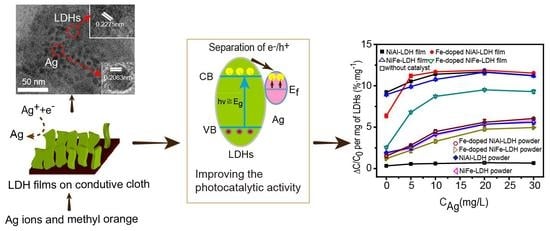
Controlled Growth of LDH Films with Enhanced Photocatalytic Activity in a Mixed Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of LDH Films
2.3. Characterization
2.4. Photocatalytic Property Measurement
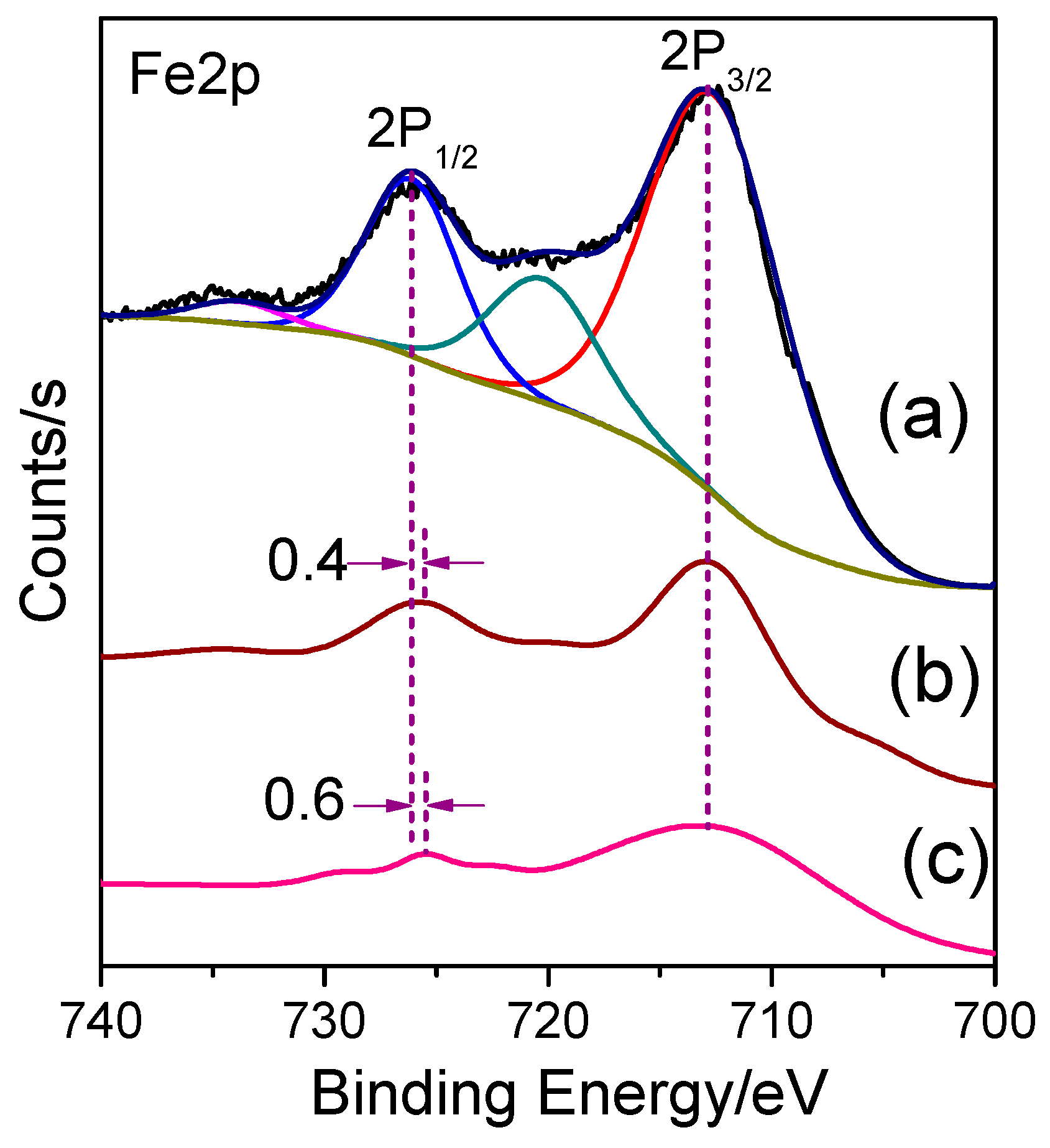
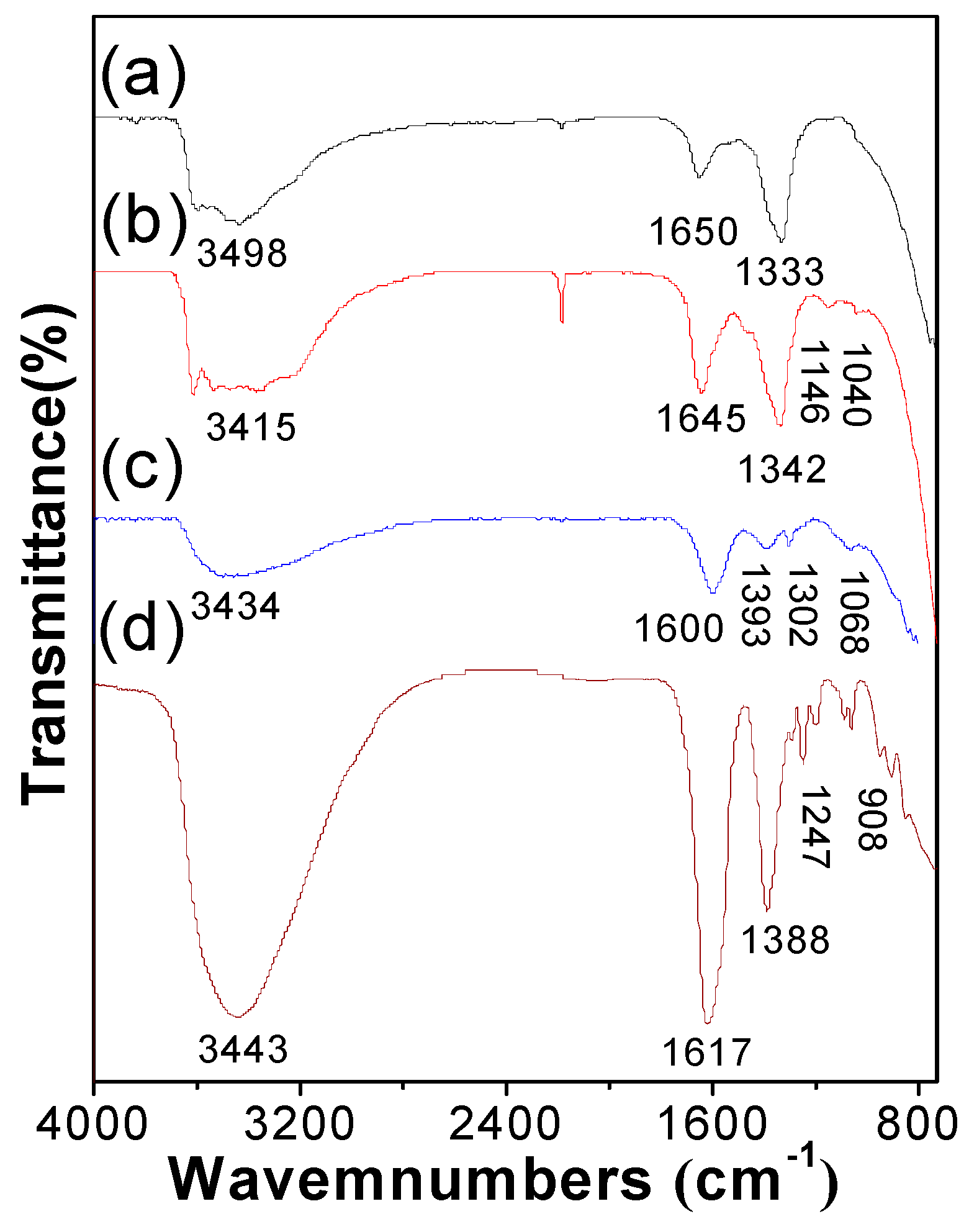
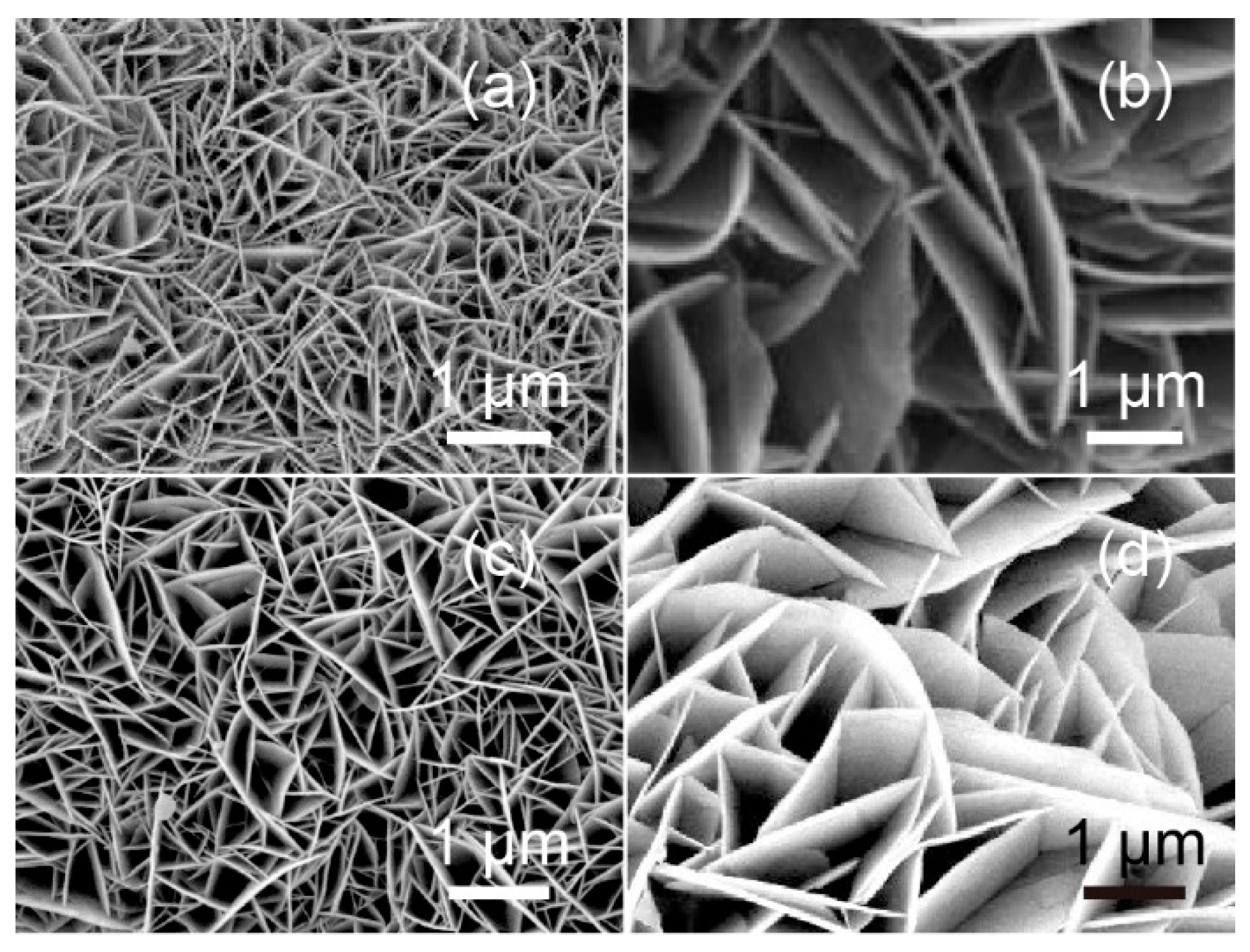
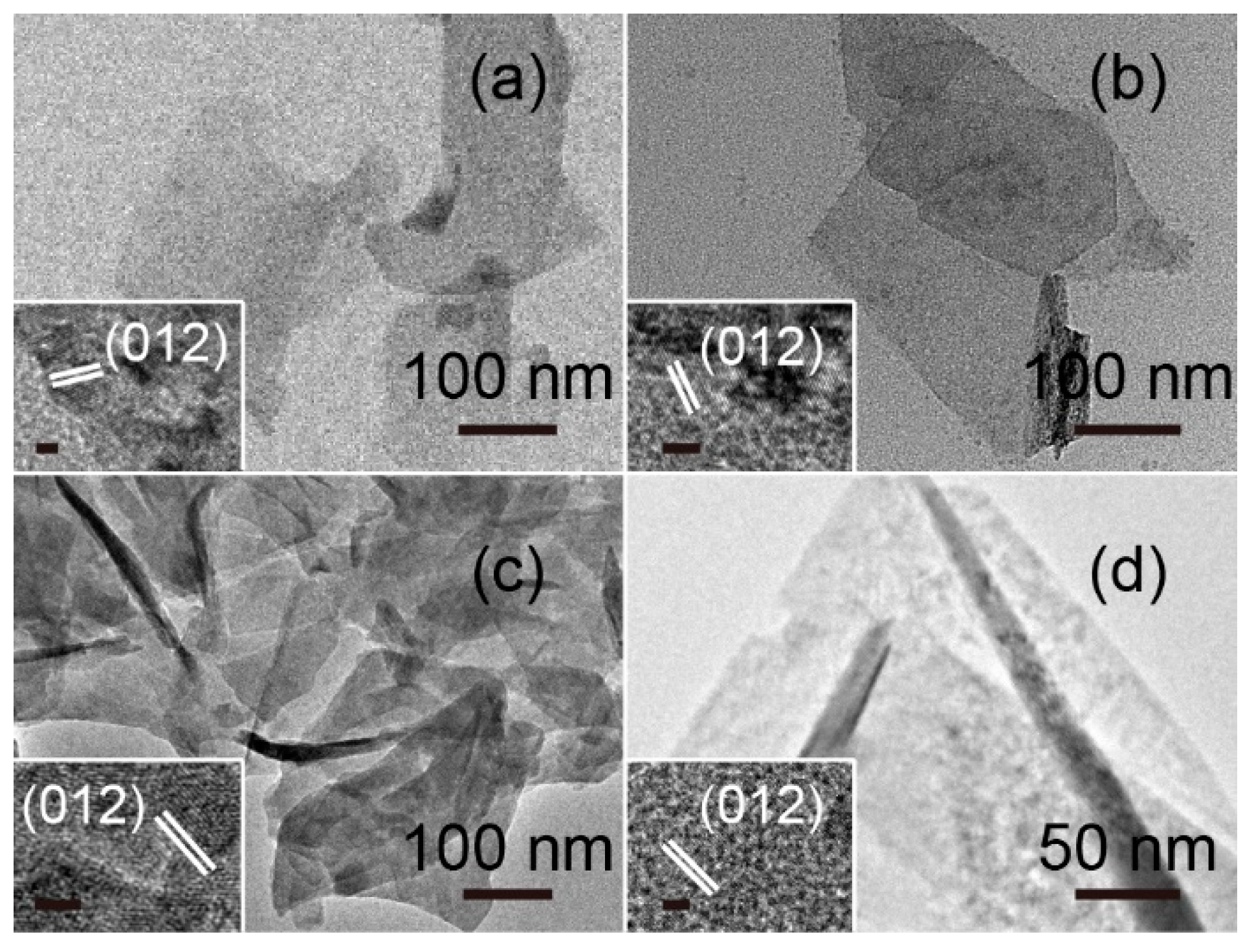
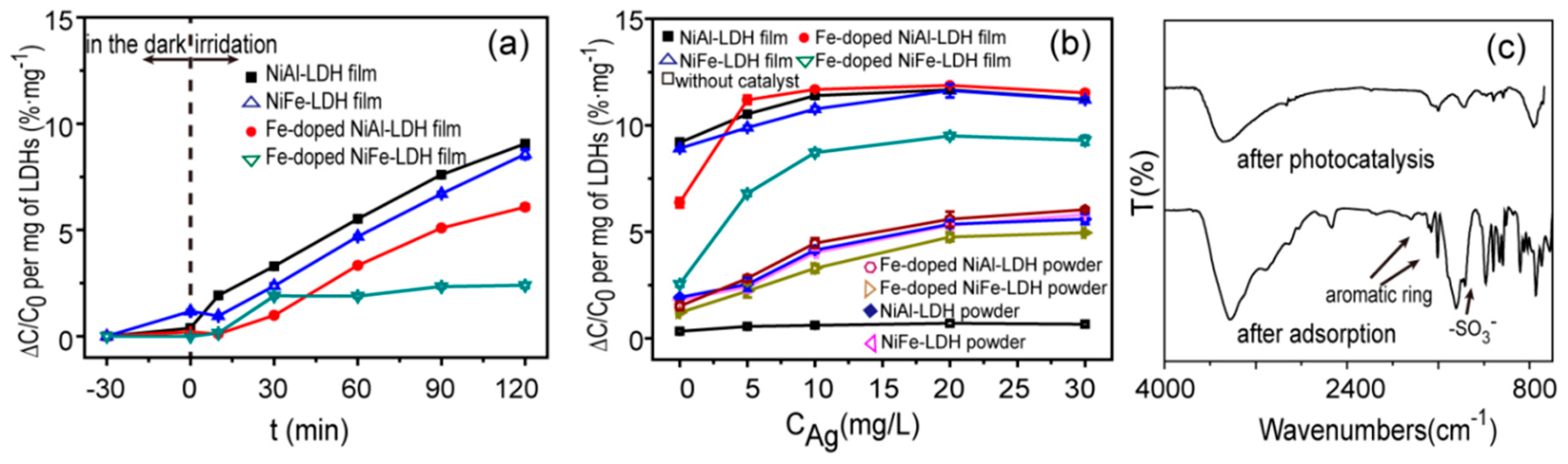
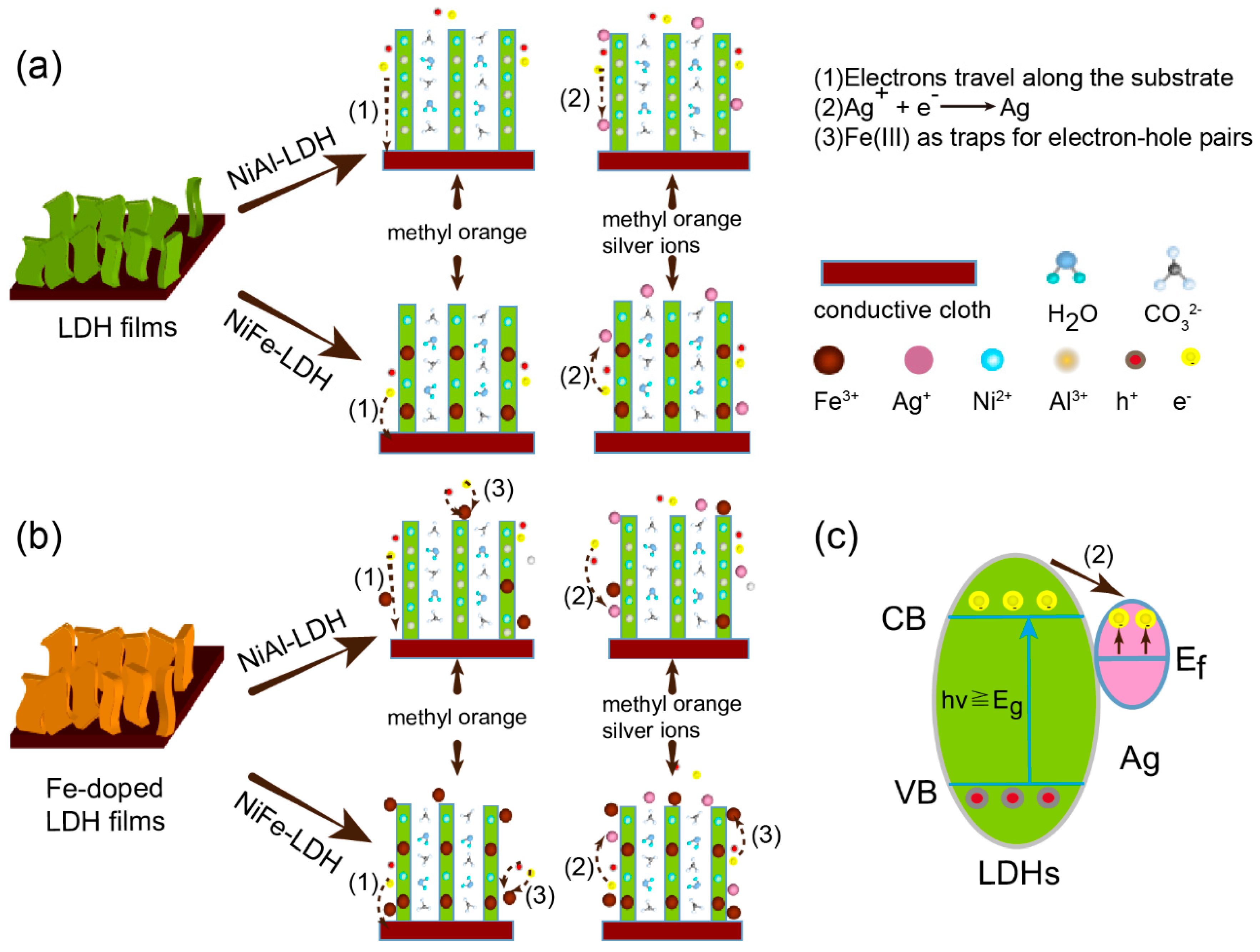
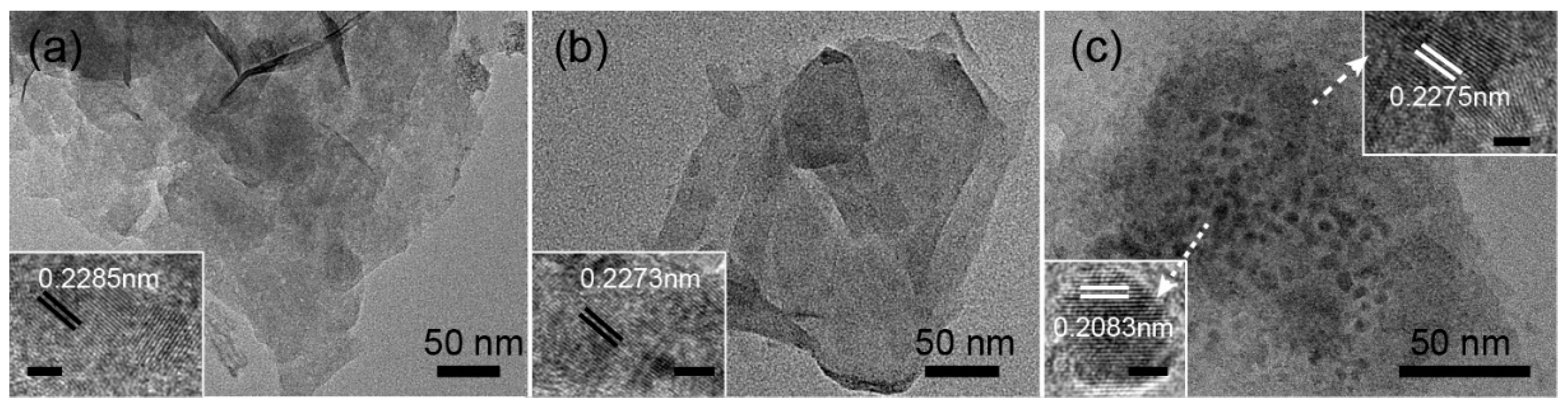
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Huang, Y.; Han, X.; Wu, Z.; Li, C.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons. Chem. Eng. J. 2018, 352, 306–315. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Deng, Q.; Ng, D.H.L.; Zhao, H. Microwave-assisted fabrication of nanoparticulate TiO2 microspheres for synergistic photocatalytic removal of Cr (VI) and methyl orange. ACS Appl. Mater. Interfaces 2014, 6, 3008–3015. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, C.; Wen, T.; Zhuang, L.; Wang, X.; Song, G.; Chen, D.; Ai, Y.; Hayat, T.; et al. Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions. Chem. Eng. J. 2018, 336, 241–252. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, Y.; Zhao, Y.; Wen, T.; Wang, X.; Chen, Z.; Sheng, G.; Chen, C.; Wang, X. Enhanced photocatalytic simultaneous removals of Cr(VI) and bisphenol A over Co(II)-modified TiO2. Langmuir 2019, 35, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Parida, K.M. Dynamics of charge-transfer behavior in a plasmon-induced quasi-type-II p–n/n–n dual heterojunction in Ag@Ag3PO4/g-C3N4/NiFe LDH nanocomposites for photocatalytic Cr(VI) reduction and phenol oxidation. ACS Omega 2018, 3, 7324–7343. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Jing, R.; Wu, M.; Zhao, S.; Liu, A.; Liu, Y.; Meng, Z. Superlattice assembly of two dimensional CoFe-LDHs nanosheets and titania nanosheets nanohybrids for high visible light photocatalytic activity. Mater. Lett. 2019, 236, 374–377. [Google Scholar] [CrossRef]
- Salehi, G.; Abazari, R.; Mahjoub, A.R. Visible-light-induced graphitic–C3N4 @nickel-aluminum layered double hydroxide nanocomposites with enhanced photocatalytic activity for removal of dyes in water. Inorg. Chem. 2018, 57, 8681–8691. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D hybrid heterojunction for high-performance photocatalytic reduction of CO2 into renewable fuels. ACS Appl. Mater. Interfaces 2018, 10, 2667–2678. [Google Scholar] [CrossRef]
- Li, P.; Huang, P.; Wei, F.; Sun, Y.; Cao, C.; Song, W. Monodispersed Pd clusters generated in situ by their own reductive support for high activity and stability in cross-coupling reactions. J. Mater. Chem. A 2014, 2, 12739–12745. [Google Scholar] [CrossRef]
