |
| WASO | Wake after sleep onset |
References
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Iranzo, A.; Cochen De Cock, V.; Fantini, M.L.; Perez-Carbonell, L.; Trotti, L.M. Sleep and sleep disorders in people with Parkinson’s disease. Lancet Neurol. 2024, 23, 925–937. [Google Scholar] [CrossRef]
- Schrempf, W.; Brandt, M.D.; Storch, A.; Reichmann, H. Sleep disorders in Parkinson’s disease. J. Park. Dis. 2014, 4, 211–221. [Google Scholar] [CrossRef]
- Schaeffer, E.; Berg, D. Dopaminergic Therapies for Non-motor Symptoms in Parkinson’s Disease. CNS Drugs 2017, 31, 551–570. [Google Scholar] [CrossRef]
- Maetzler, W.; Domingos, J.; Srulijes, K.; Ferreira, J.J.; Bloem, B.R. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov. Disord. 2013, 28, 1628–1637. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Milane, T.; Hansen, C.; Correno, M.B.; Chardon, M.; Barbieri, F.A.; Bianchini, E.; Vuillerme, N. Comparison of sleep characteristics between Parkinson’s disease with and without freezing of gait: A systematic review. Sleep Med. 2024, 114, 24–41. [Google Scholar] [CrossRef]
- Madrid-Navarro, C.J.; Puertas Cuesta, F.J.; Escamilla-Sevilla, F.; Campos, M.; Ruiz Abellan, F.; Rol, M.A.; Madrid, J.A. Validation of a Device for the Ambulatory Monitoring of Sleep Patterns: A Pilot Study on Parkinson’s Disease. Front. Neurol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Wu, J.Q.; Cronin-Golomb, A. Temporal Associations between Sleep and Daytime Functioning in Parkinson’s Disease: A Smartphone-Based Ecological Momentary Assessment. Behav. Sleep Med. 2019, 18, 560–569. [Google Scholar] [CrossRef]
- Sringean, J.; Anan, C.; Thanawattano, C.; Bhidayasiri, R. Time for a strategy in night-time dopaminergic therapy? An objective sensor-based analysis of nocturnal hypokinesia and sleeping positions in Parkinson’s disease. J. Neurol. Sci. 2017, 373, 244–248. [Google Scholar] [CrossRef]
- Mirelman, A.; Hillel, I.; Rochester, L.; Del Din, S.; Bloem, B.R.; Avanzino, L.; Nieuwboer, A.; Maidan, I.; Herman, T.; Thaler, A.; et al. Tossing and Turning in Bed: Nocturnal Movements in Parkinson’s Disease. Mov. Disord. 2020, 35, 959–968. [Google Scholar] [CrossRef]
- Prudon, B.; Duncan, G.W.; Khoo, T.K.; Yarnall, A.J.; Anderson, K.N. Primary sleep disorder prevalence in patients with newly diagnosed Parkinson’s disease. Mov. Disord. 2013, 29, 259–262. [Google Scholar] [CrossRef]
- Madrid-Navarro, C.J.; Escamilla-Sevilla, F.; Minguez-Castellanos, A.; Campos, M.; Ruiz-Abellan, F.; Madrid, J.A.; Rol, M.A. Multidimensional Circadian Monitoring by Wearable Biosensors in Parkinson’s Disease. Front. Neurol. 2018, 9, 157. [Google Scholar] [CrossRef]
- Oz, S.; Dagay, A.; Katzav, S.; Wasserman, D.; Tauman, R.; Gerston, A.; Duncan, I.; Hanein, Y.; Mirelman, A. Monitoring sleep stages with a soft electrode array: Comparison against vPSG and home-based detection of REM sleep without atonia. J. Sleep Res. 2023, 32, e13909. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Sringean, J.; Thanawattano, C. Impaired bed mobility: Quantitative torque analysis with axial inertial sensors. Neurodegener. Dis. Manag. 2017, 7, 235–243. [Google Scholar] [CrossRef]
- Qian, X.; Hao, H.; Chen, Y.; Li, L. Wake/Sleep Identification Based on Body Movement for Parkinson’s Disease Patients. J. Med. Biol. Eng. 2015, 35, 517–527. [Google Scholar] [CrossRef]
- Mikulec, M.; Galaz, Z.; Mekyska, J.; Mucha, J.; Brabenec, L.; Moravkova, I.; Rektorova, I. Prodromal Diagnosis of Lewy Body Diseases Based on Actigraphy. In Proceedings of the 2022 45th International Conference on Telecommunications and Signal Processing (TSP), Prague, Czech Republic, 13–15 July 2022. [Google Scholar] [CrossRef]
