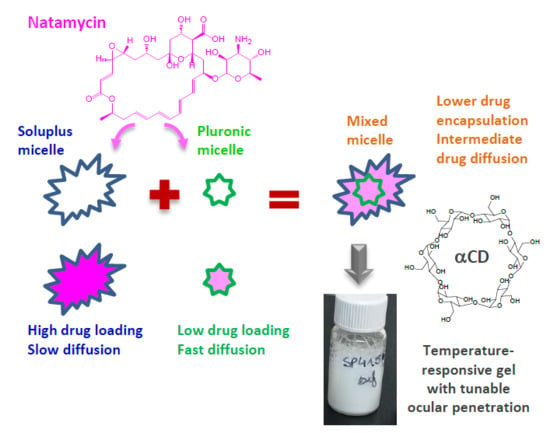
Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
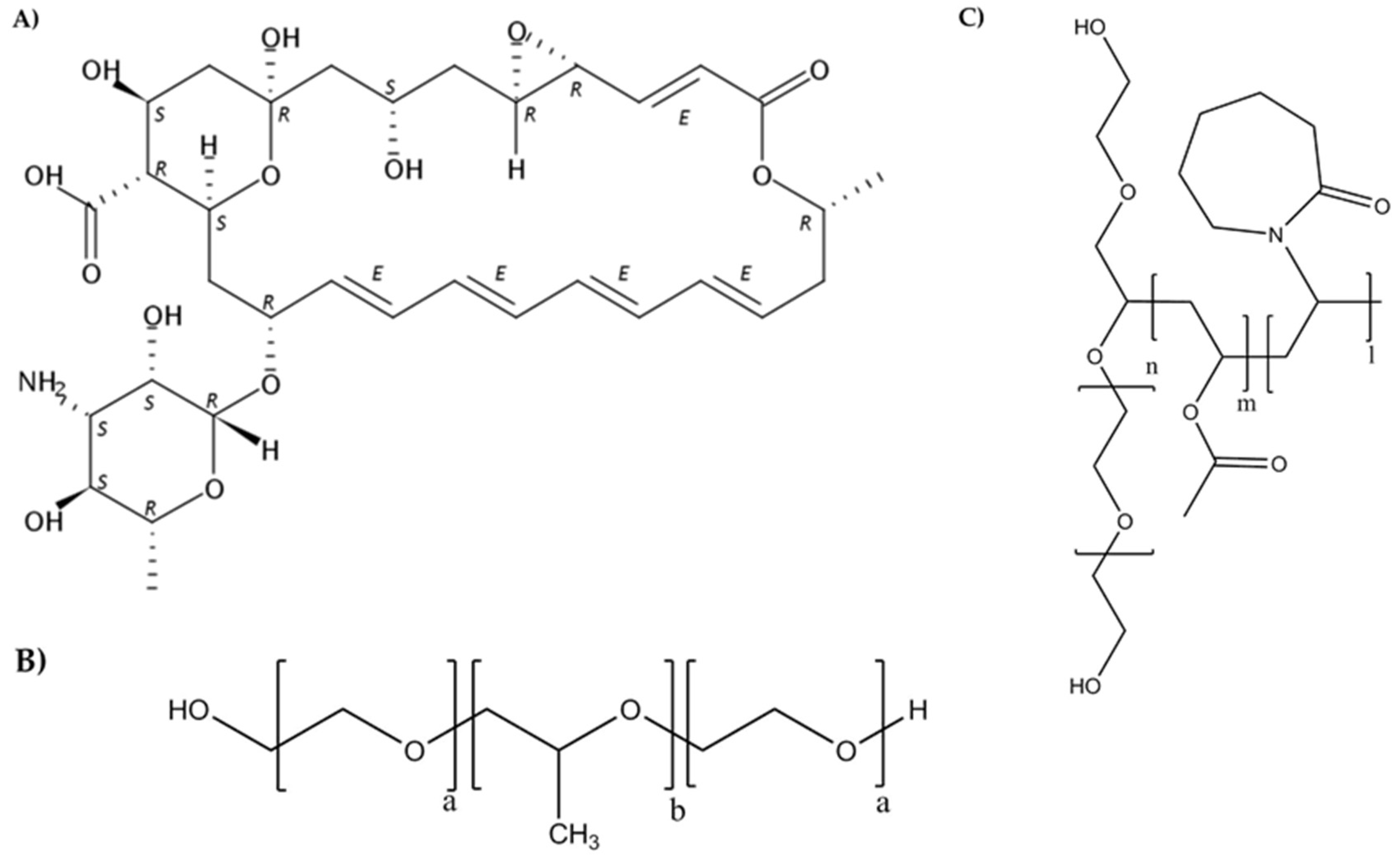
2.2. Micelles Preparation and Characterization
2.3. Solubility of Natamycin in Micelle Dispersions
2.4. Solubility of Natamycin in αCD
2.5. Preparation of Poly(pseudo)rotaxanes
2.6. Rheological Characterization
2.7. Diffusion Assays
2.8. Ocular Tolerance Test (HET-CAM Test)
2.9. Ex-Vivo Corneal and Sclera Permeability Study
2.10. Statistical Analysis
3. Results and Discussion
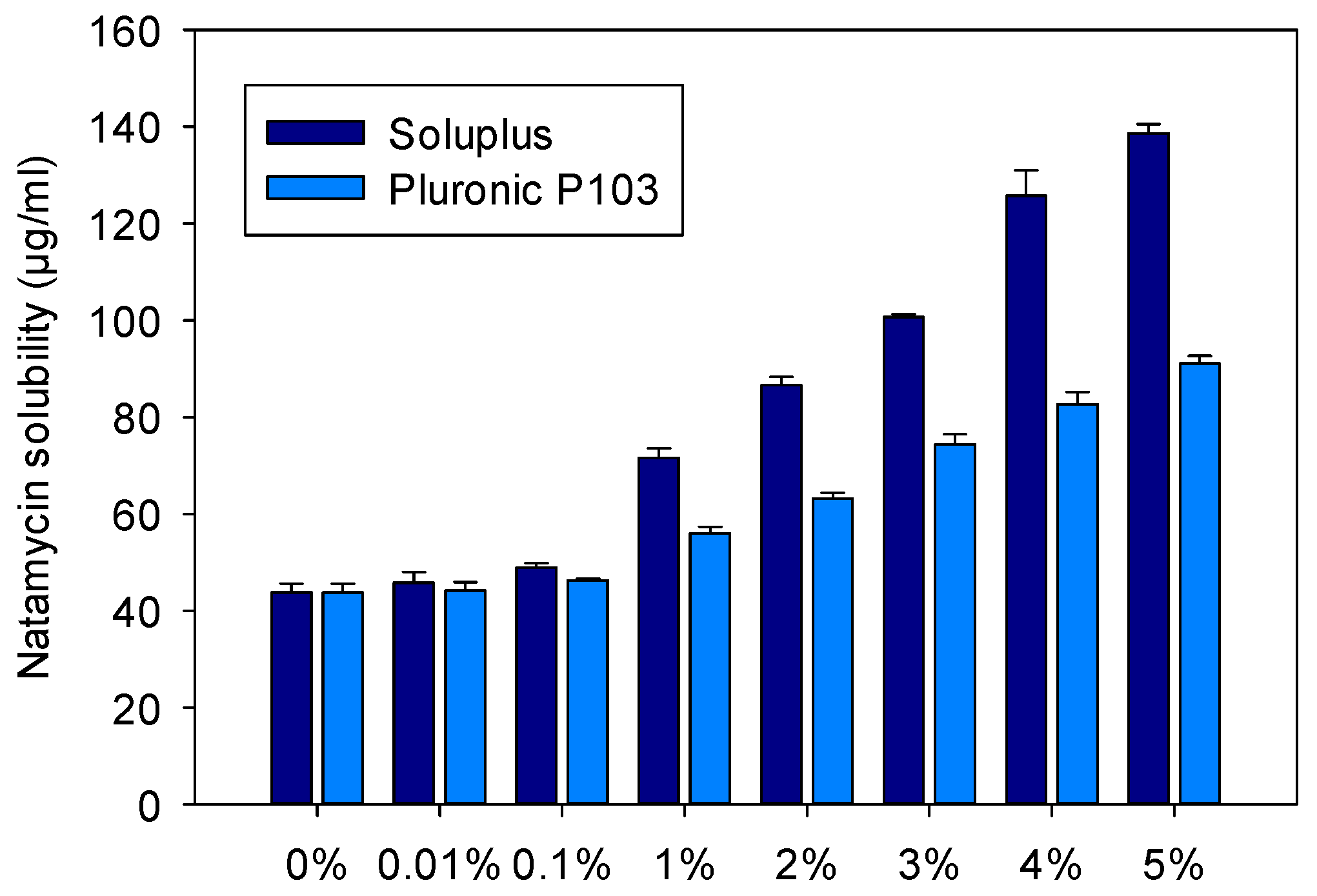
3.1. Micelles Preparation and Natamycin Solubilization
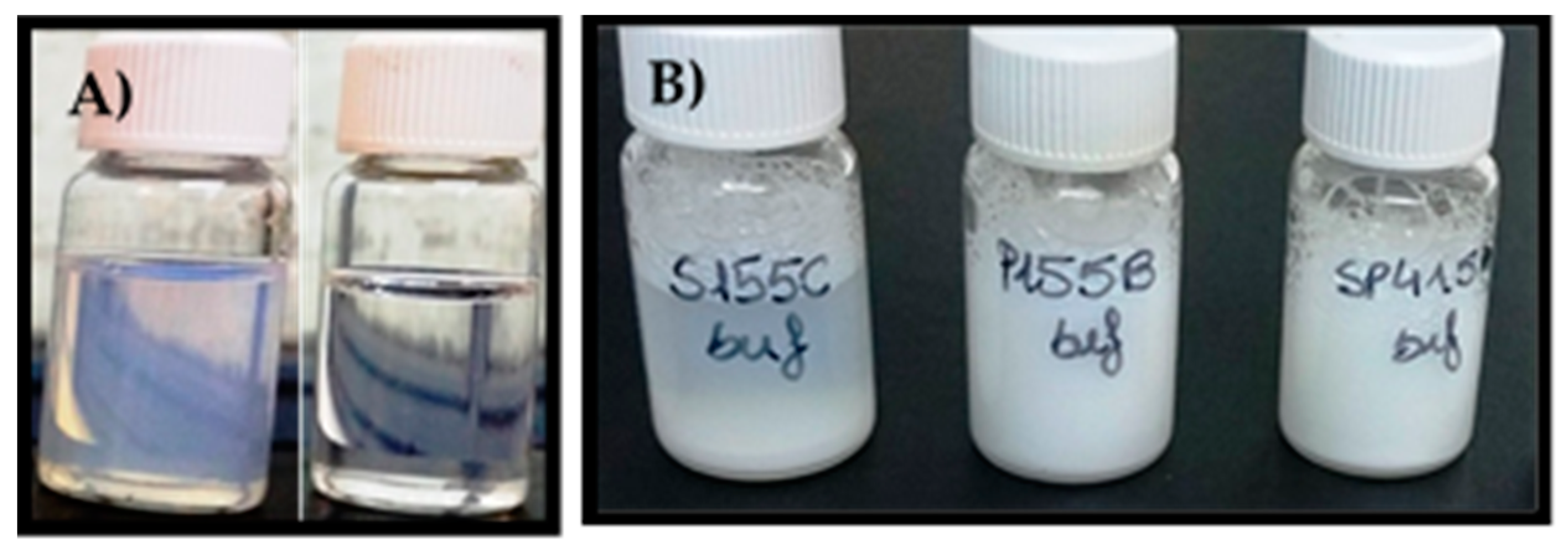
3.2. Poly(pseudo)rotaxane Formation
3.3. HET-CAM Assay
3.4. Natamycin Diffusion
3.5. Ex Vivo Permeation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, H.Y.; Chodosh, J. Diagnostic and therapeutic considerations in fungal keratitis. Int. Ophthalmol. Clin. 2011, 51, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Masoomi, A.; Ahmadikia, K.; Tabatabaei, S.A.; Soleimani, M.; Rezaie, S.; Ghahvechian, H.; Banafsheafshan, A. Fungal keratitis: An overview of clinical and laboratory aspects. Mycoses 2018, 61, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Dart, J.K.; Stapleton, F.; Minassian, D. Contact lenses and other risk factors in microbial keratitis. Lancet 1991, 338, 650–653. [Google Scholar] [CrossRef]
- Green, M.; Apel, A.; Stapleton, F. Risk factors and causative organisms in microbial keratitis. Cornea 2008, 27, 22–27. [Google Scholar] [CrossRef]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the management of infectious keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef]
- Qiu, S.; Zhao, G.Q.; Lin, J.; Wang, X.; Hu, L.T.; Du, Z.D.; Wang, Q.; Zhu, C.C. Natamycin in the treatment of fungal keratitis: A systematic review and Meta-analysis. Int. J. Ophthalmol. 2015, 8, 597–602. [Google Scholar] [CrossRef]
- Patil, A.; Lakhani, P.; Majumdar, S. Current perspectives on natamycin in ocular fungal infections. J. Drug Deliv. Sci. Technol. 2017, 41, 206–212. [Google Scholar] [CrossRef]
- Arora, R.; Gupta, D.; Goyal, J.; Kaur, R. Voriconazole versus natamycin as primary treatment in fungal corneal ulcers. Clin. Exp. Ophthalmol. 2011, 39, 434–440. [Google Scholar] [CrossRef]
- O’Day, D.M.; Head, W.S.; Robinson, R.D.; Clanton, J.A. Corneal penetration of topical amphotericin B and natamycin. Curr. Eye Res. 1986, 5, 877–882. [Google Scholar] [CrossRef]
- Segura, T.; Puga, A.M.; Burillo, G.; Llovo, J.; Brackman, G.; Coenye, T.; Concheiro, A.; Alvarez-Lorenzo, C. Materials with fungi-bioinspired surface for efficient binding and fungi-sensitive release of antifungal agents. Biomacromolecules 2014, 15, 1860–1870. [Google Scholar] [CrossRef]
