3.1. The Influence of Polymerization Solution pH on PANI/GOx, Ppy/GOx and PTh/GOx Composite Nanoparticles Formation
The large number of research papers is dealing with the chemical and electrochemical polymerization of aniline, pyrrole, and thiophene, but only few researches are dealing with the enzymatic formation of these CPs. The enzymatic formation of these CPs can be based on the application of four major compounds: corresponding monomer, glucose oxidase, glucose, and oxygen, which is naturally dissolved in used solution (
Figure 1).
GOx generates hydrogen peroxide and gluconolactone in the presence of glucose and dissolved oxygen, which is hydrolyzed to gluconic acid in an aqueous solution. Hydrogen peroxide as a strong oxidizer generates radical cation of pyrrole, aniline [
18,
19,
25], and, in such a way, it initiates the polymerization reaction in acidic environment that is created by gluconic acid [
27]. These free radical cations undergo coupling and, in this way, oligomers and polymeric structures are formed [
18,
19]. The diffusional permeability of formed polymeric structures is sufficient; therefore, a decent substrate and product mobility towards/outwards the enzyme, which is encapsulated within the formed polymer layer, is retained [
27].
PANI and Ppy are very often used for the immobilization of biomolecules [
1]. Neutral pH values are required for the enzymatic synthesis of PANI- and Ppy-based composites [
21,
24,
39]. However, good polymerization efficiency at neutral pHs is difficult to achieve; in addition, PANI and Ppy usually are better conducting and are more electroactive only in their protonated form, which is formed at low pH [
1]. The concentration of the reactant and the velocity of oxidation process both increase at low values of pH [
36]. At pH > 4.6, both aniline and pyrrole monomers and corresponding polymer chains are not protonated and, in such case, only neutral nitrogen-containing species are involved in PANI or Ppy formation reactions which are characterized by low oxidation potential [
6]. Therefore, the pH value of the polymerization solution is a very important parameter for the formation of PANI or Ppy [
18].
The enzymatic formation of PANI-, Ppy-, and PTh-based composite nanoparticles with embedded glucose oxidase was monitored by UV/Vis spectrophotometry in the pH interval from 1.0 to 12. During the first few minutes, the polymerization bulk solutions containing aniline, pyrrole, or thiophene were colourless and any absorbance peaks were observed in the visible part of spectra. The formation of polymeric nanoparticles (PANI/GOx, Ppy/GOx, or PTh/GOx, respectively) was observed after 24 h of polymerization. In the case of the formation of PANI/GOx and Ppy/GOx, the solutions become coloured yellow-brown and black-grey, respectively. It was later observed that the intensity of colour and the value of optical absorbance were dependent on the pH value of the polymerization solution. Preliminary spectrophotometric investigations showed that the enzymatic polymerization of aniline [
21], pyrrole [
24,
25] and thiophene [
12,
15] was followed by the formation of peaks with optical absorbance maximum (
λmax) at 440 nm, 460 nm, and 450 nm, respectively (
Figure 2A,
Figure 3A and
Figure 4A). These
λmax corresponds to the formation of aniline, pyrrole and thiophene oligomers. The optical absorbance peaks at
λmax = 260 nm were registered in all cases; this absorbance can be explained by a presence of protein—glucose oxidase—in the polymerization solutions, because amino acid phenylalanine optical absorbance peak is at
λmax = 260 nm.
PANI/GOx colloidal solution after 48 h lasting enzymatic polymerization was characterised by one for PANI characteristic optical absorbance peak with maximum
λmax = 440 nm (
Figure 2A), which is in agreement with another investigations related to synthesis of PANI [
8,
11,
28]. Some of the authors reported the optical absorbance peak at
λmax = 360 nm, which is attributed to emeraldine salt, corresponding to
π-
π* transition of PANI benzene ring delocalized on nitrogen atoms and related to the formation of cyclic aniline dimer 5,10-dihydrophenazine [
8,
11,
28]. It was reported that optical absorbance peaks at
λmax =360 nm and
λmax = 420 nm are able to combine into a flat or distorted single peak with a new maximum being formed between these initial two peaks [
28]. The oxidation of 5,10-dihydrophenazine generates phenazylium radical-cation, which absorbs at
λmax = 440 nm. Such a phenazylium radical-cation is usually insoluble in the most organic solvents and in aqueous medium of low pH value [
6]. All of the PANI/GOx composite nanoparticles that formed in polymerization bulk solution in the pH range from 5.0 to 8.0 were formed in the emeraldine oxidation form, which has been confirmed by the formation of brown-coloured precipitate.