- Goncalves, B.S.; Cavalcanti, P.R.; Tavares, G.R.; Campos, T.F.; Araujo, J.F. Nonparametric methods in actigraphy: An update. Sleep Sci. 2014, 7, 158–164. [Google Scholar] [CrossRef]
- Sringean, J.; Taechalertpaisarn, P.; Thanawattano, C.; Bhidayasiri, R. How well do Parkinson’s disease patients turn in bed? Quantitative analysis of nocturnal hypokinesia using multisite wearable inertial sensors. Park. Relat. Disord. 2016, 23, 10–16. [Google Scholar] [CrossRef]
- Rechichi, I.; Gangi, L.D.; Zibetti, M.; Olmo, G. Home Monitoring of Sleep Disturbances in Parkinson’s Disease: A Wearable Solution. In Proceedings of the 2024 IEEE International Conference on Pervasive Computing and Communications Workshops and other Affiliated Events (PerCom Workshops), Biarritz, France, 11–15 March 2024. [Google Scholar] [CrossRef]
- Gnarra, O.; Calvello, C.; Schirinzi, T.; Beozzo, F.; De Masi, C.; Spanetta, M.; Fernandes, M.; Grillo, P.; Cerroni, R.; Pierantozzi, M.; et al. Exploring the Association Linking Head Position and Sleep Architecture to Motor Impairment in Parkinson’s Disease: An Exploratory Study. J. Pers. Med. 2023, 13, 1591. [Google Scholar] [CrossRef]
- Schalkamp, A.K.; Harrison, N.A.; Peall, K.J.; Sandor, C. Digital outcome measures from smartwatch data relate to non-motor features of Parkinson’s disease. NPJ Park. Dis 2024, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Giganti, F.; Ramat, S.; Zilli, I.; Guidi, S.; Raglione, L.M.; Sorbi, S.; Salzarulo, P. Daytime course of sleepiness in de novo Parkinson’s disease patients. J. Sleep Res. 2013, 22, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Tai, Y.; Yamagami, Y.; Esaki, Y.; Yoshikawa, T.; Sugie, K.; Kataoka, H. Daily light exposure profiles and the association with objective sleep quality in patients with Parkinson’s disease: The PHASE study. Sleep 2024, 47, zsae036. [Google Scholar] [CrossRef] [PubMed]
- Boroojerdi, B.; Ghaffari, R.; Mahadevan, N.; Markowitz, M.; Melton, K.; Morey, B.; Otoul, C.; Patel, S.; Phillips, J.; Sen-Gupta, E.; et al. Clinical feasibility of a wearable, conformable sensor patch to monitor motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2018, 61, 70–76. [Google Scholar] [CrossRef]
- Niwa, F.; Kuriyama, N.; Nakagawa, M.; Imanishi, J. Circadian rhythm of rest activity and autonomic nervous system activity at different stages in Parkinson’s disease. Auton. Neurosci. 2011, 165, 195–200. [Google Scholar] [CrossRef]
- Whitehead, D.L.; Davies, A.D.; Playfer, J.R.; Turnbull, C.J. Circadian rest-activity rhythm is altered in Parkinson’s disease patients with hallucinations. Mov. Disord. 2008, 23, 1137–1145. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Sringean, J.; Taechalertpaisarn, P.; Thanawattano, C. Capturing nighttime symptoms in Parkinson disease: Technical development and experimental verification of inertial sensors for nocturnal hypokinesia. J. Rehabil. Res. Dev. 2016, 53, 487–498. [Google Scholar] [CrossRef]
- Raschella, F.; Scafa, S.; Puiatti, A.; Martin Moraud, E.; Ratti, P.L. Actigraphy Enables Home Screening of Rapid Eye Movement Behavior Disorder in Parkinson’s Disease. Ann. Neurol. 2023, 93, 317–329. [Google Scholar] [CrossRef]
- Kotschet, K.; Johnson, W.; McGregor, S.; Kettlewell, J.; Kyoong, A.; O’Driscoll, D.M.; Turton, A.R.; Griffiths, R.I.; Horne, M.K. Daytime sleep in Parkinson’s disease measured by episodes of immobility. Park. Relat. Disord. 2014, 20, 578–583. [Google Scholar] [CrossRef]
- Cai, G.; Huang, Y.; Luo, S.; Lin, Z.; Dai, H.; Ye, Q. Continuous quantitative monitoring of physical activity in Parkinson’s disease patients by using wearable devices: A case-control study. Neurol. Sci. 2017, 38, 1657–1663. [Google Scholar] [CrossRef]
