Recent Advances in Fluorescent Methods for Polyamine Detection and the Polyamine Suppressing Strategy in Tumor Treatment
Abstract
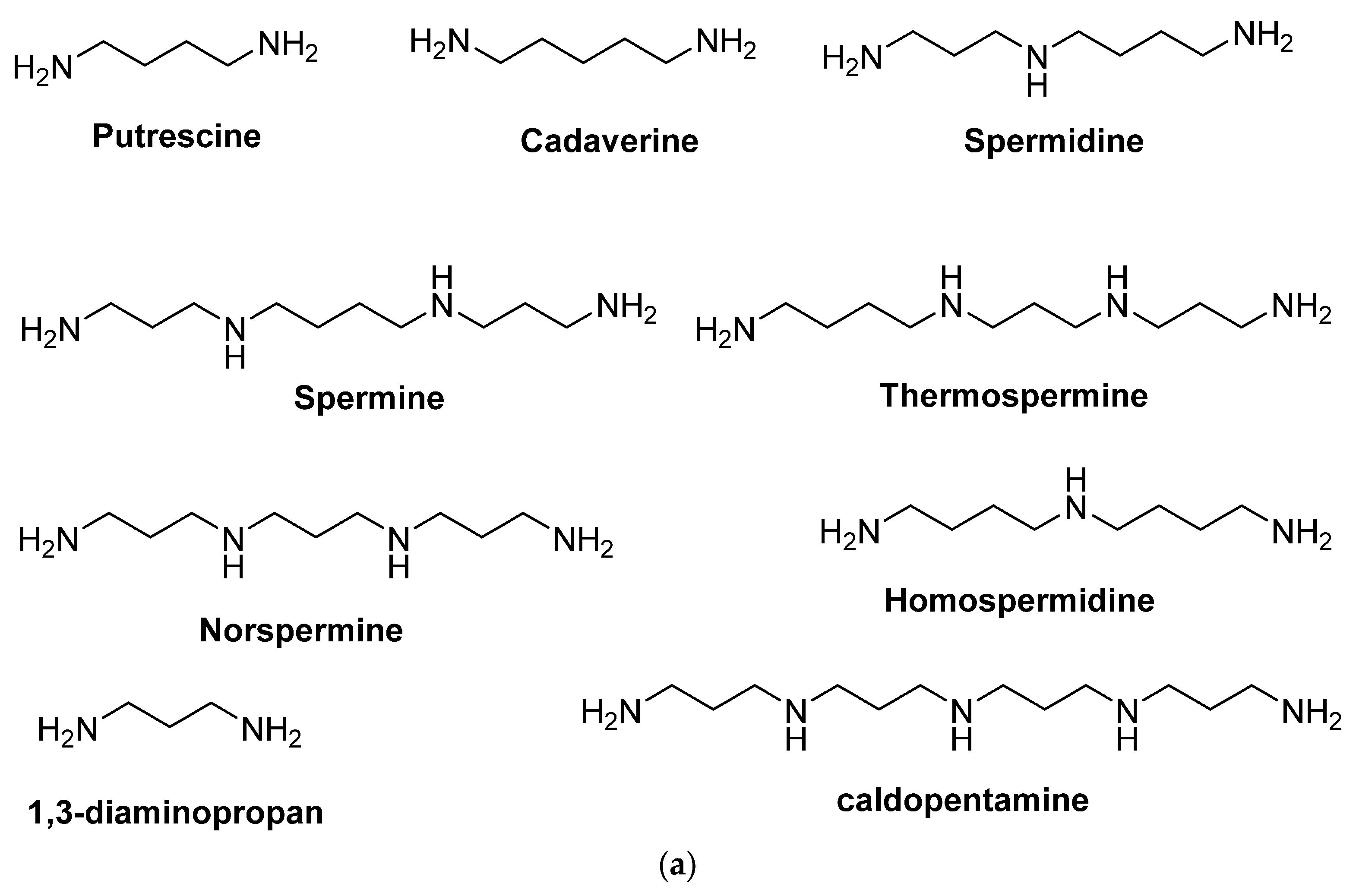
:1. Introduction
2. Polyamine Detection Methods
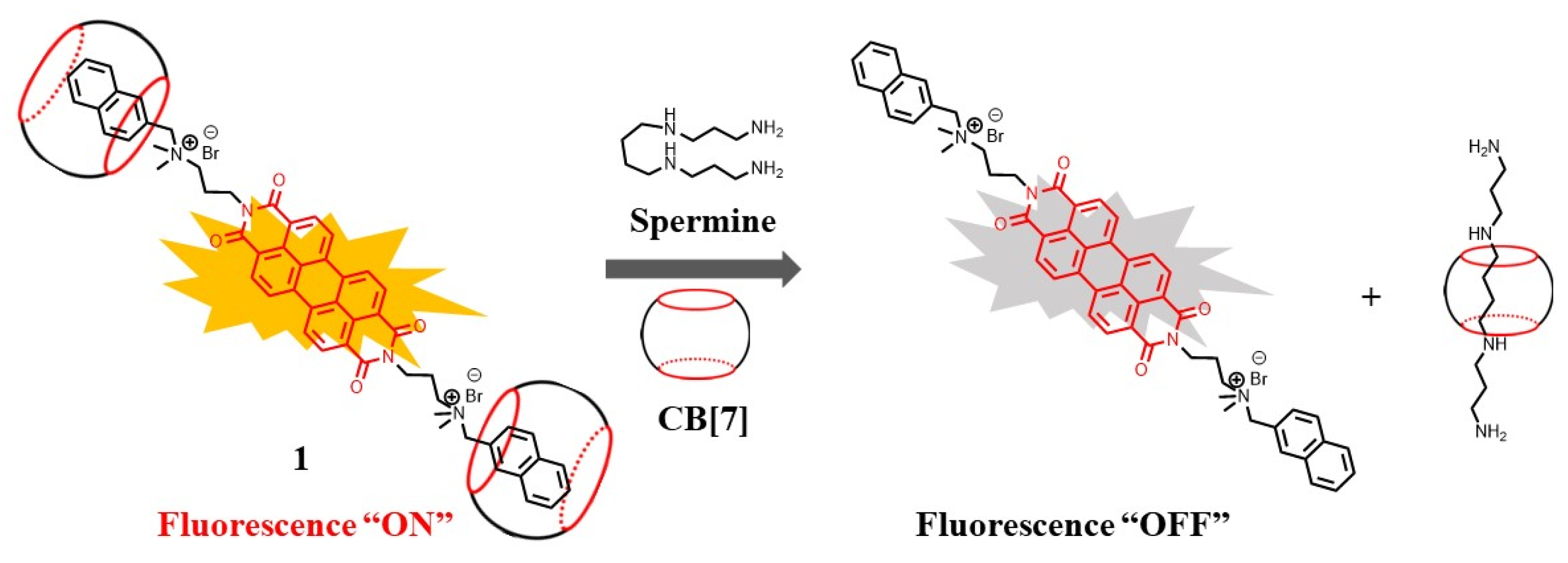
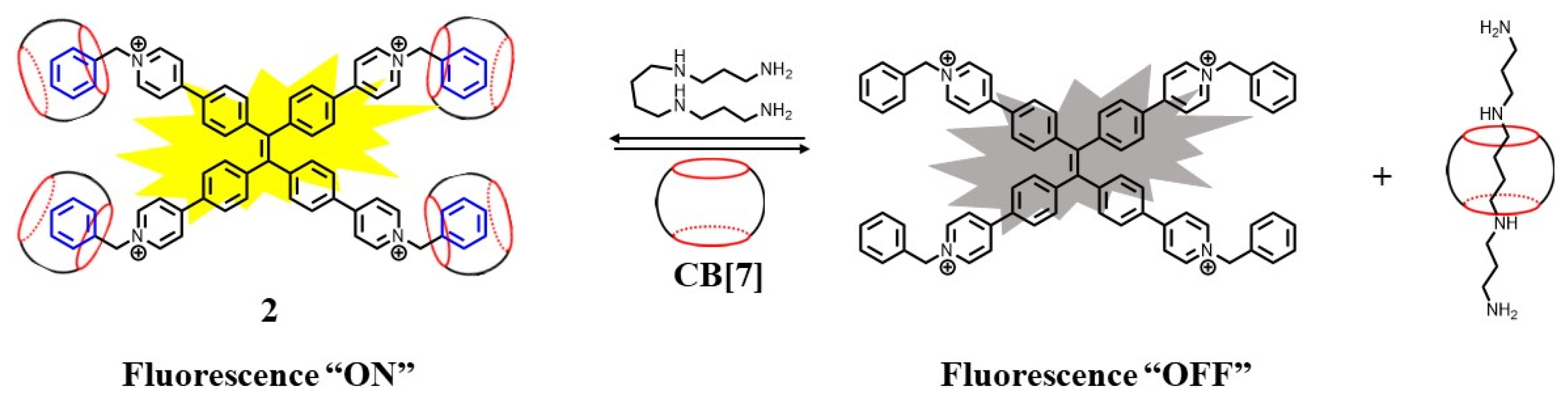
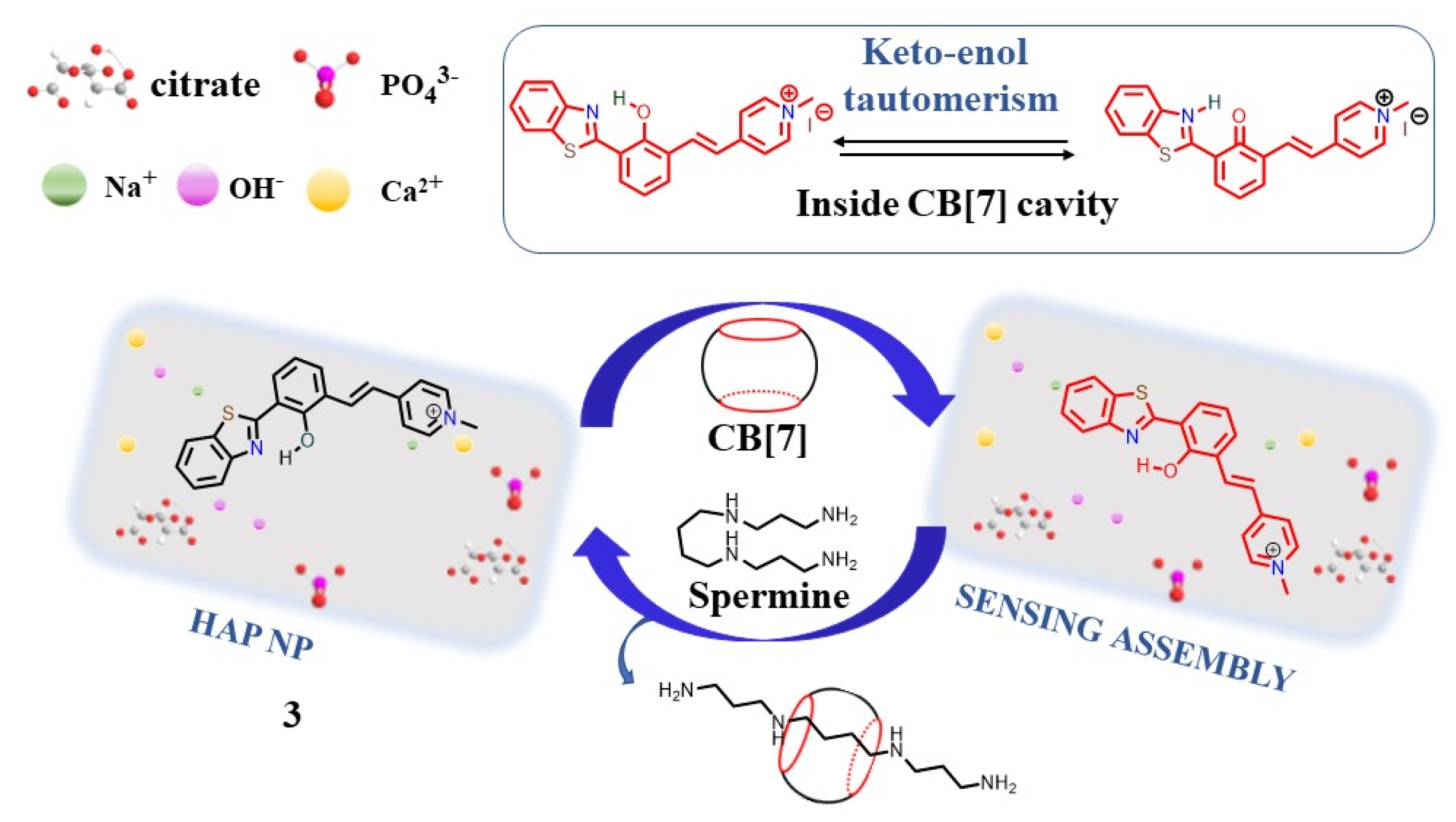
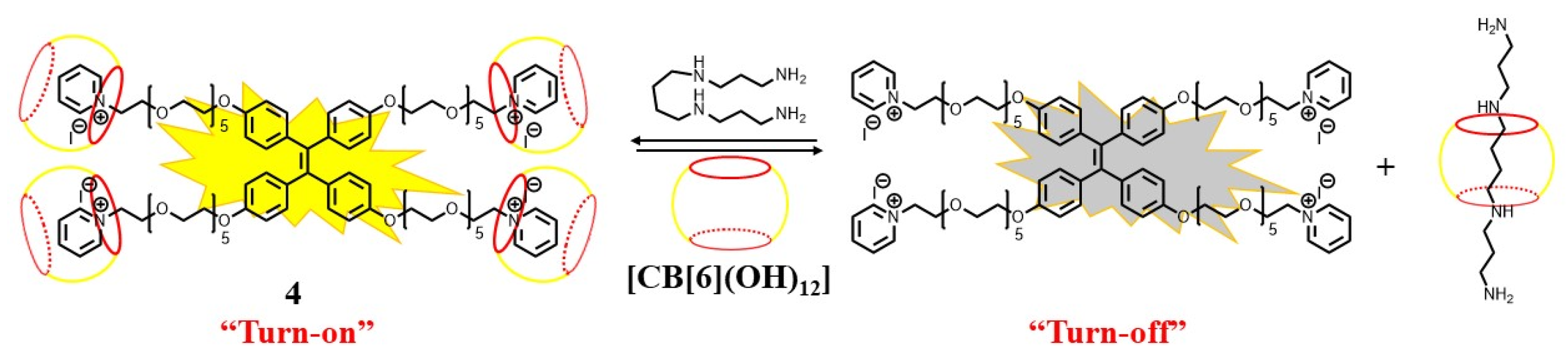
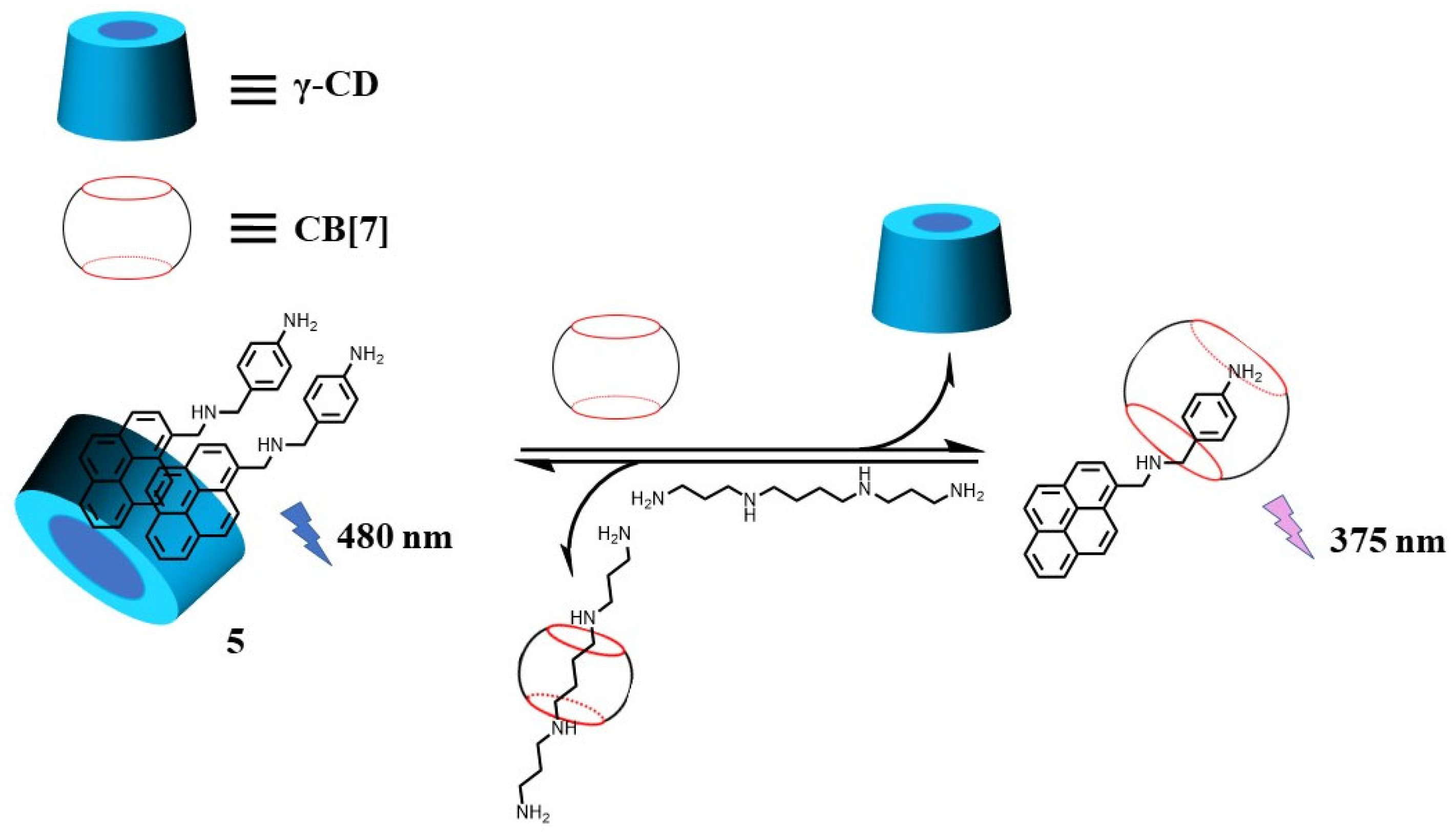
2.1. Supramolecular Sensing System for Polyamine Detection
2.2. Polyamine Detection Based on Chromophore Reaction
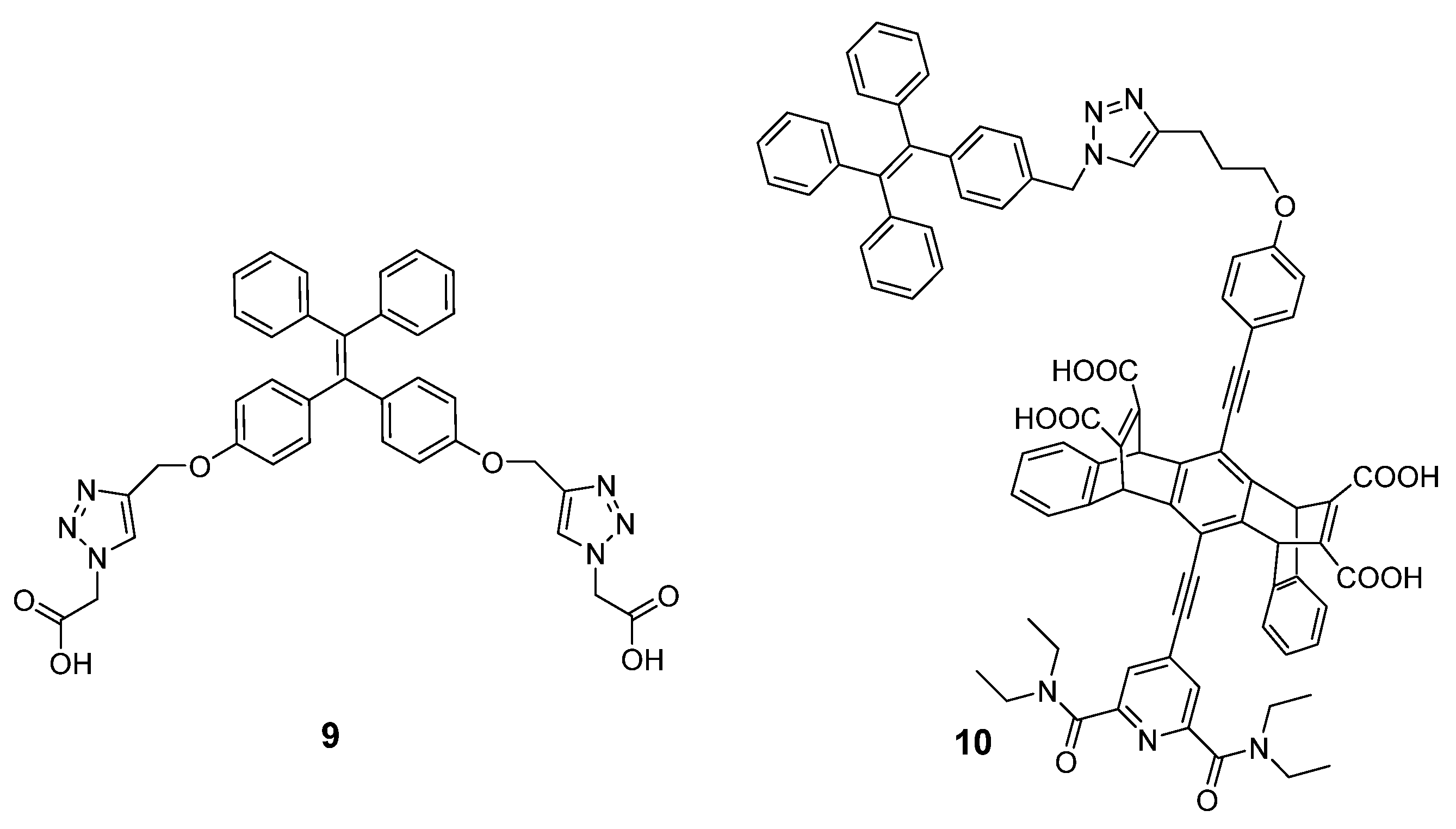
2.3. Fluorescent Small Molecules for Polyamine Detection
2.4. Fluorescent Nanoparticles for Polyamine Detection
3. Tumor Polyamine-Suppressing Strategy
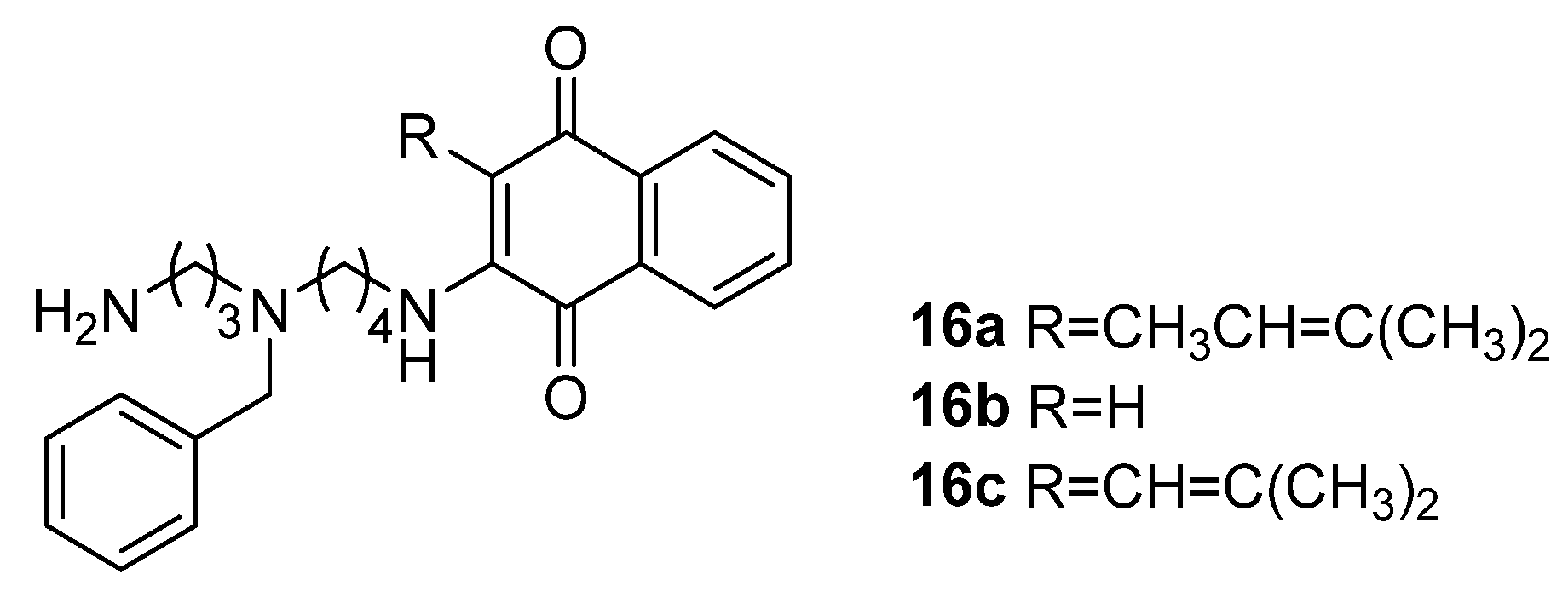
3.1. Small Molecules Target Polyamine Metabolism
3.1.1. Polyamine Analogs
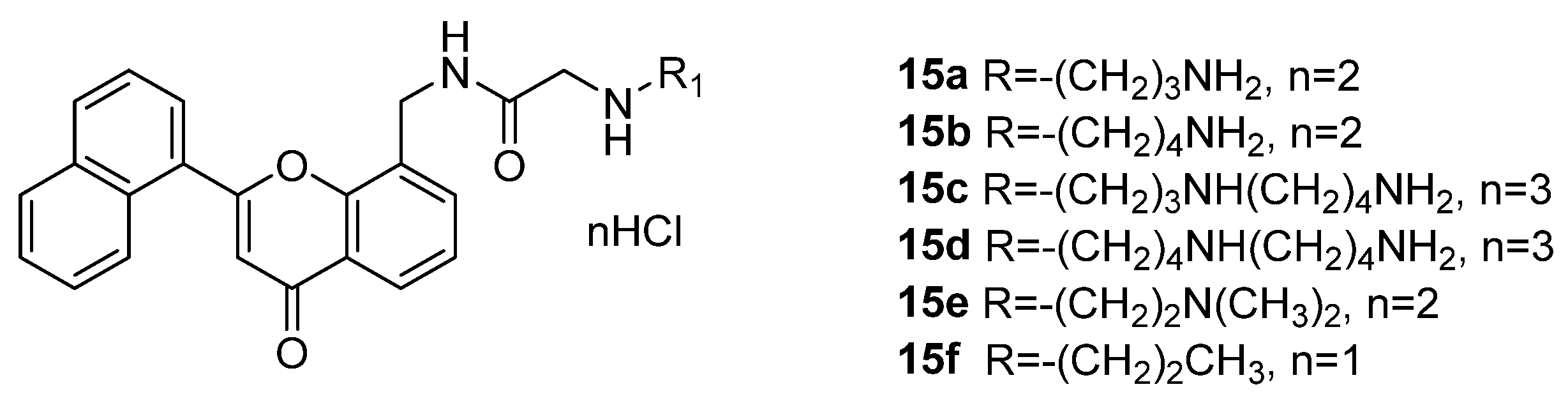
3.1.2. Polyamine Conjugates
3.2. Combinations Target Multiple Components
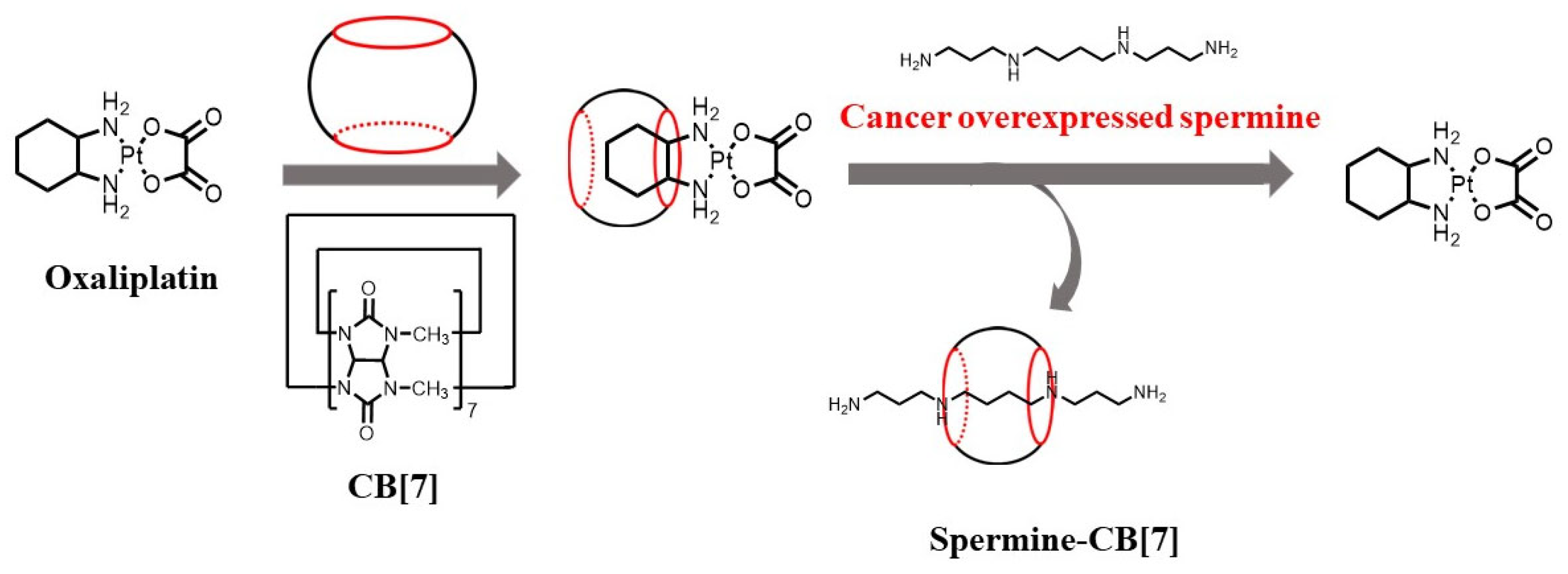
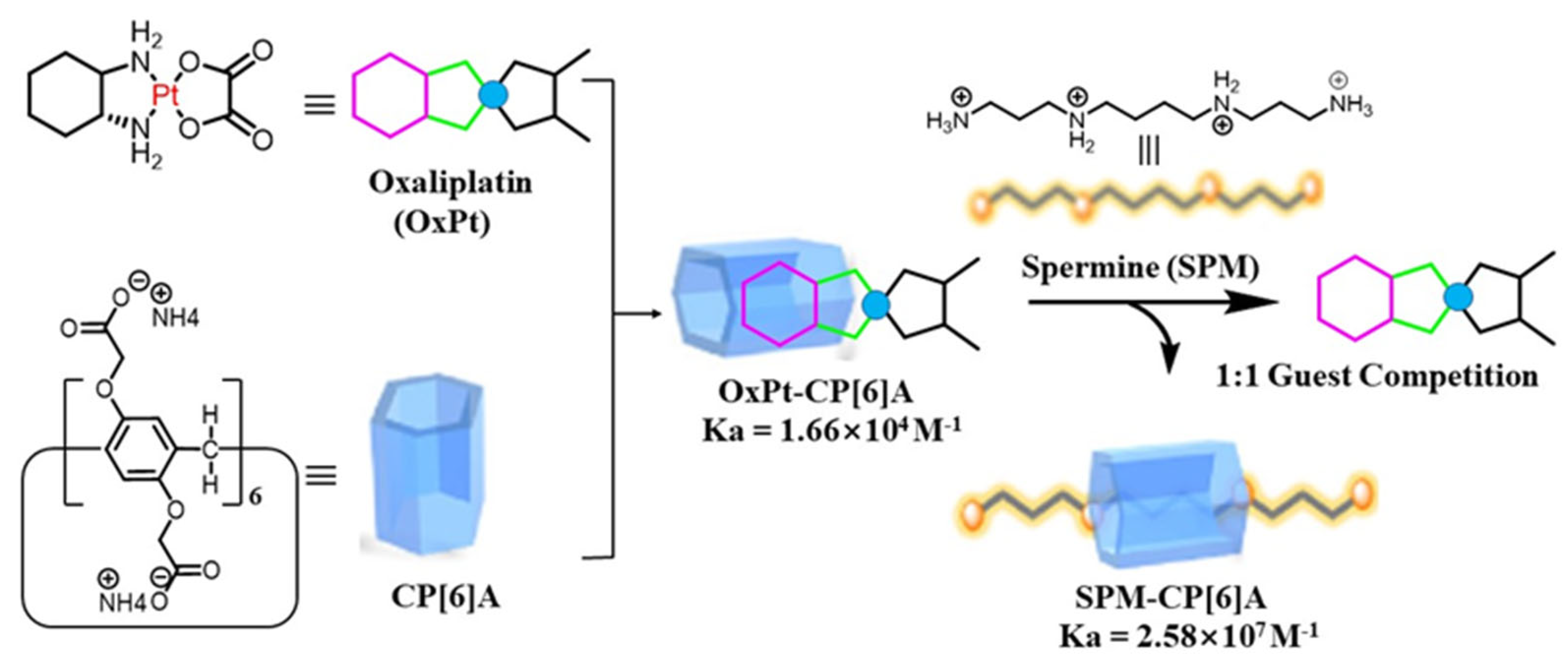
3.2.1. Spermine-Responsive Supramolecular Chemotherapy
3.2.2. Combination of Polyamine Consumption and Photodynamic Therapy (PDT)
4. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | Polyamines |
| ELSA | Enzyme-linked immunosorbent assay |
| GC-MS | Gas chromatography-mass spectrometry |
| HPLC | High-performance liquid chromatography |
| LC-MS | Liquid chromatography-mass spectrometry |
| ADA | Antibody-dependent assay |
| NMR | Nuclear magnetic resonances |
| OFP | Organic fluorescent probe |
| CB[7] | Cucurbit[7]uril |
| ODC | Ornithine decarboxylase |
| DFMO | α-difluoromethylornithine |
| AMD1 | S-adenosylmethionine decarboxylase 1 |
| SMOX | Spermine oxidase |
| PTS | Polyamine transport systems |
| ROS | Reactive oxygen species |
| Topo2α | Topoisomerase II-α |
| DOX | Doxorubicin hydrochloride |
| Oxpt | Oxaliplatin |
| PDT | Photodynamic therapy |
References
- Pegg, A.E. Toxicity of Polyamines and Their Metabolic Products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef]
- Jastrzab, R.; Kaczmarek, M.T.; Nowak, M.; Trojanowska, A.; Zabiszak, M. Complexes of Polyamines and Their Derivatives as Living System Active Compounds. Coord. Chem. Rev. 2017, 351, 32–44. [Google Scholar] [CrossRef]
- Minois, N. Molecular Basis of the ’Anti-Aging’ Effect of Spermidine and Other Natural Polyamines—A Mini-Review. Gerontology 2014, 60, 319–326. [Google Scholar] [CrossRef]
- Nichugovskiy, A.; Tron, G.C.; Maslov, M. Recent Advances in the Synthesis of Polyamine Derivatives and Their Applications. Molecules 2021, 26, 6579. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.R.; Woster, P.M.; Casero, R.A. Targeting Polyamine Metabolism for Cancer Therapy and Prevention. Biochem. J. 2016, 473, 2937–2953. [Google Scholar] [CrossRef]
- Terui, Y.; Yoshida, T.; Sakamoto, A.; Saito, D.; Oshima, T.; Kawazoe, M.; Yokoyama, S.; Igarashi, K.; Kashiwagi, K. Polyamines Protect Nucleic Acids against Depurination. Int. J. Biochem. Cell Biol. 2018, 99, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Esumi, M.; Sakurai, S.; Tanaka, M. The Effect of Spermidine on Guanine Decomposition Via Photoinduced Electron Transfer in DNA. Org. Biomol. Chem. 2019, 18, 47–51. [Google Scholar] [CrossRef]
- Kurata, H.T.; Marton, L.J.; Nichols, C.G. The Polyamine Binding Site in Inward Rectifier K+ Channels. J. Gen. Physiol. 2006, 127, 467–480. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Marton, L.J. Targeting Polyamine Metabolism and Function in Cancer and Other Hyperproliferative Diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Francis, S. Influence of Polyamines on Membrane Functions. Biochem. J. 1989, 260, 1–10. [Google Scholar]