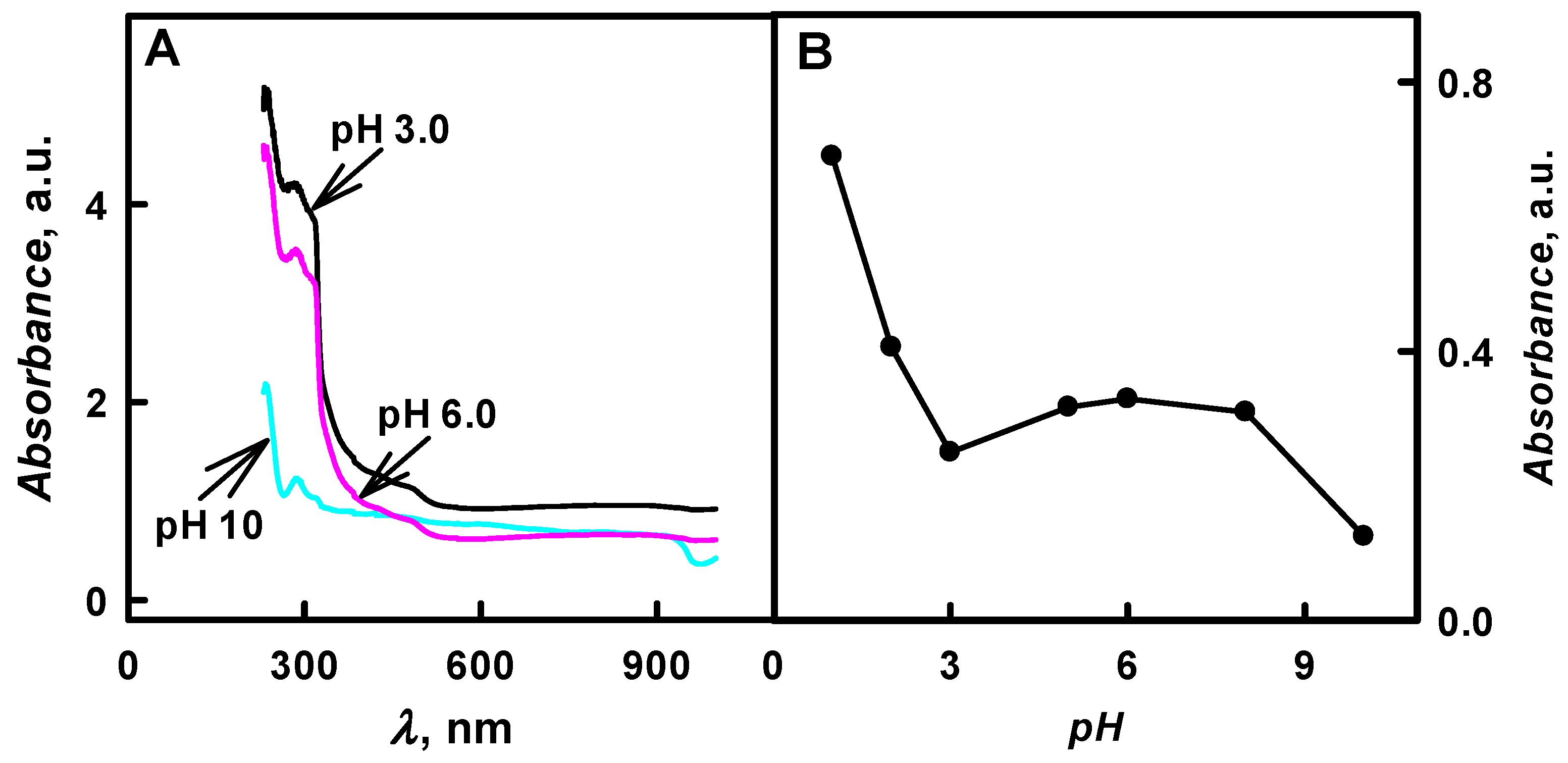
Figure 2B presents the influence of pH value of polymerization solution on the formation of PANI/GOx composite nanoparticles. It was determined that, by the increase of pH value from 1.0 to 10, the optical absorbance of formed colloidal PANI/GOx nanoparticle solution at
λmax = 440 nm has decreased 5.52 times (from 0.690 to 0.125 a.u.). The highest value of optical absorbance (0.690 a.u. at
λmax = 440 nm) was registered in the solution of pH 1.0 after 48 h lasting polymerization of aniline. However, the pH 1.0 is not suitable for PANI/GOx formation due to the inactivation of GOx and due to the non-enzymatic polymerization at this extreme pH value. In the research that was performed by another authors, it was presented that, by the increase of pH value, the polaron optical absorbance at
λmax = 420 nm and
λmax = 823 nm gradually decreases and a strong optical absorbance is observed due to the transition of the quinoid rings, which emerges at
λ = 560–600 nm [
22]. The most suitable solution for the formation of emeraldine form of PANI/GOx composite nanoparticles was SA buffer solution, pH 6.0 (absorbance value of 0.329 a.u. at
λmax = 440 nm was observed).
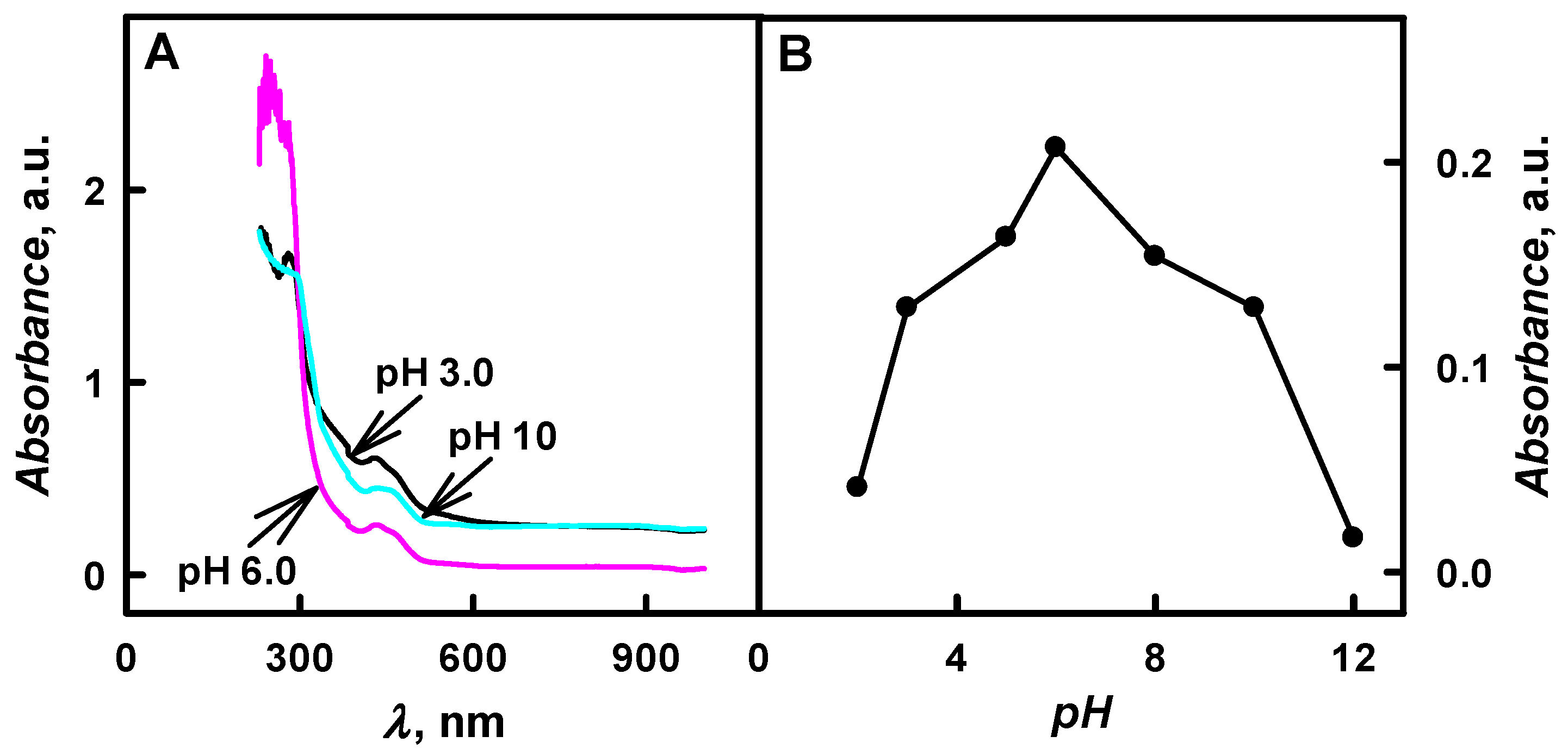
The relationship between the optical absorbance of colloidal solution of Ppy/GOx composite nanoparticles and the pH values in the range from 2.0 to 12 of polymerization solution is presented in
Figure 3. The optical absorbance peak at
λmax = 460 nm has been observed during the formation of Ppy/GOx composite nanoparticles, which is in an agreement with the results that were obtained during the chemical polymerization of pyrrole [
7]. Optical absorbance at
λmax = 460 nm is attributed to a transition from valence bond to the polaron or bipolaron state [
7]. The peak of optical absorbance with
λmax = 460 nm was observed at the early stage of pyrrole oxidation, and it was identified that it belongs to the intermediate that formed during the oxidative polymerization of pyrrole. Some authors mentioned the optical absorbance peak at
λmax = 360 nm, which is related to
π-
π* transition [