- Hoglund, A.; Hagell, P.; Broman, J.E.; Palhagen, S.; Sorjonen, K.; Fredrikson, S.; Svenningsson, P. Associations Between Fluctuations in Daytime Sleepiness and Motor and Non-Motor Symptoms in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2020, 8, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Nass, A.; Nass, R.D. Actigraphic evidence for night-time hyperkinesia in Parkinson’s disease. Int. J. Neurosci. 2008, 118, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Di, C.; Xiao, L.; Evenson, K.R.; LaCroix, A.Z.; Crainiceanu, C.M.; Buchner, D.M. An Activity Index for Raw Accelerometry Data and Its Comparison with Other Activity Metrics. PLoS ONE 2016, 11, e0160644. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex differences in Parkinson’s disease. Front. Neuroendocr. 2014, 35, 370–384. [Google Scholar] [CrossRef]
- Redline, S.; Kirchner, H.L.; Quan, S.F.; Gottlieb, D.J.; Kapur, V.; Newman, A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch. Intern. Med. 2004, 164, 406–418. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- Louter, M.; Maetzler, W.; Prinzen, J.; van Lummel, R.C.; Hobert, M.; Arends, J.B.; Bloem, B.R.; Streffer, J.; Berg, D.; Overeem, S.; et al. Accelerometer-based quantitative analysis of axial nocturnal movements differentiates patients with Parkinson’s disease, but not high-risk individuals, from controls. J. Neurol. Neurosurg. Psychiatry 2015, 86, 32–37. [Google Scholar] [CrossRef]



| Topic | Pubmed Query |
|---|---|
| Wearable sensors | (“Wearable Electronic Devices”[MeSH] or “Actigraphy”[MeSH] or “Accelerometry”[MeSH] or Wearable* or body-worn or accelerometer* or gyro* or actigraph* or smartphone* or remote sensing or smartwatch* or inertial measurement unit* or IMU or IMUs or mobile device* or sensor*) |
| AND | |
| Parkinson’s disease | (“Parkinson Disease”[MeSH] or Parkinson’s disease or Parkinson’s or Parkinson or Parkinson disease) |
| AND | |
| Sleep | (“Sleep Wake Disorders”[MeSH] or sleep[MeSH] or sleep or insomnia or dyssomnia or REM or RBD or restless legs or EDS or nocturnal) |
| AND | |
| Ambulatory monitoring | (“Monitoring, Ambulatory”[MeSH] or ambulatory or home or free living or homebased or ecological* or continuous or unsupervised or remote) |
| Study | Year | Main Goal | Key Takeaways | Sensing Modalities | Accelerometer Position | Wrist Side | Monitoring Duration |
|---|---|---|---|---|---|---|---|
| [9] | 2019 | Validate an ambulatory monitoring device for the detection of sleep and wake states using PSG and study sleep quality in patients with PD. | There was no significant difference between sleep parameters detected via the device and PSG. PD is associated with lower distal skin temperature, sleep efficiency and sleep time and greater WASO, activity during sleep and duration of naps, and a worse circadian function index. | TemperatureAccelerometerLightWrist position | Wrist | LA | 7 |
| [10] | 2019 | Investigate temporal associations between objective and subjective sleep measures and daytime functioning using smartphone-based questionnaires and actigraphy. | Objective sleep did not predict any daytime variables. Subjective sleep quality was predicted via positive affect, but objective sleep quantity and quality were not. | Accelerometer | Wrist | NS | 14 |
| [11] | 2017 | Assess the severity of nocturnal hypokinesia and sleep positions in PD patients and their spouses. | PD patients had significantly fewer episodes of turns in bed, slower turning speed and acceleration, and turned fewer degrees than their spouses. These differences were more significant in the second half of the night. PD patients spent more time in a supine position than their spouses. | AccelerometerGyroscope | WristAnkleChest | Both | 1 |
