A Wearable Molecularly Imprinted Electrochemical Sensor for Cortisol Stable Monitoring in Sweat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
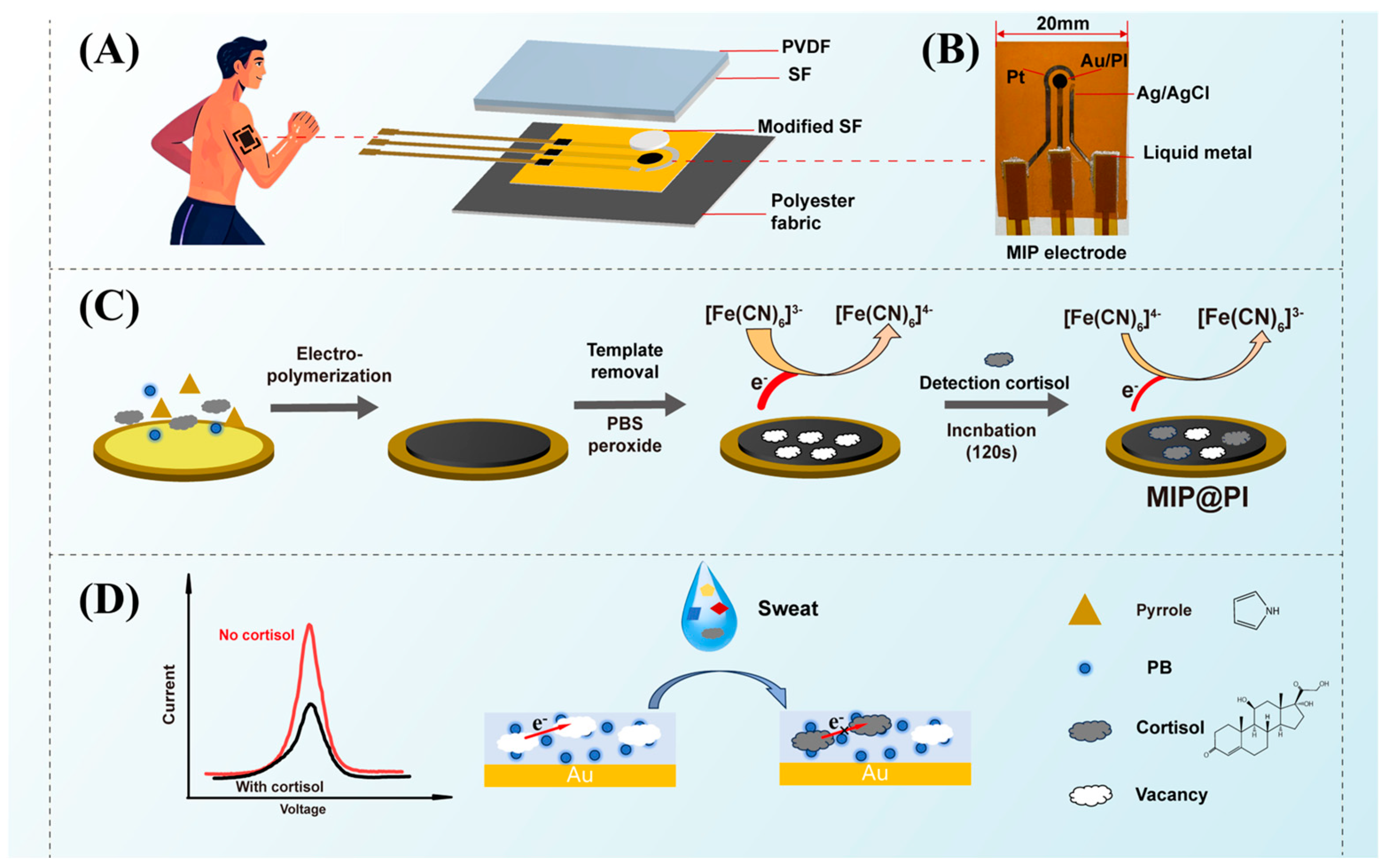
2.2. Preparation of the Electrochemical Three-Electrode System
2.3. Preparation of Silk/PVDF Composite Film
2.4. Fabrication of MIP Electrochemical Sensor
2.5. Characterization and Detection
3. Results and Discussion
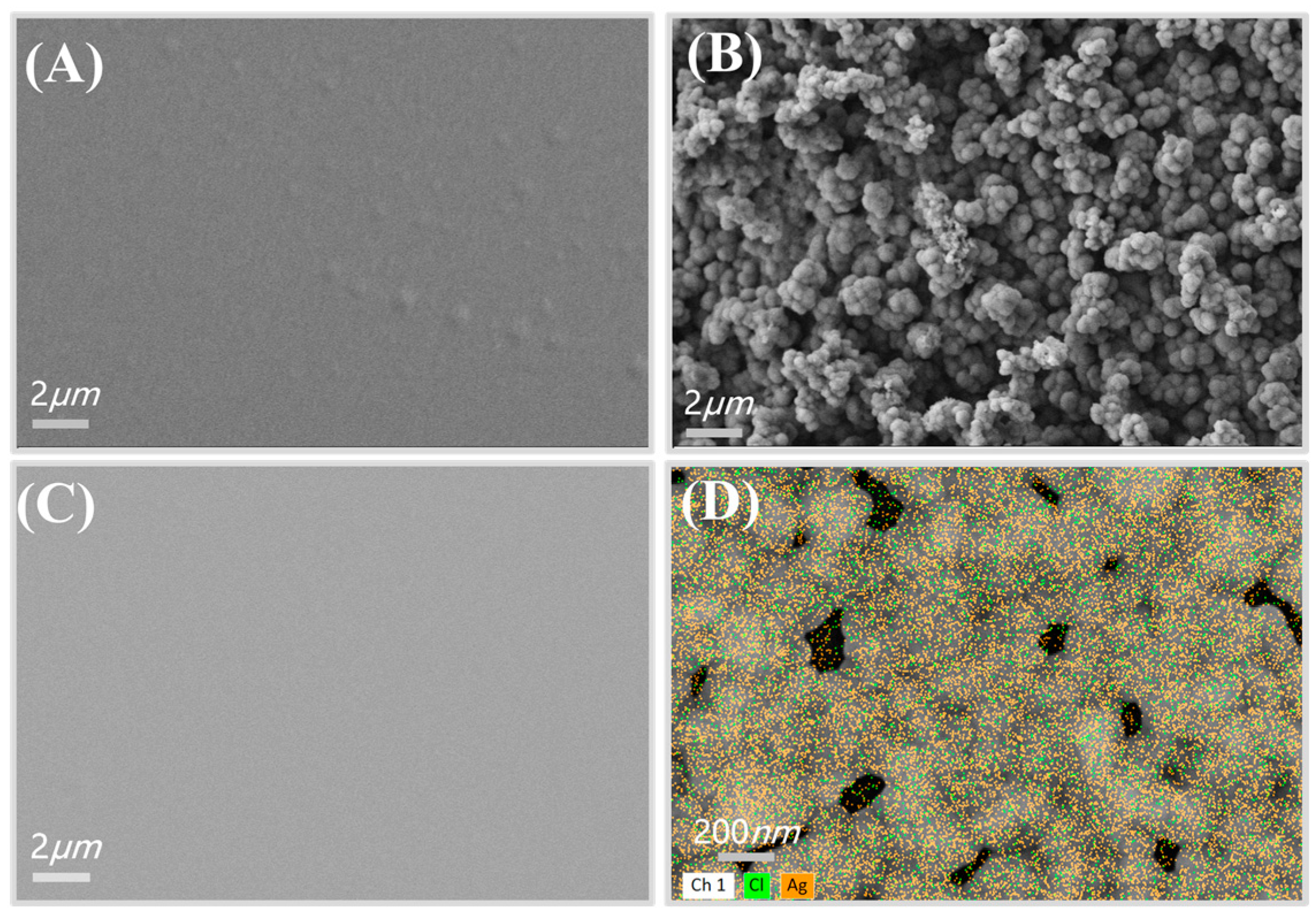
3.1. Characterization of the Electrochemical Three-Electrode System
3.2. Parameter Optimization of Membrane Process of Py-PB Films
3.3. Optimization of Template Removal Parameter and Incubation Time
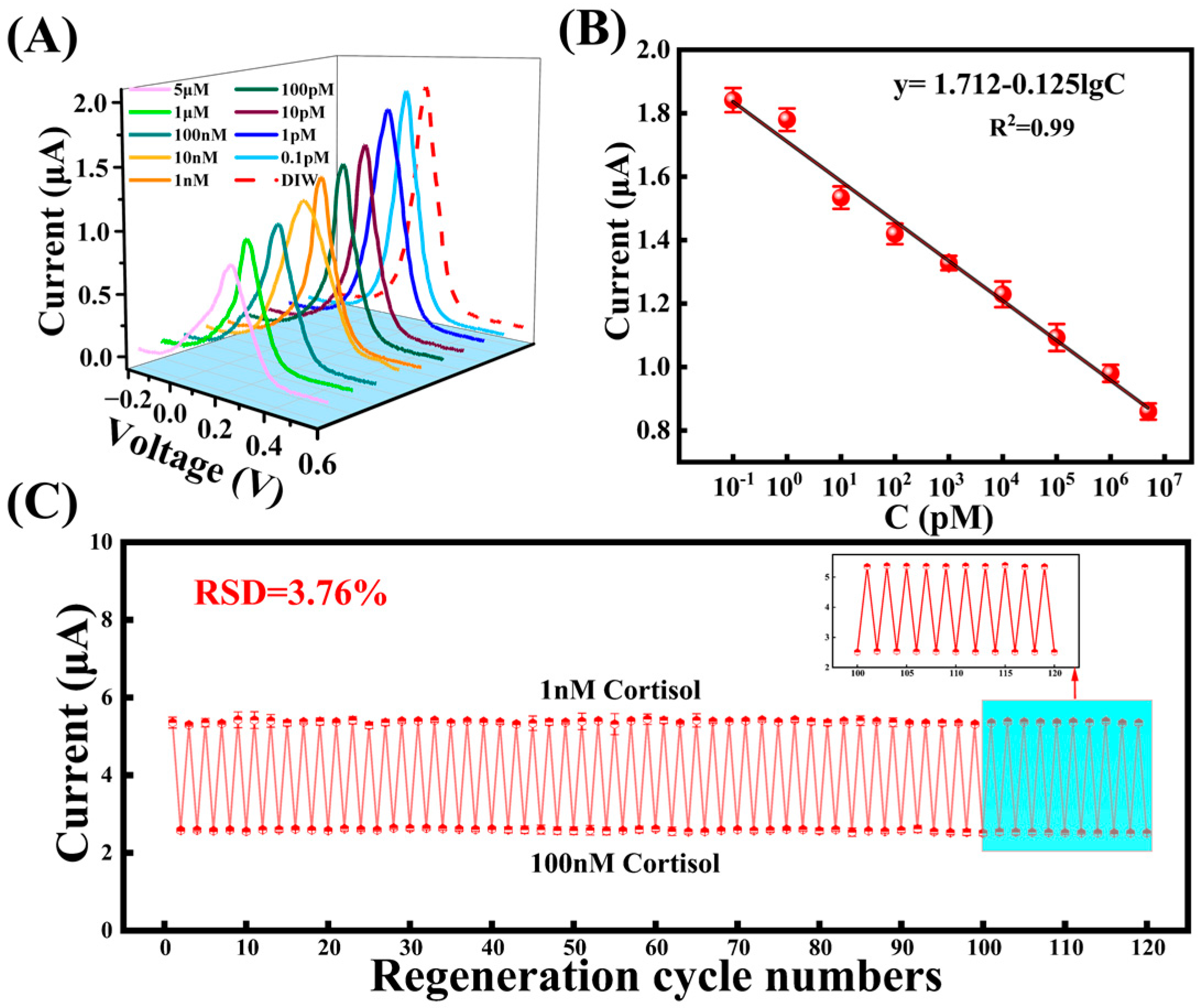
3.4. The MIP Sensor for Cortisol Detection
3.5. Performance Characterization of MIP Electrode and Sensor Stability Test
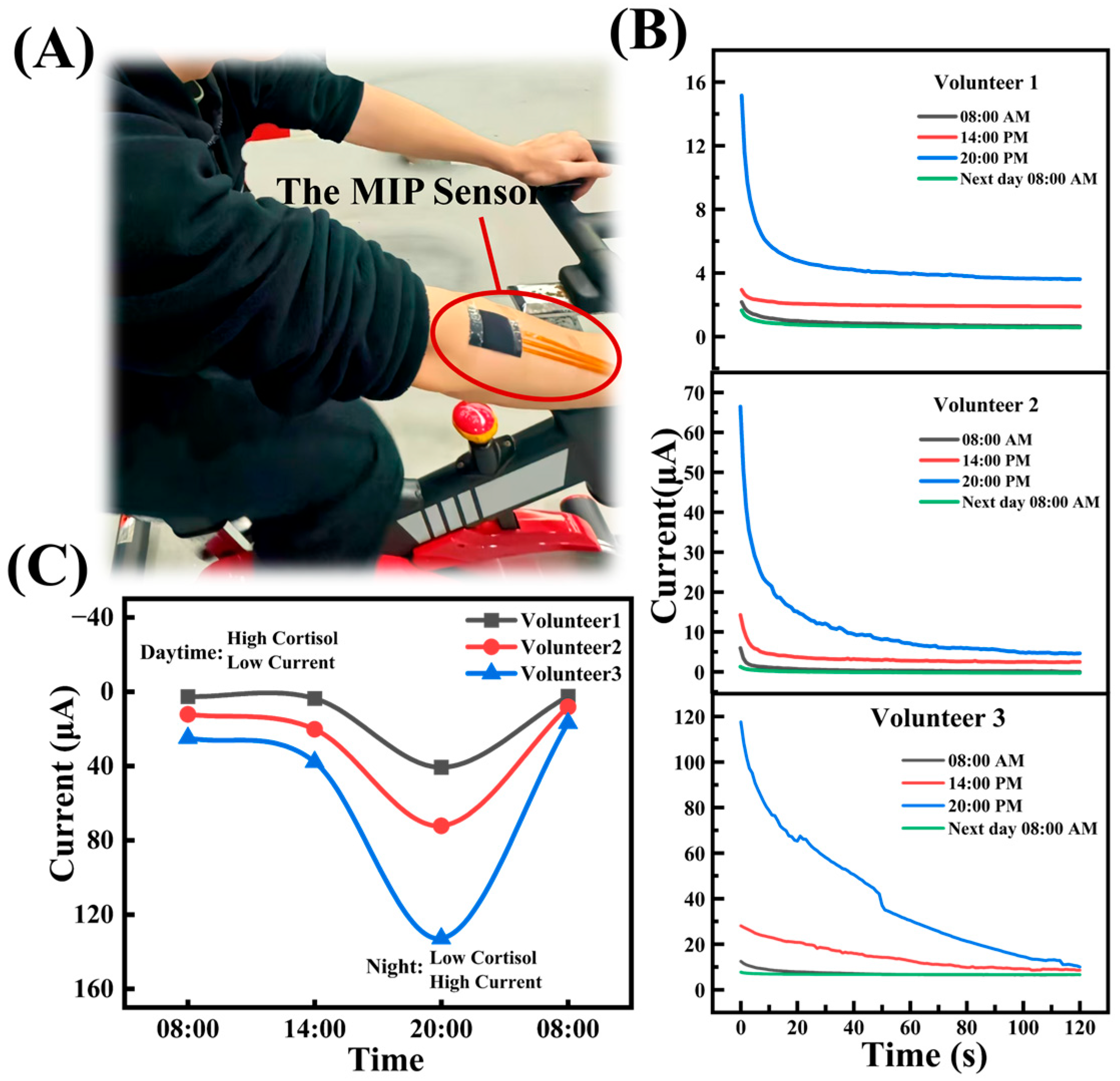
3.6. Surface Sweat Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Qi, T.; Hu, T.; Ge, Q.-Q.; Zhou, X.-N.; Li, J.-M.; Jiang, C.-L.; Wang, W. COVID-19 Pandemic Related Long-Term Chronic Stress on the Prevalence of Depression and Anxiety in the General Population. BMC Psychiatry 2021, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.; Tennant, C. Life Event Stress and Psychiatric Illness. Psychol. Med. 1978, 8, 545–549. [Google Scholar] [CrossRef]
- Kelly, J.; Mangos, G.; Williamson, P.; Whitworth, J. Cortisol and hypertension. Clin. Exp. Pharmacol. Physiol. 1998, 25, 545–549. [Google Scholar] [CrossRef]
- McEwen, B.S. Cortisol, Cushing’s Syndrome, and a Shrinking Brain--New Evidence for Reversibility. J. Clin. Endocrinol. Metab. 2002, 87, 1947–1948. [Google Scholar]