- Cevher, E.; Sensoy, D.; Zloh, M.; Mulazimoglu, L. Preparation and characterisation of natamycin: Gamma-cyclodextrin inclusion complex and its evaluation in vaginal mucoadhesive formulations. J. Pharm. Sci. 2008, 97, 4319–4335. [Google Scholar] [CrossRef]
- Saeed Nihad, A.H.; Salami, M. Study of storage conditions effect (light-heat) on natamycin content and stability in some dairy products (cheese-yoghurt). Clin. Pharmacol. Biopharm. 2017, 6, 177. [Google Scholar] [CrossRef]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Chandasana, H.; Prasad, Y.D.; Chhonker, Y.S.; Chaitanya, T.K.; Mishra, N.N.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Corneal targeted nanoparticles for sustained natamycin delivery and their PK/PD indices: An approach to reduce dose and dosing frequency. Int. J. Pharm. 2014, 477, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Lakhani, P.; Taskar, P.; Wu, K.W.; Sweeney, C.; Avula, B.; Wang, Y.H.; Khan, I.A.; Majumdar, S. Formulation development, optimization, and in vitro-in vivo characterization of natamycin-loaded PEGylated nano-lipid carriers for ocular applications. J. Pharm. Sci. 2018, 107, 2160–2171. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 1–12. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.N.; Jones, L. In vitro uptake and release of natamycin from conventional and silicone hydrogel contact lens materials. Eye Contact Lens 2013, 39, 162–168. [Google Scholar] [CrossRef]
- Koontz, J.L.; Marcy, J.E. Formation of natamycin:cyclodextrin inclusion complexes and their characterization. J. Agric. Food Chem. 2003, 51, 7106–7110. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.N.; Jones, L. In vitro drug release of natamycin from beta-cyclodextrin and 2-hydroxypropyl-beta-cyclodextrin-functionalized contact lens materials. J. Biomater. Sci. Polym. Ed. 2014, 25, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Stefansson, E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int. J. Pharm. 2017, 531, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Gan, H.X.; Wang, H.; Tan, S.J.E.; Neoh, K.Y.; Tan, S.S.J.; Diong, H.F.; Kim, J.J.; Lee, W.L.S.; Fang, X.; et al. New thermogelling poly(ether carbonate urethane)s based on Pluronics F127 and poly(polytetrahydrofuran carbonate). J. Appl. Polym. Sci. 2014, 131, 39924. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Fernandez-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. alpha-Lipoic acid in Soluplus((R)) polymeric nanomicelles for ocular treatment of diabetes-associated corneal diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef]