| [12] | 2020 | Study the prevalence of sleep disturbances and nocturnal hypokinesia in different stages of PD and their relation to nonmotor symptoms and medication dose. | Patients with advanced PD had more upright periods, fewer turns, and a slower turning velocity. Turning duration, degree, and velocity were already altered in recently diagnosed patients compared to controls. Reduced nocturnal movements were associated with PD motor severity, dysautonomia, cognition, and dopaminergic medication. | Accelerometer | Lumbar | NA | 2 |
| [13] | 2013 | Assess the prevalence of sleep disturbance in newly diagnosed PD patients using questionnaires, respiratory home monitoring, and actigraphy. | Subjective sleep measures were not associated with objective sleep-disordered breathing or periodic limb movements of sleep (PLMS). PLMS was associated with PD severity. | Accelerometer | Foot | NA | 3 |
| [14] | 2018 | Test a wrist-worn device with machine-learning processing to assess PD patients based on circadian rhythm, motor, and autonomic disruption. | The device reliably collected reliable information about motor (acceleration and time in movement) and non-motor (sleep and skin temperature rhythms) features. Acceleration during the daytime, time in movement during sleep, and their ratio (A/T) were the best indexes to characterise PD symptoms. | TemperatureAccelerometerLightWrist position | Wrist | LA | 7 |
| [15] | 2023 | Validate a new wearable system, composed of an array of dry electrodes, to accurately measure sleep at home using PSG. | The average total agreement between sleep stage classification via the two systems was 77.25%. Agreement did not vary between the PD and the control group. The system detected RSWA with a sensitivity of 85.7%. WASO was significantly higher when measured in the sleep lab when compared with the at-home recording. | EEGEOGEMG | NA | NA | 1 |
| [16] | 2017 | Develop an objective assessment of a patient’s ability to turn in bed in their own home environment. | The number, degree, velocity, acceleration, and torque of axial rotations of PD patients in bed were significantly less than those of their spouses. Significant correlations were observed between the torque of turning in bed and UPDRS and total nocturnal akinesia dystonia and cramp scores. | AccelerometerGyroscope | Chest | NA | 2 |
| [17] | 2015 | Identify the wake/sleep status in PD patients for closed-loop deep brain stimulation. | The wake/sleep state identification for the chest algorithm, when compared with video recordings, achieved an accuracy of 85.78% and 82.74%, respectively, for patients with DBS on and DBS off. The algorithm performance for the chest was comparable to that of the commonly used location on the wrist. | Accelerometer | WristChest | ND | 1 |
| [18] | 2022 | Develop a tool for an automated diagnosis of the prodromal state of Parkinsonian syndromes based on sleep actigraphy. | The sleep/wake classifier achieved an accuracy of 83%. The developed diagnosis method distinguishes participants with prodromal Parkinsonian syndromes and healthy controls with 94% accuracy, 100% sensitivity, and 91% specificity. | TemperatureLightAccelerometer | Wrist | NS | 6 † |
| [19] | 2014 | Study the interdaily stability (IS) and intradaily variability to describe rest–activity rhythm using simulated and actigraphic data. | Rhythmic synchronisation of activity and rest was significantly higher in young adults than adults with PD when the average IS was considered; however, this difference was not seen when using the IS calculated with a sample frequency of 60 min. PD patients showed reduced activity compared to young individuals. | Accelerometer | Wrist | NS | 7 |