- Nowotarski, S.L.; Woster, P.M.; Casero, R.A. Polyamines and Cancer: Implications for Chemotherapy and Chemoprevention. Expert Rev. Mol. Med. 2013, 15, e3. [Google Scholar] [CrossRef] [PubMed]
- Veen-van, S.; Martin, S.; Benoy, V.; Lyons, J.; Vanhoutte, R.; Kahler, J.P.; Gelders, G.; Lambie, E.; Zielich, J.; Annaert, W.; et al. Atp13a2 Deficiency Disrupts Lysosomal Polyamine Export. Nature 2020, 578, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. The Mechanisms by Which Polyamines Accelerate Tumor Spread. J. Exp. Clin. Cancer Res. 2011, 30, 95. [Google Scholar] [CrossRef] [PubMed]
- Uehara, N.; Shirakawa, S.; Uchino, H.; Saeki, Y. Elevated Contents of Spermidine and Spermine in the Erythrocytes of Cancer Patients. Cancer 1980, 45, 108–111. [Google Scholar] [CrossRef]
- Bassiri, H.; Benavides, A.; Haber, M.; Gilmour, S.K.; Norris, M.D.; Hogarty, M.D. Translational Development of Difluoromethylornithine (Dfmo) for the Treatment of Neuroblastoma. Transl. Pediatr. 2015, 4, 226–238. [Google Scholar]
- Gerner, E.W.; Bruckheimer, E.; Cohen, A. Cancer Pharmacoprevention: Targeting Polyamine Metabolism to Manage Risk Factors for Colon Cancer. J. Biol. Chem. 2018, 293, 18770–18778. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, F.; Medina, M.A.; Villalobos-Rueda, L.; Urdiales, J.L. Polyamines in Mammalian Pathophysiology. Cell. Mol. Life Sci. 2019, 76, 3987–4008. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, T.J. Polyamine Metabolism and Cancer. J. Cell. Mol. Med. 2003, 7, 113–126. [Google Scholar] [CrossRef]
- Matsuoka, A.; Sakamoto, T. [Establishment of an Elisa System of N1,N12-Diacetylspermine in Human Urine]. Rinsho Byori Jpn. J. Clin. Pathol. 2004, 52, 328–331. [Google Scholar]
- Yu, Z.; Huang, H.; Zhang, H.; Kessler, B.M. Improved Profiling of Polyamines Using Two-Dimensional Gas Chromatography Mass Spectrometry. Talanta 2019, 199, 184–188. [Google Scholar] [CrossRef]
- Balcerzak, W.; Pokajewicz, K.; Wieczorek, P. A Useful Procedure for Detection of Polyamines in Biological Samples as a Potential Diagnostic Tool in Cancer Diagnosis. Appl. Cancer Res. 2017, 37, 23. [Google Scholar] [CrossRef]
- Venlinen, M.; Roine, A.N.; Hkkinen, M.; Vepslinen, J.; Rantanen, T.K. Altered Polyamine Profiles in Colorectal Cancer. Anticancer Res. 2018, 38, 3601–3607. [Google Scholar] [CrossRef]
- Takahashi, Y.; Horio, H.; Sakaguchi, K.; Hiramatsu, K.; Kawakita, M. Significant Correlation between Urinary N1, N12-Diacetylspermine and Tumor Invasiveness in Patients with Clinical Stage Ia Non-Small Cell Lung Cancer. BMC Cancer 2015, 15, 65. [Google Scholar] [CrossRef]
- Patin, F.; Corcia, P.; Vourc’h, P.; Baranek, T.; Goossens, J.F.; Marouillat, S.; Dessein, A.F.; Descat, A.; Bruno, C.; Leman, S.; et al. Omics to Explore Amyotrophic Lateral Sclerosis Evolution: The Central Role of Arginine and Proline Metabolism. Mol. Neurobiol. 2017, 54, 5361–5374. [Google Scholar] [CrossRef]
- Nohta, H.; Satozono, H.; Koiso, K.; Yoshida, H.; Ishida, J.; Yamaguchi, M. Highly Selective Fluorometric Determination of Polyamines Based on Intramolecular Excimer-Forming Derivatization with a Pyrene-Labeling Reagent. Anal. Chem. 2000, 72, 4199–4204. [Google Scholar] [CrossRef]
- Lee, B.; Scopelliti, R.; Severin, K. A Molecular Probe for the Optical Detection of Biogenic Amines. Chem. Commun. 2011, 47, 9639–9641. [Google Scholar] [CrossRef]