- Simoes, S.M.; Figueiras, A.R.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert Opin. Drug Deliv. 2015, 12, 297–318. [Google Scholar] [CrossRef]
- Dey, S.; Patel, J.; Anand, B.S.; Jain-Vakkalagadda, B.; Kaliki, P.; Pal, D.; Ganapathy, V.; Mitra, A.K. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2909–2918. [Google Scholar] [CrossRef]
- Marcos, X.; Perez-Casas, S.; Llovo, J.; Concheiro, A.; Alvarez-Lorenzo, C. Poloxamer-hydroxyethyl cellulose-alpha-cyclodextrin supramolecular gels for sustained release of griseofulvin. Int. J. Pharm. 2016, 500, 11–19. [Google Scholar] [CrossRef]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 2406. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, B.; Xue, L.; San, W. Soluplus((R)) micelles as a potential drug delivery system for reversal of resistant tumor. Biomed. Pharmacother. 2015, 69, 388–395. [Google Scholar] [CrossRef]
- Takahashi, C.; Saito, S.; Suda, A.; Ogawa, N.; Kawashima, Y.; Yamamoto, H. Antibacterial activities of polymeric poly(dl-lactide-co-glycolide) nanoparticles and Soluplus® micelles against Staphylococcus epidermidis biofilm and their characterization. RSC Adv. 2015, 5, 71709–71717. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Cui, C.C.; Wei, F.; Lv, H.X. Improved solubility and oral bioavailability of apigenin via Soluplus/Pluronic F127 binary mixed micelles system. Drug Dev. Ind. Pharm. 2017, 43, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.C.; Zhang, Z.H.; Wu, H.; Jia, X.B.; Wang, Y.J.E. Optimization and evaluation of Oridonin-loaded Soluplus (R)-Pluronic P105 mixed micelles for oral administration. Int. J. Pharm. 2017, 518, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Taveira, S.F.; Varela-Garcia, A.; Dos Santos Souza, B.; Marreto, R.N.; Martin-Pastor, M.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclodextrin-based poly(pseudo)rotaxanes for transdermal delivery of carvedilol. Carbohydr. Polym. 2018, 200, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Puig-Rigall, J.; Serra-Gomez, R.; Stead, I.; Grillo, I.; Dreiss, C.A.; Gonzalez-Gaitano, G. Pseudo-polyrotaxanes of cyclodextrins with direct and reverse x-shaped block copolymers: A kinetic and structural study. Macromolecules 2019, 52, 1458–1468. [Google Scholar] [CrossRef]
- Simoes, S.M.N.; Veiga, F.; Torres-Labandeira, J.J.; Ribeiro, A.C.F.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable self-assembled cyclodextrin gels for drug delivery. Curr. Top. Med. Chem. 2014, 14, 494–509. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus®—TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Kadama, Y.; Bharatiya, B.; Hassan, P.A.; Verma, G.; Aswal, V.K.; Bahadur, P. Effect of an amphiphilic diol (Surfynol®) on the micellar characteristics of PEO–PPO–PEO block copolymers in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 110–118. [Google Scholar] [CrossRef]