| [20] | 2016 | Employ multiple inertial measurement units to compare nocturnal movements between PD patients and their spouses and correlate these metrics with disease severity scores. | PD patients had fewer turning movements and turned with a lower degree, velocity, and acceleration than their spouses. However, PD patients had more episodes of getting out of bed. Nocturnal hypokinesia was correlated with daytime axial and nonmotor symptoms. Leg movements were correlated with clinical scores. Nocturia was correlated with medication dose. | AccelerometerGyroscope | WristAnkleChest | Both | 1 |
| [21] | 2024 | Extract metrics that characterise nighttime motility and develop a method for the automatic assessment of sleep quality. | SVM was the best-performing classifier (96.2% accuracy) at distinguishing between HC and PD. XGBoost achieved 85.7% accuracy at differentiating between good and bad sleep quality. | AccelerometerGyroscopeMagnetometer | Chest | NA | 1 |
| [22] | 2023 | Analyse sleep architecture in PD patients and correlate sleep data with head position and motor and non-motor symptoms. | Sleep architecture was consistent across nights. Sleep was predominantly performed in the supine position. REM sleep in the supine decubitus was associated with disease duration and motor symptoms. No correlation was found between sleeping position and medication dose. | AccelerometerEEGEOGEMGMicrophonePPG | Head | NA | 3 |
| [23] | 2024 | Analyse the correlation between clinical scores of non-motor symptoms and passively collected digital data related to activity, sleep, and vital signs. | Digital measures of sleep correlated with clinical measures of cognition, autonomic function, and medication but did not correlate with psychiatric or motor clinical measures. Digital data could not predict scores of questionnaires or scales using linear regression. Digital outcome measures were significantly better at detecting change than clinical ones. | AccelerometerGyroscopePPGECG | Wrist | NS | 6 |
| [24] | 2013 | Evaluate the time–course of the sleepiness level during the wakefulness period in untreated patients with early-stage Parkinson’s disease. | A higher level of sleepiness was found in the patients than the controls in the hours following awakening and in the early afternoon. | Accelerometer | Wrist | NS | 3 |
| [25] | 2024 | Compare daily light exposure between patients with PD and non-PD older adults and evaluate the association of daily light exposure with objective sleep measures in patients with PD. | Greater daytime light exposure and lower nighttime light exposure were significantly associated with better objective sleep measures in patients with PD. | AccelerometerLight | Wrist | ND hand | 7 |
| [26] | 2018 | Evaluate the accuracy of the NIMBLE wearable biosensor patch to record body movements in clinic and home environments versus clinical measurement of motor symptoms. | No discernable relationship was identified between the total amounts of motor activity, or total time lying down during sleep and the quality of the sleep pattern descriptors reported by participants via a diary app. | AccelerometerEMG | ChestShinForearmHand | MA | 3 |
| [27] | 2011 | Evaluate the alteration of circadian rhythm in PD patients, by investigating rest activities and autonomic function. | PD patients have lower activity levels when out of bed and higher activity levels when in bed, and the circadian rest–activity rhythm in PD decreases with disease severity. HRV showed that the total frequency component and low-frequency/high-frequency ratio were low in PD patients, suggesting that autonomic activities and the circadian rhythm of the sympathetic nervous system are attenuated in PD. | AccelerometerECG | Wrist | LA | 7 |