7]. During enzymatic polymerization, the formed Ppy/GOx composite nanoparticles were of intensive black colour when polymerization was performed at pH 2.0 and pH 3.0; and, intensive dark-grey—in pH range from 5.0 to 12. Black colour is the most characteristic for the delocalised
π-electron system of doped Ppy [
25].
It was determined that, by the increase of pH value of polymerization bulk solution from 3.0 to 6.0, the absorbance at
λmax = 460 nm has increased by 1.60 times (
Figure 3B). Additionally, the decrease of the absorbance was followed by the increase of pH value from 8.0 to 12, and it is in agreement with investigations of other researchers [
18]. The absorbance at
λmax = 460 nm has decreased by 9.17 times when polymerization was performed in pHs ranging from 8.0 to 12 (
Figure 3B). Alkaline solutions were considered to be less suitable for Ppy formation. According to investigations [
18], the neutral radical that formed in strong bases is not able to form the dimer that consists of two pyrrole molecules. The highest value of the absorbance (0.207 a.u.) was registered at pH 6.0 after 48 h of enzymatic polymerization in all cases.
The main charge carriers in PTh are polarons and bipolarons [
15].
Figure 4 presents the optical absorbance spectra of formed PTh/GOx composite nanoparticles in the pH interval from 1.0 to 12. The UV/Vis absorbance spectra of colloidal solutions of PTh/GOx are characterized by optical absorbance peak at
λmax = 450 nm. Optical absorbance peak (
λmax = 450 nm) is attributed to the
π-
π* transition for large conjugated structure [
12,
34]. Some authors mentioned optical absorbance peak at
λmax = 890 nm, which is related to the bipolaron state [
15]. The appearance of optical absorbance peak at
λmax = 450 nm was in agreement with the investigations related to chemical-oxidative formation of: (i) PTh-based 60–100 nm nanoparticles in water solution containing copper (II) ions and H
2O
2 as oxidizer [
12] and (ii) PTh-based 25–45 nm nanoparticles in the presence of FeCl
3 as an oxidizer [
15].
As presented in
Figure 4B, the absorbance at
λmax = 450 nm was dramatically changed (4.96 times), from 0.124 to 0.025 a.u. by the increase of pH value from 1.0 to 12. The absorbance of 0.023 a.u. at
λmax = 450 nm was registered at pH 6.0 after 48 h of enzymatic polymerization.