- Díaz-Tomé, V.; Luaces-Rodríguez, A.; Silva-Rodríguez, J.; Blanco-Dorado, S.; García-Quintanilla, L.; Llovo-Taboada, J.; Blanco-Méndez, J.; García-Otero, X.; Varela-Fernández, R.; Herranz, M.; et al. Ophthalmic econazole hydrogels for the treatment of fungal keratitis. J. Pharm. Sci. 2018, 107, 1342–1351. [Google Scholar] [CrossRef]
- European Medicines Agency. Background Review for Cyclodextrins Used as Excipients. EMA/CHMP/333892/2013. Available online: https://www.ema.europa.eu/en/documents/report/background-review-cyclodextrins-used-excipients-context-revision-guideline-excipients-label-package_en.pdf (accessed on 1 April 2019).
- Segredo-Morales, E.; Martin-Pastor, M.; Salas, A.; Évora, C.; Concheiro, A.; Alvarez-Lorenzo, C.; Delgado, A. mobility of water and polymer species and rheological properties of supramolecular polypseudorotaxane gels suitable for bone regeneration. Bioconjug. Chem. 2018, 29, 503–516. [Google Scholar] [CrossRef]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop(R) and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef]
- Abdelkader, H.; Ismail, S.; Hussein, A.; Wu, Z.; Al-Kassas, R.; Alany, R.G. Conjunctival and corneal tolerability assessment of ocular naltrexone niosomes and their ingredients on the hen’s egg chorioallantoic membrane and excised bovine cornea models. Int. J. Pharm. 2012, 432, 1–10. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Gomez-Amoza, J.L.; Martinez-Pacheco, R.; Souto, C.; Concheiro, A. Microviscosity of hydroxypropylcellulose gels as a basis for prediction of drug diffusion rates. Int. J. Pharm. 1999, 180, 91–105. [Google Scholar] [CrossRef]
- Sun, C.Q.; Lalitha, P.; Prajna, N.V.; Karpagam, R.; Geetha, M.; O’Brien, K.S.; Oldenburg, C.E.; Ray, K.J.; McLeod, S.D.; Acharya, N.R.; et al. Association between in vitro susceptibility to natamycin and voriconazole and clinical outcomes in fungal keratitis. Ophthalmology 2014, 121, 1495–1500. [Google Scholar] [CrossRef]
- Pescina, S.; Carra, F.; Padula, C.; Santi, P.; Nicoli, S. Effect of pH and penetration enhancers on cysteamine stability and trans-corneal transport. Eur. J. Pharm. Biopharm. 2016, 107, 171–179. [Google Scholar] [CrossRef]
- Siefert, B.; Keipert, S. Influence of alpha-cyclodextrin and hydroxyalkylated beta-cyclodextrin derivatives on the in vitro corneal uptake and permeation of aqueous pilocarpine-HCl solutions. J. Pharm. Sci. 1997, 86, 716–720. [Google Scholar] [CrossRef]
- PubChem. Natacyn. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/natamycin#section=Solubility (accessed on 1 April 2019).
- Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Karia, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Progr. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef]

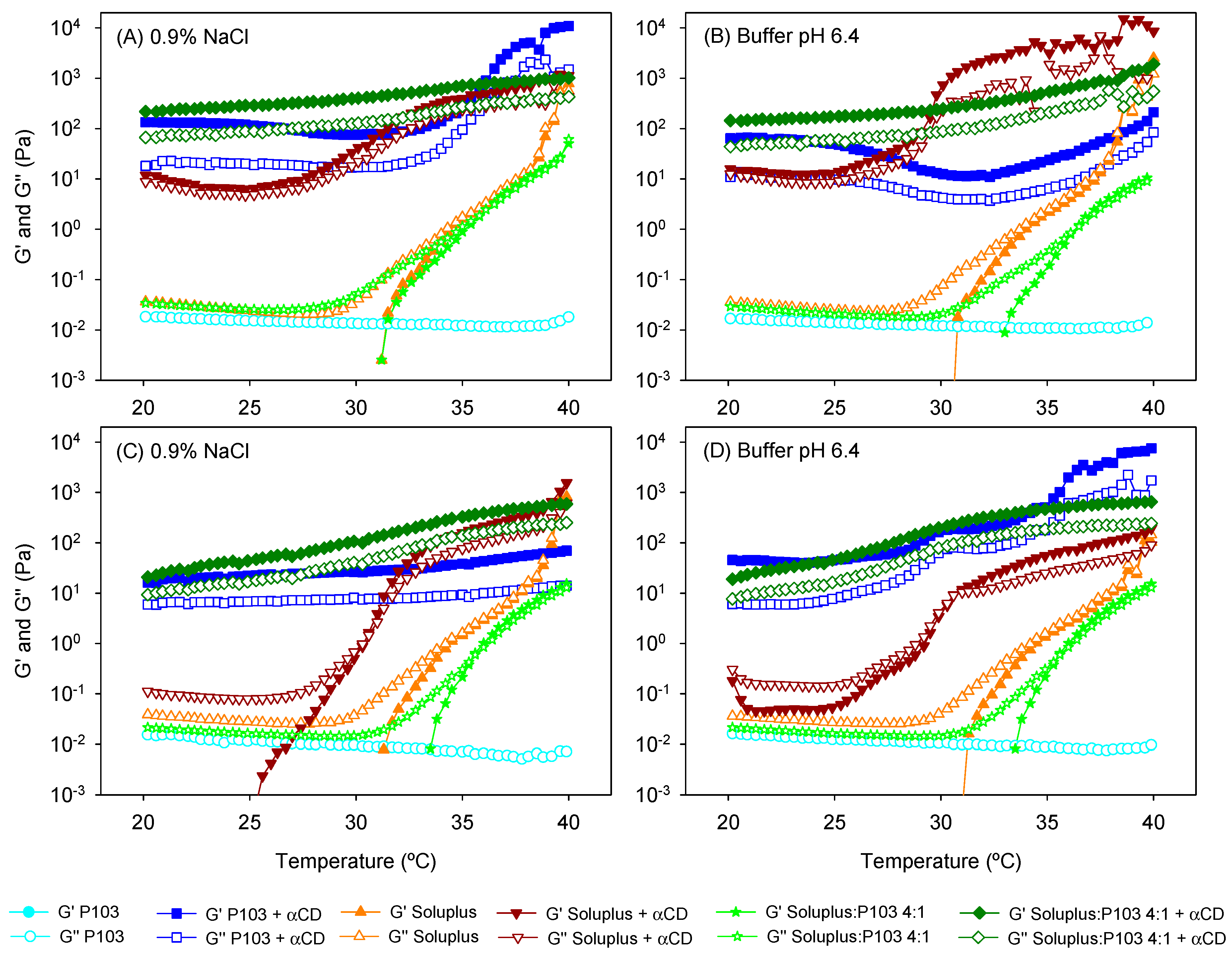
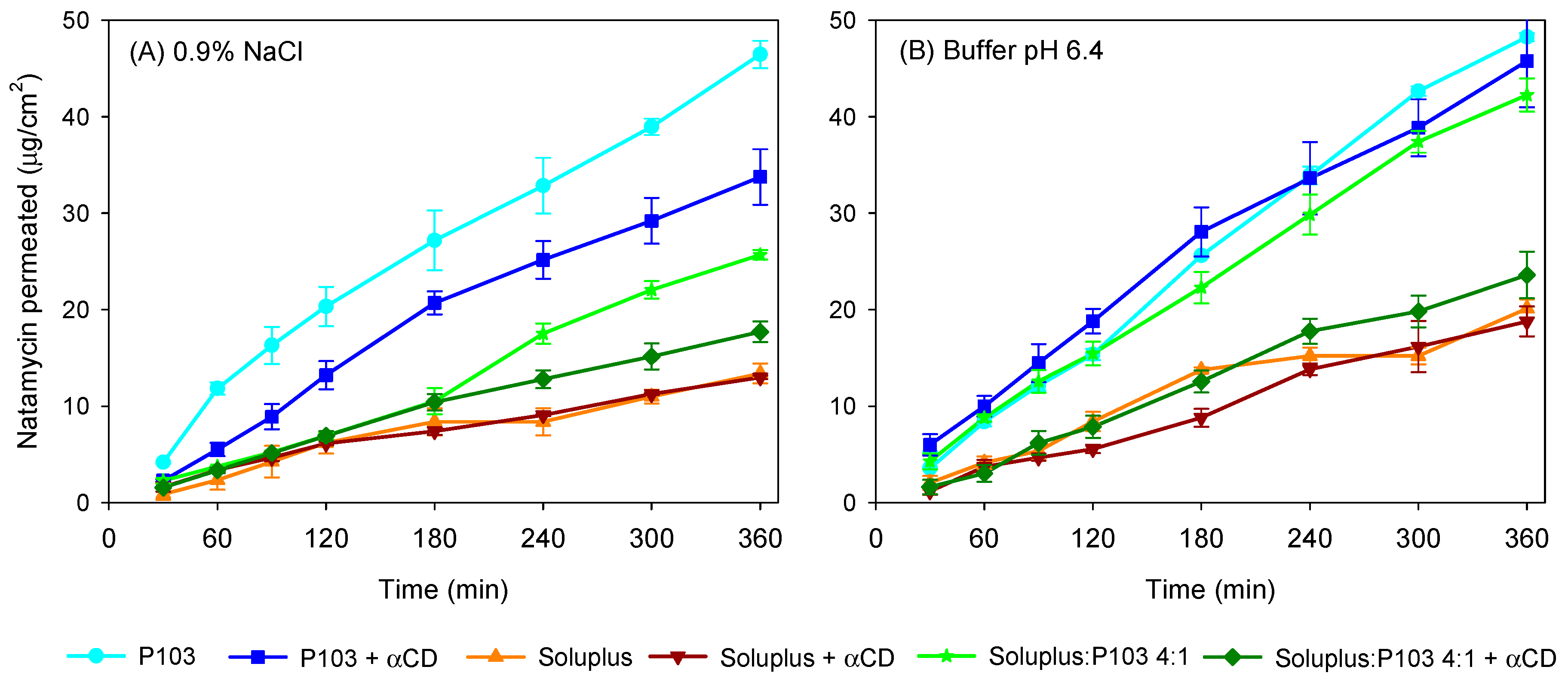
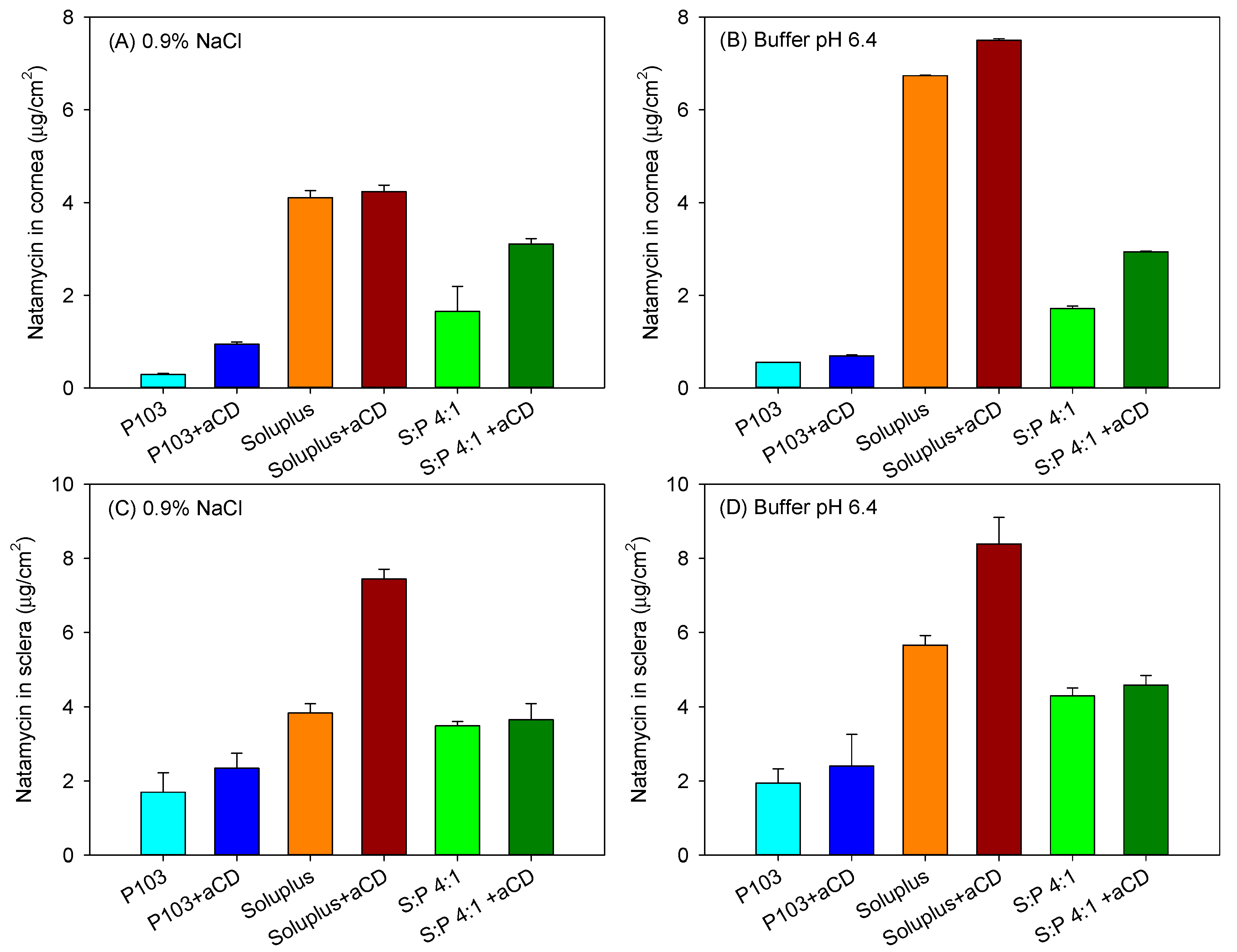
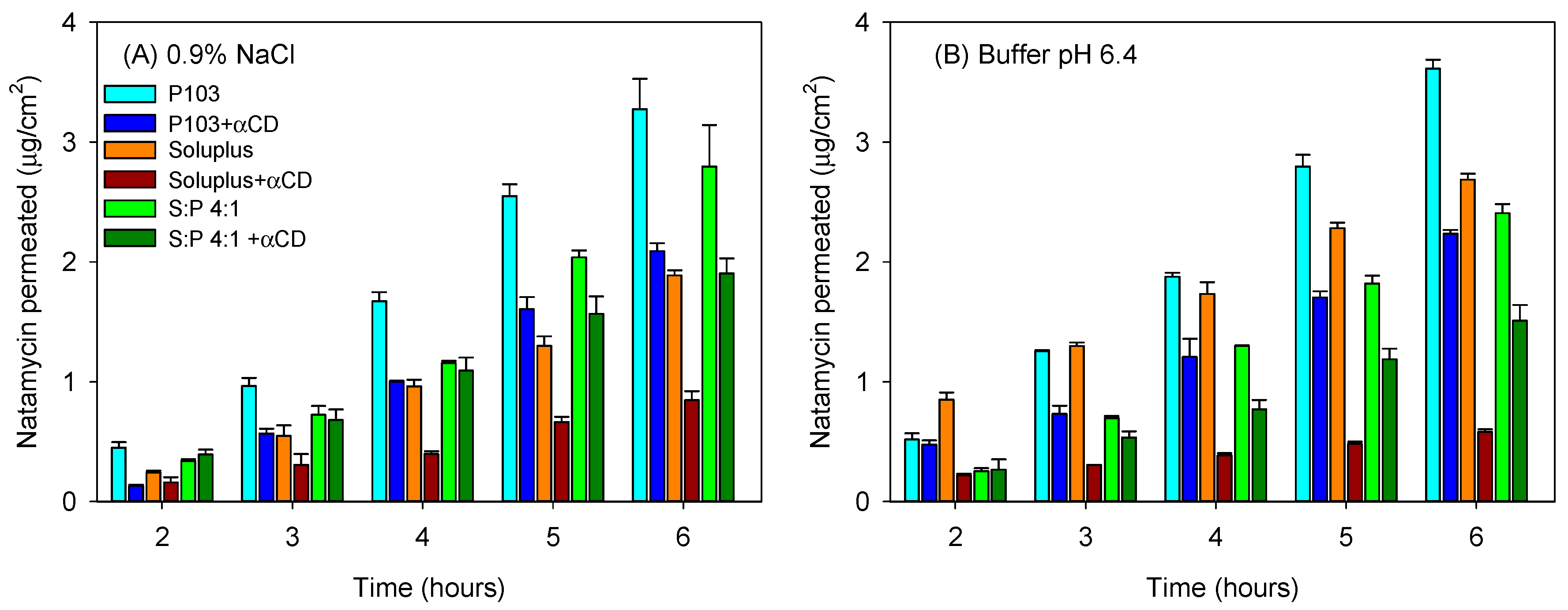
| 0.9% NaCl | ||||
| Copolymer (%w/v) | pH | Diameter (nm) | PDI | Zeta Potential (mV) |
| Soluplus (10%) | 3.34 | 90.0 ± 1.3 | 0.168 ± 0.010 | −0.40 ± 0.19 |
| Pluronic (10%) | 6.34 | 20.5 ± 0.6 | 0.238 ± 0.003 | 1.03 ± 0.47 |
| Soluplus/Pluronic P103 (1:4) | 4.67 | 129.6 ± 2.9 | 0.246 ± 0.019 | −0.66 ± 0.20 |
| Soluplus/Pluronic P103 (2:3) | 3.89 | 131.0 ± 3.0 | 0.214 ± 0.011 | −0.81 ± 0.08 |
| Soluplus/Pluronic P103 (3:2) | 3.70 | 121.7 ± 1.0 | 0.190 ± 0.017 | −1.49 ± 0.46 |
| Soluplus/Pluronic P103 (4:1) | 3.52 | 110.7 ± 1.8 | 0.209 ± 0.008 | −0.56 ± 0.41 |
| Buffer pH 6.4 | ||||
| Copolymer (%w/v) | pH | Diameter (nm) | PDI | Zeta Potential (mV) |
| Soluplus (10%) | 6.08 | 102.8 ± 1.0 | 0.189 ± 0.018 | −0.14 ± 0.36 |
| Pluronic (10%) | 6.36 | 16.1 ± 0.4 | 0.226 ± 0.018 | 1.15 ± 0.28 |
| Soluplus/Pluronic P103 (1:4) | 6.49 | 150.8 ± 4.5 | 0.217 ± 0.011 | 0.48 ± 0.06 |
| Soluplus/Pluronic P103 (2:3) | 6.47 | 140.5 ± 0.7 | 0.176 ± 0.008 | −0.02 ± 0.15 |