| [28] | 2008 | Compare rest–activity rhythms in healthy older adults and PD patients with and without hallucinations. | PD patients demonstrated a reduced amplitude of activity and increased intradaily variability compared to healthy older adults, independently of age and cognitive status. Hallucinators showed lower interdaily stability, significantly greater activity during the ‘‘nighttime’’, and a significantly reduced relative amplitude of activity compared to nonhallucinators, independently of clinical factors including motor fluctuations. | Accelerometer | Wrist | LA | 7 |
| [29] | 2016 | Develop an inertial sensor that can provide quantitative monitoring of axial rotation of patients with PD and their spouses while in bed. | Patients with PD rolled over significantly fewer times than their spouses, and the position change was significantly smaller in patients with PD. Patients with PD rolled over at a significantly slower speed and acceleration than their spouses. In contrast, patients with PD got out of bed significantly more often than their spouses. It is technically feasible to develop an easy-to-use, portable, and accurate device that can assess nocturnal movements of patients with PD. | AccelerometerGyroscope | Chest | NA | 1 |
| [30] | 2023 | Explore the use of wrist actigraphy to enable automatic RBD diagnoses in home settings. | SVM achieved the best performance in distinguishing between RBD and non-RBD patients, with an accuracy of 92.9% for in-lab data. Maximum performance was achieved with the actigraph on the wrist of the most affected side. Over 7 days of at-home data, the classifier achieved 100% accuracy for PD patients. | AccelerometerLight | Wrist | MA | 14 |
| [31] | 2014 | Study the relation between daytime immobility and sleepiness using actigraphy. | There was concordance between immobility and PSG scores in 85.6% epochs. PD patients with high ESS had significantly higher PTI than other participants. PD patients with a high PTI had more bradykinesia, less dyskinesia, and higher PDQ39 scores than those with low PTI. There was no relationship between PTI and dose or type of PD medications. PTI increased in the 30–60 min after levodopa. | Accelerometer | Wrist | MA | 10 |
| [32] | 2017 | Explore the feasibility of using wearable devices to quantitatively measure the daily activity in patients with Parkinson’s disease (PD) and to monitor medication-induced motor fluctuations. | Daily sleep time was significantly lower in PD patients than in the control group. | Accelerometer | Wrist | NS | 3 |
| [33] | 2020 | Investigate the relationship between daytime sleepiness and other non-motor and motor fluctuations in people with PD. | Episodes of daytime sleepiness, as reported by home diaries, were associated with other self-reported non-motor and motor fluctuations but were not supported with PKG data. | Accelerometer | Wrist | MA | 6 |
| [34] | 2008 | Find out how the daytime and night-time motor activity levels in individuals without motor disorders differ from those of patients with Parkinson’s disease. | PD patients had 1.5–2-fold lower daytime motor activity but also showed 1.5–2-fold higher motor activity at nighttime. Older controls showed a lower daytime but similar nighttime motor activity when compared to younger controls. A ratio of nighttime to daytime motor activity could clearly distinguish controls and patients. | Accelerometer | Wrist | ND | 3 |