3.2. The Comparison of Optical Absorbance of PANI/GOx, Ppy/GOx and PTh/GOx Composite Nanoparticles Colloidal Solution and the Choice of Optimal Enzyme/Substrate Ratio
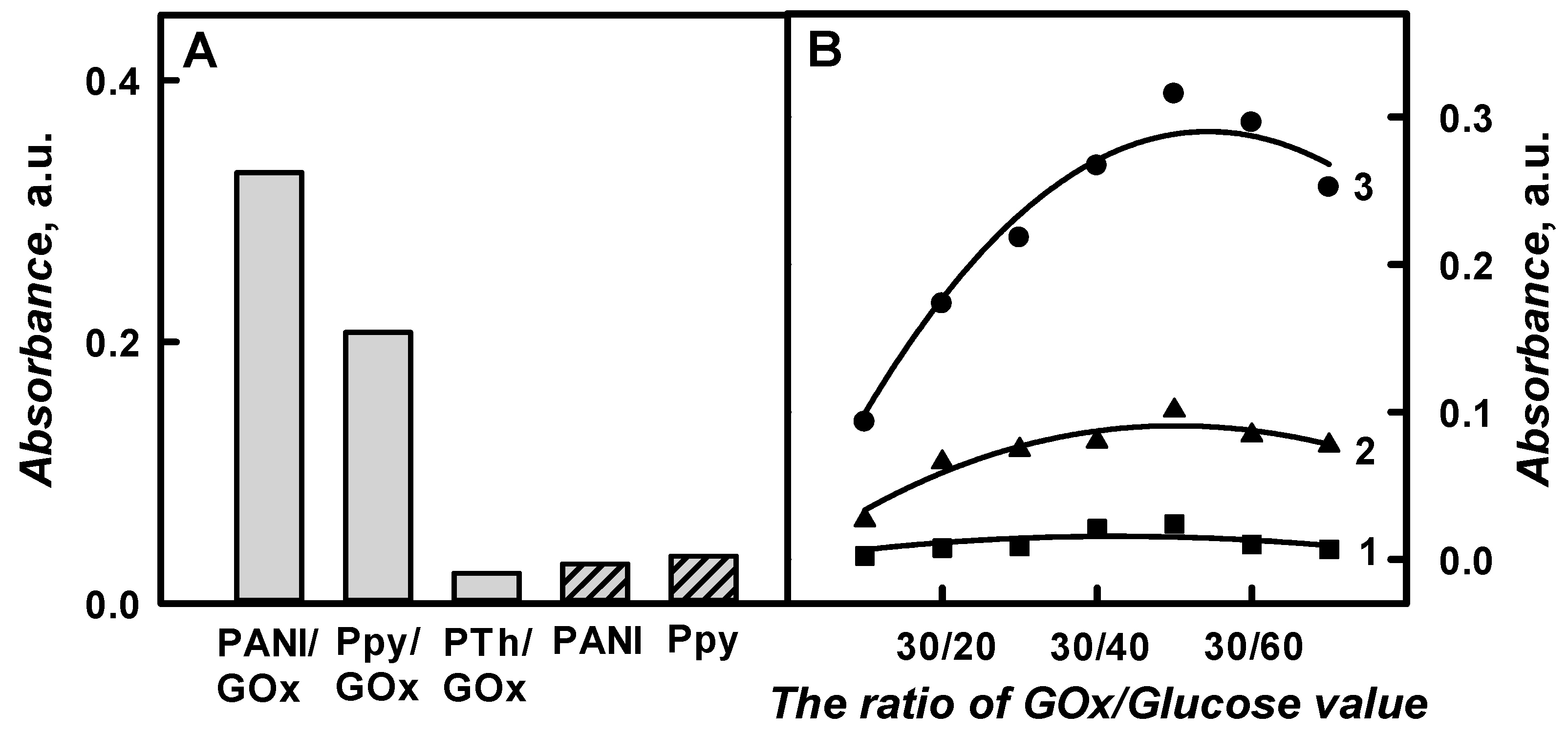
The optical absorbance of PANI/GOx, Ppy/GOx, and PTh/GOx composite nanoparticles colloidal solutions at
λmax = 440 nm,
λmax = 460nm, and
λmax = 450 nm, respectively, was assessed in SA buffer, pH 6.0, after 48 h lasting polymerization and was 0.329, 0.207, and 0.023 a.u., respectively (
Figure 5A). It was noticed that the optical absorbance of PTh/GOx composite nanoparticles after 48 h lasting enzymatic polymerization was 14.3 and 9.00 times lower than that for the PANI/GOx and Ppy/GOx composite nanoparticles. As it is presented in
Figure 5A, the absorbance of formed PANI/GOx colloidal solution after 48 h of polymerization was 1.59 times higher than that of the Ppy/GOx colloidal solution. The optical control of PTh/GOx composite nanoparticles formation was more complicated in comparison to that of PANI/GOx or Ppy/GOx composite nanoparticles formation due to the slower polymerization rate. It should be noted that it is quite difficult to prepare the solution of well-dispersed polythiophene particles, because they tend to aggregate and precipitate [
12,
34]. Therefore, further investigations of a polymerization’s duration were focused on the enzymatic formation of PANI/GOx and Ppy/GOx composite nanoparticles.
As it has been mentioned by other authors, the Ppy formation can be performed in the solution without any chemical oxidant or enzyme [
18], and this process is named autopolymerization. An experiment was performed in solutions containing only 0.05 mol L
−1 of glucose and 0.5 mol L
−1 of aniline, pyrrole, or thiophene at pH from 1.0 to 12 without GOx, to evaluate the influence of the autopolymerization on the formation of PANI, Ppy and PTh. Autopolymerization was not observed in the pH range from 1.0 to 12 for thiophene. The slow autopolymerization has only been observed at pH 1.0 for aniline and at pH below 2.0 for pyrrole and it reached 0.030 and 0.035 a.u. after 48 h lasting polymerization, respectively. This autopolymerization phenomenon was not observed at a higher pH value. The polymerization rate of aniline was 1.20 times slower in the comparison of pyrrole at pH 2.0 at pH 1.0 in the absence of GOx. The polymerization rate of pyrrole at pH 2.0 in the absence and in the presence of GOx was the same. It means that GOx was inactivated at this low pH. We have performed the polymerization at pH values higher than 3.0 in order to avoid the autopolymerization. The results of enzymatic polymerization were in an agreement with the results that were obtained for PANI [
21] and Ppy [
24]. Significant differences of the absorbance were detected in the absence and in the presence of GOx demonstrate a significant impact of the enzyme to polymerization reaction rate. The most optimal formation rate of PANI/GOx and Ppy/GOx composite nanoparticles was observed in 0.05 mol L
−1 SA buffer, pH 6.0, where GOx exhibits the maximal catalysed reaction rate and the highest stability, according to our measurements [
24,
39].
The type of oxidizing agent has significant influence on the oxidative polymerization reaction [
18]. It was determined that the polymerization is faster at higher (0.50 mol·L
−1) monomer’s concentration [
22], but further increasing of monomer’s concentration negatively influenced the formation of polymeric nanoparticles. The rate of polymerization reaction and the properties of the formed polymer not only depend on the preparation technique, selected solvent, polymerization duration, temperature, kind, and concentration of monomer, but also on the enzyme and substrate ratio [
18,
21].