- Nakamura, M.; Sanji, T.; Tanaka, M. Fluorometric Sensing of Biogenic Amines with Aggregation-Induced Emission-Active Tetraphenylethenes. Chem.-Eur. J. 2011, 17, 5344–5349. [Google Scholar] [CrossRef]
- Singh, G.; Mangat, S.; Sharma, H.; Singh, J.; Arora, A.; Singh Pannu, A.; Singh, N. Design and Syntheses of Novel Fluorescent Organosilicon-Based Chemosensors through Click Silylation: Detection of Biogenic Amines. RSC Adv. 2014, 4, 36834. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Bruck, B.S. Spermine Detection Via Metal-Mediated Ethynylarene ’Turn-on’ Fluorescence Signaling. Sens. Actuators B 2015, 207, 843–848. [Google Scholar] [CrossRef]
- Satrijo, A.; Swager, T.M. Anthryl-Doped Conjugated Polyelectrolytes as Aggregation-Based Sensors for Nonquenching Multicationic Analytes. J. Am. Chem. Soc. 2007, 129, 16020–16028. [Google Scholar] [CrossRef]
- Bao, B.; Yuwen, L.; Zheng, X.; Weng, L.; Zhu, X.; Zhan, X.; Wang, L. A Fluorescent Conjugated Polymer for Trace Detection of Diamines and Biogenic Polyamines. J. Mater. Chem. B 2010, 20, 9628–9634. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhong, D.L.; Cheng, Z.H. Calf Thymus DNA-Stabilized Polythiophene Fluorescence Probe for Label-Free Detection of Spermine. Analyst 2012, 137, 5565–5570. [Google Scholar] [CrossRef]
- Malik, A.H.; Hussain, S.; Iyer, P.K. Aggregation-Induced Fret Via Polymer-Surfactant Complexation: A New Strategy for the Detection of Spermine. Anal. Chem. 2016, 88, 7358–7363. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, X.; Zhang, Y.; Che, Y.; Zhao, J. Detection of Amines with Fluorescent Nanotubes: Applications in the Assessment of Meat Spoilage. ACS Sens. 2015, 1, 22–25. [Google Scholar] [CrossRef]
- Ikeda, M.; Yoshii, T.; Matsui, T.; Tanida, T.; Hamachi, I. Montmorillonite-Supramolecular Hydrogel Hybrid for Fluorocolorimetric Sensing of Polyamines. J. Am. Chem. Soc. 2011, 133, 1670–1673. [Google Scholar] [CrossRef]
- Koestereli, Z.; Severin, K. Fluorescence Sensing of Spermine with a Frustrated Amphiphile. Chem. Commun. 2012, 48, 5841–5843. [Google Scholar] [CrossRef]
- Tu, J.; Sun, S.; Xu, Y. A Novel Self-Assembled Platform for Ratiometric Fluorescent Detection of Spermine. Chem. Commun. 2015, 52, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Singh, J.; Kaur, H.; Singh, H.; Singh, N.; Kaur, N. Selective Chemosensing of Spermidine Based on Fluorescent Organic Nanoparticles in Aqueous Media Via a Fe3+ Displacement Assay. New J. Chem. 2015, 39, 3507–3512. [Google Scholar] [CrossRef]
- Kim, T.I.; Park, J.; Kim, Y. A Gold Nanoparticle-Based Fluorescence Turn-on Probe for Highly Sensitive Detection of Polyamines. Chem.-Eur. J. 2011, 17, 11978–11982. [Google Scholar] [CrossRef]
- Dan, Y.; Liu, J.J.; Zhi, Z.H.; Wang, N.; Zou, H.Y.; Huang, C.Z.; Wang, J. Highly Selective Detection of Spermine in Human Urine Via a Nanometal Surface Energy Transfer Platform. Talanta 2018, 188, 218–224. [Google Scholar]
- Bhamore, J.R.; Murthy, P.; Kailasa, S.K. Fluorescence Turn-Off Detection of Spermine in Biofluids Using Pepsin Mediated Synthesis of Gold Nanoclusters as a Probe. J. Mol. Liq. 2019, 280, 18–24. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Shim, J.; Biechele-Speziale, D.; Sharipov, M.; Lee, Y.I. Novel “Turn Off-on” Sensors for Highly Selective and Sensitive Detection of Spermine Based on Heparin-Quenching of Fluorescence CdTe Quantum Dots-Coated Amphiphilic Thiophene Copolymers. Sens. Actuators B 2018, 257, 734–744. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, H.; Li, Z.H.; Xu, L.; Wang, L.; Cao, D. Bio-Inspired AIE Pillar[5]Arene Probe with Multiple Binding Sites to Discriminate Alkanediamines. Chem. Commun. 