Accuracy of the Dexcom G6 Glucose Sensor during Aerobic, Resistance, and Interval Exercise in Adults with Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol
2.2. Statistical Analysis
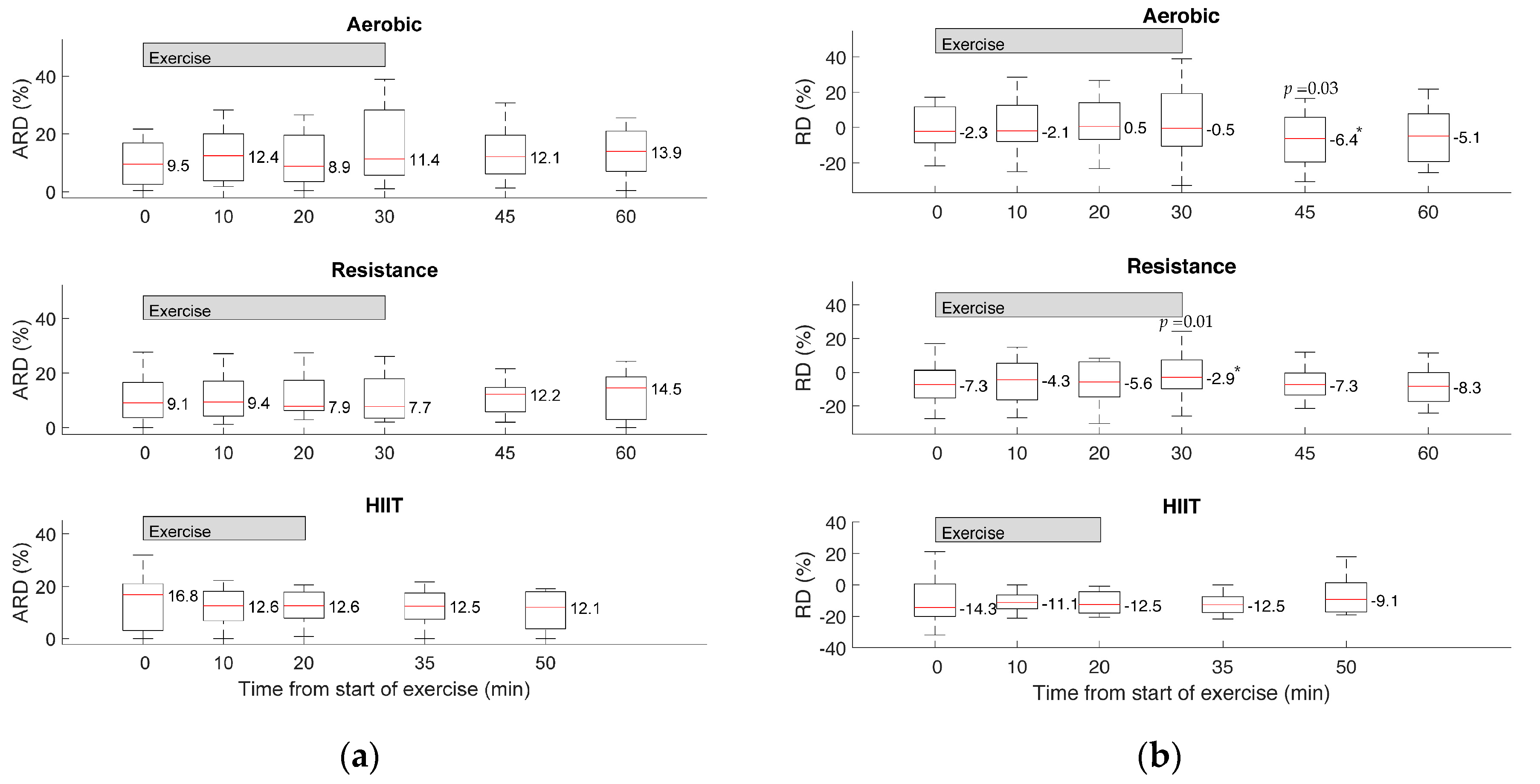
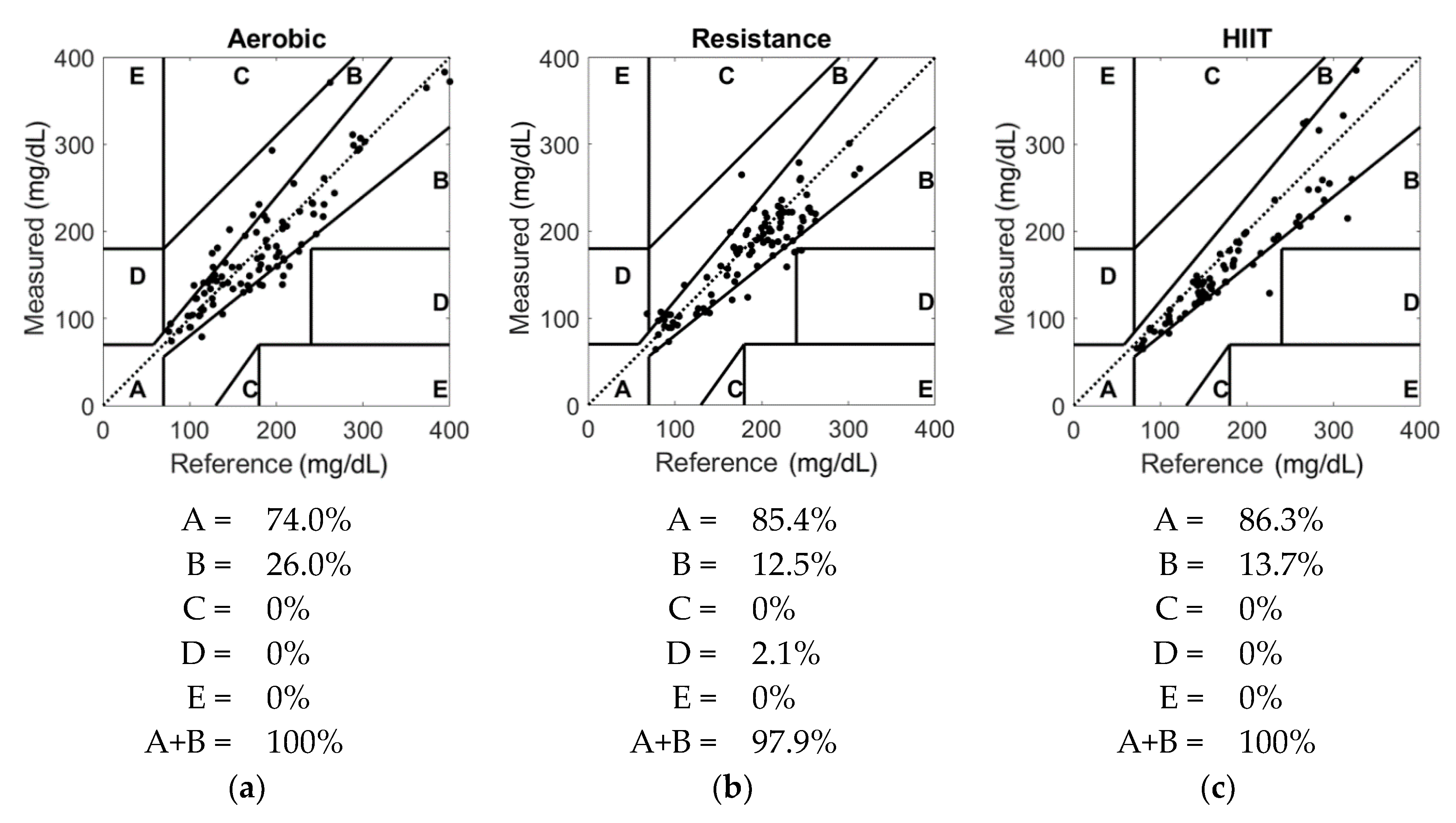
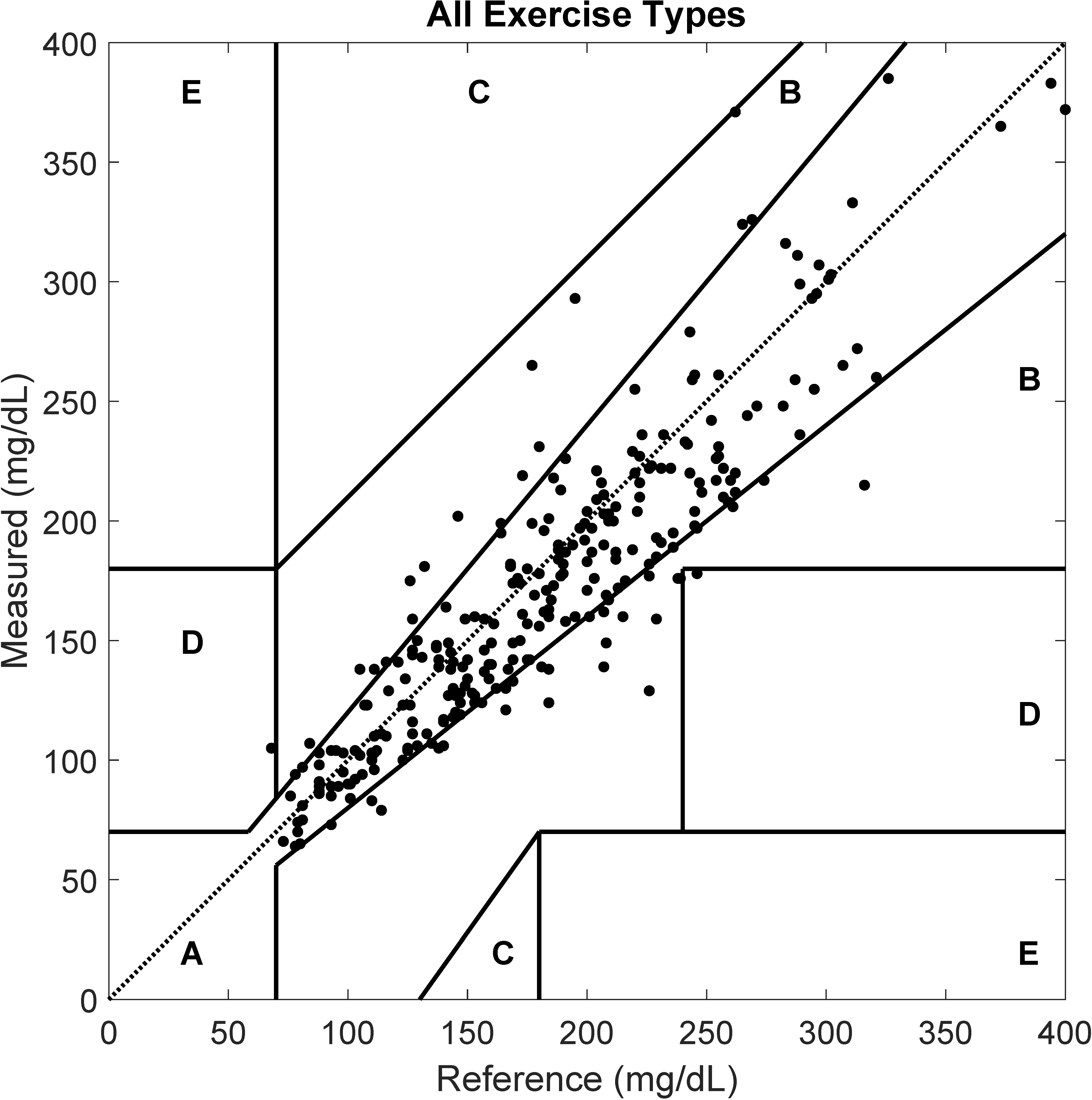
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Bohn, B.; Herbst, A.; Pfeifer, M.; Krakow, D.; Zimny, S.; Kopp, F.; Melmer, A.; Steinacker, J.M.; Holl, R.W. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: A cross-sectional multicenter study of 18,028 patients. Diabetes Care 2015, 38, 1536–1543. [Google Scholar] [CrossRef] [Green Version]
- Chimen, M.; Kennedy, A.; Nirantharakumar, K.; Pang, T.T.; Andrews, R.; Narendran, P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012, 55, 542–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, M.M.; Funk, M.; Grey, M. Cardiovascular health in adults with type 1 diabetes. Prev. Med. 2016, 91, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Brazeau, A.-S.; Rabasa-Lhoret, R.; Strychar, I.; Mircescu, H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008, 31, 2108–2109. [Google Scholar] [CrossRef] [Green Version]
- Riddell, M.C.; Zaharieva, D.P.; Tansey, M.; Tsalikian, E.; Admon, G.; Li, Z.; Kollman, C.; Beck, R.W. Individual glucose responses to prolonged moderate intensity aerobic exercise in adolescents with type 1 diabetes: The higher they start, the harder they fall. Pediatric Diabetes 2019, 20, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American diabetes association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [Green Version]
- Marliss, E.B.; Vranic, M. Intense exercise has unique effects on both insulin release and its roles in Glucoregulation: Implications for diabetes. Diabetes 2002, 51, S271–S283. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.; Gray, B.J.; Luzio, S.; Dunseath, G.; Bain, S.C.; Hanley, S.; Richards, A.; Rhydderch, D.C.; Ayles, M.; Kilduff, L.P.; et al. Similar magnitude of post-exercise hyperglycemia despite manipulating resistance exercise intensity in type 1 diabetes individuals. Scand. J. Med. Sci. Sports 2016, 26, 404–412. [Google Scholar] [CrossRef]