- Iqbal, T.; Simpkin, A.J.; Roshan, D.; Glynn, N.; Killilea, J.; Walsh, J.; Molloy, G.; Ganly, S.; Ryman, H.; Coen, E.; et al. Stress Monitoring Using Wearable Sensors: A Pilot Study and Stress-Predict Dataset. Sensors 2022, 22, 8135. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Teicher, M.H.; Trestman, R.L.; Levengood, R.A.; Siever, L.J. Cortisol Regulation in Posttraumatic Stress Disorder and Major Depression: A Chronobiological Analysis. Biol. Psychiatry 1996, 40, 79–88. [Google Scholar] [CrossRef]
- Taves, M.D.; Gomez-Sanchez, C.E.; Soma, K.K. Extra-Adrenal Glucocorticoids and Mineralocorticoids: Evidence for Local Synthesis, Regulation, and Function. Am. J. Physiol. -Endocrinol. Metab. 2011, 301, E11–E24. [Google Scholar] [CrossRef]
- Holsboer, F.; Ising, M. Stress Hormone Regulation: Biological Role and Translation into Therapy. Annu. Rev. Psychol. 2010, 61, 81–109. [Google Scholar] [CrossRef]
- Djuric, Z.; Bird, C.E.; Furumoto-Dawson, A.; Rauscher, G.H.; Ruffin Iv, M.T.; Stowe, R.P.; Tucker, K.L.; Masi, C.M. Biomarkers of Psychological Stress in Health Disparities Research. Open Biomark. J. 2008, 1, 7–19. [Google Scholar] [CrossRef]
- Levine, A.; Zagoory-Sharon, O.; Feldman, R.; Lewis, J.G.; Weller, A. Measuring Cortisol in Human Psychobiological Studies. Physiol. Behav. 2007, 90, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory Routines Cause Animal Stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar] [PubMed]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xie, Q.; Jin, J.; Qiao, T.; Wang, H.; Chen, L.; Deng, H.; Lu, Z. HPLC-FLU Detection of Cortisol Distribution in Human Hair. Clin. Biochem. 2010, 43, 677–682. [Google Scholar] [CrossRef]
- Appel, D.; Schmid, R.D.; Dragan, C.-A.; Bureik, M.; Urlacher, V.B. A Fluorimetric Assay for Cortisol. Anal. Bioanal. Chem. 2005, 383, 182–186. [Google Scholar] [CrossRef]