| Soluplus/Pluronic P103 (3:2) | 6.20 | 127.8 ± 0.9 | 0.171 ± 0.011 | −0.12 ± 0.01 |
| Soluplus/Pluronic P103 (4:1) | 6.48 | 114.7 ± 2.4 | 0.170 ± 0.011 | −0.18 ± 0.13 |
| Copolymer (% w/w) | Soluplus (M) | NAT (M) | NAT (µg/mL) | χ | P | PM | ΔG (KJ/mol) | mf |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.87 × 10−5 | 0.71 × 10−4 | 46.99 | 0.52 | 0.07 | 7869.6 | −22,227.0 | 0.06 |
| 1 | 8.70 × 10−5 | 1.04 × 10−4 | 69.31 | 0.44 | 0.58 | 6619.2 | −21,798.2 | 0.37 |
| 2 | 1.74 × 10−4 | 1.26 × 10−4 | 84.03 | 0.35 | 0.91 | 5233.1 | −21,216.1 | 0.48 |
| 3 | 2.61 × 10−4 | 1.47 × 10−4 | 97.93 | 0.31 | 1.23 | 4700.0 | −20,949.8 | 0.55 |
| 4 | 3.48 × 10−4 | 1.84 × 10−4 | 122.51 | 0.34 | 1.78 | 5131.2 | −21,167.3 | 0.64 |
| 5 | 4.35 × 10−4 | 2.03 × 10−4 | 135.23 | 0.31 | 2.07 | 4769.5 | −20,986.2 | 0.67 |
| 10 | 8.70 × 10−4 | 2.98 × 10−4 | 198.49 | 0.27 | 3.51 | 4038.0 | −20,573.7 | 0.78 |
| 10 (buffer) * | 8.70 × 10−4 | 3.96 × 10−4 | 263.73 | 0.38 | 4.99 | 5743.2 | −21,446.5 | 0.83 |
| Copolymer (% w/w) | Pluronic P103 (M) | NAT (M) | NAT (µg/mL) | χ | P | PM | ΔG (KJ/mol) | mf |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.20 × 10−3 | 0.70 × 10−4 | 46.27 | 5.59 × 10−2 | 0.05 | 845.5 | −16,699.6 | 0.05 |