- Colberg, S.R.; Hernandez, M.J.; Shahzad, F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care 2013, 36, e177. [Google Scholar] [CrossRef] [Green Version]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef] [PubMed]
- DeSalvo, D.J.; Miller, K.M.; Hermann, J.M.; Maahs, D.M.; Hofer, S.E.; Clements, M.A.; Lilienthal, E.; Sherr, J.L.; Tauschmann, M.; Holl, R.W. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr. Diabetes 2018, 19, 1271–1275. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA 2017, 317, 371–378. [Google Scholar] [CrossRef]
- Haak, T.; Hanaire, H.; Ajjan, R.; Hermanns, N.; Riveline, J.-P.; Rayman, G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: A multicenter, open-label randomized controlled trial. Diabetes Ther. 2017, 8, 55–73. [Google Scholar] [CrossRef] [Green Version]
- Ólafsdóttir, A.F.; Polonsky, W.; Bolinder, J.; Hirsch, I.B.; Dahlqvist, S.; Wedel, H.; Nyström, T.; Wijkman, M.; Schwarcz, E.; Hellman, J.; et al. A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD-3). Diabetes Technol. Ther. 2018, 20, 274–284. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Signoriello, S.; Maio, A.; Chiodini, P.; Bellastella, G.; Scappaticcio, L.; Longo, M.; Giugliano, D.; Esposito, K. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: A systematic review with meta-analysis of randomized controlled trials. Diabetes Care 2020, 43, 1146–1156. [Google Scholar] [CrossRef]
- Brown, S.A.; Kovatchev, B.P.; Raghinaru, D.; Lum, J.W.; Buckingham, B.A.; Kudva, Y.C.; Laffel, L.M.; Levy, C.J.; Pinsker, J.E.; Wadwa, R.P.; et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N. Engl. J. Med. 2019, 381, 1707–1717. [Google Scholar] [CrossRef]