- Arcadio, F.; Seggio, M.; Pitruzzella, R.; Zeni, L.; Bossi, A.M.; Cennamo, N. An Efficient Bio-Receptor Layer Combined with a Plasmonic Plastic Optical Fiber Probe for Cortisol Detection in Saliva. Biosensors 2024, 14, 351. [Google Scholar] [CrossRef]
- Assalve, G.; Lunetti, P.; Di Cagno, A.; De Luca, E.W.; Aldegheri, S.; Zara, V.; Ferramosca, A. Advanced Wearable Devices for Monitoring Sweat Biochemical Markers in Athletic Performance: A Comprehensive Review. Biosensors 2024, 14, 574. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Oliveira, O.N. Plasmonic Biosensing: Focus Review. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent Advances in Cortisol Sensing Technologies for Point-of-Care Application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef]
- Haq, N.; Araque, K.; Kanegusuku, A.; Wei, B.; Soldin, S. Are Serum Cortisol Measurements by Immunoassays Reliable? Med. Res. Arch. 2020, 8, 2128. [Google Scholar] [CrossRef]
- Sekar, M.; Pandiaraj, M.; Bhansali, S.; Ponpandian, N.; Viswanathan, C. Carbon Fiber Based Electrochemical Sensor for Sweat Cortisol Measurement. Sci. Rep. 2019, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, C.-E.; Koukouvinos, G.; Goustouridis, D.; Raptis, I.; Kakabakos, S.; Petrou, P.; Livaniou, E. Cortisol Immunosensors: A Literature Review. Biosensors 2023, 13, 285. [Google Scholar] [CrossRef]
- Yue Jing, L.; Fan, Y.; Zhi Chen, B.; Li, D.; Ting He, Y.; Liang Zhang, G.; Liang, L.; Du, J.; Wang, Y.; Dong Guo, X. An Aptamer-Integrated Conductive Microneedle Biosensor for Real-Time Transdermal Cortisol Monitoring. Chem. Eng. J. 2024, 502, 157488. [Google Scholar] [CrossRef]