2021, 57, 13114–13117. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, T. Analyte-Directed Formation of Emissive Excimers for the Selective Detection of Polyamines. Chem. Commun. 2016, 52, 10648. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhu, W.; Chen, Q.; Li, X.; Zhang, G. Selective Fluorescent Probes for Spermine and 1-Adamantanamine Based on the Supramolecular Structure Formed between AIE-Active Molecule and Cucurbit[N]Urils. Sens. Actuators B 2018, 261, 602. [Google Scholar] [CrossRef]
- Bhosle, A.; Banerjee, M.; Barooah, N.; Bhasikuttan, A.C.; Kadu, K.; Ramanan, S.R.; Chatterjee, A. ESIPT-Active Hydroxybenzothiazole-Picolinium@Cb[7]-HAP Nps Based Supramolecular Sensing Assembly for Spermine, Spermidine and Cadaverine: Application in Monitoring Cancer Biomarkers and Food Spoilage. J. Photochem. Photobiol. A 2022, 426, 113770. [Google Scholar] [CrossRef]
- Naik, V.G.; Kumar, V.; Bhasikuttan, A.C.; Kadu, K.; Ramanan, S.R.; Bhosle, A.A.; Banerjee, M.; Chatterjee, A. Solid-supported amplification of aggregation emission: A tetraphenylethylene–cucurbit[6]uril@hydroxyapatite-based supramolecular sensing assembly for the detection of spermine and spermidine in human urine and blood. ACS Appl. Bio Mater. 2021, 4, 1813–1822. [Google Scholar] [CrossRef]
- Tripathi, N.; Singh, P.; Luxami, V.; Mahajan, D.; Kumar, S. Spermine Detection from Urine and Blood Serum Using Ionic Self-assembly of Benzimidazolium Based Dipod and Dodecylsulfate. Sens. Actuators B 2018, 270, 552–561. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Huy, B.T.; Phong, P.T.; Han, J.S.; Kwon, D.H.; Lee, Y. Simple Fluorescence Optosensing Probe for Spermine Based on Ciprofloxacin-Tb3+ Complexation. PLoS ONE. 2021, 16, e0251306. [Google Scholar]
- Tian, H.; Chang, Y.; Hu, X.; Li, X.; Guo, D. Supramolecular Imaging of Spermine in Cancer Cells. Nanoscale 2021, 13, 15362–15368. [Google Scholar] [CrossRef]
- Tian, H.; Yu, X.; Yao, J.; Gao, G.; Wu, W.; Yang, C. Supramolecular Spectral/Visual Detection of Urinary Polyamines through Synergetic/Competitive Complexation with Gamma-CD and CB[7]. Chem. Commun. 2021, 57, 1806–1809. [Google Scholar] [CrossRef]
- Wang, L.; Ran, X.; Tang, H.; Cao, D. Recent Advances on Reaction-Based Amine Fluorescent Probes. Dyes Pigm. 2021, 194, 109634. [Google Scholar] [CrossRef]
- Du, J.; Hu, M.; Fan, J.; Peng, X. Fluorescent Chemodosimeters Using “Mild” Chemical Events for the Detection of Small Anions and Cations in Biological and Environmental Media. Chem. Soc. Rev. 2012, 41, 4511–4535. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, D.; Sakabe, M.; Kamiya, M.; Yamamoto, K.; Urano, Y. Sensitive Β-Galactosidase-Targeting Fluorescence Probe for Visualizing Small Peritoneal Metastatic Tumours in Vivo. Nat. Commun. 2015, 6, 6463. [Google Scholar] [CrossRef]
- Uno, S.N.; Kamiya, M.; Yoshihara, T.; Sugawara, K.; Okabe, K.; Tarhan, M.C.; Fujita, H.; Funatsu, T.; Okada, Y.; Tobita, S. A Spontaneously Blinking Fluorophore Based on Intramolecular Spirocyclization for Live-Cell Super-Resolution Imaging. Nat. Chem. 2015, 6, 681–689. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, T.; Li, S.; Yu, T.; Zhang, X.; Hu, R.; Feng, J.; Wang, S.; Liang, T.; Chen, J. Ultrasensitive Reversible Chromophore Reaction of Bodipy Functions as High Ratio Double Turn on Probe. Nat. Commun. 2018, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, S.; Tang, H.; Cao, D. An efficient probe for sensing different concentration ranges of glutathione based on AIE-active Schiff base nanoaggregates with distinct reaction mechanism. Sens. Actuators B 2018, 273, 1085–1090. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Ran, X.; Wang, L.; Tang, H.; Cao, D. A Highly Efficient, Colorimetric and Fluorescent Probe for Recognition of Aliphatic Primary Amines Based on a Unique Cascade Chromophore Reaction. Chem. Commun. 2019, 55, 9789–9792. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Z.; Ran, X.; Tang, H.; Cao, D. Recent Advances of Nir Dyes of Pyrrolopyrrole Cyanine and Pyrrolopyrrole Aza-Bodipy: Synthesis and Application. Dyes Pigm. 2022, 198, 110040. [Google Scholar] [CrossRef]
- Shimizu, S.; Lino, T.; Araki, Y.; Kobayashi, N. Pyrrolopyrrole Aza-Bodipy Analogues: A Facile Synthesis and Intense Fluorescence. Chem. Commun. 2013, 49, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Iino, T.; Saeki, A.; Seki, S.; Kobayashi, N. Rational Molecular Design Towards Vis/Nir Absorption and Fluorescence by Using Pyrrolopyrrole Aza -Bodipy and Its Highly Conjugated Structures for Organic Photovoltaics. Chem.-Eur. J. 2014, 21, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Wang, L.; Tang, H.; Ran, X. Pyrrolopyrrole Aza-Bodipy Dyes for Ultrasensitive and Highly Selective Biogenic Diamine Detection. Sens. Actuators B 2020, 312, 127953. [Google Scholar] [CrossRef]
- Wang, L.; Ding, H.; Tang, H.; Cao, D.; Ran, X. A Novel and Efficient Chromophore Reaction Based on a Lactam-Fused Aza-Bodipy for Polyamine Detection. Anal. Chim. Acta 2020, 1135, 38–46. [Google Scholar] [CrossRef]
- Wang, L.; Xin, S.; Zhang, C.; Ran, X.; Tang, H.; Cao, D. Development of a Novel Chromophore Reaction-Based Fluorescent Probe for Biogenic Amines Detection. J. Mater. Chem. B 2021, 9, 9383–9394. [Google Scholar] [CrossRef]
- Barros, M.; Ceballos, S.; Arroyo, P.; Sáez, J.A.; Parra, M.; Gil, S.; Costero, A.M.; Gaviña, P. Spermine and Spermidine Detection through Restricted Intramolecular Rotations in a Tetraphenylethylene Derivative. Chemosensors 2022, 10, 8. [Google Scholar] [CrossRef]
- Huang, J.; Ye, W.; Zha, S.; Tao, Y.; Yang, M.; Huang, K.; Liu, J.; Fung, J.; Li, Y.; Zhu, L.; et al. Sensitive and Responsive Pentiptycene-based Molecular Fluorescence Chemosensor for Detection of Polyamines. J. Lumin. 2021, 232, 117856. [Google Scholar] [CrossRef]
- Samira Abbasi-Moayed, S.; Bigdeli, A.; Hormozi-Nezhad, M.R. Determination of Spermine and Spermidine in Meat with a Ratiometric Fluorescence Nanoprobe and a Combinational Logic Gate. Food Chem. 2022, 384, 132459. [Google Scholar] [CrossRef]
- Khan, S.A.; Misra, T.K. Novel Gluconate Stabilized Gold Nanoparticles as a Colorimetric Sensor for Quantitative Evaluation of Spermine. Colloids Surf. A 2022, 648, 129146. [Google Scholar] [CrossRef]
- Leelasree, T.; Aggarwa, H. MOF Sensors for Food Safety: Ultralow Detection of Putrescine and Cadaverine in Protein Rich Foods. J. Mater. Chem. C. 2022, 10, 2121–2127. [Google Scholar] [CrossRef]
- Li, Q.Z.; Zuo, Z.W.; Zhou, Z.R.; Ji, Y. Polyamine Homeostasis-Based Strategies for Cancer: The Role of Combination Regimens. Eur. J. Pharmacol. 2021, 910, 174456. [Google Scholar] [CrossRef]