- Kumareswaran, K.; Elleri, D.; Allen, J.M.; Caldwell, K.; Nodale, M.; Wilinska, M.E.; Amiel, S.A.; Hovorka, R.; Murphy, H.R. Accuracy of continuous glucose monitoring during exercise in type 1 diabetes pregnancy. Diabetes Technol. Ther. 2013, 15, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Herrington, S.J.; Gee, D.L.; Dow, S.D.; Monosky, K.A.; Davis, E.; Pritchett, K.L. Comparison of glucose monitoring methods during steady-state exercise in women. Nutrients 2012, 4, 1282–1292. [Google Scholar] [CrossRef]
- Pleus, S.; Schoemaker, M.; Morgenstern, K.; Schmelzeisen-Redeker, G.; Haug, C.; Link, M.; Zschornack, E.; Freckmann, G. Rate-of-change dependence of the performance of two CGM systems during induced glucose swings. J. Diabetes Sci. Technol. 2015, 9, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Facchinetti, A.; Del Favero, S.; Sparacino, G.; Cobelli, C. Model of glucose sensor error components: Identification and assessment for new Dexcom G4 generation devices. Med. Biol. Eng. Comput. 2015, 53, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Cobelli, C.; Schiavon, M.; Dalla Man, C.; Basu, A.; Basu, R. Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror of blood glucose: Insight from an in Silico study. Diabetes Technol. Ther. 2016, 18, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Dube, S.; Slama, M.; Errazuriz, I.; Amezcua, J.C.; Kudva, Y.C.; Peyser, T.; Carter, R.E.; Cobelli, C.; Basu, R. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 2013, 62, 4083–4087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, O.; Yardley, J.E.; Bracken, R.M. Interstitial glucose and physical exercise in type 1 diabetes: Integrative physiology, technology, and the gap in-between. Nutrients 2018, 10, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, R.; Johnson, M.L.; Kudva, Y.C.; Basu, A. Exercise, hypoglycemia, and type 1 diabetes. Diabetes Technol. Ther. 2014, 16, 331–337. [Google Scholar] [CrossRef]
