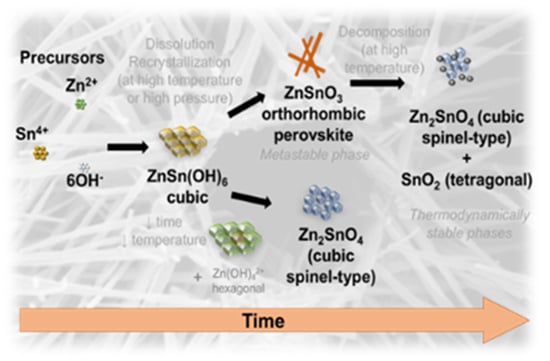
Growth Mechanism of Seed-Layer Free ZnSnO3 Nanowires: Effect of Physical Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanostructures’ Synthesis
2.2. Nanostructures’ Characterization
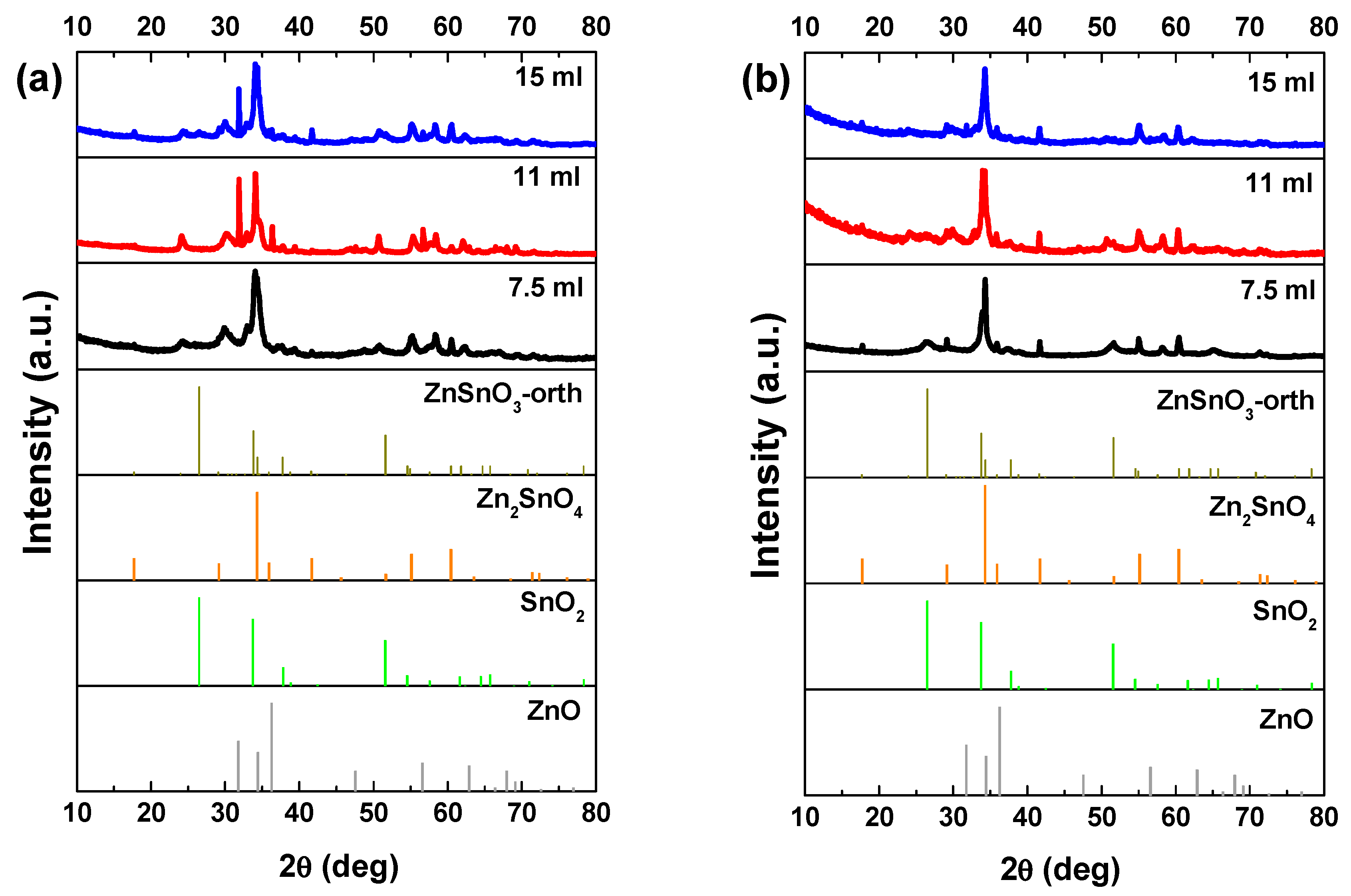
3. Results and Discussion
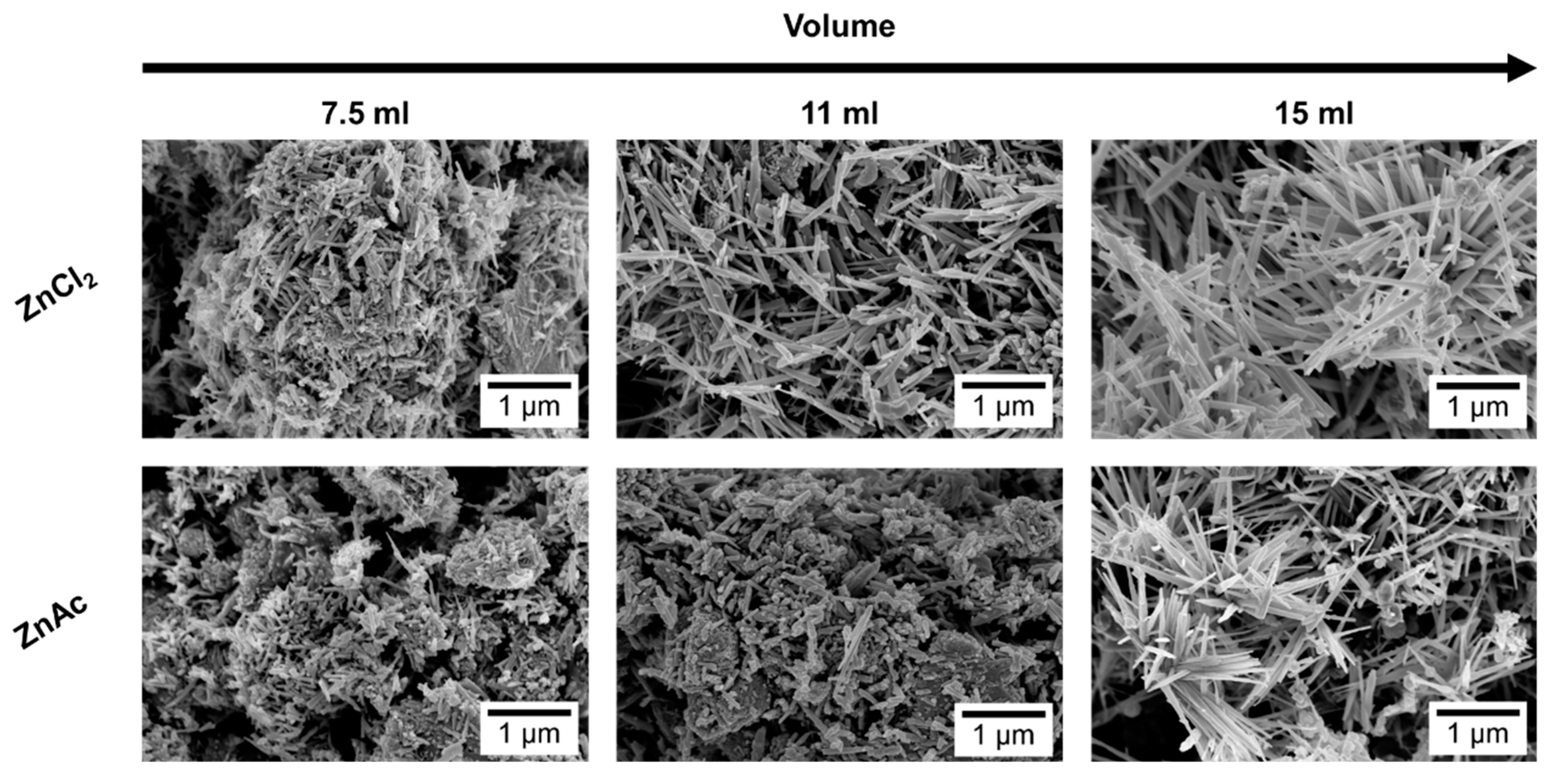
3.1. Reaction Mixture Volume
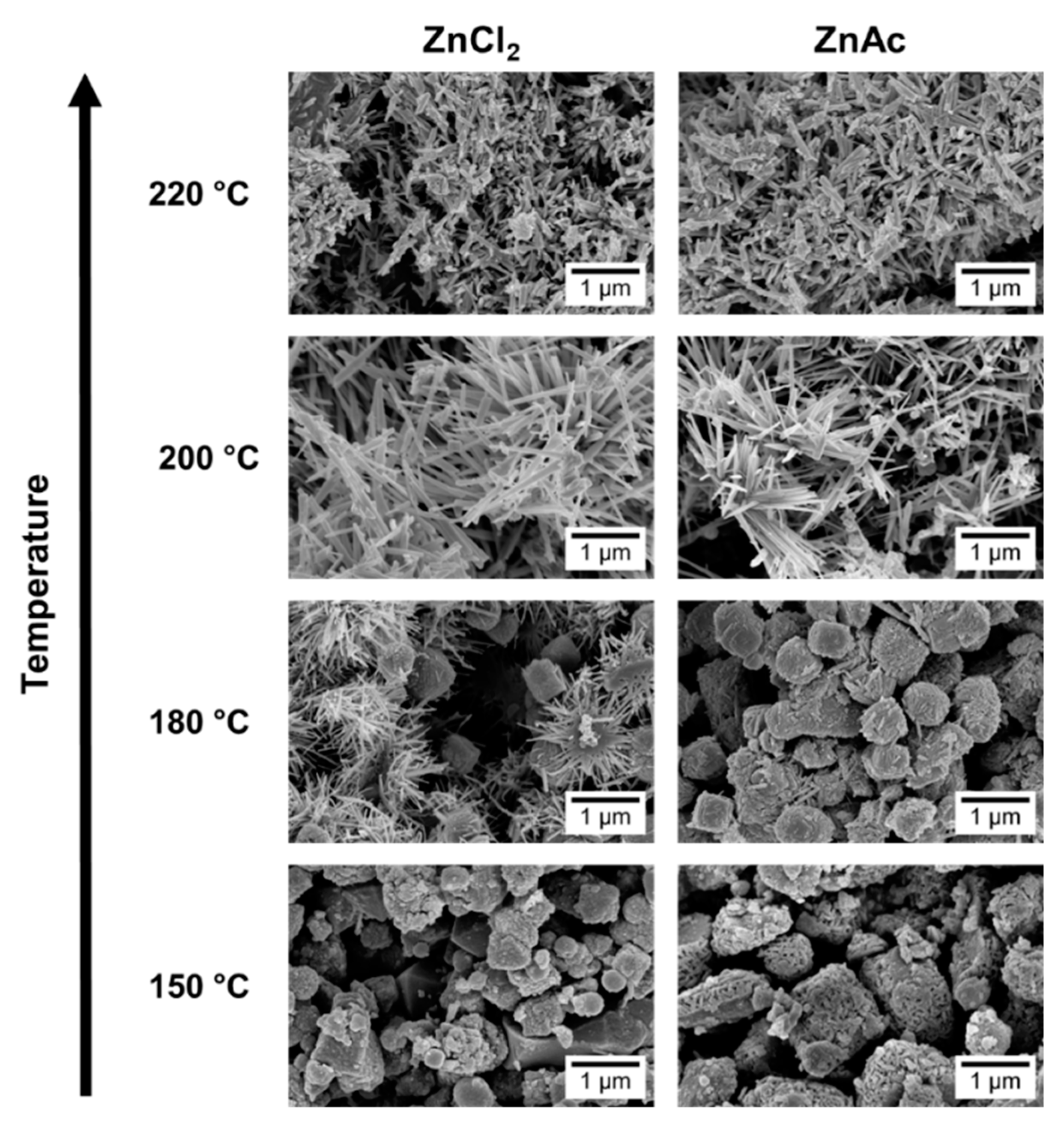
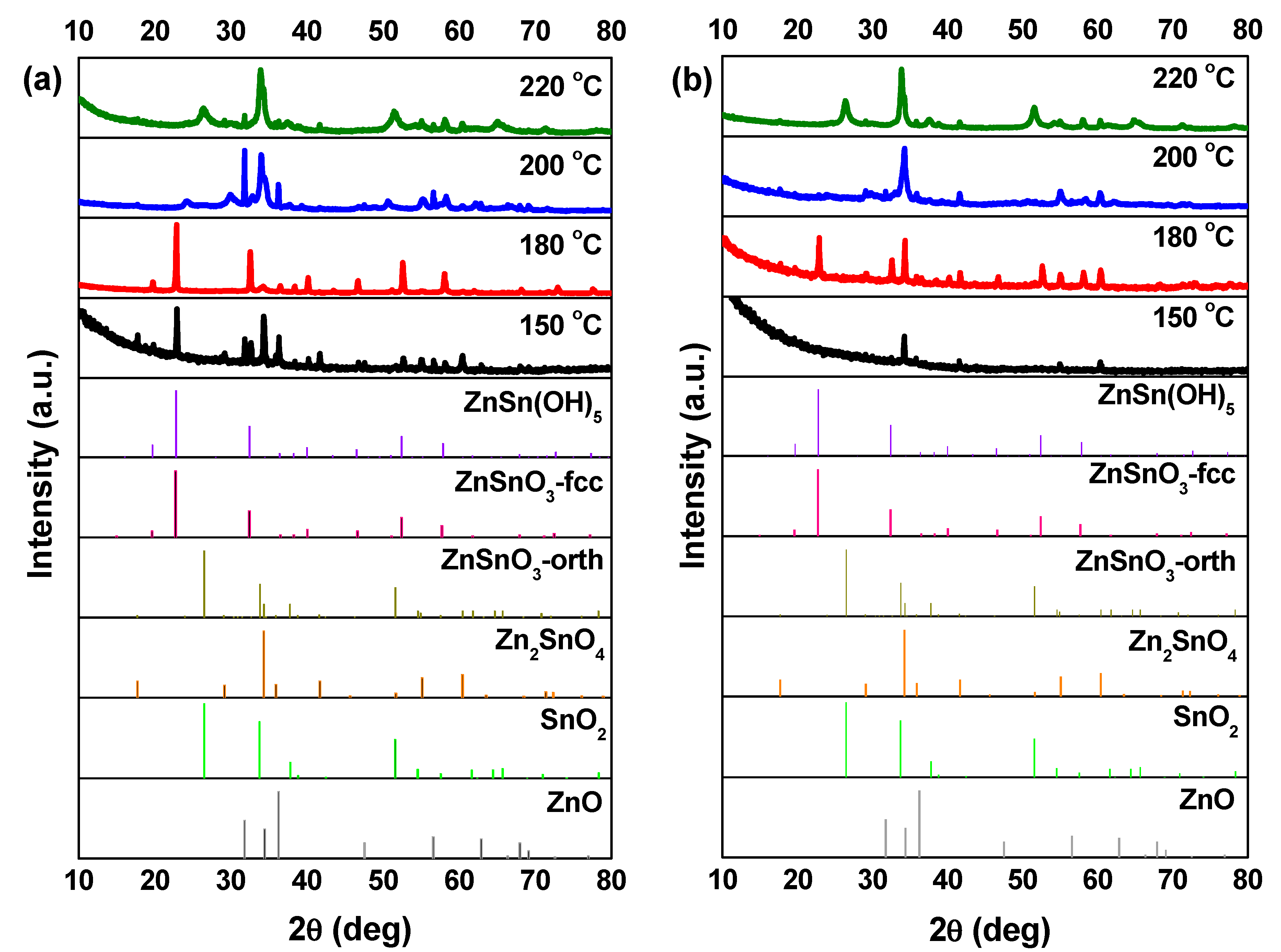
3.2. Synthesis Temperature
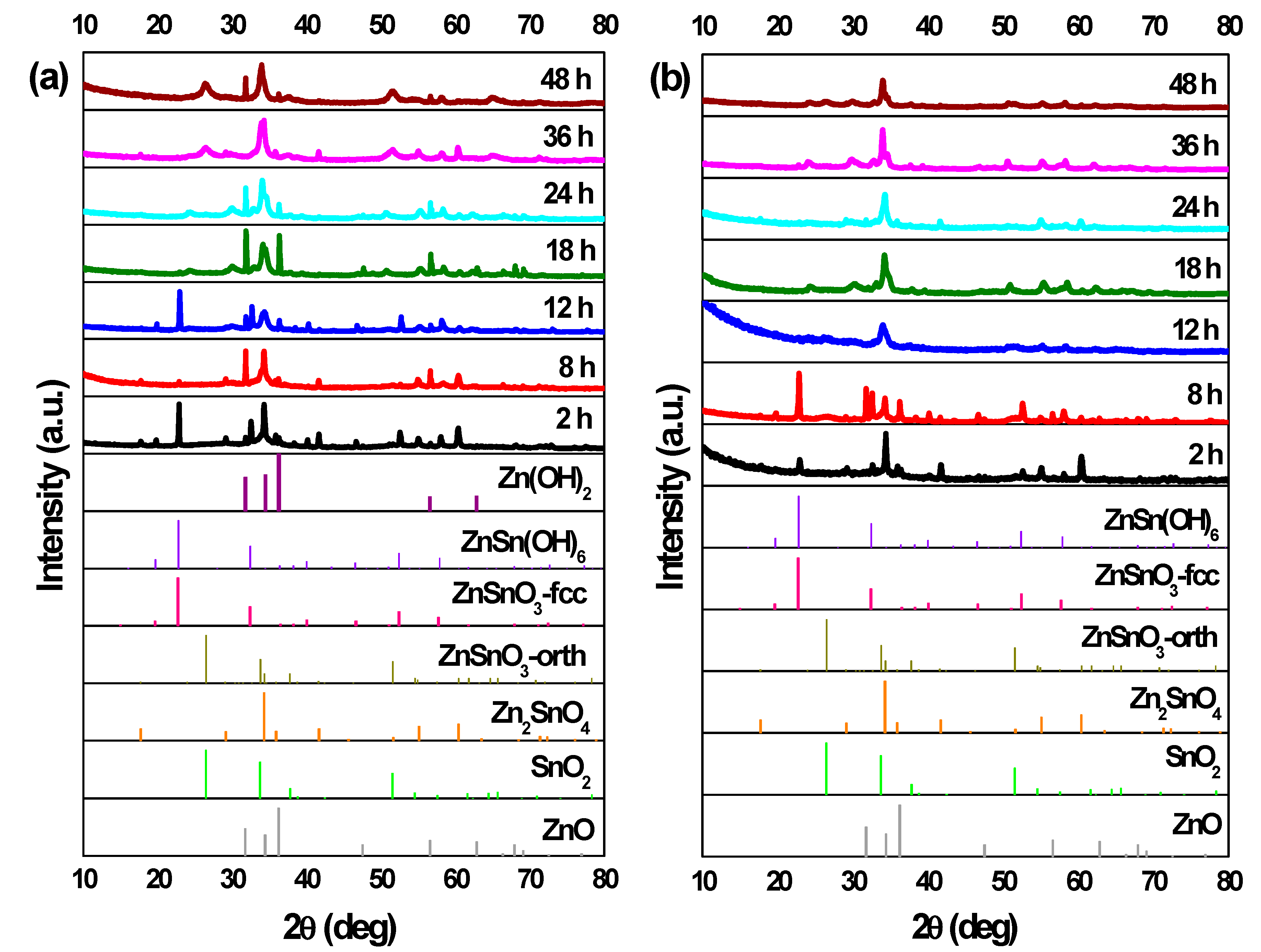
3.3. Reaction Time
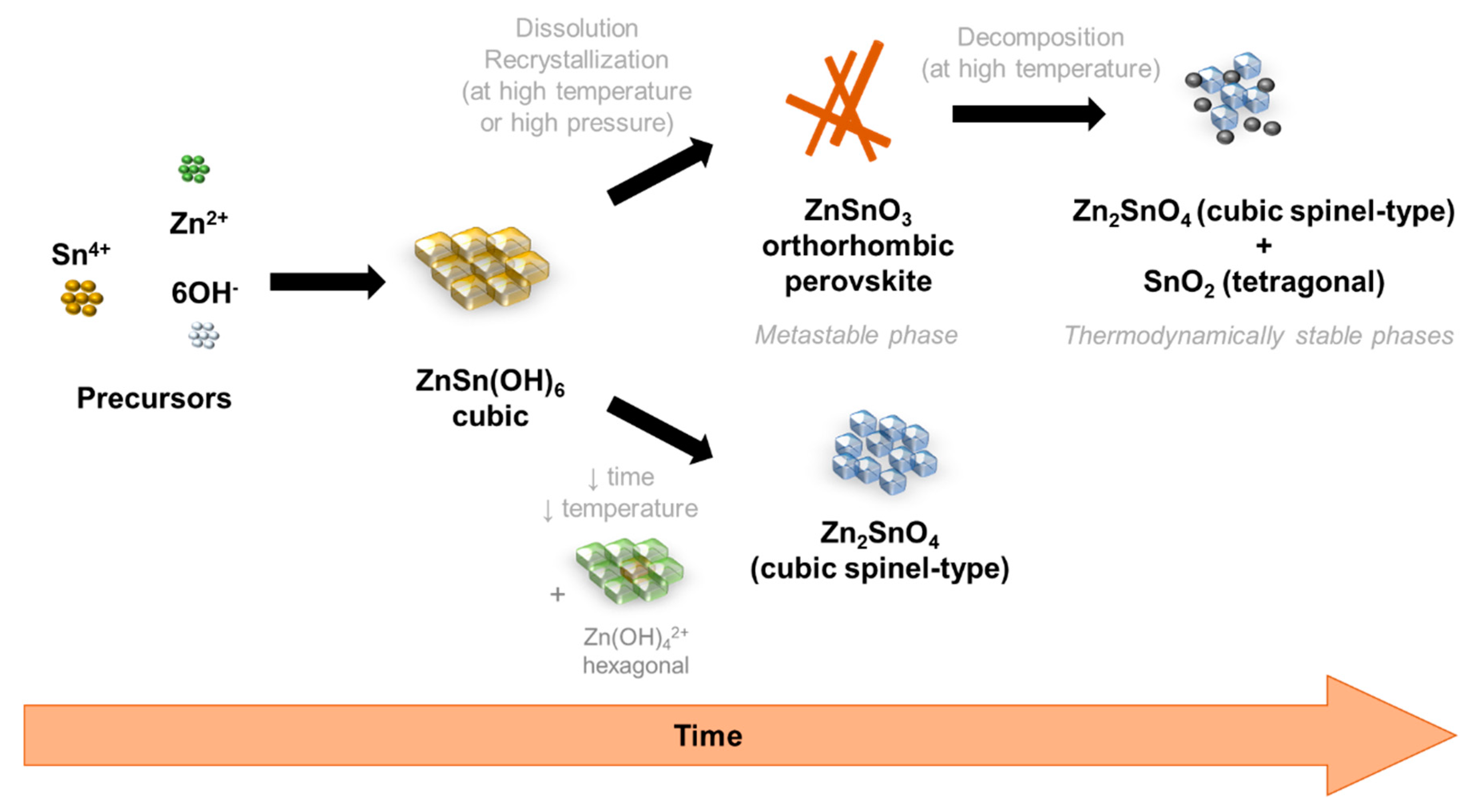
3.4. Growth Mechanism of ZnSnO3 Nanowires
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—Nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Pimentel, A.; Ferreira, S.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave synthesized ZnO nanorod arrays for UV sensors: A seed layer annealing temperature study. Materials 2016, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-fast microwave synthesis of ZnO nanorods on cellulose substrates for UV sensor applications. Materials 2017, 10, 1308. [Google Scholar] [CrossRef]
- Kiazadeh, A.; Gomes, H.L.; Barquinha, P.; Martins, J.; Rovisco, A.; Pinto, J.V.; Martins, R.; Fortunato, E. Improving positive and negative bias illumination stress stability in parylene passivated IGZO transistors. Appl. Phys. Lett. 2016, 109, 051606. [Google Scholar] [CrossRef]
- Bahubalindruni, P.G.; Martins, J.; Santa, A.; Tavares, V.; Martins, R.; Fortunato, E.; Barquinha, P. High-Gain transimpedance amplifier for flexible radiation dosimetry using ingazno tfts. IEEE J. Electron Devices Soc. 2018, 6, 760–765. [Google Scholar] [CrossRef]
- Ide, K.; Nomura, K.; Hosono, H.; Kamiya, T. Electronic defects in amorphous oxide semiconductors: A review. Phys. Status Solidi Appl. Mater. Sci. 2019, 1800372, 1–28. [Google Scholar] [CrossRef]
- Mancini, L.; Sala, S.; Recchioni, M.; Benini, L.; Goralczyk, M.; Pennington, D. Potential of life cycle assessment for supporting the management of critical raw materials. Int. J. Life Cycle Assess. 2015, 20, 100–116. [Google Scholar] [CrossRef]