- Zea, M.; Bellagambi, F.G.; Ben Halima, H.; Zine, N.; Jaffrezic-Renault, N.; Villa, R.; Gabriel, G.; Errachid, A. Electrochemical Sensors for Cortisol Detections: Almost There. TrAC Trends Anal. Chem. 2020, 132, 116058. [Google Scholar] [CrossRef]
- Dash, R.J.; England, B.G.; Midgley, A.R.; Niswender, G.D. A Specific, Non-Chromatographic Radioimmunoassay for Human Plasma Cortisol. Steroids 1975, 26, 647–661. [Google Scholar] [CrossRef]
- Manickam, P.; Pasha, S.K.; Snipes, S.A.; Bhansali, S. A Reusable Electrochemical Biosensor for Monitoring of Small Molecules (Cortisol) Using Molecularly Imprinted Polymers. J. Electrochem. Soc. 2017, 164, B54–B59. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, Y.; Dong, W.; Zhao, Y.; Sun, L. Bio-Inspired Imprinting Materials for Biomedical Applications. Adv. Sci. 2022, 9, 2202038. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Naik, A.R.; Zhou, Y.; Dey, A.A.; Arellano, D.L.G.; Okoroanyanwu, U.; Secor, E.B.; Hersam, M.C.; Morse, J.; Rothstein, J.P.; Carter, K.R.; et al. Printed Microfluidic Sweat Sensing Platform for Cortisol and Glucose Detection. Lab. Chip 2021, 22, 156–169. [Google Scholar] [CrossRef]
- Yeasmin, S.; Wu, B.; Liu, Y.; Ullah, A.; Cheng, L.-J. Nano Gold-Doped Molecularly Imprinted Electrochemical Sensor for Rapid and Ultrasensitive Cortisol Detection. Biosens. Bioelectron. 2022, 206, 114142. [Google Scholar] [CrossRef]
- Mei, X.; Yang, J.; Yu, X.; Peng, Z.; Zhang, G.; Li, Y. Wearable Molecularly Imprinted Electrochemical Sensor with Integrated Nanofiber-Based Microfluidic Chip for in Situ Monitoring of Cortisol in Sweat. Sens. Actuators B Chem. 2023, 381, 133451. [Google Scholar] [CrossRef]