- Castle, J.R.; Rodbard, D. How well do continuous glucose monitoring systems perform during exercise? Diabetes Technol. Ther. 2019, 21, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Riddell, M.C.; Potashner, D.; Brown, R.E.; Aronson, R. Time lag and accuracy of continuous glucose monitoring during high intensity interval training in adults with type 1 diabetes. Diabetes Technol. Ther. 2019, 21, 286–294. [Google Scholar] [CrossRef]
- Larose, S.; Rabasa-Lhoret, R.; Roy-Fleming, A.; Suppère, C.; Tagougui, S.; Messier, V.; Taleb, N. Changes in accuracy of continuous glucose monitoring using Dexcom G4 platinum over the course of moderate intensity aerobic exercise in type 1 diabetes. Diabetes Technol. Ther. 2019, 21, 364–369. [Google Scholar] [CrossRef]
- Zaharieva, D.P.; Turksoy, K.; McGaugh, S.M.; Pooni, R.; Vienneau, T.; Ly, T.; Riddell, M.C. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol. Ther. 2019, 21, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Balo, A. The accuracy and efficacy of the dexcom g4 platinum continuous glucose monitoring system. J. Diabetes Sci. Technol. 2015, 9, 1021–1026. [Google Scholar] [CrossRef]
- Matuleviciene, V.; Joseph, J.I.; Andelin, M.; Hirsch, I.B.; Attvall, S.; Pivodic, A.; Dahlqvist, S.; Klonoff, D.; Haraldsson, B.; Lind, M. A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 System) and enlite sensor (Guardian REAL-Time System) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol. Ther. 2014, 16, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.N.; Laffel, L.M.; Wadwa, R.P.; Garg, S.K. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol. Ther. 2018, 20, 428–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castorino, K.; Polsky, S.; O’Malley, G.; Levister, C.; Nelson, K.; Farfan, C.; Brackett, S.; Puhr, S.; Levy, C. Performance of the Dexcom G6 continuous glucose monitoring system in pregnant women with diabetes. Diabetes Technol. Ther. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castle, J.R.; Guillot, F.; Gabo, V.; Branigan, D.; Tyler, N.; Riddell, M.; Jacobs, P.G. Accuracy of the Dexcom G6 system during aerobic, resistance, and interval exercise in adults with type 1 diabetes. In Proceedings of the Official Journal of ATTD Advanced Technologies & Treatments for Diabetes Conference, Madrid, Spain, 19–22 February 2020; Diabetes Technologies & Therpeutics: Madrid, Spain, 2020; Volume 22. [Google Scholar]