- Fernandes, C.; Santa, A.; Santos, Â.; Bahubalindruni, P.; Deuermeier, J.; Martins, R.; Fortunato, E.; Barquinha, P. A sustainable approach to flexible electronics with zinc-tin oxide thin-film transistors. Adv. Electron. Mater. 2018, 4, 1800032. [Google Scholar] [CrossRef]
- Salgueiro, D.; Kiazadeh, A.; Branquinho, R.; Santos, L.; Barquinha, P.; Martins, R.; Fortunato, E. Solution based zinc tin oxide TFTs: The dual role of the organic solvent. J. Phys. D Appl. Phys. 2017, 50, 065106. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S. Morphological zinc stannate: Synthesis, fundamental properties and applications. J. Mater. Chem. A 2017, 5, 20534–20560. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Zinc stannate nanostructures: Hydrothermal synthesis. Sci. Technol. Adv. Mater. 2011, 12, 013004. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Guo, Y.; Duan, H.; Li, H.; Liu, H. Synthesis of orthorhombic perovskite-type ZnSnO3 single-crystal nanoplates and their application in energy harvesting. ACS Appl. Mater. Interfaces 2017, 9, 8271–8279. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Chen, C.-Y.; Zhang, Y.; Chen, K.-H.; Yang, Y.; Hu, Y.; He, J.-H.; Wang, Z.L. Ultrahigh sensitive piezotronic strain sensors based on a ZnSnO3 nanowire/microwire. ACS Nano 2012, 6, 4369–4374. [Google Scholar] [CrossRef] [PubMed]
- Men, H.; Gao, P.; Zhou, B.; Chen, Y.; Zhu, C.; Xiao, G.; Wang, L.; Zhang, M. Fast synthesis of ultra-thin ZnSnO3 nanorods with high ethanol sensing properties. Chem. Commun. 2010, 46, 7581. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, T.; Zopes, D.; Mathur, S. Phase-selective microwave synthesis and inkjet printing applications of Zn2SnO4 (ZTO) quantum dots. J. Mater. Chem. 2012, 22, 17732. [Google Scholar] [CrossRef]
- Lo, M.-K.; Lee, S.-Y.; Chang, K.-S. Study of ZnSnO3-nanowire piezophotocatalyst using two-step hydrothermal synthesis. J. Phys. Chem. C 2015, 119, 5218–5224. [Google Scholar] [CrossRef]
- Mao, Y.; Park, T.-J.; Wong, S.S. Synthesis of classes of ternary metal oxide nanostructures. Chem. Commun. 2005, 5721–5735. [Google Scholar] [CrossRef]