| Reporting | External Validity | Internal Validity—Bias | Internal Validity—Confounding | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 5 | 6 | 7 | 9 | 10 | 11 | 12 | 16 | 17 | 18 | 20 | 21 | 22 | 25 | 26 | Total (%) |
| [9] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 1 | NA | 73% |
| [10] | 1 | 0 | 1 | NA | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 0 | NA | 0 | NA | 62% |
| [11] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 1 | 0 | 1 | NA | 81% |
| [12] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 1 | NA | 75% |
| [13] | 1 | 1 | 1 | 1 | 1 | 1 | NA | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 0 | 0 | NA | 69% |
| [14] | 1 | 1 | 1 | 2 | 0 | 1 | NA | 0 | 0 | 0 | 1 | NA | 1 | 0 | 0 | 0 | 1 | NA | 56% |
| [15] | 1 | 1 | 1 | NA | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 1 | NA | 71% |
| [16] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 1 | 0 | 1 | NA | 81% |
| [17] | 1 | 1 | 0 | 0 | 1 | 1 | NA | NA | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 0 | NA | 47% |
| [18] | 1 | 0 | 0 | 0 | 1 | 1 | NA | NA | 0 | 0 | 1 | NA | 1 | 1 | NA | NA | 0 | NA | 46% |
| [19] | 1 | 0 | 0 | 0 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 0 | NA | 44% |
| [20] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | 88% |
| [21] | 1 | 1 | 0 | 2 | 1 | 1 | NA | 0 | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 0 | NA | 56% |
| [22] | 1 | 1 | 1 | NA | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | NA | NA | NA | NA | 82% |
| [23] | 1 | 0 | 0 | NA | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | NA | NA | NA | 0 | 57% |
| [24] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 0 | 1 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 1 | NA | 75% |
| [25] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 0 | NA | 69% |
| [26] | 1 | 0 | 1 | NA | 1 | 1 | NA | 0 | 0 | 0 | 1 | NA | 1 | 1 | NA | NA | NA | NA | 64% |
| [27] | 1 | 0 | 1 | 2 | 1 | 1 | NA | 1 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | 88% |
| [28] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 0 | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 1 | NA | 69% |
| [29] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 0 | NA | 81% |
| [30] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 1 | 1 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 1 | NA | 81% |
| [31] | 0 | 0 | 0 | 1 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 1 | 0 | 1 | NA | 56% |
| [32] | 1 | 0 | 1 | 2 | 1 | 1 | NA | 1 | 0 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 1 | NA | 69% |
| [33] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 0 | 0 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | 81% |
| [34] | 1 | 1 | 1 | 2 | 1 | 1 | NA | 0 | 0 | 0 | 1 | NA | 1 | 1 | 1 | 0 | 1 | NA | 75% |
| Total (%) | 96% | 69% | 77% | 81% | 96% | 100% | 0% | 67% | 15% | 0% | 100% | 100% | 100% | 96% | 41% | 19% | 65% | 0% | |
| Study | No. of PDP | No. of Controls | Age of PDP | Age of Controls | PDP% Female | Control% Female | MinH&Y | MaxH&Y | Countries |
|---|---|---|---|---|---|---|---|---|---|
| [9] | 15 | HC: n = 15Sleep Disorders: n = 70 | 65.53 ± 2.19 † y | 60.71 ± 1.97 † y | 20 | 20 | NS | NS | Spain |
| [10] | 20 | 0 | 66.5 ± 9.3 y | NA | 35.0 | NA | 1 | 4 | USA |
| [11] | 18 | 18 | 64.9 ± 7.6 y | 63.8 ± 8.5 y | 22.2 | 77.8 | NS | NS | Thailand |
| [12] | 304 | 205 | 68.4 ± 8.5 y | 66.3 ± 13.3 y | 35 | 66 | 1 | 3 | Belgium, Israel, Italy, Netherlands, UK |
| [13] | 106 | 99 | 66.5 ‡(60.1–74.1) y | 67.9 ‡ y | 36.8 | 45.5 | 1 | 3 | UK |
| [14] | 12 | 12 | 65.8 ± 2.67 † y | 69.41 ± 1.90 † y | 25.0 | 25.0 | 2 | 3 | Spain |