- Tyler, N.S.; Mosquera-Lopez, C.M.; Wilson, L.M.; Dodier, R.H.; Branigan, D.L.; Gabo, V.B.; Guillot, F.H.; Hilts, W.W.; El Youssef, J.; Castle, J.R.; et al. An artificial intelligence decision support system for the management of type 1 diabetes. Nat. Metab. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ekhlaspour, L.; Mondesir, D.; Lautsch, N.; Balliro, C.; Hillard, M.; Magyar, K.; Radocchia, L.G.; Esmaeili, A.; Sinha, M.; Russell, S.J. Comparative accuracy of 17 point-of-care glucose meters. J. Diabetes Sci. Technol. 2017, 11, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxon, F. Individual comparisons by ranking methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef]
- Richardson, J.T.E. The analysis of 2 × 2 contingency tables—Yet again. Stat. Med. 2011, 30, 890. [Google Scholar] [CrossRef]
- Campbell, I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef]
- Schmelzeisen-Redeker, G.; Schoemaker, M.; Kirchsteiger, H.; Freckmann, G.; Heinemann, L.; del Re, L. Time delay of CGM sensors. J. Diabetes Sci. Technol. 2015, 9, 1006–1015. [Google Scholar] [CrossRef] [Green Version]
- MATLAB. Version 9.7.0.1296695 (R2019b); The MathWorks Inc.: Natick, MA, USA, 2020. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LLC: College Station, TX, USA, 2017. [Google Scholar]
- Taleb, N.; Emami, A.; Suppere, C.; Messier, V.; Legault, L.; Chiasson, J.-L.; Rabasa-Lhoret, R.; Haidar, A. Comparison of two continuous glucose monitoring systems, Dexcom G4 Platinum and Medtronic Paradigm Veo Enlite System, at rest and during exercise. Diabetes Technol. Ther. 2016, 18, 561–567. [Google Scholar] [CrossRef]