The effect of enzyme/substrate ratio on the rate of PANI/GOx, Ppy/GOx, and PTh/GOx composite nanoparticles formation was investigated in the next set of spectrophotometric measurements. The investigations were performed during 48 h in the polymerization solution, pH 6.0, containing 0.50 mol L
−1 of aniline, pyrrole, or thiophene, 0.75 mg mL
−1 of GOx and glucose concentration varying from 0.015 to 0.05 mol L
−1.
Figure 5B presents the influence of the ratio of GOx/Glucose on the registered absorbance.
The registered absorbance significantly increased by increasing glucose concentration from 0.015 mol L−1 in the polymerization solution up to 0.05 mol L−1. The absorbance of PANI/GOx, Ppy/GOx, and PTh/GOx composite nanoparticles, which were prepared using 0.75 mg mL−1 of GOx and 0.05 mol L−1 of glucose, increased by 3.39, 3.79, and 10.1 times in a comparison to the absorbance that was obtained for polymeric nanoparticles with embedded GOx using 0.015 mol L−1 of glucose. By the increase of glucose concentration up to 0.015 mol L−1, the absorbance of PANI/GOx, Ppy/GOx, and PTh/GOx decreased 1.25, 1.31, and 3.53 times, if compared with the results that were obtained for the absorbance with 0.75 mg mL−1 of GOx and 0.05 mol L−1 of glucose. Thus, the ratio of GOx/Glucose 0.75 mg mL−1/0.05 mol L−1 was selected as the most optimal for polymeric nanoparticle composites formation.
3.3. The Evaluation of PANI/GOx, Ppy/GOx and PTh/GOx Composite Nanoparticles Formation by DLS and the Morphology of PANI/GOx, Ppy/GOx Nanocomposites
Depending on pH, the negatively or positively charged enzymes can be entrapped within the CPs during oxidation/reduction reactions [
20]. Enzymatic polymerization strongly depends on the solution’s pH. For instance, the conducting form of linear PANI is synthesized at pH 4.0–4.5, wherein, at pH 6.0 or higher, more branched and insulating form of PANI is formed [
11,
22].
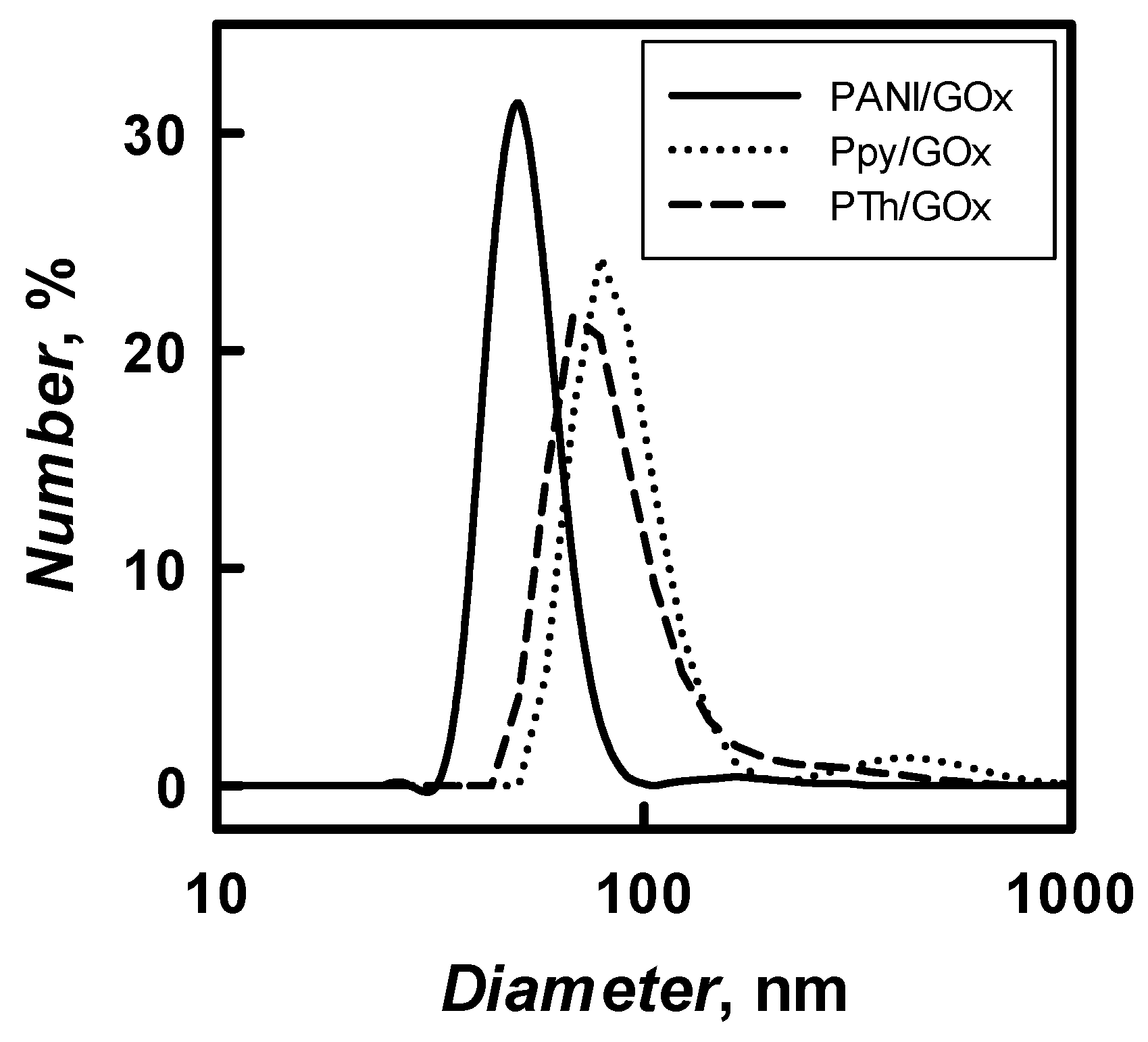
The diameter of 48 h enzymatically synthesized PANI-, Ppy-, and PTh-based nanocomposites with embedded GOx in solution of pH 2.0–8.0 was evaluated by the DLS technique. The results of PANI/GOx, Ppy/GOx, and PTh/GOx composite nanoparticles diameter and their distribution are presented in
Table 1 and
Figure 6, respectively. The diameter of PANI/GOx, Ppy/GOx, and PTh/GOx composites nanoparticles depended on 5h3 pH of polymerization solution and it decreased by the increase of pH. It was determined that, in SA buffer, pH 6.0, PANI/GOx, Ppy/GOx, and PTh/GOx composite nanoparticles of 54, 86, and 98 nm diameter were formed, respectively. The DLS results (
Table 1) represent that formed PANI/GOx composite nanoparticles were 1.59 and 1.81 times smaller if compared with Ppy/GOx and PTh/GOx. Due to the relatively large (535 nm) diameters of PTh/GOx, which were formed at pH 8.0, we decided that such relatively large particle colloidal solution will be not very stable and mostly focused on the investigation of PANI/GOx and Ppy/GOx composite nanoparticles, which are smaller; therefore, it was expected that colloidal solution of these nanoparticles will be more stable.