- Saridjan, N.S.; Henrichs, J.; Schenk, J.J.; Jaddoe, V.W.V.; Hofman, A.; Kirschbaum, C.; Verhulst, F.C.; Tiemeier, H. Diurnal Cortisol Rhythm and Cognitive Functioning in Toddlers: The Generation R Study. Child Neuropsychol. 2014, 20, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Ramgir, N.; Bhansali, S. An Immunoelectrochemical Sensor for Salivary Cortisol Measurement. Sens. Actuators B Chem. 2008, 133, 533–537. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Wang, X.; Jia, K.; Zhang, H.; Wang, Y.; Chu, H.; Zhong, X.; Lin, M.; Chen, P.; et al. Wearable and Regenerable Electrochemical Fabric Sensing System Based on Molecularly Imprinted Polymers for Real-Time Stress Management. Adv. Funct. Mater. 2024, 34, 2312897. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Recent Advancements in Nanobiosensors: Current Trends, Challenges, Applications, and Future Scope. Biosensors 2022, 12, 892. [Google Scholar] [CrossRef]
- Beluomini, M.A.; Da Silva, J.L.; Sedenho, G.C.; Stradiotto, N.R. D-Mannitol Sensor Based on Molecularly Imprinted Polymer on Electrode Modified with Reduced Graphene Oxide Decorated with Gold Nanoparticles. Talanta 2017, 165, 231–239. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, M.; Li, J.; Liu, L.; Yu, J.; Li, Z.; Ding, B. Wearable Biosensor for Sensitive Detection of Uric Acid in Artificial Sweat Enabled by a Fiber Structured Sensing Interface. Nano Energy 2021, 85, 106031. [Google Scholar] [CrossRef]
- Tang, W.; Yin, L.; Sempionatto, J.R.; Moon, J.-M.; Teymourian, H.; Wang, J. Touch-Based Stressless Cortisol Sensing. Adv. Mater. 2021, 33, 2008465. [Google Scholar] [CrossRef]
- Buffon, E.; Stradiotto, N.R. Electrochemical Sensor Based on Molecularly Imprinted Poly(Ortho-Phenylenediamine) for Determination of Hexahydrofarnesol in Aviation Biokerosene. Sens. Actuators B Chem. 2019, 287, 371–379. [Google Scholar] [CrossRef]