- Liu, P.; Degirmenci, V.; Hensen, E. Unraveling the synergy between gold nanoparticles and chromium-hydrotalcites in aerobic oxidation of alcohols. J. Catal. 2014, 313, 80–91. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Zhu, G.; Wang, M.; Chen, Y.; Zhu, J.; Xi, Y.; He, H. Plasmonic Ag coated Zn/Ti-LDH with excellent photocatalytic activity. Appl. Surf. Sci. 2018, 433, 458–467. [Google Scholar] [CrossRef]
- Coronado, E.; Martí-Gastaldo, C.; Navarro-Moratalla, E.; Ribera, A. Intercalation of [M(ox)3]3− (M=Cr, Rh) complexes into NiIIFeIII-LDH. Appl. Clay Sci. 2010, 48, 228–234. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Wang, X.; Wang, L. Fluorescence enhancement of Tb3+-doped CaAl-LDH by cytosine. J. Lumin. 2018, 204, 42–50. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Shangguan, W. Metal (oxide) modified (M¼ Pd, Ag, Au and Cu) H2SrTa2O7 for photocatalytic CO2 reduction with H2O: the effect of cocatalysts on promoting activity toward CO and H2 evolution. Int. J. Hydrogen Energ. 2019, 44, 4123–4132. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyla, S.; Dibenedetto, A.; Aresta, M.; Macyk, W. Zinc sulfide functionalized with ruthenium nanoparticles for photocatalytic reduction of CO2. Appl. Catal. B-Enviro. 2015, 178, 170–176. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, Y.; Li, X. Synergistic effects of CuO and Au nanodomains on Cu2O cubes for improving photocatalytic activity and stability. Chinese J. Catal. 2019, 40, 105–113. [Google Scholar] [CrossRef]