Some of the researchers evaluated the diameter of synthesized polymer nanocomposites by FE-SEM. During the enzymatic polymerization of aniline using chitosan and poly(
N-isopropylacrylamide) as steric stabilizers 50 nm PANI nanoparticles were synthesized [
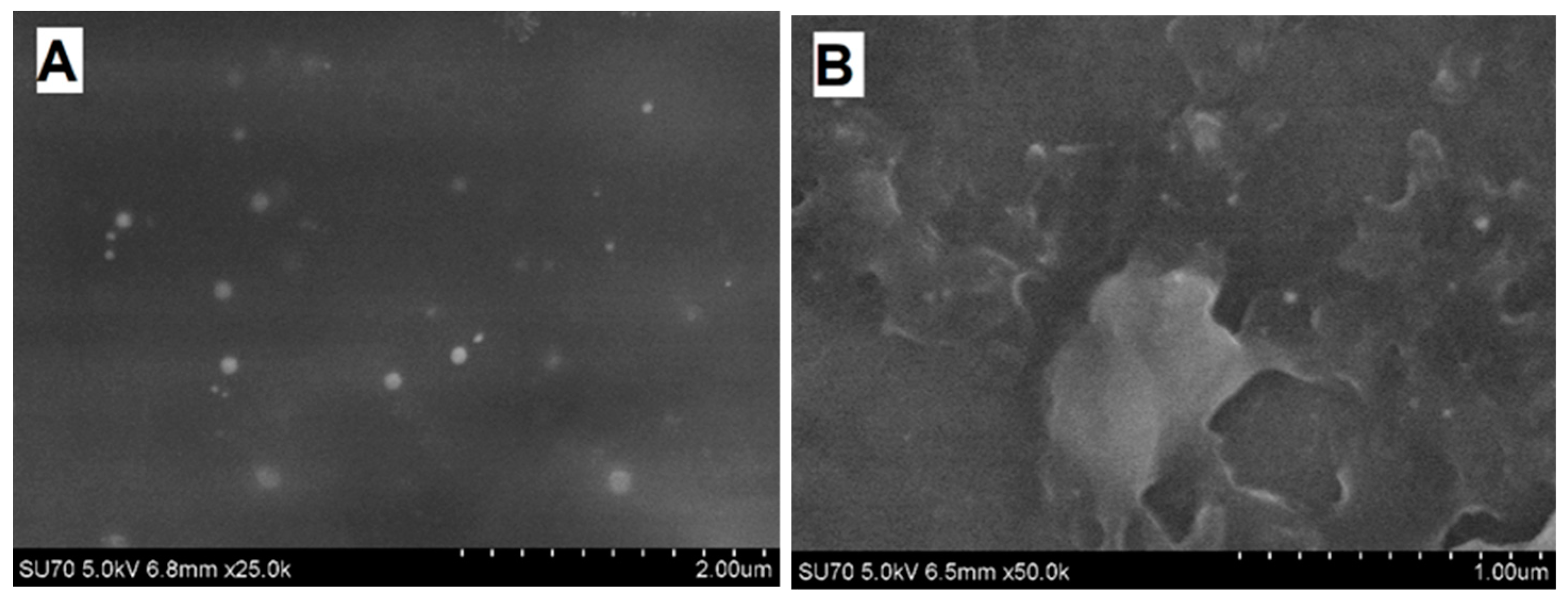
11]. In our study, PANI/GOx and Ppy/GOx composite nanoparticles formed during 24 h lasting enzymatic polymerization were investigated by FE-SEM (
Figure 7).
Spherical PANI/GOx and Ppy/GOx composite nanoparticles were formed during enzymatic polymerization. It is seen that the PANI/GOx (
Figure 7A) nanoparticles were randomly distributed on the surface of graphite rod used for investigations. The Ppy/GOx nanoparticles were densely stuck together (
Figure 7B). The diameter of nanoparticles depends on the kind of polymerized monomer. It was determined that the diameter of PANI/GOx and Ppy/GOx composites was in the range of 38–50 and 50–100 nm, respectively.