- Choi, K.H.; Siddiqui, G.U.; Yang, B.; Mustafa, M. Synthesis of ZnSnO3 nanocubes and thin film fabrication of (ZnSnO3/PMMA) composite through electrospray deposition. J. Mater. Sci. Mater. Electron. 2015, 26, 5690–5696. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Bao, C.; Xue, G.; Zhang, J.; Liu, J.; Yu, T.; Zou, Z. Vertically building Zn2SnO4 nanowire arrays on stainless steel mesh toward fabrication of large-area, flexible dye-sensitized solar cells. Nanoscale 2012, 4, 3490–3494. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Janes, D.B.; Ju, S. Interface studies of N2 plasma-treated ZnSnO nanowire transistors using low-frequency noise measurements. Nanotechnology 2013, 24, 305201. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Nogami, M.; Yoshida, M.; Katsumata, T.; Inaguma, Y. First-principles studies on novel polar oxide ZnSnO3; pressure-induced phase transition and electric properties. Adv. Mater. 2010, 22, 2579–2582. [Google Scholar] [CrossRef] [PubMed]
- Dal Corso, A.; Posternak, M.; Resta, R.; Baldereschi, A. Ab initio study of piezoelectricity and spontaneous polarization in ZnO. Phys. Rev. B 1994, 50, 10715–10721. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Mukherjee, D.; Kons, C.; Witanachchi, S.; Mukherjee, P. Evidence of superior ferroelectricity in structurally welded ZnSnO3 nanowire arrays. Small 2014, 10, 4093–4099. [Google Scholar] [PubMed]
- Miyauchi, M.; Liu, Z.; Zhao, Z.-G.; Anandan, S.; Hara, K. Single crystalline zinc stannate nanoparticles for efficient photo-electrochemical devices. Chem. Commun. 2010, 46, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Sheng, Y.; Wan, L.; Bi, K.; Huang, K.; Jia, R.; Liu, J.; Wang, Y. A novel self-catalytic route to zinc stannate nanowires and cathodoluminescence and electrical transport properties of a single nanowire. J. Alloy. Compd. 2016, 657, 394–399. [Google Scholar] [CrossRef]
- Hwang, J.K.; Cho, S.; Dang, J.M.; Kwak, E.B.; Song, K.; Moon, J.; Sung, M.M. Direct nanoprinting by liquid-bridge-mediated nanotransfer moulding. Nat. Nanotechnol. 2010, 5, 742–748. [Google Scholar] [CrossRef]
