MWCNTs-CTAB and HFs-Lac Nanocomposite-Modified Glassy Carbon Electrode for Rutin Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus and Measurements
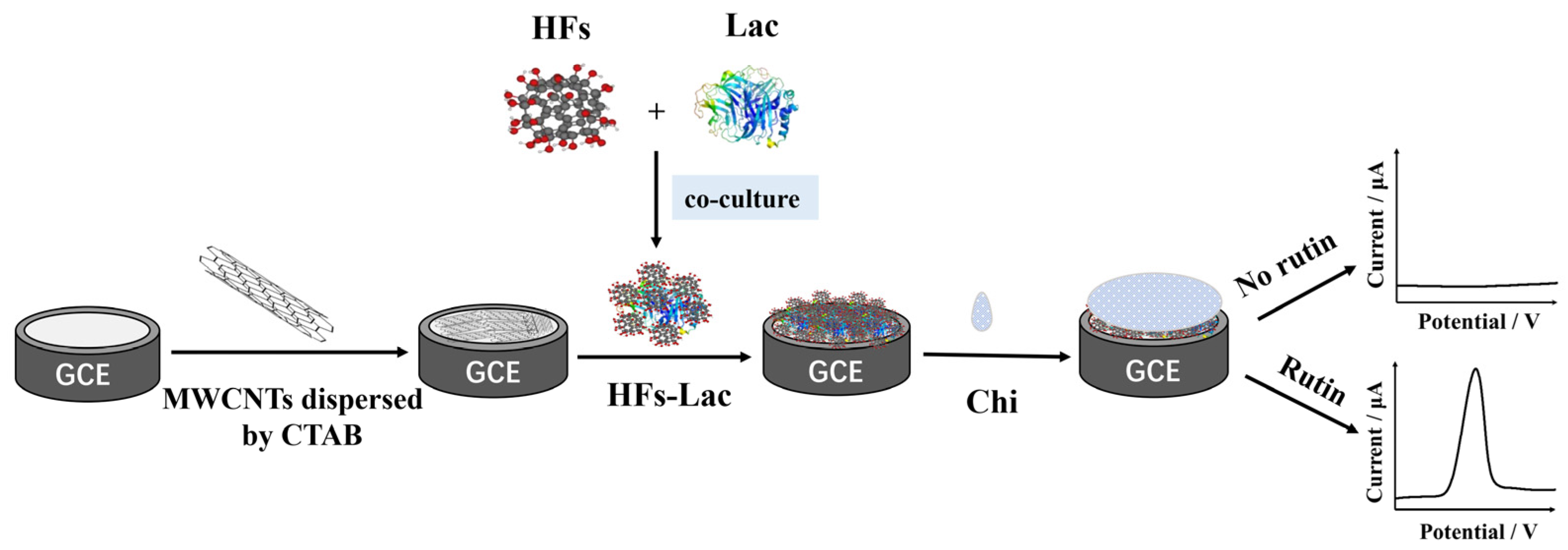
2.3. Preparation of Modified Electrode
2.4. Preparation of Samples
3. Results and Discussion
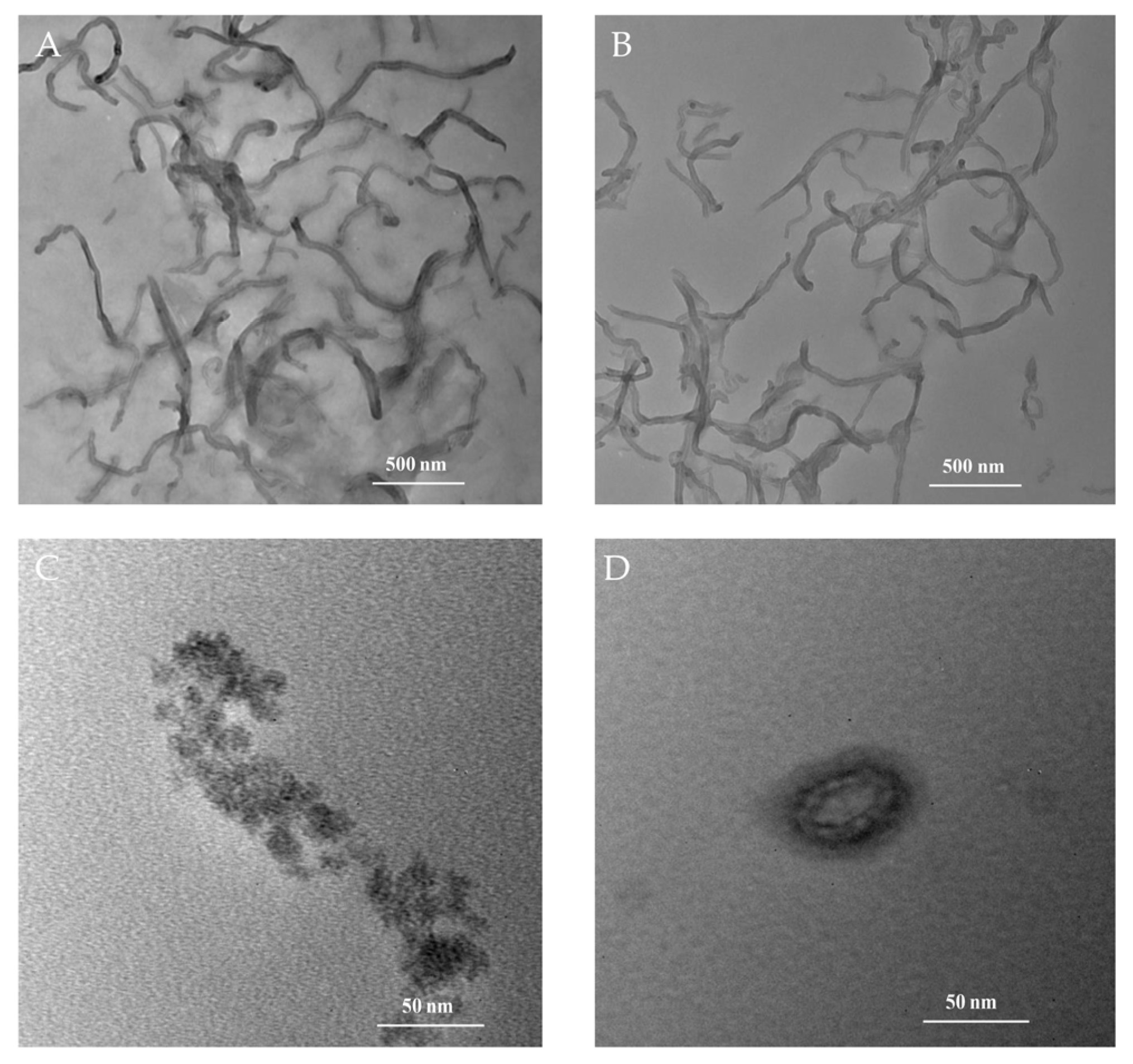
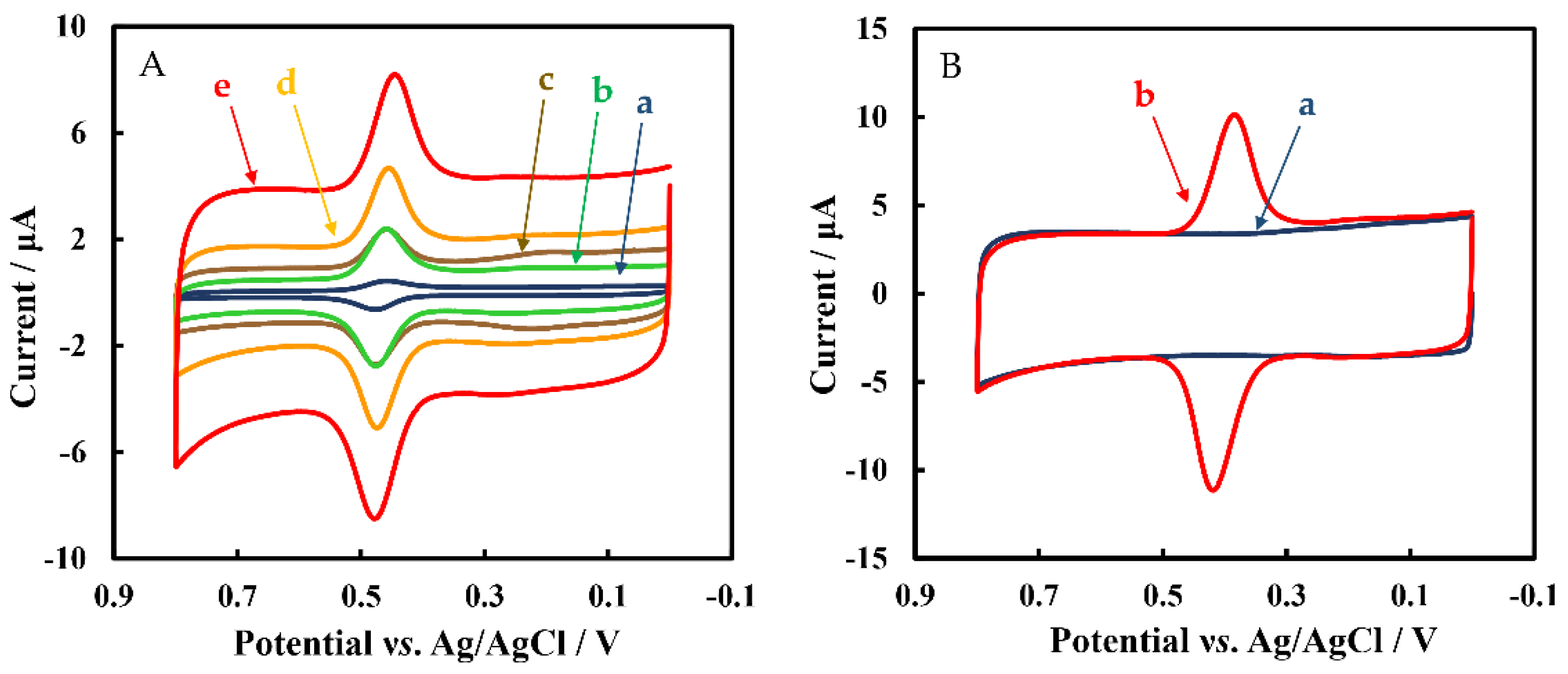
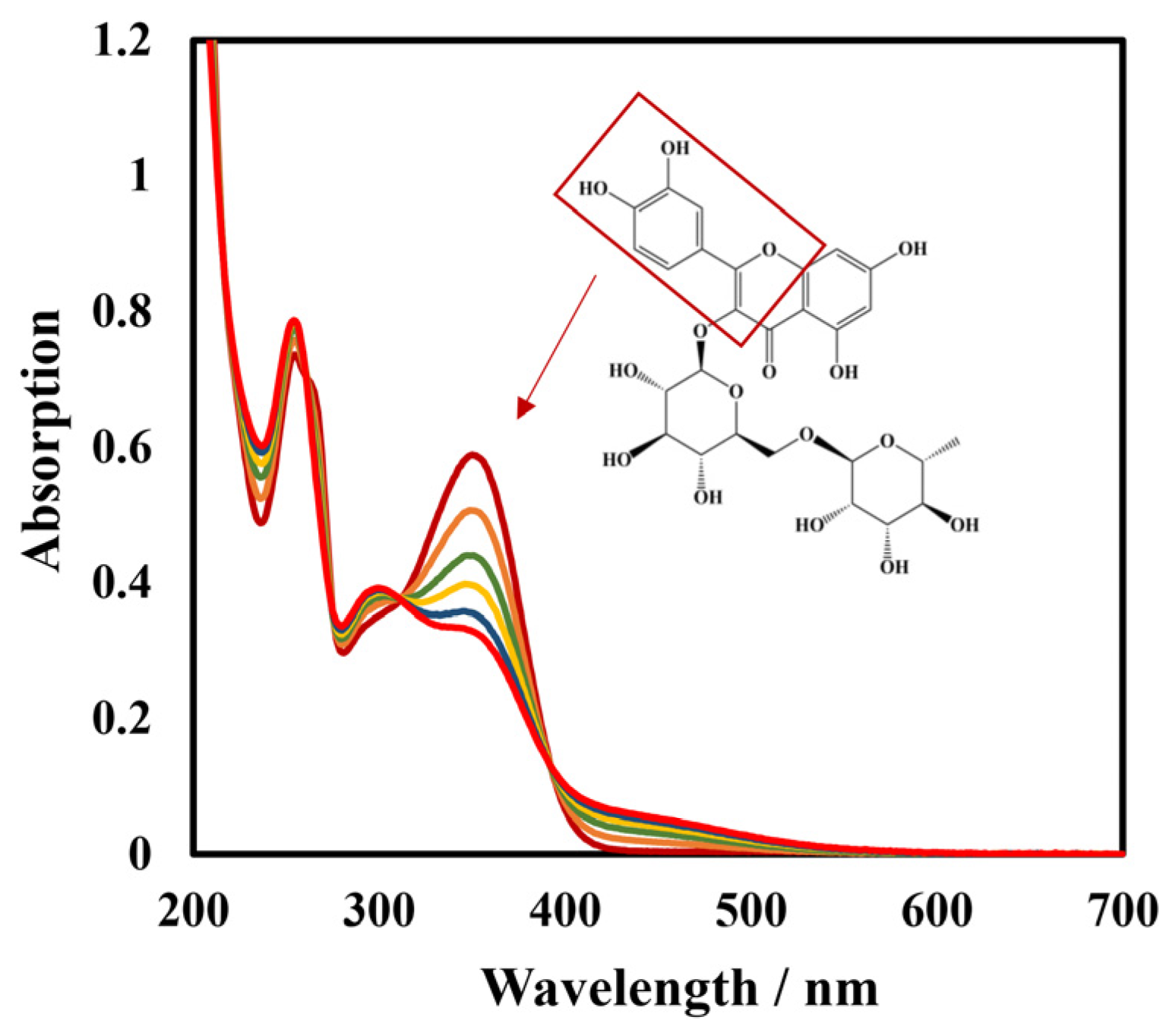
3.1. Characteristics of Modified Materials
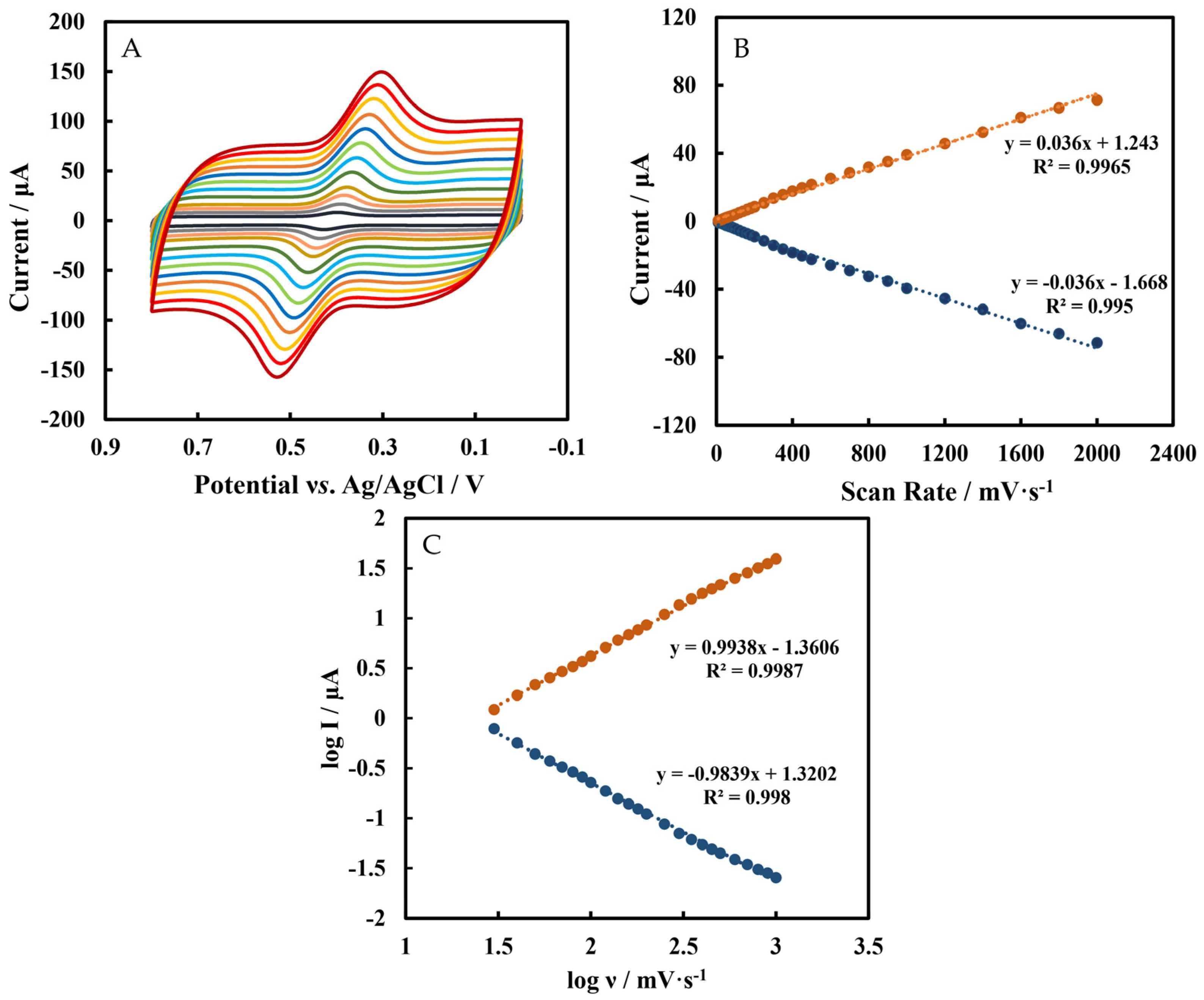
3.2. Effects of Scan Rates
3.3. Effects of pH Values
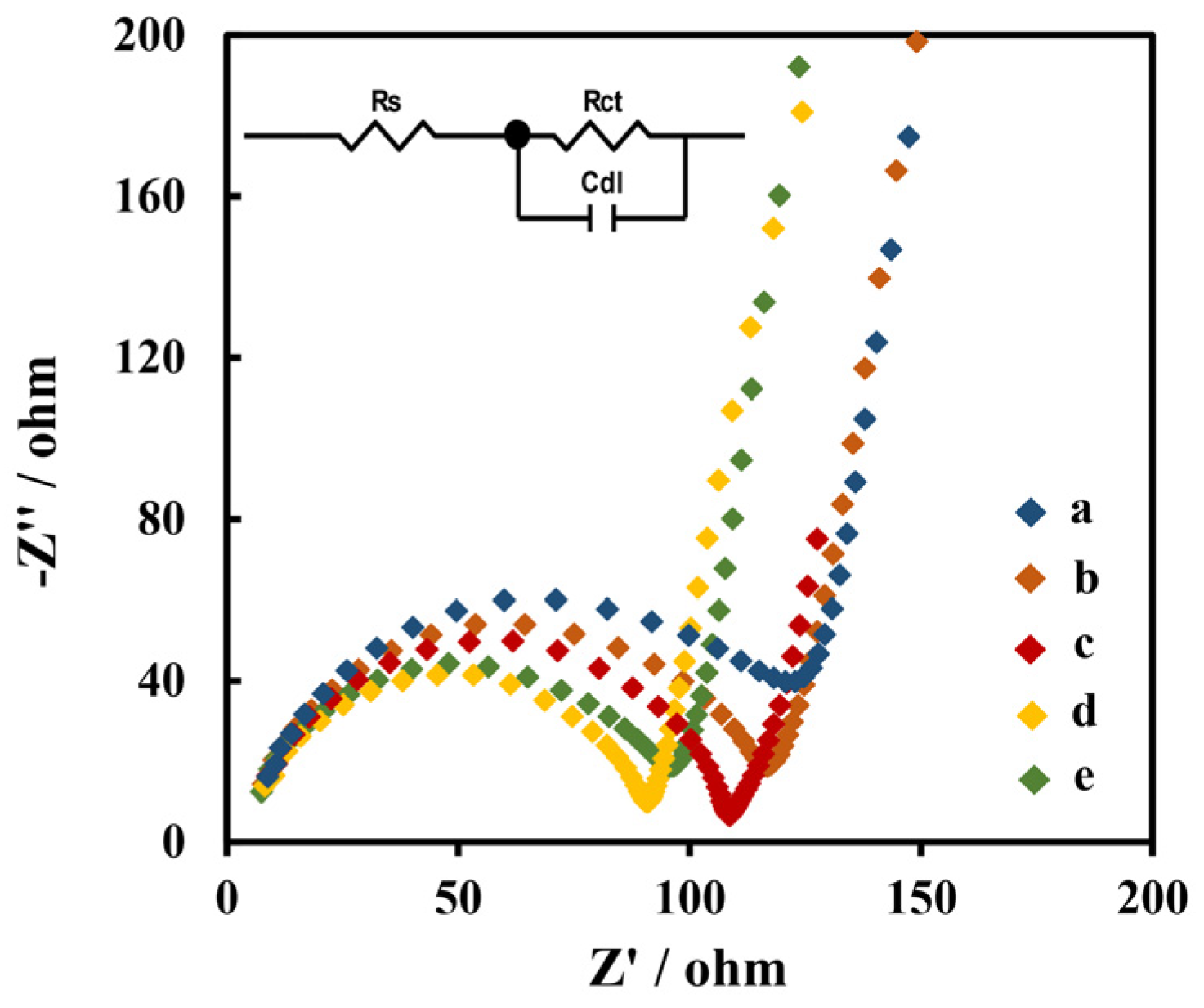
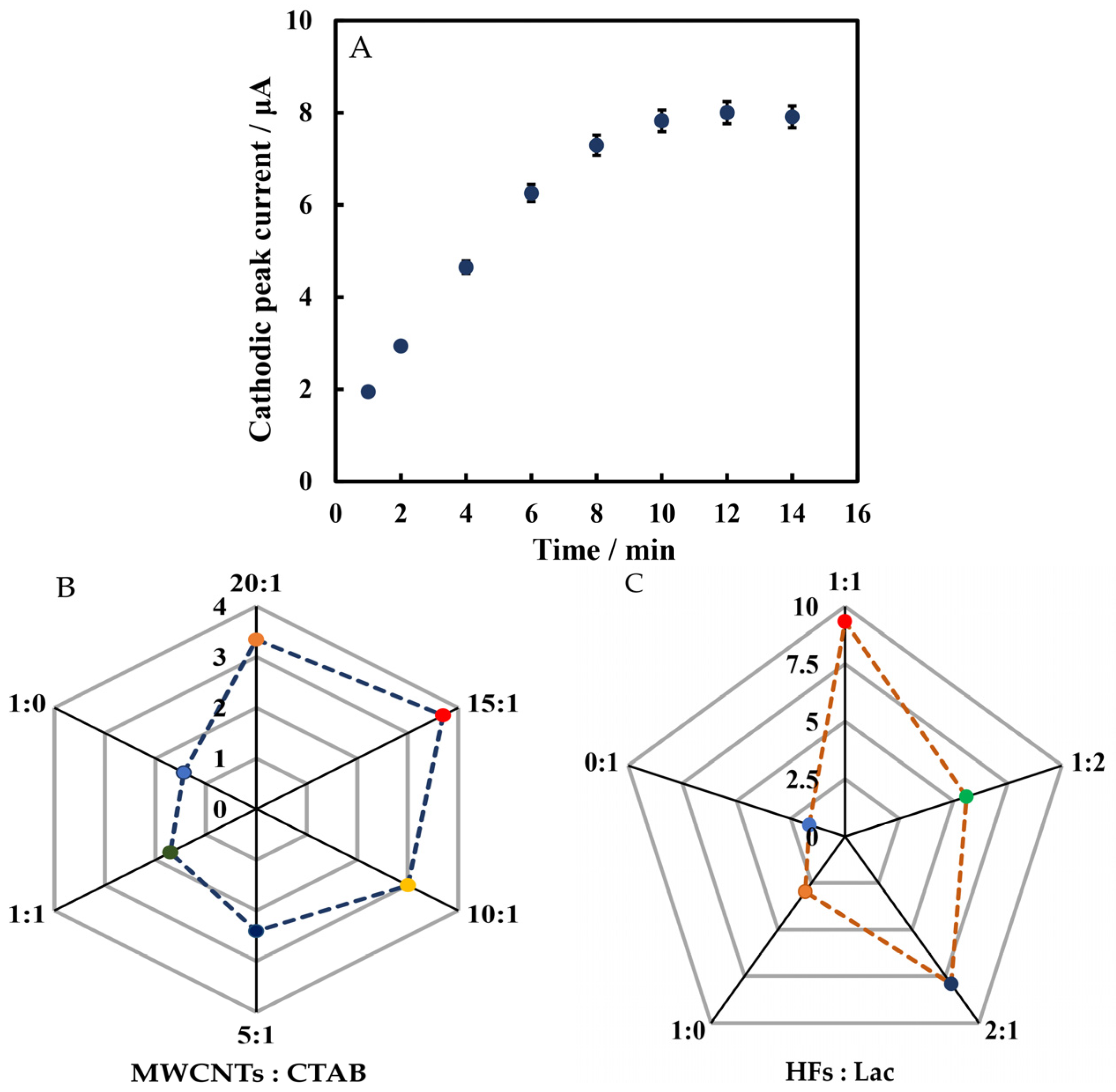
3.4. Optimization of Experimental Conditions
3.5. Calibration Plot of Rutin
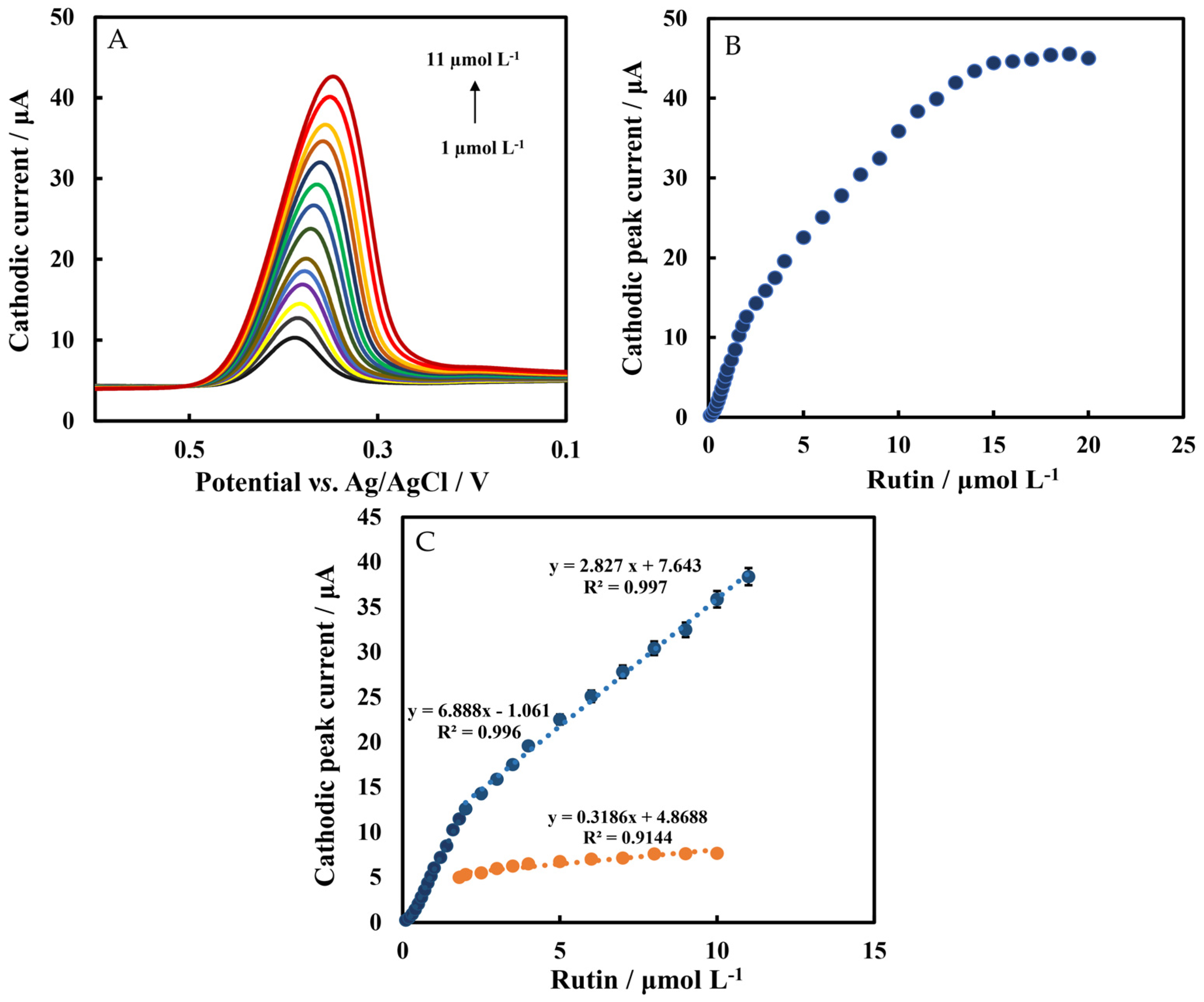
| Working Electrode | Liner Range (μmol L−1) | LOD (nmol L−1) | Sensitivity (μA μM−1 ) | Reference |
|---|---|---|---|---|
| Lac/GCE | 0.1–0.7 0.7–1.3 | -- | 5.85 2.47 | [14] |
| Ir NPs-IL-clay/PPO-Lac/CPE | 0.0917–3.1 | 30.9 | 4.26 | [17] |
| AuNPs-CD-Lac/CPE | 0.3–2.97 | 170 | 4.51 | [18] |
| MWCNTs-IL-Gel/GCE | 0.072–6 | 20 | 3.666 | [19] |
| MIPIL/IL-GR | 0.03–1 | 10 | 0.183 | [20] |
| Fe2.5Cr0.2Ce0.3O4-rGO/GCE | 0.075–12 | 52 | 2.988 | [21] |
| Chi-Lac-TPP-CPE | 0.599–3.92 5.82–13.1 | 0.0623 0.712 | 3.195 0.77 | [34] |
| MWCNTs-CD-Fe3O4/GCE | 0.02–10 | 16.4 | 1.24 | [51] |
| Gr/GNR | 0.032–0.1 | 7.86 | - | [56] |
| MIP/AuNPs-MoS2-GN/GCE | 0.01–45 | 4 | 1.146 | [57] |
| Chi/HFs-Lac/MWCNTs-CTAB/GCE | 0.1–2 2–11 | 30 95.5 | 6.888 2.827 | This work |
3.6. Anti-Interference Ability and Stability of Biosensor

| Interfering Substance | Ethanol | Glu | Vit C | Gly | UA |
|---|---|---|---|---|---|
| IF (%) | 0.03 | 0.01 | 0.03 | 0.03 | 0.02 |
3.7. Determination of Rutin in Tablets and Serum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Loescher, C.M.; Morton, D.W.; Razic, S.; Agatonovic-Kustrin, S. High performance thin layer chromatography (HPTLC) and high performance liquid chromatography (HPLC) for the qualitative and quantitative analysis of Calendula officinalis—Advantages and limitations. J. Pharm. Biomed. Anal. 2014, 98, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kraujalis, P.; Venskutonis, P.R.; Ibáñez, E.; Herrero, M. Optimization of rutin isolation from Amaranthus paniculatus leaves by high pressure extraction and fractionation techniques. J. Supercrit. Fluids 2015, 104, 234–242. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Das, M.K. Rutin-phospholipid complex in polymer matrix for long-term delivery of rutin via skin for the treatment of inflammatory diseases. Artif. Cells Nanomed. Biotechnol. 2018, 46, 41–56. [Google Scholar] [CrossRef]
- Paudel, K.R.; Wadhwa, R.; Tew, X.N.; Lau, N.J.X.; Madheswaran, T.; Panneerselvam, J.; Zeeshan, F.; Kumar, P.; Gupta, G.; Anand, K.; et al. Rutin loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Life Sci. 2021, 276, 119436. [Google Scholar] [CrossRef] [PubMed]
- Filipsky, T.; Riha, M.; Haskova, P.; Pilarova, V.; Novakova, L.; Semecky, V.; Vavrova, J.; Holeckova, M.; Palicka, V.; Simunek, T.; et al. Intravenous rutin in rat exacerbates isoprenaline-induced cardiotoxicity likely due to intracellular oxidative stress. Redox Rep. 2017, 22, 78–90. [Google Scholar] [CrossRef]
- Chen, I.L.; Tsai, Y.J.; Huang, C.M.; Tsai, T.H. Lymphatic absorption of quercetin and rutin in rat and their pharmacokinetics in systemic plasma. J. Agric. Food Chem. 2010, 58, 546–551. [Google Scholar] [CrossRef]
- Mohamed, S.; Youssef, A.; Issa, M.; Abdel Salam, H.; El-Ansary, A. Validated HPLC Method for Quantitative Analysis of Gallic Acid and Rutin in Leaves of Moringa Oleifera Grown in Egypt. Egypt. J. Chem. 2020, 64, 1583–1591. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Y.; Xu, X.; Sun, Y.; Li, Y.; Du, J. A covalent triazine framework as an oxidase mimetic in the luminol chemiluminescence system: Application to the determination of the antioxidant rutin. Microchim. Acta 2019, 187, 42. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.F.; Solangi, A.R.; Memon, S.Q.; Mallah, A.; Memon, N.; Memon, A.A. Simultaneous Determination of Quercetin, Rutin, Naringin, and Naringenin in Different Fruits by Capillary Zone Electrophoresis. Food Anal. Methods 2016, 10, 83–91. [Google Scholar] [CrossRef]