- Rodríguez, N.A.; Savateev, A.; Grela, M.A.; Dontsova, D. Facile Synthesis of Potassium Poly(heptazine imide) (PHIK)/Ti-Based Metal−Organic Framework (MIL-125-NH2) Composites for Photocatalytic Applications. ACS Appl. Mater. Interfaces 2017, 9, 22941–22949. [Google Scholar] [CrossRef]
- Han, D.; Li, B.; Yang, S.; Wang, X.; Gao, W.; Si, Z.; Zuo, Q.; Li, Y.; Li, Y.; Duan, Q.; et al. Engineering Charge Transfer Characteristics in Hierarchical Cu2S QDs @ ZnO Nanoneedles with p–n Heterojunctions: Towards Highly Efficient and Recyclable Photocatalysts. Nanomaterials 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B-Enviro. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Zhu, B.; Yu, J.; Xu, J. CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl. Catal. B-Enviro. 2018, 230, 194–202. [Google Scholar] [CrossRef]
- Zhou, R.; Guzman, M.I. CO2 Reduction under Periodic Illumination of ZnS. J. Phys. Chem. C 2014, 118, 11649–11656. [Google Scholar] [CrossRef]
- Lan, M.; Fan, G.; Yang, L.; Li, F. Significantly enhanced visible-light-induced photocatalytic performance of hybrid Zn-Cr layered double hydroxide/graphene nanocomposite and the mechanism study. Ind. Eng. Chem. Res. 2014, 53, 12943–12952. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Bhattacharyya, K.G. Ni/Co/Ti layered double hydroxide for highly efficient photocatalytic degradation of Rhodamine B and Acid Red G: A comparative study. Photochem. Photobiol. Sci. 2017, 16, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ci, C.; Du, Y.; Liu, X.; Li, X.; Xie, X. Facile synthesis of NiAl-LDHs with tunable establishment of acid-base activity sites. Mater. Chem. Phys. 2018, 211, 72–78. [Google Scholar] [CrossRef]