- Lim, T.; Kim, H.; Meyyappan, M.; Ju, S. Photostable Zn2SnO4 nanowire transistors for transparent displays. ACS Nano 2012, 6, 4912–4920. [Google Scholar] [CrossRef]
- Siddiqui, G.U.; Rehman, M.M.; Choi, K.H. Enhanced resistive switching in all-printed, hybrid and flexible memory device based on perovskite ZnSnO3 via PVOH polymer. Polymer 2016, 100, 102–110. [Google Scholar] [CrossRef]
- Yang, Y.J.; Rehman, M.M.; Siddiqui, G.U.; Na, K.H.; Choi, K.H. Effect of adding a polymer and varying device size on the resistive switching characteristics of perovskite nanocubes heterojunction. Curr. Appl. Phys. 2017, 17, 1733–1741. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, K.-H.; Zhang, Y.; Wang, Z.L. A self-powered piezotronic strain sensor based on single ZnSnO3 microbelts. RSC Adv. 2013, 3, 25184. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, D.; Lee, J.H.; Kim, T.Y.; Gupta, M.K.; Kim, S.W. Unidirectional high-power generation via stress-induced dipole alignment from ZnSnO3 nanocubes/polymer hybrid piezoelectric nanogenerator. Adv. Funct. Mater. 2014, 24, 37–43. [Google Scholar] [CrossRef]
- Wang, G.; Xi, Y.; Xuan, H.; Liu, R.; Chen, X.; Cheng, L. Hybrid nanogenerators based on triboelectrification of a dielectric composite made of lead-free ZnSnO3 nanocubes. Nano Energy 2015, 18, 28–36. [Google Scholar] [CrossRef]
- Wu, J.M.; Xu, C.; Zhang, Y.; Yang, Y.; Zhou, Y.; Wang, Z.L. Flexible and transparent nanogenerators based on a composite of lead-free ZnSnO3 triangular-belts. Adv. Mater. 2012, 24, 6094–6099. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Xu, C.; Zhang, Y.; Wang, Z.L. Lead-free nanogenerator made from single ZnSnO3 microbelt. ACS Nano 2012, 6, 4335–4340. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, X.; Long, J.; Ding, Z.; Yan, T.; Zhang, G.; Zhang, Z.; Lin, H.; Fu, X. Hydrothermal synthesis, characterization, and photocatalytic properties of Zn2SnO4. J. Solid State Chem. 2009, 182, 517–524. [Google Scholar] [CrossRef]
- Khan, M.N.; Jaisai, M.; Dutta, J. Photocatalytic inactivation of Escherichia coli using zinc stannate nanostructures under visible light. Adv. Mater. Res. 2015, 1131, 203–209. [Google Scholar] [CrossRef]