- Malinowski, S.; Wardak, C.; Jaroszynska-Wolinska, J.; Herbert, P.A.F.; Panek, R. Cold Plasma as an Innovative Construction Method of Voltammetric Biosensor Based on Laccase. Sensors 2018, 18, 4086. [Google Scholar] [CrossRef]
- Yang, X.; Long, J.; Sun, D. Highly-sensitive Electrochemical Determination of Rutin Using NMP-Exfoliated Graphene Nanosheets-Modified Electrode. Electroanalysis 2016, 28, 83–87. [Google Scholar] [CrossRef]
- Antoniazzi, C.; de Lima, C.A.; Marangoni, R.; de Castro, E.G.; Santana, E.R.; Spinelli, A. Molybdenum trioxide incorporated in a carbon paste as a sensitive device for bisphenol A monitoring. Microchem. J. 2020, 159, 105528. [Google Scholar] [CrossRef]
- Zapp, E.; Brondani, D.; Vieira, I.C.; Dupont, J.; Scheeren, C.W. Bioelectroanalytical Determination of Rutin Based on bi-Enzymatic Sensor Containing Iridium Nanoparticles in Ionic Liquid Phase Supported in Clay. Electroanalysis 2010, 23, 764–776. [Google Scholar] [CrossRef]
- Brugnerotto, P.; Silva, T.R.; Brondani, D.; Zapp, E.; Vieira, I.C. Gold Nanoparticles Stabilized in β-Cyclodextrin and Decorated with Laccase Applied in the Construction of a Biosensor for Rutin. Electroanalysis 2017, 29, 1031–1037. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Zhao, X.; Lu, X. Electrochemical behavior of rutin on a multi-walled carbon nanotube and ionic liquid composite film modified electrode. Colloids Surf. B 2010, 81, 344–349. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, J.; Zeng, Y.; Zhu, Y.; Wang, H.; Lei, X.; Huang, S.; Guo, L.; Li, L. Electrochemical determination of rutin based on molecularly imprinted poly (ionic liquid) with ionic liquid-graphene as a sensitive element. Sens. Actuators B Chem. 2020, 311, 127911. [Google Scholar] [CrossRef]
- Şenocak, A.; Korkmaz, E.; Khataee, A.; Demirbas, E. A facile and synergetic strategy for electrochemical sensing of rutin antioxidant by Ce–Cr doped magnetite@rGO. Mater. Chem. Phys. 2022, 275, 125298. [Google Scholar] [CrossRef]