- Jiang, D.B.; Jing, C.; Yuan, Y.; Feng, L.; Liu, X.; Dong, F.; Dong, B.; Zhang, Y.X. 2D-2D growth of NiFe LDH nanoflakes on montmorillonite for cationic and anionic dye adsorption performance. J. Colloid. Interf. Sci. 2019, 540, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.; Sahoo, M.; Singha, S. A novel approach towards solvent-free epoxidation of cyclohexene by Ti(IV)-Schiff base complex-intercalated LDH using H2O2 as oxidant. J. Catal. 2010, 276, 161–169. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Zhang, X.; Wang, X.; Zhu, N.; Wu, J.; Li, P. Intercalation of Fe(III) complexes into layered double hydroxides: Synthesis and structural preservation. Appl. Clay Sci. 2012, 65–66, 87–94. [Google Scholar] [CrossRef]
- Rajeshkhanna, G.; Singh, T.I.; Kim, N.H.; Lee, J.H. Remarkable bifunctional oxygen and hydrogen evolution electrocatalytic activities with trace-Level Fe doping in Ni- and Co-layered double hydroxides for overall water-splitting. ACS Appl. Mater. Interfaces 2018, 10, 42453–42468. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, Z.; Zhang, P.; Ma, H.; Gao, Z.; Wang, H.; Lu, Y.; He, J.; Zhao, Y. Transition metal ions regulated oxygen evolution reaction performance of Ni-based hydroxides hierarchical nanoarrays. Sci. Rep. 2017, 7, 46154. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, X.; Li, J.; Zhang, H.; Chen, S.; Han, W.; Yang, D. Surface modification of hematite photoanode by NiFe layered double hydroxide for boosting photoelectrocatalytic water oxidation. J. Alloys Compd. 2018, 764, 341–346. [Google Scholar] [CrossRef]
- Youn, D.H.; Park, Y.B.; Kim, J.Y.; Magesh, G.; Jang, Y.J.; Lee, J.S. One-pot synthesis of NiFe layered double hydroxide/reduced graphene oxide composite as an efficient electrocatalyst for electrochemical and photoelectrochemical water oxidation. J. Power Sources 2015, 294, 437–443. [Google Scholar] [CrossRef]
- Bajnóczi, E.G.; Balázs, N.; Mogyorósi, K.; Srankó, D.F.; Pap, Z.; Ambrus, Z.; Canton, S.; Norén, K.; Kuzmann, E.; Vértes, A.; et al. The influence of the local structure of Fe(III) on the photocatalytic activity of doped TiO2 photocatalysts-An EXAFS, XPS and Mósbauer spectroscopic study. Appl. Catal. B-Enviro. 2011, 103, 232–239. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Nocchetti, M.; Latterini, L.; Pagano, C.; Massetti, E.; Rossi, C. Immobilization of kojic acid in ZnAl-hydrotalcite like compounds. J. Phys. Chem. Solids. 2012, 73, 94–98. [Google Scholar] [CrossRef]
- Inamuddin. Xanthan gum/titanium dioxide nanocomposite for photocatalytic degradation of methyl orange dye. Int. J. Biol. Macromol. 2019, 121, 1046–1053. [Google Scholar] [CrossRef]