- Zhao, Q.; Ju, D.; Song, X.; Deng, X.; Ding, M.; Xu, X.; Zeng, H. Polyhedral Zn2SnO4: Synthesis, enhanced gas sensing and photocatalytic performance. Sens. Actuators B Chem. 2016, 229, 627–634. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Chang, K.-S. Piezopotential-induced schottky behavior of Zn1−xSnO3 nanowire arrays and piezophotocatalytic applications. J. Am. Ceram. Soc. 2016, 99, 2593–2600. [Google Scholar] [CrossRef]
- Biswas, A.; Saha, S.; Jana, N.R. ZnSnO3 nanoparticle-based piezocatalysts for ultrasound-assisted degradation of organic pollutants. ACS Appl. Nano Mater. 2019, 2, 1120–1128. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, M.; Hu, C. Novel Zn2SnO4 hierarchical nanostructures and their gas sensing properties toward ethanol. J. Phys. Chem. C 2011, 115, 5522–5529. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, A.K.; Gautam, C. Synthesis and humidity sensing investigations of nanostructured ZnSnO3. J. Sens. Technol. 2011, 1, 116–124. [Google Scholar] [CrossRef]
- Tharsika, T.; Haseeb, A.S.M.A.; Akbar, S.A.; Sabri, M.F.M.; Wong, Y.H. Gas sensing properties of zinc stannate (Zn2SnO4) nanowires prepared by carbon assisted thermal evaporation process. J. Alloys Compd. 2015, 618, 455–462. [Google Scholar] [CrossRef]
- Pang, C.; Yan, B.; Liao, L.; Liu, B.; Zheng, Z.; Wu, T.; Sun, H.; Yu, T. Synthesis, characterization and opto-electrical properties of ternary Zn2SnO4 nanowires. Nanotechnology 2010, 21, 465706. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, Z.; Liang, B.; Wang, X.; Shen, G. Transparent metal oxide nanowire transistors. Nanoscale 2012, 4, 3001–3012. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.S.; Raychaudhuri, A.K. Fabrication of nanowires of multicomponent oxides: Review of recent advances. Mater. Sci. Eng. C 2005, 25, 738–751. [Google Scholar] [CrossRef]
- Ji, X.; Huang, X.; Liu, J.; Jiang, J.; Li, X.; Ding, R.; Hu, Y.; Wu, F.; Li, Q. Hydrothermal synthesis of novel Zn2SnO4 octahedron microstructures assembled with hexagon nanoplates. J. Alloys Compd. 2010, 503, L21–L25. [Google Scholar] [CrossRef]