- Bally, L.; Zueger, T.; Pasi, N.; Carlos, C.; Paganini, D.; Stettler, C. Accuracy of continuous glucose monitoring during differing exercise conditions. Diabetes Res. Clin. Pract. 2016, 112, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Moser, O.; Eckstein, M.L.; Mueller, A.; Birnbaumer, P.; Aberer, F.; Koehler, G.; Sourij, C.; Kojzar, H.; Holler, P.; Simi, H.; et al. Impact of physical exercise on sensor performance of the FreeStyle Libre intermittently viewed continuous glucose monitoring system in people with Type 1 diabetes: A randomized crossover trial. Diabet. Med. 2019, 36, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Sigal, R.J.; Kenny, G.P.; Riddell, M.C.; Lovblom, L.E.; Perkins, B.A. Point accuracy of interstitial continuous glucose monitoring during exercise in type 1 diabetes. Diabetes Technol. Ther. 2013, 15, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Emhoff, C.-A.W.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J. Appl. Physiol. 2013, 114, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, B.C.; Horning, M.A.; Casazza, G.A.; Wolfel, E.E.; Butterfield, G.E.; Brooks, G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E244–E251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diabetes Research in Children Network (DirecNet) Study Group. The effects of aerobic exercise on glucose and counterregulatory hormone concentrations in children with type 1 diabetes. Diabetes Care 2006, 29, 20–25. [Google Scholar] [CrossRef]
- Guelfi, K.J.; Jones, T.W.; Fournier, P.A. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 2005, 28, 1289–1294. [Google Scholar] [CrossRef] [Green Version]
- Biagi, L.; Bertachi, A.; Quirós, C.; Giménez, M.; Conget, I.; Bondia, J.; Vehí, J. Accuracy of continuous glucose monitoring before, during, and after aerobic and anaerobic exercise in patients with type 1 diabetes mellitus. Biosensors 2018, 8, 22. [Google Scholar] [CrossRef] [Green Version]
| Mean ± SD (Range) | |
|---|---|
| Age (years) | 30.5 ± 6.1 (20.0–41.3) |
| Weight (kg) | 82.4 ± 19.3 (51.8–128.0) |
| Height (cm) | 169.9 ± 17.2 (100.5–188.5) |