3.4. The Influence of Polymerization Duration on PANI/GOx and Ppy/GOx Composite Nanoparticles Formation
One of most important parameters of polymer‘s formation is the duration of a polymerization. The influence of polymerization duration (from 5 to 288 h) on the formation of PANI/GOx and Ppy/GOx composite nanoparticles was investigated. The polymerization was performed in 0.05 mol L
−1 SA buffer, pH 6.0, with 0.05 mol L
−1 of glucose, 0.50 mol L
−1 of aniline or pyrrole, and 0.75 mg mL
−1 of GOx. Visible aggregation of PANI/GOx and Ppy/GOx was observed after 48 h lasting enzymatic polymerization. Monomers are oxidized during the enzymatic polymerization and the amount of polymers is increasing by the prolongation of synthesis’s duration. It should be noted that the enzyme tends to loss catalytic activity due to long lasting polymerization [
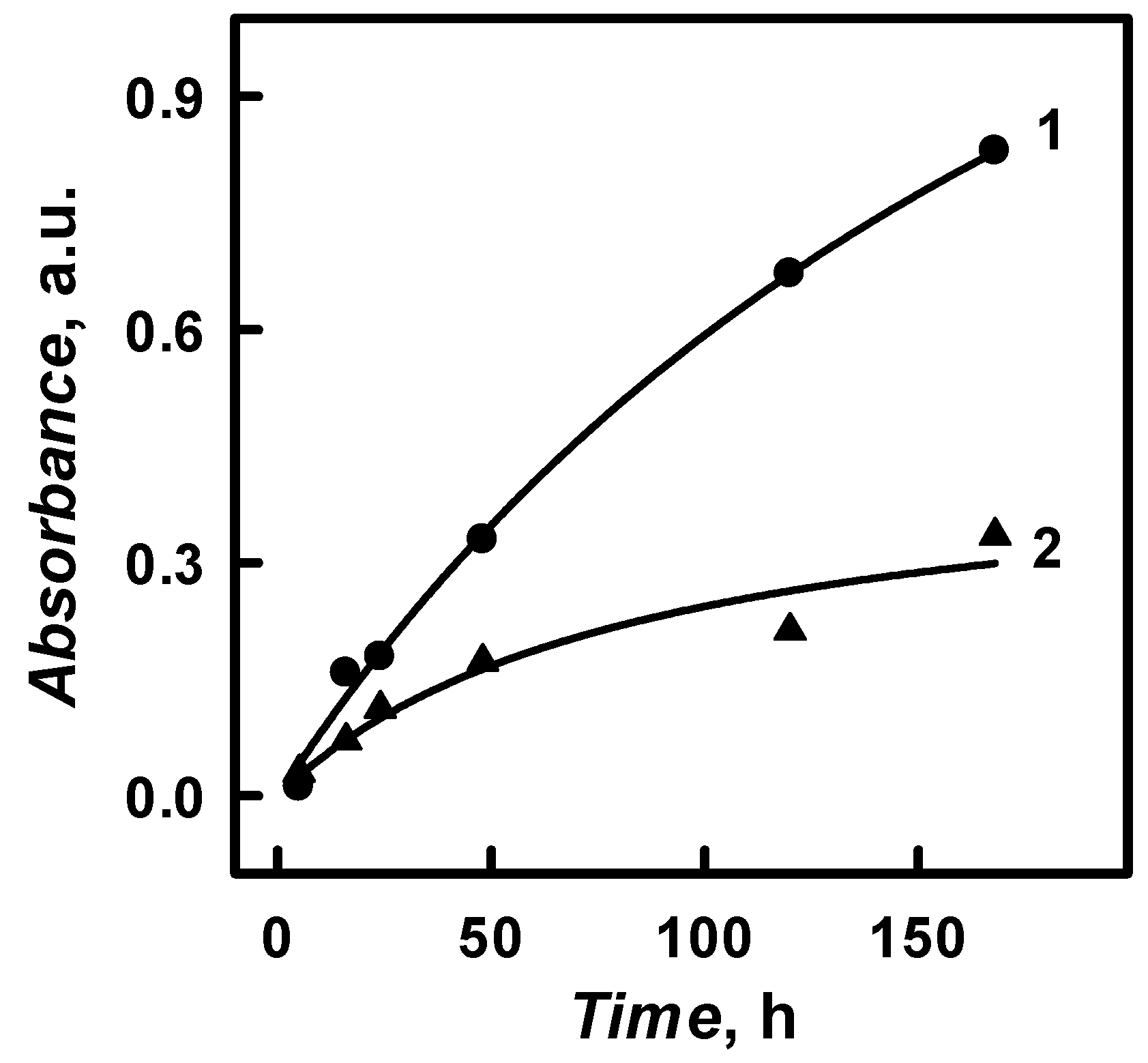
18]. The amount and the intensity of absorbance for the obtained PANI/GOx and Ppy/GOx composite nanoparticles at
λmax = 440 nm and
λmax = 460 nm increased by the prolongation of polymerization duration from 5 to 168 h (
Figure 8).
Such an effect indicated the formation of PANI/GOx and Ppy/GOx composite nanoparticles and it is in an agreement with some previous researches [
18,
24]. The maximal concentration of PANI/GOx and Ppy/GOx composite nanoparticle formation was achieved after 168 h. Optical absorbance after 168 h at
λmax = 440 nm and
λmax = 460 nm has increased by 74.7 and 11.1 times, respectively, in a comparison with the absorbance that was registered after 5 h (
Figure 8). After 168 h lasting formation of Ppy/GOx composite nanoparticles the optical absorbance at
λmax = 460 nm was 2.48 times lower than that of PANI/GOx at
λmax = 440 nm after the same duration of polymerization. It was determined that the absorbance of PANI/GOx and Ppy/GOx composite nanoparticles has decreased during long-lasting polymerization (more than 168 h). The limited solubility of synthesized polymers and the precipitation of particles or clusters of PANI/GOx and Ppy/GOx from the polymerization solution could explain this phenomenon [
18]. When the formation of PANI/GOx and Ppy/GOx composites lasted longer than 168 h, the significant part of formed nanoparticles has precipitated. Though, optical absorbance at
λmax = 440 nm and
λmax = 460 nm of PANI/GOx and Ppy/GOx after 48 h lasting polymerization was 2.52 and 1.94 times lower than that registered after 168 h lasting polymerization. 48–96 h lasting polymerization seems to be more efficient for PANI/GOx and Ppy/GOx composite nanoparticles formation, because smaller PANI/GOx and Ppy/GOx will be formed.