- Zeng, J.; Xin, M.; Li, K.; Wang, H.; Yan, H.; Zhang, W. Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals. J. Phys. Chem. C 2008, 112, 4159–4167. [Google Scholar] [CrossRef]
- Rovisco, A.; Branquinho, R.; Martins, J.; Oliveira, M.J.; Nunes, D.; Fortunato, E.; Martins, R.; Barquinha, P. Seed-layer free zinc tin oxide tailored nanostructures for nanoelectronic applications: Effect of chemical parameters. ACS Appl. Nano Mater. 2018, 1, 3986–3997. [Google Scholar] [CrossRef]
- Su, B.; Wu, Y.; Jiang, L. The art of aligning one-dimensional (1D) nanostructures. Chem. Soc. Rev. 2012, 41, 7832. [Google Scholar] [CrossRef] [PubMed]
- Christian, B.; Volk, J.; Lukàcs, I.E.; Sautieff, E.; Sturm, C.; Graillot, A.; Dauksevicius, R.; O’Reilly, E.; Ambacher, O.; Lebedev, V. Piezo-force and vibration analysis of ZnO nanowire arrays for sensor application. Procedia Eng. 2016, 168, 1192–1195. [Google Scholar] [CrossRef]
- Fu, Y.; Nie, Y.; Zhao, Y.; Wang, P.; Xing, L.; Zhang, Y.; Xue, X. Detecting Liquefied Petroleum Gas (LPG) at room temperature using ZnSnO3ZnO nanowire piezo-nanogenerator as self-powered gas sensor. ACS Appl. Mater. Interfaces 2015, 7, 10482–10490. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.Y.; Chen, Y.J.; Wang, Y.G.; Wang, T.H. Synthesis and ethanol sensing properties of ZnSnO3 nanowires. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, T.; Zhang, R.; Lou, Z.; Deng, J.; Zhang, T. Comparison of toluene sensing performances of zinc stannate with different morphology-based gas sensors. Sens. Actuators B Chem. 2016, 227, 448–455. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, T.; Zhang, R.; Deng, J.; Lu, G.; Wang, L. Highly sensitive sensing platform based on ZnSnO3 hollow cubes for detection of ethanol. Appl. Surf. Sci. 2017, 400, 262–268. [Google Scholar]
- Najam Khan, M.; Al-Hinai, M.; Al-Hinai, A.; Dutta, J. Visible light photocatalysis of mixed phase zinc stannate/zinc oxide nanostructures precipitated at room temperature in aqueous media. Ceram. Int. 2014, 40, 8743–8752. [Google Scholar] [CrossRef]