- Zapp, E.; Westphal, E.; Gallardo, H.; de Souza, B.; Cruz Vieira, I. Liquid crystal and gold nanoparticles applied to electrochemical immunosensor for cardiac biomarker. Biosens. Bioelectron. 2014, 59, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Movahedifar, F.; Tajik, S.; Jahani, S. A Review on the Effects of Introducing CNTs in the Modification Process of Electrochemical Sensors. Electroanalysis 2018, 31, 1195–1203. [Google Scholar] [CrossRef]
- Niu, N.-N.; Zhao, W.-J.; Xiao, B.-L.; Liang, Y.-C.; Meng, X.; Song, X.-Y.; Li, D.; Hong, J.; Moosavi-Movahedi, A.A. Quench treatment cytochrome c: Transformation from a classical redox protein to a peroxidase like enzyme. J. Iran. Chem. Soc. 2021, 19, 2347–2357. [Google Scholar] [CrossRef]
- Yang, Y.J.; Guo, L.; Zhang, W. The electropolymerization of CTAB on glassy carbon electrode for simultaneous determination of dopamine, uric acid, tryptophan and theophylline. J. Electroanal. Chem. 2016, 768, 102–109. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Basu, S.; Aminabhavi, T.M. Versatile fullerenes as sensor materials. Mater. Today Chem. 2021, 20, 100454. [Google Scholar] [CrossRef]
- Brahman, P.K.; Pandey, N.; Topkaya, S.N.; Singhai, R. Fullerene-C60-MWCNT composite film based ultrasensitive electrochemical sensing platform for the trace analysis of pyruvic acid in biological fluids. Talanta 2015, 134, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Rashidi, A.; Kooti, M.; Askarieh, M.; Pourhashem, S.; Ghasemy, E.; Izadi, N. A novel highly sensitive and selective H2S gas sensor at low temperatures based on SnO2 quantum dots-C60 nanohybrid: Experimental and theory study. Talanta 2018, 188, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.M.; Ali, M.A.; Al-Hemaid, F.M. Palladium nanoparticles decorated on activated fullerene modified screen printed carbon electrode for enhanced electrochemical sensing of dopamine. J. Colloid Interface Sci. 2015, 448, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Lu, Z.; Gao, X. Syntheses, Structures and Antioxidant Activities of Fullerenols: Knowledge Learned at the Atomistic Level. J. Clust. Sci. 2015, 26, 375–388. [Google Scholar] [CrossRef]