| [15] | 29 | 21 | 65.4 ± 7.6 y | 56.6 ± 8.4 y | 34.5 | 38.1 | 1 | 3 | NS |
| [16] | 17 | 17 | 64.9 ± 7.9 y | 64.3 ± 8.6 y | 29.4 | 70.6 | NS | NS | Thailand |
| [17] | 12 | 13 | 64.5 ± 2.0 y | Young HC: 24.8 ± 1.53 y Old HC: 61.5 ± 0.5 y | NS | NS | NS | NS | China |
| [18] | NS | HC, and participants with dementia with Lewy bodies and mild cognitive impairment | NS | NS | NS | NS | NS | NS | NS |
| [19] | 26 | 24 | (38–69) y | (18–23) y | NS | NS | NS | NS | Brazil |
| [20] | 19 | 19 | 64.63 ± 7.95 y | 64.32 ± 8.46 y | 26.3 | 73.7 | 1,5 | 3 | Thailand |
| [21] | 12 | 28 | 68 ± 4.1 y | 38 ± 10.7 y | 35.7 | 35.7 | NS | NS | Italy |
| [22] | 20 | 0 | 65.7 ± 8.6 y | NA | 50 | NA | 1 | 3 | Italy |
| [23] | 149 | 0 | 67.69 ± 7.54 y | NA | NS | NA | NS | NS | NS |
| [24] | 18 | 18 | 68.39 ± 1.89 † y | 67.22 ± 1.98 † y | 50.0 | 50.0 | 1 | 2 | Italy |
| [25] | 189 | 1101 | 71.3 ± 7.6 y | 71.9 ± 7.1 y | 46.6 | 53.2 | 1 | 5 | Japan |
| [26] | 21 | 0 | 65 ± 7 y | NA | 42.9 | NA | 2 | 3 | USA |
| [27] | 27 | 30 | 69.33 ± 7.29 y | 68.93 ± 5.12 y | 37 | 47 | 1 | 4 | Japan |
| [28] | 50 | 29 | 73.36 ± 7.54 y | 70.90 ± 5.59 y | 24 | 31 | NS | NS | UK |
| [29] | 6 | 6 | 65.5 ± 7.45 y | 66.67 ± 7.76 y | 0 | 100 | NS | NS | Thailand |
| [30] | 26 | Insomnia: n = 18 | 68.02 ± 10.6 y | 52.7 ± 15.3 y | 26.9 | 33.3 | 1.5 | 3 | Switzerland |
| [31] | 68 | 30 | 65.9 ‡(40–80) y | 65.8 ‡ y | NS | NS | NS | NS | Australia |
| [32] | 21 | 20 | 66.52 ± 9.13 y | 63.15 ± 8.70 y | 23.8 | 25.0 | 1 | 4 | China |
| [33] | 52 | 0 | 65.3 ± 10.5 y | NA | 38.5 | NA | 1 | 4 | Sweden |
| [34] | 17 | 69 | 74.88 ± 7.2 y | 53.47 ± 21.03 y | 47.1 | 65.2 | 1 | 3 | Germany |
| Sleep Disorder | Studies | Main Outcomes |
|---|---|---|
| Nocturnal hypokinesia | [11,12,16,20,29] | Number, degree, velocity, acceleration, and torque of turns is reduced in PD patients. The difference increases throughout the night and with disease severity. |
| Nocturia | [11,20,29] | Getting out of bed was more frequent in PD patients than controls and was associated with nocturia. |
| Restless legs syndrome | [13] | Periodic limb movements were not associated with symptomatic restless legs syndrome. |
| RBD | [15,23,30] | An electrode array positioned in the head allowed the detection of RBD with 92% accuracy. An RBD classifier based on actigraphic features achieved 100% accuracy. |
| Extreme daytime sleepiness | [23,31,33] | Periods of immobility measured via actigraphy were associated with extreme daytime sleepiness when measured with the ESS but not the KSS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, J.; Ramos, B.; Fernandes, J.; Hansen, C.; Maetzler, W.; Vila-Chã, N.; Maia, L.F. Wearable Sensors for Sleep Monitoring in Free-Living Environments: A Scoping Review on Parkinson’s Disease. Biosensors 2025, 15, 212. https://doi.org/10.3390/bios15040212
Matos J, Ramos B, Fernandes J, Hansen C, Maetzler W, Vila-Chã N, Maia LF. Wearable Sensors for Sleep Monitoring in Free-Living Environments: A Scoping Review on Parkinson’s Disease. Biosensors. 2025; 15(4):212. https://doi.org/10.3390/bios15040212
Chicago/Turabian StyleMatos, Joana, Beatriz Ramos, Joana Fernandes, Clint Hansen, Walter Maetzler, Nuno Vila-Chã, and Luís F. Maia. 2025. "Wearable Sensors for Sleep Monitoring in Free-Living Environments: A Scoping Review on Parkinson’s Disease" Biosensors 15, no. 4: 212. https://doi.org/10.3390/bios15040212
APA StyleMatos, J., Ramos, B., Fernandes, J., Hansen, C., Maetzler, W., Vila-Chã, N., & Maia, L. F. (2025). Wearable Sensors for Sleep Monitoring in Free-Living Environments: A Scoping Review on Parkinson’s Disease. Biosensors, 15(4), 212. https://doi.org/10.3390/bios15040212