| 1 | 2.02 × 10−3 | 0.84 × 10−4 | 56.00 | 9.59 × 10−3 | 0.27 | 145.1 | −12,332.5 | 0.21 |
| 2 | 4.04 × 10−3 | 0.95 × 10−4 | 63.21 | 7.40 × 10−3 | 0.44 | 112.0 | −11,690.4 | 0.30 |
| 3 | 6.06 × 10−3 | 1.12 × 10−4 | 74.37 | 7.71 × 10−3 | 0.69 | 116.6 | −11,790.1 | 0.41 |
| 4 | 8.08 × 10−3 | 1.24 × 10−4 | 82.63 | 7.31 × 10−3 | 0.88 | 110.6 | −11,659.3 | 0.47 |
| 5 | 1.01 × 10−2 | 1.37 × 10−4 | 91.06 | 7.10 × 10−3 | 1.07 | 107.4 | −11,586.3 | 0.52 |
| 10 | 2.02 × 10−2 | 2.16 × 10−4 | 143.49 | 7.45 × 10−3 | 2.27 | 112.7 | −11,706.4 | 0.69 |
| 10 (buffer) * | 2.02 × 10−2 | 2.15 × 10−4 | 143.39 | 7.44 × 10−3 | 2.26 | 112.6 | −11,704.0 | 0.69 |
| Formulation | 0.9% NaCl | Buffer pH 6.4 | ||
|---|---|---|---|---|
| D × 106 (cm2/min) | R2 | D × 106 (cm2/min) | R2 | |
| Pluronic P103 10% | 49.46 (3.99) | 0.992 | 65.14 (0.96) | 0.992 |
| Pluronic P103 + αCD 10% | 32.66 (4.73) | 0.990 | 50.30 (11.57) | 0.990 |
| Soluplus 10% | 5.56 (1.62) | 0.992 | 10.58 (0.53) | 0.982 |
| Soluplus 10% + αCD 10% | 3.72 (0.24) | 0.982 | 9.92 (2.38) | 0.992 |
| Soluplus/Pluronic P 103 (4:1) | 18.31 (1.24) | 0.998 | 45.68 (4.10) | 0.989 |
| Soluplus/Pluronic P 103 (4:1) + αCD 10% | 8.21 (1.22) | 0.970 | 16.14 (2.00) | 0.996 |
| Formulation | 0.9% NaCl | Buffer pH 6.4 | ||
|---|---|---|---|---|
| J (µg/(cm2·h)) | Papp × 106 (cm/s) | J (µg/(cm2·h)) | Papp × 106 (cm/s) | |