- Cai, X.; Jia, H.; Liu, Z.; Hou, B.; Luo, C.; Feng, Z.; Li, W.; Liu, J. Polyhydroxylated fullerene derivative C60(OH)24 prevents mitochondrial dysfunction and oxidative damage in an MPP+-induced cellular model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 3622–3634. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.F.; Yang, T.; Yang, X.L.; Zhang, Y.S.; Xiao, B.L.; Hong, J.; Sheibani, N.; Ghourchian, H.; Hong, T.; Moosavi-Movahedi, A.A. Direct electrochemistry of glucose oxidase and glucose biosensing on a hydroxyl fullerenes modified glassy carbon electrode. Biosens. Bioelectron. 2014, 60, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. TrAC Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef]
- Fernandes, S.C.; de Oliveira, I.R.W.Z.; Fatibello-Filho, O.; Spinelli, A.; Vieira, I.C. Biosensor based on laccase immobilized on microspheres of chitosan crosslinked with tripolyphosphate. Sens. Actuators B-Chem. 2008, 133, 202–207. [Google Scholar] [CrossRef]
- Liu, L.; Anwar, S.; Ding, H.; Xu, M.; Yin, Q.; Xiao, Y.; Yang, X.; Yan, M.; Bi, H. Electrochemical sensor based on F,N-doped carbon dots decorated laccase for detection of catechol. J. Electroanal. Chem. 2019, 840, 84–92. [Google Scholar] [CrossRef]
- Bilir, K.; Weil, M.-T.; Lochead, J.; Kök, F.N.; Werner, T. Construction of an oxygen detection-based optic laccase biosensor for polyphenolic compound detection. Turk. J. Biol. 2016, 40, 1303–1310. [Google Scholar] [CrossRef]
- Ferry, Y.; Leech, D. Amperometric Detection of Catecholamine Neurotransmitters Using Electrocatalytic Substrate Recycling at a Laccase Electrode. Electroanalysis 2005, 17, 113–119. [Google Scholar] [CrossRef]
- Lou, C.; Jing, T.; Zhou, J.; Tian, J.; Zheng, Y.; Wang, C.; Zhao, Z.; Lin, J.; Liu, H.; Zhao, C.; et al. Laccase immobilized polyaniline/magnetic graphene composite electrode for detecting hydroquinone. Int. J. Biol. Macromol. 2020, 149, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Li, D.; Wei, Q. A laccase based biosensor on AuNPs-MoS2 modified glassy carbon electrode for catechol detection. Colloids Surf. B 2020, 186, 110683. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, A.C.; Vieira, I.C.; Dupont, J.; Scheeren, C.W.; de Oliveira, L.F. Biosensor for luteolin based on silver or gold nanoparticles in ionic liquid and laccase immobilized in chitosan modified with cyanuric chloride. Analyst 2009, 134, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Boubezari, I.; Bessueille, F.; Bonhomme, A.; Raimondi, G.; Zazoua, A.; Errachid, A.; Jaffrezic-Renault, N. Laccase-Based Biosensor Encapsulated in a Galactomannan-Chitosan Composite for the Evaluation of Phenolic Compounds. Biosensors 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Villanueva, G.E.; Luna-Moreno, D.; Blanco-Gamez, E.A.; Rodriguez-Delgado, J.M.; Villarreal-Chiu, J.F.; Rodriguez-Delgado, M.M. A Novel Enzyme-Based SPR Strategy for Detection of the Antimicrobial Agent Chlorophene. Biosensors 2021, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Santana, E.R.; Martins, E.C.; Spinelli, A. Electrode modified with nitrogen-doped graphene quantum dots supported in chitosan for triclocarban monitoring. Microchem. J. 2021, 167, 106297. [Google Scholar] [CrossRef]
- Alvarado-Ramirez, L.; Rostro-Alanis, M.; Rodriguez-Rodriguez, J.; Sosa-Hernandez, J.E.; Melchor-Martinez, E.M.; Iqbal, H.M.N.; Parra-Saldivar, R. Enzyme (Single and Multiple) and Nanozyme Biosensors: Recent Developments and Their Novel Applications in the Water-Food-Health Nexus. Biosensors 2021, 11, 410. [Google Scholar] [CrossRef]
- Sherigara, B.S.; Kutner, W.; D’Souza, F. Electrocatalytic Properties and Sensor Applications of Fullerenes and Carbon Nanotubes. Electroanalysis 2003, 15, 735–772. [Google Scholar] [CrossRef]
- Wu, X.; Yang, S.T.; Wang, H.; Wang, L.; Hu, W.; Cao, A.; Liu, Y. Influences of the size and hydroxyl number of fullerenes/fullerenols on their interactions with proteins. J. Nanosci. Nanotechnol. 2010, 10, 6298–6304. [Google Scholar] [CrossRef] [PubMed]
- Araujo, K.C.; de M. B. Costa, E.M.; Pazini, F.; Valadares, M.C.; de Oliveira, V. Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products. Food Chem. Toxicol. 2013, 51, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.N.; Xiao, B.L.; Niu, N.N.; Moosavi-Movahedi, A.A.; Hong, J. Glucose Oxidase Immobilized on a Functional Polymer Modified Glassy Carbon Electrode and Its Molecule Recognition of Glucose. Polymers 2019, 11, 115. [Google Scholar] [CrossRef]