- Alexiou, G.; Lianos, G.; Ragos, V.; Galani, V.; Kyritsis, A. Difluoromethylornithine in Cancer: New Advances. Future Oncol. 2016, 13, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Ploskonos, M.V.; Syatkin, S.P.; Neborak, E.V.; Hilal, A.; Sungrapova, K.Y.; Sokuyev, R.I.; Blagonravov, M.L.; Korshunova, A.Y.; Terentyev, A.A. Polyamine Analogues of Propanediamine Series Inhibit Prostate Tumor Cell Growth and Activate the Polyamine Catabolic Pathway. Anticancer Res. 2020, 40, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Thomas, T. Cellular and Animal Model Studies on the Growth Inhibitory Effects of Polyamine Analogues on Breast Cancer. Med. Sci. 2018, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, L.; Yue, K.; Zhang, Z.; Su, S.; Chen, Y.; Yu, L.; Zhang, P.; Ma, R.; Li, Y.; et al. A Naphthalimide-Polyamine Conjugate Preferentially Accumulates in Hepatic Carcinoma Metastases as a Lysosome-Targeted Antimetastatic Agent. Eur. J. Med. Chem. 2021, 221, 113469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Li, M.; Li, M.; Xie, S.; Wang, C. Synthesis and Evaluation of Novel Amonafide-Polyamine Conjugates as Anticancer Agents. Chem. Biol. Drug Des. 2017, 89, 670–680. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Ge, C.; Chang, L.; Wang, C.; Tian, Z.; Wang, S.; Dai, F.; Zhao, L.; Xie, S. Synthesis and Biological Evaluation of Novel Alkylated Polyamine Analogues as Potential Anticancer Agents. Eur. J. Med. Chem. 2018, 143, 1732–1743. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, Y.; Luo, W.; Zhu, Z.; Zhang, X.; Xie, S.; Hong, C.; Wang, Y.; Su, Y.; Zhao, J.; et al. Synthesis and Biological Properties of Polyamine Modified Flavonoids as Hepatocellular Carcinoma Inhibitors. Eur. J. Med. Chem. 2016, 121, 110–119. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Chang, Y.; Ge, C.; Li, X.; Feng, Y.; Xie, S.; Wang, C.; Dai, F.; Luo, W. Synthesis and Biological Evaluation of Novel Asymmetric Naphthalene Diimide Derivatives as Anticancer Agents Depending on Ros Generation. MedChemComm 2018, 9, 1377–1385. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, Z.X.; Zhang, X.; Luo, W.; Chang, L.P.; Chen, S.; Wang, Y.X.; Xie, S.Q.; Chang, C.C.; Wang, C.J. The Lead Optimization of the Polyamine Conjugate of Flavonoid with a Naphthalene Motif: Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2018, 146, 564–576. [Google Scholar] [CrossRef]
- Manickam, M.; Boggu, P.R.; Pillaiyar, T.; Nam, Y.J.; Abdullah, M.; Lee, S.J.; Kang, J.S.; Jung, S.H. Design, Synthesis and Anticancer Activity of 2-Amidomethoxy-1,4-Naphthoquinones and Its Conjugates with Biotin/Polyamine. Bioorg. Med. Chem. Lett. 2021, 31, 127685. [Google Scholar] [CrossRef]
- Romao, L.; do Canto, V.P.; Netz, P.A.; Pinto, A.C.; Follmer, C. Conjugation with Polyamines Enhances the Antitumor Activity of Naphthoquinones against Human Glioblastoma Cells. Anticancer Drugs 2018, 29, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Fidanzi-Dugas, C.; Liagre, B.; Chemin, G.; Perraud, A.; Carrion, C.; Couquet, C.Y.; Granet, R.; Sol, V.; Leger, D.Y. Analysis of the in Vitro and in Vivo Effects of Photodynamic Therapy on Prostate Cancer by Using New Photosensitizers, Protoporphyrin Ix-Polyamine Derivatives. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1676–1690. [Google Scholar] [CrossRef]
- Xie, X.W.; Liu, Z.P.; Li, X. Design, Synthesis, Bioevaluation of Lfc- and Pa-Tethered Anthraquinone Analogues of Mitoxantrone. Bioorg. Chem. 2020, 101, 104005. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pinon, A.; Gamond, A.; Martin, F.; Laurent, A.; Champavier, Y.; Barette, C.; Liagre, B.; Fagnere, C.; Sol, V.; et al. Synthesis and Biological Evaluation of Chalcone-Polyamine Conjugates as Novel Vectorized Agents in Colorectal and Prostate Cancer Chemotherapy. Eur. J. Med. Chem. 2021, 222, 113586. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Z.; Xu, J.F.; Sun, Z.; Zhang, X. Cytotoxicity Regulated by Host-Guest Interactions: A Supramolecular Strategy to Realize Controlled Disguise and Exposure. ACS Appl. Mater. Interfaces 2016, 8, 22780–22784. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Z.; Zhao, H.; Xu, J.I.; Sun, Z.; Zhang, X. Supramolecular Chemotherapy: Cooperative Enhancement of Antitumor Activity by Combining Controlled Release of Oxaliplatin and Consuming of Spermine by Cucurbit[7]Uril. ACS Appl. Mater. Interfaces 2017, 9, 8602–8608. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Wu, H.; Xu, J.F.; Sun, Z.; Zhang, X. Supramolecular Polymeric Chemotherapy Based on Cucurbit[7]Uril-Peg. Copolymer. Biomater. 2018, 178, 697–705. [Google Scholar] [CrossRef]
- Ding, Y.F.; Wei, J.; Li, S.; Pan, Y.T.; Wang, R. Host–Guest Interactions Initiated Supramolecular Chitosan Nanogels for Selective Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 28665–28670. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, L.; Meng, Q.; Li, B.; Chen, R.; Sun, Z. Supramolecular Chemotherapy: Noncovalent Bond Synergy of Cu-curbit[7]uril against Human Colorectal Tumor Cells. Langmuir 2021, 37, 9547–9552. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, H.; Jiao, R.; Liu, H.; Qin, C.; Xu, L.; Chen, Y. Supramolecular Chemotherapy: Host−Guest Complexes of Heptaplatin-cucurbit[7]uril toward Colorectal Normal and Tumor Cells. Langmuir 2021, 37, 5475–5482. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, Y.; Huang, Z.; Xu, J.F.; Sun, Z.; Zhang, X. Supramolecular Chemotherapy: Carboxylated Pillar[6]Arene for Decreasing Cytotoxicity of Oxaliplatin to Normal Cells and Improving Its Anticancer Bioactivity against Colorectal Cancer. ACS Appl. Mater. Interfaces 2018, 10, 5365–5372. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Teng, K.; Ding, Y.; Yue, L.; Yang, Q.; Wang, R. Dual Stimuli-Responsive Bispillar[5]Arene-Based Nanoparticles for Precisely Selective Drug Delivery in Cancer Cells. Chem. Commun. 2019, 55, 2340–2343. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Z.; Yang, K.; Yue, L.; Cheng, Q.; Ma, Y.; Lu, S.; Chen, G.; Wang, R. Polyamine-Responsive Morphological Transformation of a Supramolecular Peptide for Specific Drug Accumulation and Retention in Cancer Cells. Small 2021, 17, 2101139. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ni, H.; Meng, Z.; Wang, J.; Li, C. Supramolecular Trap for Catching Polyamines in Cells as an Anti-Tumor Strategy. Nat. Commun. 2019, 10, 3546. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, L.; Sun, T.; Tang, H.; Bui, B.; Cao, D.; Wang, R.; Chen, W. Characterization of Nanoparticles Combining Polyamine Detection with Photodynamic Therapy. Commun. Biol. 2021, 4, 803. [Google Scholar] [CrossRef]
- Michalski, R.; Pecyna-Utylska, P.; Kernert, J. Determination of Ammonium and Biogenic Amines by Ion Chromatography. A review. J. Chromatogr. A 2021, 1651, 462319. [Google Scholar] [CrossRef]
- Zhou, T.; Yigaimu, A.; Muhammad, T.; Jian, P.; Sha, L.; Zhang, S. Novel Carrier-Mediated Membrane-Assisted Three-Phase Liquid–Liquid Extraction Coupled with Liquid Chromatography–Mass Spectrometry for the Determination of Eight Biogenic Amines in Foods. Food Chem. 2022, 387, 132857. [Google Scholar] [CrossRef]
- Ahangari, H.; Kurbanoglu, S.; Ehsani, A.; Uslu, B. Latest Trends for Biogenic Amines Detection in foods: Enzymatic Biosensors and Nanozymes Applications. Trends Food Sci. Technol. 2021, 112, 75–87. [Google Scholar] [CrossRef]
- Asano, K.; Sasaki, Y.; Zhou, Q.; Mitobe, R.; Tang, W.; Lyu, X.; Kamiko, M.; Tanaka, H.; Yamagami, A.; Hagiya, K.; et al. Detection of Polyamines by an Extended Gate-Type Organic Transistor Functionalized with a Carboxylate Attached 1,3,4-thiadiazole Derivative. J. Mater. Chem. C 2021, 9, 11690–11697. [Google Scholar] [CrossRef]