- Reszczynska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible light activity of rare earth metal doped (Er3+, Yb3+ or Er3+/Yb3+) titania photocatalysts. Appl. Catal. B-Enviro. 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Boppella, R.; Choi, C.; Moon, J.; Kim, D.H. Spatial charge separation on strongly coupled 2D-hybrid of rGO/La2Ti2O7/NiFe-LDH heterostructures for highly efficient noble metal free photocatalytic hydrogen generation. Appl. Catal. B-Enviro. 2018, 239, 178–186. [Google Scholar] [CrossRef]
- Ambrus, Z.; Balázs, N.; Alapi, T.; Wittmann, G.; Sipos, P.; Dombi, A.; Mogyorósi, K. Synthesis, structure and photocatalytic properties of Fe(III)-doped TiO2 prepared from TiCl3. Appl. Catal. B-Enviro. 2008, 81, 27–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, T.X.; Hu, Y.B.; Guo, X.L.; Peng, H.H.; Zhang, Y.X.; Feng, L.; Zheng, H.L. Delta manganese dioxide nanosheets decorated magnesium wire for the degradation of methyl orange. J. Colloid Interf. Sci. 2017, 490, 226–232. [Google Scholar] [CrossRef]
- Chen, G.; Bian, S.; Guo, C.Y.; Wu, X. Insight into the Z-scheme heterostructure WO3/g-C3N4 for enhanced photocatalytic degradation of methyl orange. Mater. Lett. 2019, 236, 596–599. [Google Scholar] [CrossRef]
- Lva, J.; Zhu, Q.; Zeng, Z.; Zhang, M.; Yang, J.; Zhao, M.; Wang, W.; Cheng, Y.; He, G.; Sun, Z. Enhanced photocurrent and photocatalytic properties of porous ZnO thin film by Ag nanoparticles. J. Phys. Chem. Solids 2017, 111, 104–109. [Google Scholar] [CrossRef]