- Ahmad, R.; Griffete, N.; Lamouri, A.; Felidj, N.; Chehimi, M.M.; Mangeney, C. Nanocomposites of Gold Nanoparticles@Molecularly Imprinted Polymers: Chemistry, Processing, and Applications in Sensors. Chem. Mater. 2015, 27, 5464–5478. [Google Scholar] [CrossRef]
- Karthika, P.; Shanmuganathan, S.; Subramanian, V.; Delerue-Matos, C. Selective Detection of Salivary Cortisol Using Screen-Printed Electrode Coated with Molecularly Imprinted Polymer. Talanta 2024, 272, 125823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wei, W.; Li, Y.; Li, X.; Liu, Y.; Wang, X.; Chen, S. Sweat and Deformation-Resistance Graphite/PVDF/PANI-Based Temperature Sensor for Real-Time Body Temperature Monitoring. Adv. Mater. Technol. 2024, 9, 2400149. [Google Scholar] [CrossRef]
- Shama, N.A.; Aşır, S.; Göktürk, I.; Yılmaz, F.; Türkmen, D.; Denizli, A. Electrochemical Detection of Cortisol by Silver Nanoparticle-Modified Molecularly Imprinted Polymer-Coated Pencil Graphite Electrodes. ACS Omega 2023, 8, 29202–29212. [Google Scholar] [CrossRef]
- Kim, M.; Park, D.; Park, J.; Park, J. Bio-Inspired Molecularly Imprinted Polymer Electrochemical Sensor for Cortisol Detection Based on O-Phenylenediamine Optimization. Biomimetics 2023, 8, 282. [Google Scholar] [CrossRef]
- Liu, H.; Qin, W.; Li, X.; Feng, L.; Gu, C.; Chen, J.; Tian, Z.; Chen, J.; Yang, M.; Qiao, H.; et al. Molecularly Imprinted Electrochemical Sensors Based on Ti3 C2 T x -MXene and Graphene Composite Modifications for Ultrasensitive Cortisol Detection. Anal. Chem. 2023, 95, 16079–16088. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Qin, Y.; Xia, Y.; Xu, X.; Sun, X.; Yu, D.; Mugo, S.M.; Wang, D.; Zhang, Q. An Integrated Wearable Sweat Sensing Patch for Passive Continuous Analysis of Stress Biomarkers at Rest. Adv. Funct. Mater. 2023, 33, 2212083. [Google Scholar] [CrossRef]