- Mali, S.S.; Su Shim, C.; Kook Hong, C. Highly porous Zinc Stannate (Zn2SnO4) nanofibers scaffold photoelectrodes for efficient methyl ammonium halide perovskite solar cells. Sci. Rep. 2015, 5, 11424. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, G.U.; Rehman, M.M.; Choi, K.H. Resistive switching phenomena induced by the heterostructure composite of ZnSnO3 nanocubes interspersed ZnO nanowires. J. Mater. Chem. C 2017, 5, 5528–5537. [Google Scholar] [CrossRef]
- Cheng, B.; Ouyang, Z.; Chen, C.; Xiao, Y.; Lei, S. Individual Zn2SnO4-sheathed ZnO heterostructure nanowires for efficient resistive switching memory controlled by interface states. Sci. Rep. 2013, 3, 3249. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Zhang, J.; Li, Z.; Wang, G.; Gao, F.; Ewing, R.C.; Lian, J. Energetic stability, structural transition, and thermodynamic properties of ZnSnO3. Appl. Phys. Lett. 2011, 98, 1–4. [Google Scholar] [CrossRef]
- Montenegro, D.N.; Hortelano, V.; Martínez, O.; Martínez-Tomas, M.C.; Sallet, V.; Muñoz-Sanjosé, V.; Jiménez, J. Non-radiative recombination centres in catalyst-free ZnO nanorods grown by atmospheric-metal organic chemical vapour deposition. J. Phys. D Appl. Phys. 2013, 46, 235302. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.; Li, Q.; Wu, Y.; Li, Q.; Wang, T. An evolution from 3D face-centered-cubic ZnSnO3 nanocubes to 2D orthorhombic ZnSnO3 nanosheets with excellent gas sensing performance. Nanotechnology 2012, 23, 415501. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, Q.; Yang, Z. Biomorphic synthesis of ZnSnO3 hollow fibers for gas sensing application. Sens. Actuators B Chem. 2011, 156, 983–989. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Bao, D.; Wang, L.; Chen, Y.; Zhou, X.; Yang, P.; Sun, S.; Zhang, M. One pot, two phases: Individual orthorhombic and face-centered cubic ZnSnO3 obtained synchronously in one solution. Inorg. Chem. 2014, 53, 12289–12296. [Google Scholar] [CrossRef] [PubMed]
- Placke, A.; Kumar, A.; Priya, S. Synthesis and behavior of cetyltrimethyl ammonium bromide stabilized Zn1 + xSnO3 + x (0 ≤ x ≤ 1) nano-crystallites. PLoS ONE 2016, 11, e0156246. [Google Scholar] [CrossRef]