The initial aniline and pyrrole conversion into polymer reaction rate (
V) was evaluated by formula
V = (tgα), where tgα was calculated from
Figure 8 by the evaluation of estimated/approximated absorbance of PANI/GOx and Ppy/GOx solutions (1.27 a.u. for PANI/GOx and 0.446 a.u. for Ppy/GOx) that formed when 100% of monomers, which were present in the solution, will be converted into polymer. The initial aniline and pyrrole polymerization rate was determined as 0.0037 and 0.0106 mol L
−1 h
−1, respectively. It is seen that the initial polymerization rate of aniline is 2.86 times slower than that of pyrrole.
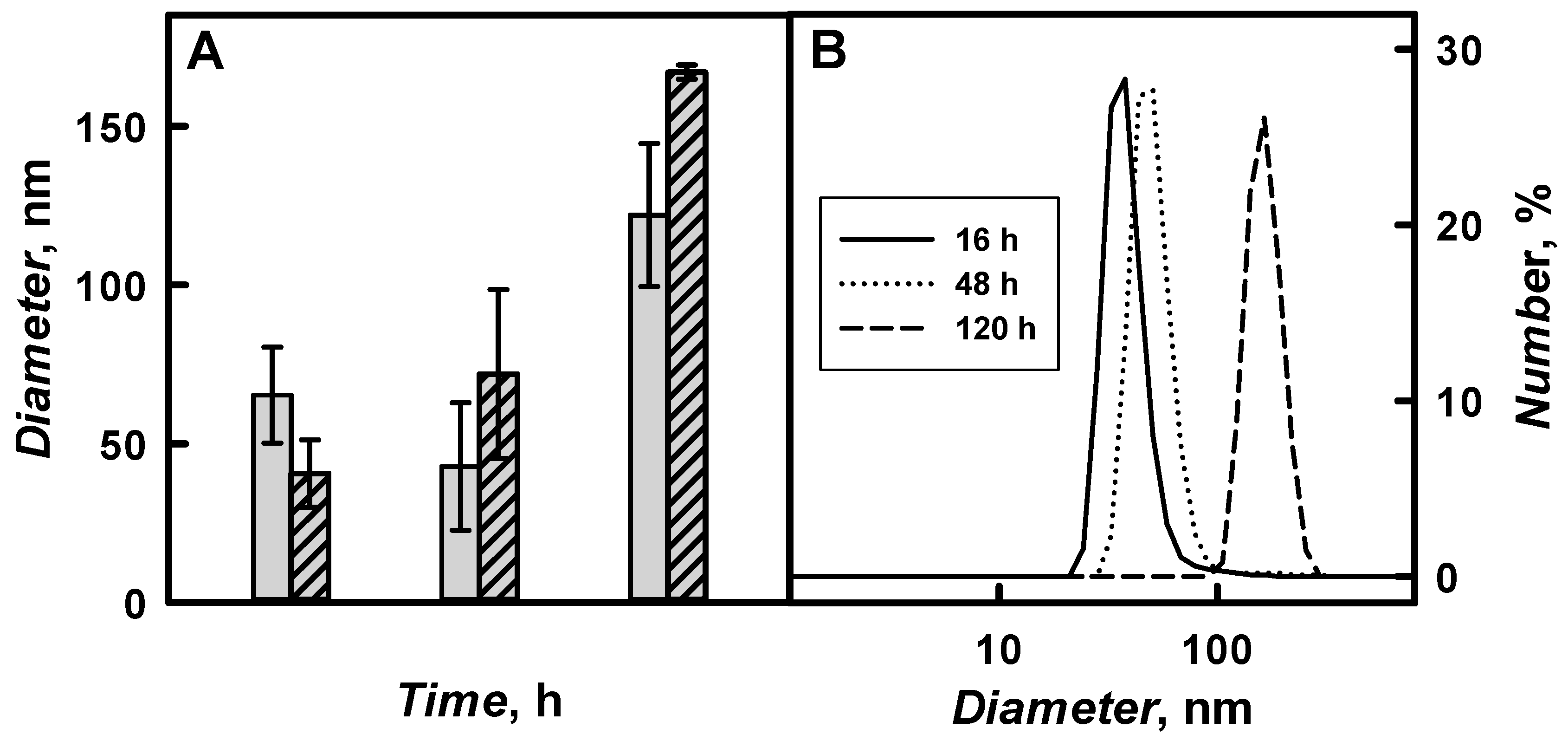
The diameter of PANI/GOx and Ppy/GOx composite nanoparticles and their aggregates was investigated by DLS and
Figure 9 and
Table 2 present the results. The diameter of polymeric nanoparticles increased by the increase of polymerization duration from 16 to 120 h. 41 nm PANI/GOx and 65 nm Ppy/GOx were formed after 16 h of enzymatic polymerization. The diameter of PANI/GOx and Ppy/GOx increased up to 167 and 122 nm, respectively, after 120 h of the polymerization.
The aggregates of PANI/GOx and Ppy/GOx composite nanoparticles were formed from the 120th hour of enzymatic polymer’s formation. During 168–288 h lasting polymerization, a lot of agglomerates that are characterized by large diameter were formed, which was in an agreement with our previous researches, where 142 nm PANI/GOx composite nanoparticles were formed after 168 h lasting polymerization [
21]. For the enzymatic synthesis of smaller polymeric nanoparticles, 48 h lasting polymerization is the most suitable, because, in such way, 72 nm PANI/GOx (
Figure 9B) and 43 nm Ppy/GOx composite nanoparticles were formed. Other authors have reported that, during enzymatic polymerization of aniline using chitosan and poly(
N-isopropylacrylamide) as steric stabilizers and horseradish peroxidase as enzyme, 50 nm PANI nanoparticles were synthesized [
11]. However, the PANI matrix doped by small anions is characterized by poor stability. Therefore, it is recommended to dope PANI by larger anions to solve this problem [
28].
The electrical charge of particles is usually expressed by zeta-potential. The zeta-potential was determined in SA buffer, pH 6.0, for polymeric nanoparticles formed by 48 h lasting polymerization in our investigations. The zeta-potentials of PANI/GOx, Ppy/GOx, and PTh/GOx nanoparticles were −6.2, −9.4, and −8.6 mV, respectively.