| Pluronic P103 10% | 0.724 (0.042) | 1.67 (0.09) | 0.774 (0.032) | 1.79 (0.07) |
| Pluronic P103 + αCD 10% | 0.496 (0.023) | 1.15 (0.05) | 0.449 (0.012) | 1.04 (0.03) |
| Soluplus 10% | 0.403 (0.006) | 0.93 (0.01) | 0.466 (0.024) | 1.08 (0.05) |
| Soluplus 10% + αCD 10% | 0.174 (0.018) | 0.40 (0.04) | 0.090 (0.008) | 0.27 (0.02) |
| Soluplus/Pluronic P 103 (4:1) | 0.623 (0.065) | 1.44 (0.15) | 0.543 (0.021) | 1.26 (0.05) |
| Soluplus/Pluronic P 103 (4:1) + αCD 10% | 0.391 (0.023) | 0.91 (0.05) | 0.313 (0.016) | 0.73 (0.04) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials 2019, 9, 745. https://doi.org/10.3390/nano9050745
Lorenzo-Veiga B, Sigurdsson HH, Loftsson T, Alvarez-Lorenzo C. Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials. 2019; 9(5):745. https://doi.org/10.3390/nano9050745
Chicago/Turabian StyleLorenzo-Veiga, Blanca, Hakon Hrafn Sigurdsson, Thorsteinn Loftsson, and Carmen Alvarez-Lorenzo. 2019. "Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin" Nanomaterials 9, no. 5: 745. https://doi.org/10.3390/nano9050745
APA StyleLorenzo-Veiga, B., Sigurdsson, H. H., Loftsson, T., & Alvarez-Lorenzo, C. (2019). Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials, 9(5), 745. https://doi.org/10.3390/nano9050745