| Sex | 10 Male–14 Female |
| HbA1c (%) | 8.8 ± 1.4 (7.1–12.7) |
| HbA1c (mmol/mol) | 73 ± 15.3 (54–115) |
| Duration of diabetes (years) | 15.7 ± 7.0 (3–33) |
| VO2 max (mL/kg/min) | 33.8 ± 8.4 (20.4–51.4) |
| Race—no. (%) | |
| White | 18 (75.0) |
| Black | 4 (16.7) |
| Other | 2 (8.3) |
| Mean RD (Linear) | Mean ARD (Gamma) | |||||
|---|---|---|---|---|---|---|
| Measures | Estimate (%) | p | 95% CI | Estimate (Ratio 1) | p | 95% CI |
| Intercept | −0.19 | 0.96 | −7.2–6.8 | 10.85 | <0.001 | 6.97–16.90 |
| Exercise type (vs. aerobic): | ||||||
| HIIT | −11.3 | 0.040 | −22.0–−0.5 | 0.980 | 0.93 | 0.593–1.617 |
| Resistance | −4.6 | 0.38 | −15.1–5.8 | 0.933 | 0.78 | 0.565–1.541 |
| Reference Meter 2 | −0.74 | 0.003 | −1.2–−0.3 | 0.997 | 0.83 | 0.971–1.024 |
| Glucose drop 2 | 3.1 | <0.001 | 2.3–3.9 | 1.035 | 0.31 | 0.969–1.105 |
| Time since start | −0.28 | 0.43 | −1.0–0.4 | 1.027 | 0.40 | 0.966–1.092 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillot, F.H.; Jacobs, P.G.; Wilson, L.M.; Youssef, J.E.; Gabo, V.B.; Branigan, D.L.; Tyler, N.S.; Ramsey, K.; Riddell, M.C.; Castle, J.R. Accuracy of the Dexcom G6 Glucose Sensor during Aerobic, Resistance, and Interval Exercise in Adults with Type 1 Diabetes. Biosensors 2020, 10, 138. https://doi.org/10.3390/bios10100138
Guillot FH, Jacobs PG, Wilson LM, Youssef JE, Gabo VB, Branigan DL, Tyler NS, Ramsey K, Riddell MC, Castle JR. Accuracy of the Dexcom G6 Glucose Sensor during Aerobic, Resistance, and Interval Exercise in Adults with Type 1 Diabetes. Biosensors. 2020; 10(10):138. https://doi.org/10.3390/bios10100138
Chicago/Turabian StyleGuillot, Florian H., Peter G. Jacobs, Leah M. Wilson, Joseph El Youssef, Virginia B. Gabo, Deborah L. Branigan, Nichole S. Tyler, Katrina Ramsey, Michael C. Riddell, and Jessica R. Castle. 2020. "Accuracy of the Dexcom G6 Glucose Sensor during Aerobic, Resistance, and Interval Exercise in Adults with Type 1 Diabetes" Biosensors 10, no. 10: 138. https://doi.org/10.3390/bios10100138