- Al-Qurain, A.A.; Williams, D.B.; Mackenzie, L.; Roberts, M.S.; Wiese, M.D. Simultaneous LC-MS/MS quantification of oxycodone, tramadol and fentanyl and their metabolites (noroxycodone, oxymorphone, O-desmethyltramadol, N-desmethyltramadol, and norfentanyl) in human plasma and whole blood collected via venepuncture and volumetric absorptive micro sampling. J. Pharm. Biomed. Anal. 2021, 203, 114171. [Google Scholar]
- Karabiberoğlu, Ş.U.; Koçak, Ç.C.; Dursun, Z. An over-oxidized poly(Rutin) modified electrode for selective and sensitive determination of catechol and hydroquinone. J. Electroanal. Chem. 2019, 850, 113415. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Xiao, B.-L.; Song, X.-Y.; Meng, X.; Ma, X.-X.; Li, Y.-Y.; Hong, J.; Moosavi-Movahedi, A.A. A Highly Sensitive Electrochemical Sensor Based on β-cyclodextrin Functionalized Multi-Wall Carbon Nanotubes and Fe3O4 Nanoparticles for Rutin Detection. J. Electrochem, Soc. 2022, 169, 047509. [Google Scholar] [CrossRef]
- Yang, X.-L.; Zhang, Y.-S.; Yang, T.; Geng, F.-Y.; Li, D.; Xiao, B.-L.; Hong, J.; Moosavi-Movahedi, A.A.; Ghourchian, H. A soft-template nanostructured peroxidase based on cytochrome c and sodium decyl sulfate and its electrochemical properties on hydroxyl fullerenes modified glassy carbon electrode. J. Iran. Chem. Soc. 2015, 13, 471–479. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, Q.; Li, L.; Wang, Y.; Ye, B. A new voltammetric sensor and its application in pharmaceutical analysis for rutin. J. Environ. Sci. Health A 2020, 55, 837–846. [Google Scholar] [CrossRef]
- Martins, L.O.; Durao, P.; Brissos, V.; Lindley, P.F. Laccases of prokaryotic origin: Enzymes at the interface of protein science and protein technology. Cell. Mol. Life Sci. 2015, 72, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Taraba, A.; Szymczyk, K. Quercetin and rutin mixtures with alcohols: Spectroscopic and conductometric studies. J. Mol. Struct. 2020, 1203, 127464. [Google Scholar] [CrossRef]