- Gross, E.M.; Lowry, E.R.; Schaffer, L.V.; Henry, C.S. Electrogenerated Chemiluminescent Detection of Polyamines on a Microfluidic Device Using Micromolded Carbon Paste Microelectrodes. Electroanalysis 2022, 34, 410. [Google Scholar] [CrossRef]
- Duan, Q.; Shi, H.; Tan, L.; Liu, Z.; Huang, Q.; Shen, W.; Cao, L.; Lee, H.; Tang, S. Ultrahigh-Performance Supercritical Fluid Chromatography and Detection of Multiple Biogenic Amines in Gentamicin Sulfate: Method Development Using Computer-Assisted Modeling. Anal. Chem. 2022, 94, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, C.S.; Park, S.J.; Kim, J.; Seo, S.E.; An, J.E.; Ha, S.; Bae, J.; Phyo, S.; Lee, J.; et al. In-situ Food Spoilage Monitoring Using a Wireless Chemical Receptor-Conjugated Graphene Electronic Nose. Biosens. Bioelectron. 2022, 200, 113908. [Google Scholar] [CrossRef]

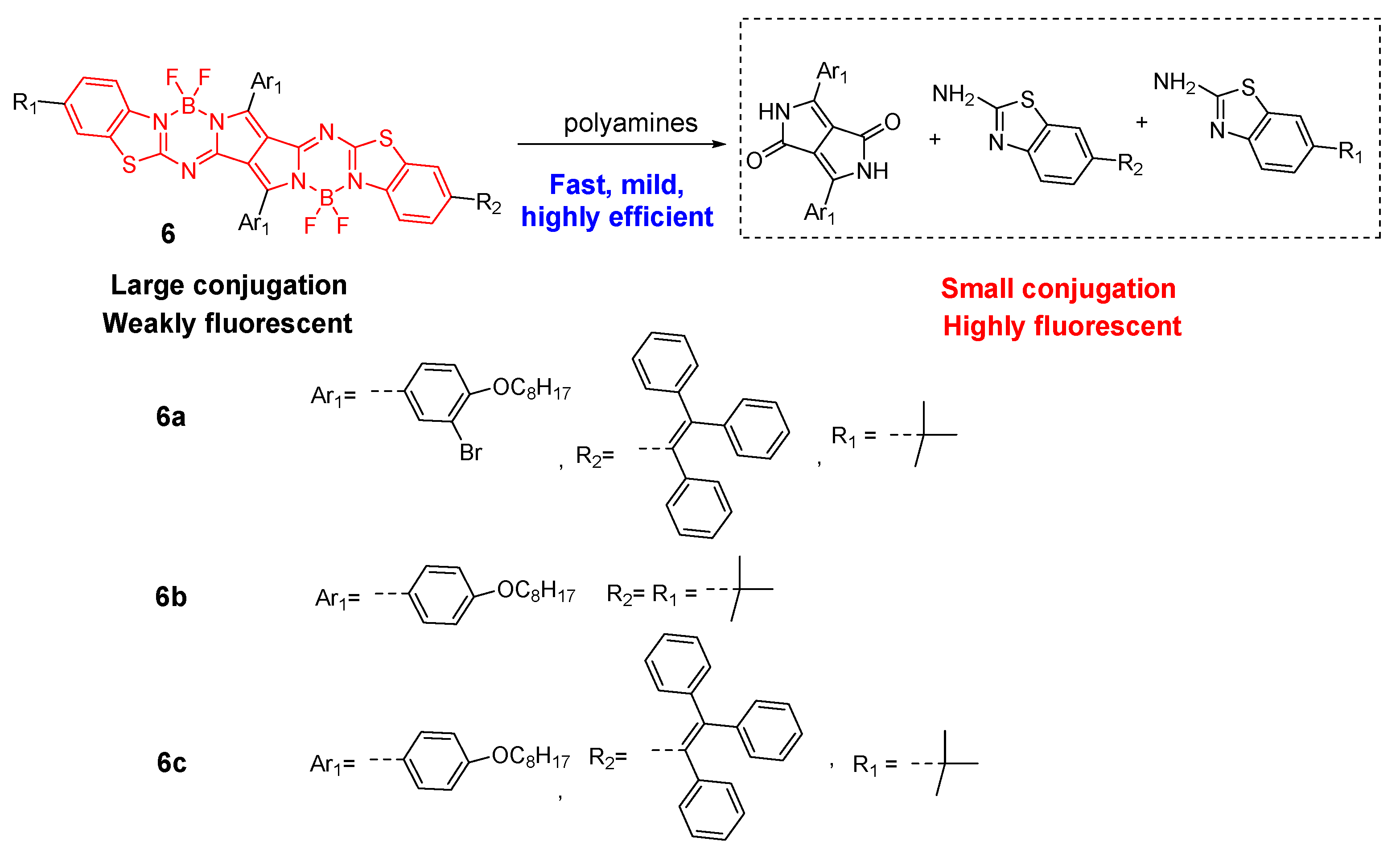
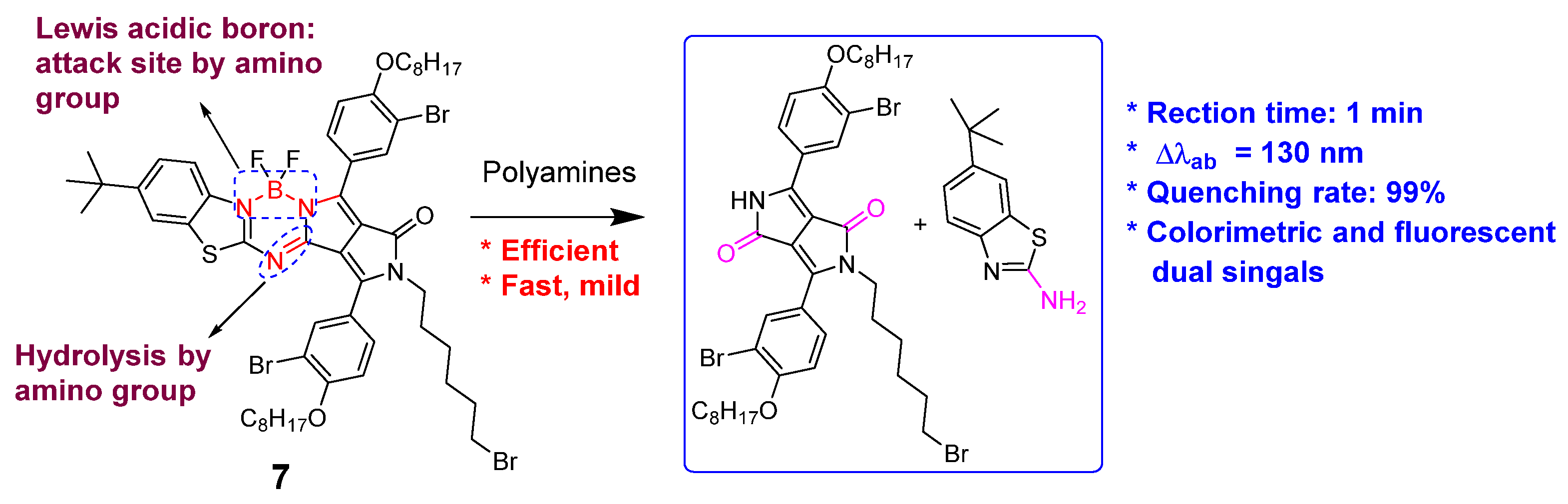
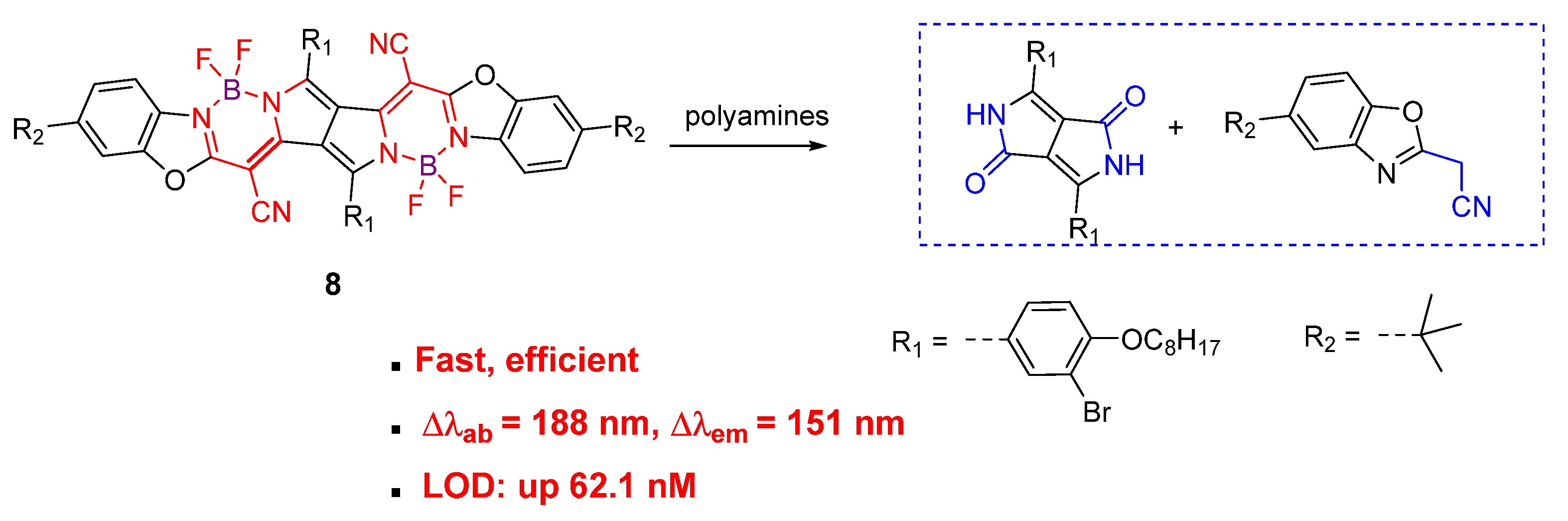
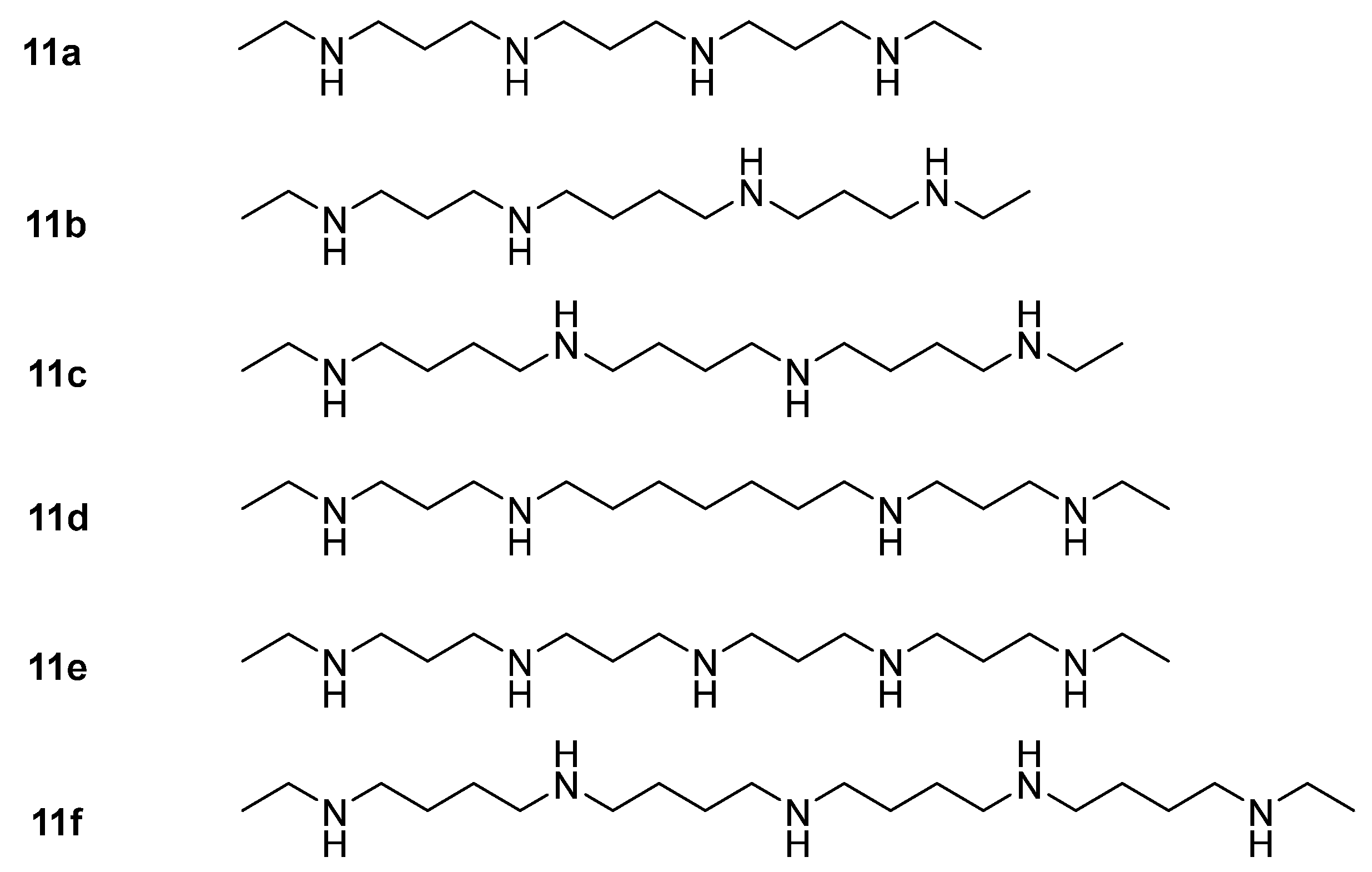


| Detection Methods | Advantages | Disadvantages |
|---|---|---|
| Immunoassays | High specificity, rapid and easy operation, good reproducibility | Poor quantitative accuracy, requirements for high purity antibody and analytes, need of antibody, largely dependent on the source and affinity of the antibody |
| HPLC, GC-MS LC-MS | Fast analysis speed, broad applicability, high accuracy | Complex technical support, high equipment cost, need for professional staff, long and tedious sample pretreatment step, chemical derivatization processes |
| Chromophore reaction-based fluorescent probes | High specificity, colorimetric and fluorescence change, naked-eye detection | Several synthesis steps, need for optimal probes’ structure, poor water solubility, a short to long detection time |
| Organic fluorescent probes | Based on non-covalent interaction with high sensitivity, real-time, and high throughput | Poor water solubility, medium selectivity, susceptible to the microenvironment, several synthesis steps |
| Supramolecular sensing system | High selectivity, low LOD, high throughput | Needs several components, susceptible to external factors, enough large binding-affinity difference between polyamine–host and guest–host |
| Fluorescent nanoparticle | Facile fabrication, easy to control fluorescence performance | Toxicity of metal ions, instability of fluorescence, poor data reproducibility |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.; Wang, L.; Ran, X.; Tang, H.; Cao, D. Recent Advances in Fluorescent Methods for Polyamine Detection and the Polyamine Suppressing Strategy in Tumor Treatment. Biosensors 2022, 12, 633. https://doi.org/10.3390/bios12080633
Lu B, Wang L, Ran X, Tang H, Cao D. Recent Advances in Fluorescent Methods for Polyamine Detection and the Polyamine Suppressing Strategy in Tumor Treatment. Biosensors. 2022; 12(8):633. https://doi.org/10.3390/bios12080633
Chicago/Turabian StyleLu, Bingli, Lingyun Wang, Xueguang Ran, Hao Tang, and Derong Cao. 2022. "Recent Advances in Fluorescent Methods for Polyamine Detection and the Polyamine Suppressing Strategy in Tumor Treatment" Biosensors 12, no. 8: 633. https://doi.org/10.3390/bios12080633
APA StyleLu, B., Wang, L., Ran, X., Tang, H., & Cao, D. (2022). Recent Advances in Fluorescent Methods for Polyamine Detection and the Polyamine Suppressing Strategy in Tumor Treatment. Biosensors, 12(8), 633. https://doi.org/10.3390/bios12080633