- Hoque, M.A.; Guzman, M.I. Photocatalytic activity: experimental features to report in heterogeneous photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Bahnemann, D.W. Undesired role of sacrificial reagents in photocatalysis. J. Phys. Chem. Lett. 2013, 4, 3479–3483. [Google Scholar] [CrossRef]
- Kamat, P.V.; Jin, S. Semiconductor photocatalysis: “tell us the complete story!”. ACS Energy Lett. 2018, 3, 622–623. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Fang, P.; Kumar, P.; Wang, W.; Liu, B.; Li, J. Controlled Growth of LDH Films with Enhanced Photocatalytic Activity in a Mixed Wastewater Treatment. Nanomaterials 2019, 9, 807. https://doi.org/10.3390/nano9060807
Wang Z, Fang P, Kumar P, Wang W, Liu B, Li J. Controlled Growth of LDH Films with Enhanced Photocatalytic Activity in a Mixed Wastewater Treatment. Nanomaterials. 2019; 9(6):807. https://doi.org/10.3390/nano9060807
Chicago/Turabian StyleWang, Zhongchuan, Pengfei Fang, Parveen Kumar, Weiwei Wang, Bo Liu, and Jiao Li. 2019. "Controlled Growth of LDH Films with Enhanced Photocatalytic Activity in a Mixed Wastewater Treatment" Nanomaterials 9, no. 6: 807. https://doi.org/10.3390/nano9060807
APA StyleWang, Z., Fang, P., Kumar, P., Wang, W., Liu, B., & Li, J. (2019). Controlled Growth of LDH Films with Enhanced Photocatalytic Activity in a Mixed Wastewater Treatment. Nanomaterials, 9(6), 807. https://doi.org/10.3390/nano9060807