- Swamy, N.K.; Mohana, K.N.S.; Hegde, M.B.; Madhusudana, A.M.; Rajitha, K.; Nayak, S.R. Fabrication of graphene nanoribbon-based enzyme-free electrochemical sensor for the sensitive and selective analysis of rutin in tablets. J. Appl. Electronchem. 2021, 51, 1047–1057. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Tang, Y.; Zhao, F.; Zeng, B. Fabrication and application of a rutin electrochemical sensor based on rose-like AuNPs-MoS2-GN composite and molecularly imprinted chitosan. Microchem. J. 2021, 168, 106505. [Google Scholar] [CrossRef]


| Concentration (μmol L−1) | Biosensor (μmol L−1) | Recovery (%) | RSD (%) | UHPLC (μmol L−1) | Recovery (%) |
|---|---|---|---|---|---|
| 1 | 0.98 | 98 | 1.14 | 0.98 | 98 |
| 5 | 4.84 | 96.8 | 2.76 | 4.91 | 98.2 |
| 10 | 9.86 | 98.6 | 2.37 | 9.83 | 98.3 |
| Concentration (μmol L−1) | Biosensor (μmol L−1) | Recovery (%) | RSD (%) | UHPLC (μmol L−1) | Recovery (%) |
|---|---|---|---|---|---|
| 0.5 | 0.472 | 94.4 | 2.69 | 0.515 | 103 |
| 1 | 1.03 | 103 | 2.14 | 1.15 | 115 |
| 2.5 | 2.44 | 97.7 | 1.88 | 2.64 | 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.-Y.; Meng, X.; Xiao, B.-L.; Li, Y.-Y.; Ma, X.-X.; Moosavi-Movahedi, A.A.; Hong, J. MWCNTs-CTAB and HFs-Lac Nanocomposite-Modified Glassy Carbon Electrode for Rutin Determination. Biosensors 2022, 12, 632. https://doi.org/10.3390/bios12080632
Song X-Y, Meng X, Xiao B-L, Li Y-Y, Ma X-X, Moosavi-Movahedi AA, Hong J. MWCNTs-CTAB and HFs-Lac Nanocomposite-Modified Glassy Carbon Electrode for Rutin Determination. Biosensors. 2022; 12(8):632. https://doi.org/10.3390/bios12080632
Chicago/Turabian StyleSong, Xin-Yan, Xin Meng, Bao-Lin Xiao, Yang-Yang Li, Xin-Xin Ma, Ali Akbar Moosavi-Movahedi, and Jun Hong. 2022. "MWCNTs-CTAB and HFs-Lac Nanocomposite-Modified Glassy Carbon Electrode for Rutin Determination" Biosensors 12, no. 8: 632. https://doi.org/10.3390/bios12080632
APA StyleSong, X.-Y., Meng, X., Xiao, B.-L., Li, Y.-Y., Ma, X.-X., Moosavi-Movahedi, A. A., & Hong, J. (2022). MWCNTs-CTAB and HFs-Lac Nanocomposite-Modified Glassy Carbon Electrode for Rutin Determination. Biosensors, 12(8), 632. https://doi.org/10.3390/bios12080632