- Bora, T.; Al-Hinai, M.H.; Al-Hinai, A.T.; Dutta, J. Phase transformation of metastable ZnSnO3 upon thermal decomposition by in-situ temperature-dependent raman spectroscopy. J. Am. Ceram. Soc. 2015, 98, 4044–4049. [Google Scholar] [CrossRef]
- Kumari, V.; Patra, A.K.; Bhaumik, A. Self-assembled ultra-small zinc stannate nanocrystals with mesoscopic voids via a salicylate templating pathway and their photocatalytic properties. RSC Adv. 2014, 4, 13626–13634. [Google Scholar] [CrossRef]
- Saafi, I.; Dridi, R.; Mimouni, R.; Amlouk, A.; Yumak, A.; Boubaker, K.; Petkova, P.; Amlouk, M. Microstructural and optical properties of SnO2-ZnSnO3 ceramics. Ceram. Int. 2016, 42, 6273–6281. [Google Scholar] [CrossRef]
- Mary Jaculine, M.; Justin Raj, C.; Jerome Das, S. Hydrothermal synthesis of highly crystalline Zn2SnO4 nanoflowers and their optical properties. J. Alloys Compd. 2013, 577, 131–137. [Google Scholar] [CrossRef]
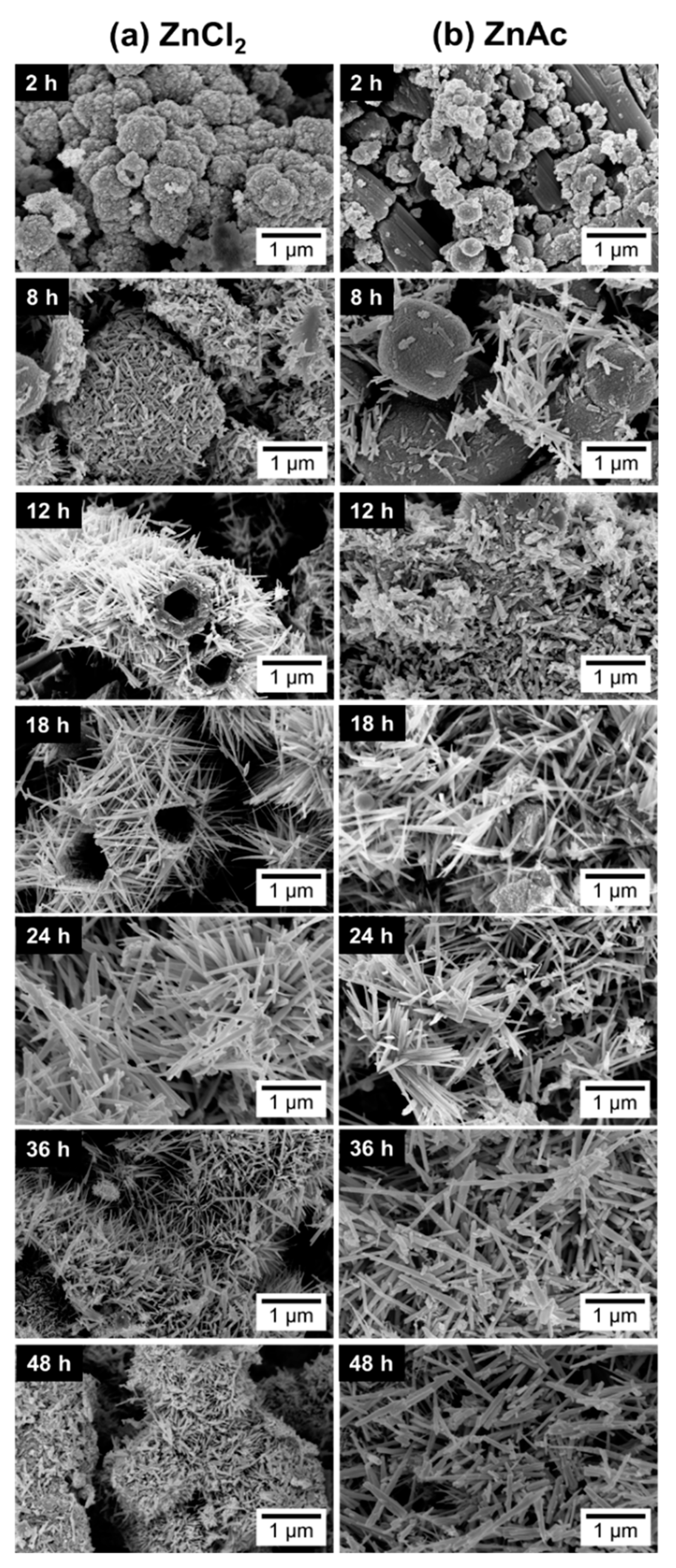
| Reaction Time (h) | Nanowires’ Length (nm) | |
|---|---|---|
| ZnCl2 | ZnAc | |
| 24 | 605 ± 75 | 540 ± 175 |
| 36 | 420 ± 115 | 1090 ± 250 |
| 48 | 220 ± 35 | 950 ± 190 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovisco, A.; Branquinho, R.; Martins, J.; Fortunato, E.; Martins, R.; Barquinha, P. Growth Mechanism of Seed-Layer Free ZnSnO3 Nanowires: Effect of Physical Parameters. Nanomaterials 2019, 9, 1002. https://doi.org/10.3390/nano9071002
Rovisco A, Branquinho R, Martins J, Fortunato E, Martins R, Barquinha P. Growth Mechanism of Seed-Layer Free ZnSnO3 Nanowires: Effect of Physical Parameters. Nanomaterials. 2019; 9(7):1002. https://doi.org/10.3390/nano9071002
Chicago/Turabian StyleRovisco, Ana, Rita Branquinho, Jorge Martins, Elvira Fortunato, Rodrigo Martins, and Pedro Barquinha. 2019. "Growth Mechanism of Seed-Layer Free ZnSnO3 Nanowires: Effect of Physical Parameters" Nanomaterials 9, no. 7: 1002. https://doi.org/10.3390/nano9071002
APA StyleRovisco, A., Branquinho, R., Martins, J., Fortunato, E., Martins, R., & Barquinha, P. (2019). Growth Mechanism of Seed-Layer Free ZnSnO3 Nanowires: Effect of Physical Parameters. Nanomaterials, 9(7), 1002. https://doi.org/10.3390/nano9071002