- Mugo, S.M.; Robertson, S.V.; Lu, W. A Molecularly Imprinted Screen-Printed Carbon Electrode for Electrochemical Epinephrine, Lactate, and Cortisol Metabolites Detection in Human Sweat. Anal. Chim. Acta 2023, 1278, 341714. [Google Scholar] [CrossRef]
- Gillan, L.; Jansson, E. Molecularly Imprinted Polymer on Roll-to-Roll Printed Electrodes as a Single Use Sensor for Monitoring of Cortisol in Sweat. Flex. Print. Electron. 2022, 7, 025014. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; He, Z.; Wu, Y.; Bai, X.; Li, Y.; Yang, W.; Liu, Y.; Li, R.-W. A Wearable Molecularly Imprinted Electrochemical Sensor for Cortisol Stable Monitoring in Sweat. Biosensors 2025, 15, 194. https://doi.org/10.3390/bios15030194
Chen Y, He Z, Wu Y, Bai X, Li Y, Yang W, Liu Y, Li R-W. A Wearable Molecularly Imprinted Electrochemical Sensor for Cortisol Stable Monitoring in Sweat. Biosensors. 2025; 15(3):194. https://doi.org/10.3390/bios15030194
Chicago/Turabian StyleChen, Yitao, Zidong He, Yuanzhao Wu, Xinyu Bai, Yuancheng Li, Weiwei Yang, Yiwei Liu, and Run-Wei Li. 2025. "A Wearable Molecularly Imprinted Electrochemical Sensor for Cortisol Stable Monitoring in Sweat" Biosensors 15, no. 3: 194. https://doi.org/10.3390/bios15030194
APA StyleChen, Y., He, Z., Wu, Y., Bai, X., Li, Y., Yang, W., Liu, Y., & Li, R.-W. (2025). A Wearable Molecularly Imprinted Electrochemical Sensor for Cortisol Stable Monitoring in Sweat. Biosensors, 15(3), 194. https://doi.org/10.3390/bios15030194




